Abstract
Gene expression of reproductive system of the black tiger shrimp (Peneaus monodon) has been widely studied to address poor maturation problem in captivity. However, a systematic evaluation of reference genes in quantitative real-time PCR (qPCR) for P. monodon reproductive organs is lacking. In this study, the stability of four potential reference genes (18s rRNA, GAPDH, β-actin, and EF1-α) was examined in the reproductive tissues in various conditions using bioinformatic tools: NormFinder and geNorm. For NormFinder, EF1-α and GAPDH ranked first and second as the most stable genes in testis groups whereas GAPDH and EF1-α were for ovaries from wild-caught broodstock and domesticated groups. EF1-α and β-actin ranked first and second for the eyestalk ablated ovaries. For geNorm, EF1-α and GAPDH had the best stability in all testis and ovaries from domesticated groups whereas EF1-α and β-actin were the best for ovaries from wild-caught and eyestalk ablated groups. Moreover, the expression levels of two well-known reproductive genes, Dmc1 and Vitellogenin, were used to validate these reference genes. When normalized to EF1-α, the expected expression patterns were obtained in all cases. Therefore, this work suggests that EF1-α is more versatile as reference genes in qPCR analysis for reproductive system in P. monodon.
Introduction
Quantitative real-time polymerase chain reaction (qPCR) is a useful technique to measure gene expression levels due to its high sensitivity, accuracy, and reproducibility. To employ qPCR for gene expression analysis, housekeeping genes are used as internal control to normalize expression levels of other genes of interest. Therefore, it is important to select a reference gene whose expression level is constitutive and constant under different experimental conditions or biological samples for a particular study.
Several internal control genes have been validated for qPCR in different experimental conditions in many organisms such as human tissues [1], [2], [3], [4], [5], Pimephales promelas [6], Oryzias latipes [7], Solea senegalensis and Hippoglossus hippoglossus [8], Danio rerio [9], rice Oryza sativa L. ssp. Indica var. IR64 [10], soybean Glycine max [L.] Merr. [11], and Leptospira [12]. In the Pacific blue shrimp Penaeus stylirostris, elongation factor 1 alpha (EF-1α) and glyceraldehydes-3 phosphate dehygrogenase (GAPDH) have been validated as reference genes for expression analysis of immune genes [13]. In the black tiger shrimp (P. monodon), several housekeeping genes, such as β-actin [14], [15], [16], EF-1α [17], [18], [19], [20], GAPDH [19], 40S rRNA [21], 18S rRNA [16], [22], and elongation factor 2 [23] have been used as an internal control for qPCR. However, to date, no study has validated their suitability as an internal control for gene expression analysis using qPCR in P. monodon.
Recently, the reproductive system of both male and female P. monodon has been extensively studied because poor reproductive maturation in captivity presents a serious threat to the shrimp farming industries. Although several studies employed qPCR to examine gene expression profiles during reproductive maturation [18], [20], [24], [25], [26], [27], [28], the gene expression studies for the reproductive system of this organism can be inaccurate without using appropriate internal control genes. In this study, we validated four commonly used reference genes (18S rRNA, GAPDH, β-actin, and EF-1α) to be used as an internal control in qPCR analysis of reproductive samples with various conditions. Gene expression levels of these four genes in three ovary sample groups (wild-caught broodstock, domesticated shrimp, and eyestalk ablated broodstock) and two testis sample groups (wild-caught broodstock and domesticated shrimp) were measured by qPCR and two computational analysis tools (geNorm and NormFinder) were used to compare expression stability of the four candidate reference genes. Moreover, relative expression levels of two reproductive genes, Dmc1 for testicular development [25] and Vitellogenin for ovarian maturation [29], [30], were also measured using the four candidate reference genes for normalization.
Results and Discussion
Expression Levels of Housekeeping Genes in Reproductive Organ of Penaeus monodon by Quantitative Real-time PCR (qPCR)
Due to its accuracy, sensitivity, fast speed and reproducibility, quantitative real-time PCR (qPCR) has become a useful method for gene expression analysis. However, its accuracy relies upon a good reference gene whose expression levels should remain stable across tissues and different environmental conditions. Nevertheless, there is no an ultimate gene to be used as an internal control for all cell types or all experimental conditions. For gene expression analysis of the reproductive system in the black tiger shrimp Peneaus monodon, samples differ between individuals, tissues, growth stages and developmental stages; yet no previous study has examined the most appropriate genes to be used as an internal control gene.
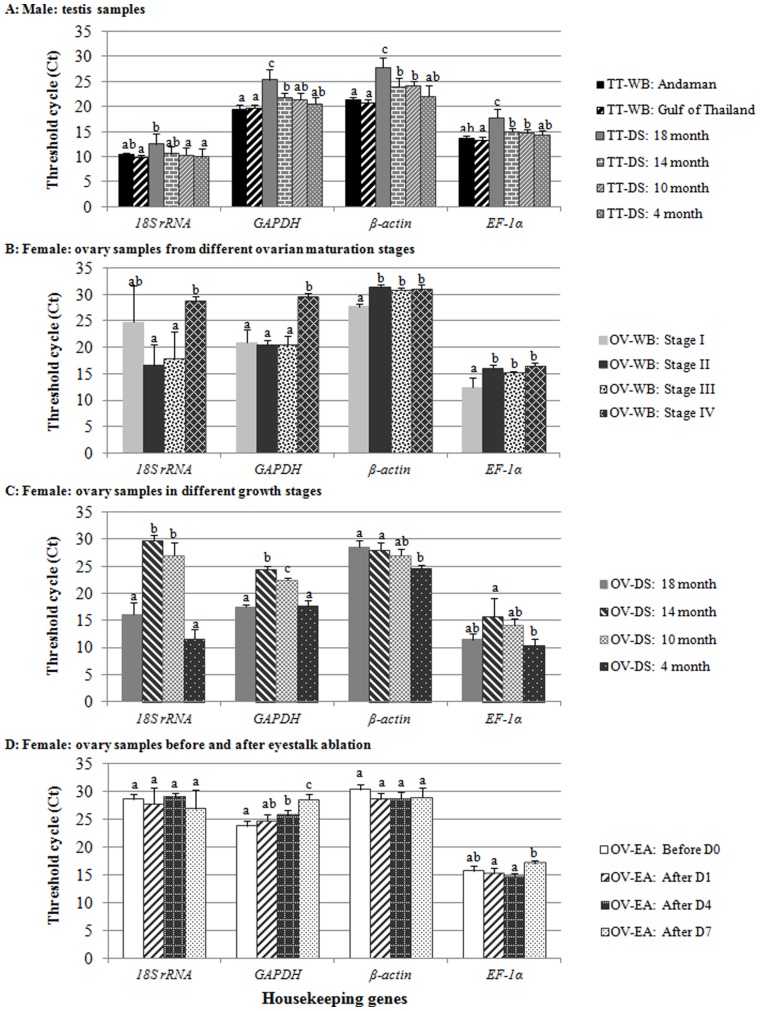
In this study, four commonly used housekeeping genes (18S rRNA, GAPDH, β-actin, and EF-1α) in qPCR gene expression analysis were validated for their suitability as a reference gene for reproductive organs of P. monodon. Male samples tested in this study were testes (TT) of wild brooders (WB) from Andaman Sea and Gulf of Thailand and of domesticated shrimp (DS) at 4-, 10-, 14-, and 18-month-old). Female samples were ovaries (OV) from wild brooders (WB) with various degree of reproductive maturation (Stages I–IV), from domesticated shrimp (DS) at 4-, 10-, 14-, and 18-month-old, and from domesticated broodstock before and after eyestalk ablation (EA; an eyestalk ablation is a common practice to induce ovarian maturation) at day 1, 4, and 7 (Table 1). To determine expression profiles of these housekeeping genes in the shrimp reproductive system, threshold cycle (Ct) values of all sample groups were measured (Fig. 1).
Table 1. Testis and ovary samples from P. monodon used in this study.
| No. | Samples | GSI* |
| Ovary samples | ||
| I | Wild broodstock (OV-WB) | |
| Stage I | n = 5; GSI = 0.69±0.27% | |
| Stage II | n = 5; GSI = 2.45±0.32% | |
| Stage III | n = 5; GSI = 4.80±1.12% | |
| Stage IV | n = 5; GSI = 11.43±1.29% | |
| II | Domesticated shrimp (OV-DS) | |
| 18-month-old domesticated shrimp (DS) | n = 7; GSI = 0.88±0.29% | |
| 14-month-old DS | n = 5; GSI = 1.04±0.29% | |
| 10-month-old DS | n = 5; GSI = 0.66±0.30% | |
| 4-month-old DS | n = 5; GSI = NA | |
| III | Eyestalk ablated 14-month-old domesticated broodstock (OV-EA) | |
| Before eyestalk ablation: Day 0 (D0) | n = 5; GSI* = 1.14±0.17% | |
| After eyestalk ablation: Day 1 (D1) | n = 5; GSI* = 1.22±0.14%, | |
| Day 4 (D4) | n = 5; GSI* = 1.31±0.33% | |
| Day 7 (D7) | n = 5; GSI* = 5.18±2.31% | |
| Testis samples | ||
| I | Wild broodstock (TT-WB) | |
| Andaman Sea (West) | n = 5; GSI = 1.14±0.26% | |
| Gulf of Thailand (East) | n = 5; GSI = 0.74±0.12% | |
| II | Domesticated shrimp (TT-DS) | |
| 18-month-old domesticated shrimp (DS) | n = 7; GSI = 0.53±0.21% | |
| 14-month-old DS | n = 5; GSI = 0.51±0.05% | |
| 10-month-old DS | n = 5; GSI = 0.69±0.25% | |
| 4-month-old DS | n = 5; GSI = NA | |
GSI is gonadosomatic index calculate as a percentage of testis weight by total body weight.
Figure 1. Threshold cycle values (Ct) of four housekeeping genes (18s rRNA, GAPDH, β-actin, and EF1-α) determined by qPCR from (A) testis samples of wild broodstock from different locations (Andaman sea and Gulf of Thailand) and domesticated shrimp with different growth stages (18-, 14-, 10-, and 4-month-old domesticated shrimp, DS), (B) ovary samples from wild broodstock with different ovarian maturation stages (Stages I–IV), (C) ovary samples of domesticated shrimp from different growth stages (18-, 14-, 10-, and 4-month-old domesticated shrimp, DS), and (D) ovary samples from 14-month-old domesticated broodstock before and after eyestalk ablation for 1, 4 and 7 days.
Different letters above the bars signify statistical differences.
In all testis sample groups, the expression patterns of four housekeeping genes were similar with the significantly higher Ct values found in 18-month-old domesticated broodstock (TT-DS: 18 month) than the other groups. GAPDH and β-actin genes showed similar expression levels ranging from 20–30 cycles, whereas 18S rRNA and EF-1α were expressed lower from 10–20 cycles (Fig. 1A).
In the female group, the ovary samples were categorized into three groups: different ovarian maturation stages, different growth stages, and before and after eyestalk ablation (Figs. 1B–1D). For different ovarian maturation stages, two distinct expression patterns were observed. The β-actin and EF-1α expression patterns showed the similar trend with the lowest levels in Stage I and became higher but at a constant level during Stages II–IV. In contrast, the expression profiles of 18S rRNA and GAPDH showed different patterns with higher variation in expression levels throughout different stages (Fig. 1B). For different growth stages, similar expression profiles of 18S rRNA, GAPDH, and EF-1α were observed but EF-1α has the lowest variation of expression levels among these three genes. Although β-actin had a distinct pattern from the rest, its expression levels throughout growth stages were more constant (Fig. 1C). In the case of ovaries from non-ablated and ablated broodstock, the expression profiles of all housekeeping genes were similar, except for that of GAPDH whose levels were significantly different after the eyestalk ablation for 7 days (Fig. 1D).
In addition, when the expression profiles (Ct) of the four housekeeping genes were compared in all sample groups, 18S rRNA and GAPDH showed high variation of the Ct values ranging from 10–30 cycles, while β-actin (Ct = 25–30 cycles) and EF-1α (Ct = 10–15 cycles) were expressed with less variation. Although GAPDH, an important gene encoding for a glycolytic pathway enzyme in carbohydrate metabolism, was frequently used as an internal control for qPCR analysis, it seems to be a good internal control only for lowly expressed genes [31]. Some studies showed GAPDH was unsuitable as an internal control due to its significant variation of expression levels between different individuals during pregnancy [32], with developmental stages [33], [34] and during the cell cycle of human cells [35], which agrees with our result when mRNA from different individuals and developmental stages were examined. For the case of 18S rRNA, this ribosomal subunit gene was previously used as internal control in the gene expression studies of rice with environmental stresses [36] and the fathead minnow fish with environmental estrogens exposure [37]. However, there are two main drawbacks that 18S rRNA cannot be used for normalization: (1) rRNA can be lost during mRNA purification, and (2) it is expressed at much greater levels than target mRNAs [38]. Perhaps, the biological functions of proteins encoded by GAPDH and 18S rRNA suggest that their transcript levels are significantly regulated by various experimental settings and variable in different tissues and thus unstable [39], [40].
Unlike GAPDH and 18S rRNA, the expression levels of EF-1α and β-actin were found to be more stable with lower variation in threshold cycles (Ct) in this study. Considering the Ct values, EF-1α is more suitable for normalization than β-actin because of its lower threshold cycle than that of β-actin. As a matter of fact, β-actin, encoding a cytoskeletal protein, was previously reported to have wide variation in its transcript levels in response to experimental manipulation in human breast epithelial cells [41], and blastomeres [42], as well as in various porcine tissues [43] and canine myocardium [44]. Its expression levels also varied in sample sets from embryonic, larval, and post-larval stages and gonad of the Kuruma shrimp [45]. In addition, the presence of β-actin pseudogenes can interfere with the interpretation of expression results as the same primer will detect both β-actin mRNA and DNA from this pseudogene [46]. For EF-1α, this transcriptional factor gene was employed as an internal control gene in gene expression studies of different tissues from the Atlantic salmon [47], [48], samples from different developmental conditions in the desert locust [49], and samples during larval development in the flatfish [50]. EF-1α was also the most suitable internal control for measuring the highly expressed genes [13].
Stability in Expression Levels of the Housekeeping Genes
To systematically examine the stability in expression levels of the four housekeeping genes, two computational methods were employed: NormFinder and geNorm. The Ct values of each gene in both testis and ovary samples were converted into copy numbers using their standard curves. The algorithms of both methods aim to identify genes whose expression levels are most stable by assigning the highest stability value for the maximum number of time points.
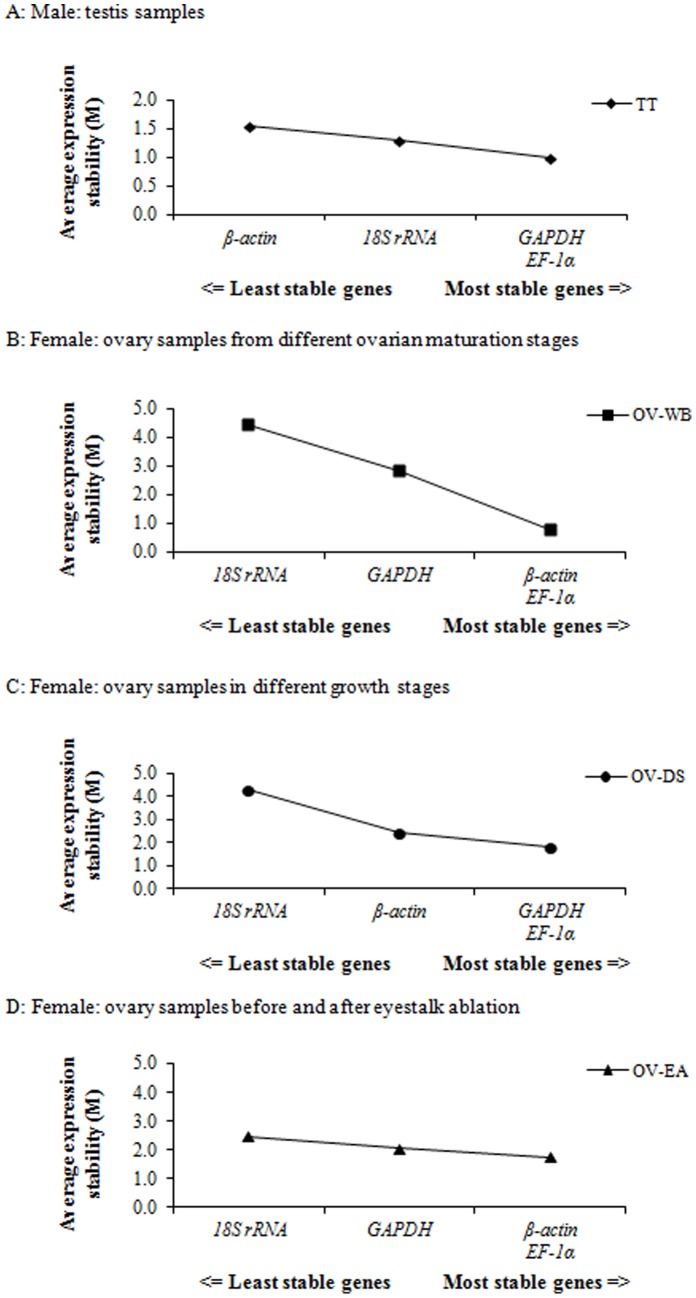
The first method, geNorm, was used to calculate an average expression stability values (M values) by averaging pair-wise variation of a particular gene across all examined reference genes. It allows the most appropriate reference gene to be chosen by using the geometric mean of the expression of the candidate cDNA [51]. However, the program provides the final result as the two most stable genes for a multivariate data set. As a result, the two most stable genes with the lowest M value were given. For testis samples (TT) and female domesticated shrimp with different growth stages (OV-DS), GAPDH and EF-1α genes had the lowest M values suggesting most stable expression levels (Fig. 2A and 2C), whereas β-actin and EF-1α genes were the most stable pair for OV-WB and OV-EA groups (Fig. 2B and 2D). Another method, NormFinder, was separately used to confirm the results from geNorm. Not only does it measure the variation of expression levels, but it also ranks potential reference genes by how much they differ between study groups; in another word, it measures the extent by which they are affected by the experimental conditions [52]. It estimates the expression variation among candidate genes using a model-based approach to calculate a stability value for each gene and identify the single best reference gene with the highest stability in expression level indicated by the lowest value. Stability values and ranking order of the candidate reference genes in a given sample group were shown in Table 2. In the testis samples (TT) and ovary samples during the eyestalk ablation (OV-EA), EF-1α has the best stability value, whereas GAPDH has the best value for female wild broodstock from different ovarian maturation stages (OV-WB) and domesticated shrimp from different growth stages (OV-DS). However, the GAPDH stability was lowest in samples from the eyestalk ablation experiment.
Figure 2. Average expression stability values (M), which is the mean pair-wise variation between an individual gene and all other tested genes, determined by geNorm software.
(A) Average M value of 46 testis samples in P. monodon (TT), (B) Average M value of ovary samples from wild broodstock with different ovarian maturation stages (OV-WB), (C) Average M value of ovary samples from domesticated shrimp with different growth stages (OV-DS) and (D) Average M value of ovary samples from domesticated broodstock before and after eyestalk-ablation (OV-EA) in P. monodon.
Table 2. Stability values and ranking order (in parentheses) of the candidate reference genes measured by the NormFinder software.
| Stability Values | ||||
| Genes | Male | Female | ||
| (TT) | OV-WB | OV-DS | OV-EA | |
| 18S rRNA | 1.05 (3) | 4.08 (4) | 3.97 (4) | 1.31 (3) |
| GAPDH | 0.34 (2) | 1.16 (1) | 0.79 (1) | 1.37 (4) |
| β-actin | 1.09 (4) | 2.19 (3) | 1.91 (3) | 1.07 (2) |
| EF-1α | 0.09 (1) | 1.63 (2) | 1.23 (2) | 0.55 (1) |
The genes with the highest stability values were hightlighted in each case. TT = testis samples, OV-WB = ovary samples from different ovarian maturation stages, OV-DS = ovary samples in different growth stages, and OV-EA = ovary samples before and after eyestalk ablation.
When compare between two methods, we found that EF-1α appeared to have most stable expression levels for testis samples (TT) and ovary samples during the eyestalk ablation (OV-EA). The only sample group from wild broodstock ovaries (OV-WB) and female domesticated shrimp with different growth stages (OV-DS) gave varied result. Although the most stable genes from NormFinder method found in OV-WB and OV-DS groups belonged to GAPDH, the second rank of stable genes belonged to EF-1α which correlated to the most stable genes from geNorm method. From geNorm method, EF-1α was only one gene found to be the most stable genes in all four sample groups (TT, OV-WB, OV-DS, and OV-ES). Moreover, EF-1α was in the first and second ranks of stable gene from NormFinder method, whereas GAPDH and β-actin ranked the forth (OV-EA) and the third (OV-WB and OV-DS) of stable gene, respectively. Therefore, the appropriate internal control for reproductive system of P. monodon seemed to be EF-1α due to its most stable expression levels across samples.
One caution to be considered is that both software algorithms rely upon an assumption that the expression of these reference genes should remain constant across the sample groups. However, this might not be the case in all conditions. Therefore, it is noteworthy to also consider other algorithms based on normalization software tools in this type of evaluation [53], [54].
Validation of Housekeeping Genes with Reproductive-relevant Genes in Reproductive System in P. monodon
To validate whether these housekeeping genes are suitable as internal controls for qPCR analysis of reproductive gene expression in P. monodon, they were used as a reference gene for expression analysis of two known reproductive-relevant genes (Dmc1 for testis and Vg for ovary) whose expression patterns were previously reported. The relative expression value and the absolute copy number were measured from standard curves of Dmc1 and Vg using these reference genes.
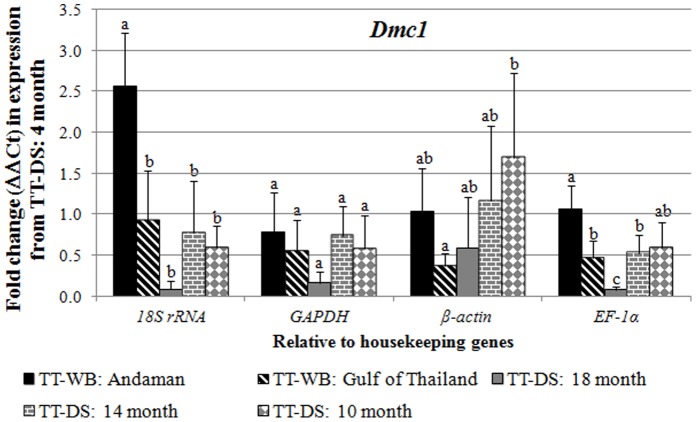
Dmc1, a RAC A-like recombinase, is known to be a specific factor for meiotic recombination and has been identified as a molecular marker for initial stages of meiosis because it was specifically expressed during the early meiotic prophase [55]. Moreover, Dmc1 is reportedly to be essential for meiosis as found in several species such as humans [56], mice [56], mouse [57], Japanese eel (Anguilla japonica) [58], whiteleg shrimp (Litopenaeus vannamei) [59], Caenorhabditis elegans, [60], rice (Oryza sativa L. ssp. japonica) [61], Arabidopsis thaliana [62], and yeast [63]. It was also used as a gene marker for particular purposes; for example, for spermatocyte-specific gene for study the role of sumoylation in vivo in mice and mouse [64], for comparing gene expression profile of TOPAZ1, which is potential marker for germ cell development [65], and for study of social status and gonadotropic signals on testis development in Nile tilapia (Oreochromis niloticus) [66]. Moreover, Dmc1 was also discovered in testis cDNA library of Crustacea, mitten crab (Eriocheir sinensis) [67], and P. monodon [26]. Furthermore, the expression levels of Dmc1 were correlated to testis maturation degrees in the P. monodon and it was also proposed to be an indicator for early stages of germ cell development in L. vannamei [25], [59]. Therefore, the Dmc1 expression level was normalized to each candidate genes to examine their suitability as an internal reference (Fig. 3). Using 18S rRNA, GAPDH, and EF-1α as reference genes, the Dmc1 exhibited similar profile as previously reported with the highest expression levels found in testis of wild broodstock from Andaman sea (TT-WB:Andaman) and the lowest found in testis of 18-month-old TT-DS while the rest of samples were expressed equally [25]. Although EF-1α was also used as a reference gene in the previous report and the both expression profiles of Dmc1 were similar, the samples used in both experiments were completely different demonstrating robustness of EF-1α as an internal reference. Moreover, when consider the fold change in expression of the samples relatively to that of 4-month-old TT-DS, statistical analysis indicated that the expression levels normalized to EF-1α gave significant differences between testis groups with lower variation than those normalized to 18S rRNA and GAPDH. On the other hand, the Dmc1 expression pattern normalized to β-actin exhibited a different pattern from the others (Fig. 3). Likewise, the expression pattern and significant level of the genes in copy number suggested the same results as in the fold change (Fig. S1).
Figure 3. Relative expression levels in term of fold change (ΔΔCt) of a known testis-relevant marker, Dmc1, to those of housekeeping genes in 5 testis sample groups; wild broodstock from Andaman sea (black), wild broodstock from Gulf of Thailand (diagonal lines), 18-month-old domesticated shrimp (DS) (gray), 14-month-old DS (horizontal lines), and 10-month-old DS (diamond), were compared with four housekeeping genes (18s rRNA, GAPDH, β-actin, and EF1-α) in 4-month-old domesticated shrimp.
Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
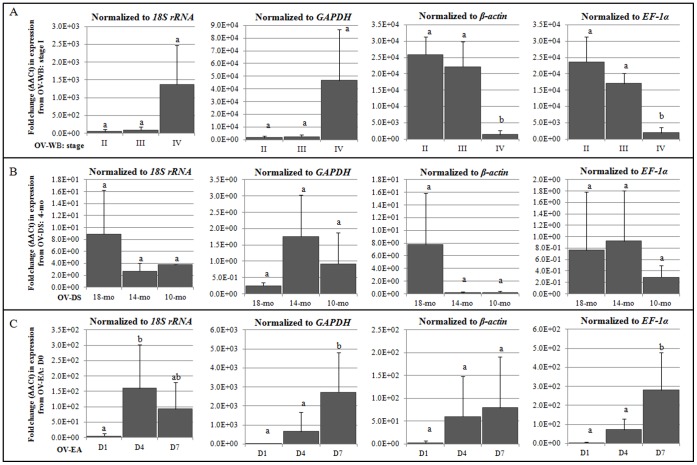
Vitellogenin (Vg) is a well-known indicator for ovarian maturation indicated by higher values in gonadosomatic index (GSI, ratio between gonad weight to body weight indicating ovarian maturation degrees) [68]. Based on the GSI value, ovarian maturation in penaeid shrimp can be categorized into four stages (I–IV): pre-vitellogenic, vitellogenesis, cortical rod, and late cortical rod [69]. Previous reports showed that the Vg expression level was low at previtellogenic stage (Stage I), increased to the highest level at vitellogenic stage (Stage II) and slightly decreased at early cortical rod (Stage III) and late cortical rod (Stage IV) in Kuruma prawn [30], [70] and Banana shrimp [29]. In our study, the fold change in the Vg expression during different maturation stages (OV-WB) relative to Stage I when normalized to EF-1α and β-actin showed similar profiles to the previously reports with significantly higher expression levels during Stages II–IV [29], [30], [70]. In contrast, the opposite expression trend was observed when normalized to GAPDH and 18S rRNA. In OV-DS group, no significant difference in expression level of Vitellogenin was observed which could be explained from their GSI values that belong to Stage I for all the samples. Therefore, it would be difficult to validate the housekeeping genes with this sample group where there is no previous report on the Vg expression pattern during growth or different age in domesticated shrimp. For the samples from eyestalk ablation experiment (OV-EA), GSI values of Day 1 and Day 4 samples belong to Stage I (1.22±0.14% and 1.31±0.33%, respectively), while GSI value of Day 7 samples belongs to Stage III (5.18±2.31%). The Vg expression pattern normalized to EF-1α, GAPDH and β-actin showed similar pattern which agreed with the previous report that suggested increasing expression levels of Vg after an eyestalk ablation. Only the Vg expression levels normalized to EF-1α or GAPDH exhibited significantly higher levels in the Day 7 samples which were accordant to previous reports [29], [30], [70]. Besides, the Vg expression pattern normalized to 18S rRNA showed a different profile with no correlation to previous reports (Fig. 4). The expression pattern and significant level of the genes in copy number suggested the same results as in the fold change (Fig. S2).
Figure 4. Relative expression levels in term of fold change (ΔΔCt) of a known ovary-relevant marker, Vitellogenin (Vg), to those of the housekeeping genes in three ovary sample groups:
(A) Wild broodstock (WB) from four different ovarian maturation stages compared to those of the housekeeping genes in WB stage 1, (B) Domesticated shrimp at 18-month-, 14-month-, and 10-month-old compared to those of the housekeeping genes in domesticated shrimp at 4-month-old, (C) Domesticated broodstock after the ablation for 1 (D1), 4 (D4), and 7 (D7) days compared to those of the housekeeping genes in before the ablation (D0). Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
When EF-1α was used, the obtained expression patterns of both testicular development marker (Dmc1) and ovarian maturation marker (Vg) agreed with the previously report to the levels of statistically significant differences in most of the cases [18], [20], [25], [29], [30].
In conclusion, an appropriate choice of an internal control gene in relative quantification for reproductive gene expression profile in the black tiger shrimp is clearly important and needed to be carefully evaluated for their robustness. We identified the most stable reference genes for qPCR gene expression analysis by comparing the stability of commonly used reference genes using two bioinformatic programs, geNorm and NormFinder. EF-1α was validated to be the most reliable internal control gene for qPCR gene expression analysis of reproductive system in the black tiger shrimp. The result from this study will help future gene expression studies to use an appropriate internal control gene to avoid bias and inaccurate result.
Materials and Methods
Ethics Statement
No specific permits were required for the described field studies. The field studies did not involve endangered or protected species.
RNA Samples and Reverse Transcription
Testis and ovary sample groups from male and female P.monodon were examined in this study. Testes samples were collected from wild broodstock (TT-WB) from Andaman Sea and Gulf of Thailand: West and East and domesticated shrimp at different growth stages (TT-DS: 4-, 10-, 14-, and 18-month-old). Ovary samples were collected from wild broodstock with different ovarian maturation stages (OV-WB: Stages I–IV), domesticated shrimp with different growth stages (OV-DS: 4-, 10-, 14-, and 18-month-old) and domesticated broodstock before and after eyestalk ablation for 1, 4 and 7 days (OV-EA: D0, D1, D4, and D7) as shown in Table 1. All samples were quickly frozen in liquid nitrogen for RNA extraction. RNA samples were extracted from the tissues using TRI-REAGENT according to manufacturer’s instruction (Molecular Research Center, USA). Contaminated genomic DNA was removed by treatment with DNase I at 0.15 U/µg total RNA at 37°C for 30 min. One microgram of total RNA was reverse transcribed (RT) using RevertAid™ First Strand cDNA Synthesis Kits (Fermentas) for testis samples and ImProm-II™ Reverse Transcription System (Promega) for ovary samples according to manufacturer’s instructions. The quantity of cDNA was measured using NanoDrop (ND-8000).
Quantitative Real-time PCR (qPCR)
The expression levels of four housekeeping genes (18S rRNA, GAPDH, β-actin, and EF-1α) and testis-relevant (Dmc1) and ovary-relevant (Vitellogenin) transcripts in different shrimp conditions were measured by quantitative real-time PCR (qPCR). Primers for all the genes examined in the study were either designed from available nucleotide sequences for each transcript from the NCBI database (http://www.ncbi.nlm.nih.gov/) using Oligo analyzer (http://eu.idtdna.com/analyzer/applications/oligoanalyzer/default.aspx) or previous literature (Table 3). A single peak from melting curve of each amplicon was examined to ensure specificity of the primers (Fig. S3).
Table 3. Primer pairs for quantitative real-time PCR (qPCR).
| Gene | Source of sequence | Primer Sequence | Size (bp) | PCR Efficiencies |
| Vitellogenin | ABB89953 | F: 5′-ATTCGGAACGTGCATTTGCTGCA-3′ | 188 | 96.2% |
| R: 5′-GTTCTCAAGCATTGTGACAGGATT-3′ | ||||
| Dmc1 | Leelatanawit et al., 2008 | F: 5′-ATGTGCGAGAAGCGAAGGC-3′ | 150 | 96.8% |
| R: 5′-GCAGAGAGTGTGGGAGATTTGTG-3′ | ||||
| EF-1α | Leelatanawit et al., 2008 | F: 5′-TTCCGACTCCAAGAACGACC-3′ | 122 | 96.5% |
| R: 5′-GAGCAGTGTGGCAATCAAGC-3′ | ||||
| GAPDH | AI770197 | F: 5′-ACATCGTTGAGTCCACTGGTGTGTT-3′ | 103 | 98.7% |
| R: 5′-GCATCGGCAGAAGGAGCGG-3′ | ||||
| β-actin | Qiu et al., 2008 | F: 5′- GCCCTTGCTCCTTCCACTATC-3′ | 143 | 99.0% |
| R: 5′- CCGGACTCTTCGTACTCATCCT-3′ | ||||
| 18S rRNA | Jarasrassamee et al., 2005 | F: 5′-GAGACGGCTACCACATCTAAG -3′ | 182 | 97.6% |
| R: 5′- ATACGCTAGTGGAGCTGGA-3′ |
For construction of the standard curve for each transcript, a plasmid containing the transcript was constructed by cloning the PCR product of the transcript into a pGEM-T easy vector (Promega). The resulting vector was transformed into E. coli JM109. The plasmid was extracted and used as the template for construction of the standard curve by 10-fold serial dilutions (103–108 copy numbers).
Each qPCR reaction was performed in a 20 µl total reaction volume containing 2X iQ™ SYBR® Green Supermix (Bio-Rad), 200 ng of first strand cDNA template, and 0.2 µM of a primer pair. Cycling parameters were 95°C for 2.5 min; followed by 40 cycles of 95°C for 30 sec, 58°C for 20 sec, and 72°C for 30 sec. The specificity of PCR products was confirmed by melting curve analysis performed from 55°C–95°C with a continuous fluorescent reading with a 0.5°C increment. Expression levels of different sample groups were statistically tested by ANOVA followed by Tukey test (P<0.05).
Data Analysis
The stability of expression levels of reference genes was evaluated by NormFinder [52] and geNorm [51]. The stability value of each candidate reference gene from each sample group was assessed separately by NormFinder and geNorm methods. The copy numbers of the four candidate housekeeping genes were calculated from the threshold cycle (Ct) obtained from qPCR experiment. These values were used as input to determine expression stability using the two software-based approaches. Moreover, relative expression levels of known ovary-relevant gene Vitellogenin (Vg) and known testis-relevant gene Dmc1 were examined using each of the four housekeeping genes as a reference gene to compare with previously reported expression patterns to see the robustness of each candidate as a reference gene.
Supporting Information
Relative expression levels in term of copy numbers of a known testis-relevant marker, Dmc1 , to the expression levels of the housekeeping genes in wild broodstock from Andaman sea black), wild broodstock from Gulf of Thailand (diagonal lines), 18-month-old domesticated shrimp (DS) (gray), 14-month-old DS (horizontal lines), 10-month-old DS (diamond), and 4-month-old DS (gray spots). Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
(TIF)
Relative expression levels in term of copy numbers of a known ovary-relevant marker, Vitellogenin ( Vg ), to the expression levels of the housekeeping genes in three ovary sample groups: (A) Wild broodstock (WB) from four different ovarian maturation stages, (B) Domesticated shrimp (DS) at 18-, 14-, 10-, and 4-month-old (C) Domesticated broodstock before the ablation (D0), and after the ablation for 1 (D1), 4 (D4), and 7 (D7) days. Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
(TIF)
Melting curves of qPCR amplicons in (A) testis samples and (B) ovary samples.
(TIF)
Acknowledgments
We would like to express our gratitude to Ms. Somjai Wongtripop, Ms. Juthatip Prommoon, Mr. Panomkorn Chaitongsakul and Mr. Annop Kudech at Shrimp Genetic Improvement Center (SGIC, Thailand) for the shrimp sample and facility to conduct the eyestalk ablation experiment. We are also grateful to Prof. Dr. Morakot Tanticharoen and Dr. Kanyawim Kirtikara for their mentorship in the black tiger shrimp research program. We would also like to acknowledge Dr. Sirawut Klinbunga for his contribution to the paper.
Funding Statement
This research is supported by the National Center for Genetic Engineering and Biotechnology (BIOTEC, Thailand) and Cluster and Program Management Office of the National Science and Technology Development Agency, Thailand (Grant number: P10-10467 and URL: http://www.nstda.or.th/scholarship). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemiolo B, Trappe S (2004) Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 2. Goidin D, Mamessier A, Staquet MJ, Schmitt D, Berthier-Vergnes O (2001) Ribosomal 18S RNA prevails over glyceraldehyde-3-phosphate dehydrogenase and beta-actin genes as internal standard for quantitative comparison of mRNA levels in invasive and noninvasive human melanoma cell subpopulations. Anal Biochem 295: 17–21. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Creer A, Jemiolo B, Trappe S (2005) Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 4. He JQ, Sandford AJ, Wang IM, Stepaniants S, Knight DA, et al. (2008) Selection of housekeeping genes for real-time PCR in atopic human bronchial epithelial cells. Eur Respir J 32: 755–762. [DOI] [PubMed] [Google Scholar]
- 5. Foldager CB, Munir S, Ulrik-Vinther M, Soballe K, Bunger C, et al. (2009) Validation of suitable house keeping genes for hypoxia-cultured human chondrocytes. BMC Mol Biol 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filby AL, Tyler CR (2007) Appropriate ‘housekeeping’ genes for use in expression profiling the effects of environmental estrogens in fish. BMC Mol Biol 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Z, Hu J (2007) Development and validation of endogenous reference genes for expression profiling of medaka (Oryzias latipes) exposed to endocrine disrupting chemicals by quantitative real-time RT-PCR. Toxicol Sci 95: 356–368. [DOI] [PubMed] [Google Scholar]
- 8. Infante C, Asensio E, Canavate JP, Manchado M (2008) Molecular characterization and expression analysis of five different elongation factor 1 alpha genes in the flatfish Senegalese sole (Solea senegalensis Kaup): differential gene expression and thyroid hormones dependence during metamorphosis. BMC Mol Biol 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCurley AT, Callard GV (2008) Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol Biol 9: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun 345: 646–651. [DOI] [PubMed] [Google Scholar]
- 11. Jian B, Liu B, Bi Y, Hou W, Wu C, et al. (2008) Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol 9: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carrillo-Casas EM, Hernandez-Castro R, Suarez-Guemes F, de la Pena-Moctezuma A (2008) Selection of the internal control gene for real-time quantitative rt-PCR assays in temperature treated Leptospira. Curr Microbiol 56: 539–546. [DOI] [PubMed] [Google Scholar]
- 13. Dhar AK, Bowers RM, Licon KS, Veazey G, Read B (2009) Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Mol Immunol 46: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 14.Pongsomboon S, Tang S, Boonda S, Aoki T, Hirono I, et al.. (2008) Differentially expressed genes in Penaeus monodon hemocytes following infection with yellow head virus. [DOI] [PubMed]
- 15. Zhou F, Jiang S, Huang J, Qiu L, Zhang D, et al. (2011) Molecular analysis of the QM gene from Penaeus monodon and its expression on the different ovarian stages of development. Molecular Biology Reports 38: 1921–1927. [DOI] [PubMed] [Google Scholar]
- 16. Antony SP, Singh ISB, Sudheer NS, Vrinda S, Priyaja P, et al. (2011) Molecular characterization of a crustin-like antimicrobial peptide in the giant tiger shrimp, Penaeus monodon, and its expression profile in response to various immunostimulants and challenge with WSSV. Immunobiology 216: 184–194. [DOI] [PubMed] [Google Scholar]
- 17.Somboonwiwat K, Supungul P, Rimphanitchayakit V, Aoki T, Hirono I, et al.. (2006) Differentially expressed genes in hemocytes of Vibrio harveyi-challenged shrimp Penaeus monodon. [DOI] [PubMed]
- 18. Leelatanawit R, Uawisetwathana U, Klinbunga S, Karoonuthaisiri N (2011) A cDNA microarray, UniShrimpChip, for identification of genes relevant to testicular development in the black tiger shrimp (Penaeus monodon). BMC Mol Biol 12: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Underwood DJ, Cowley JA, Sellars MJ, Barnes AC, van Hulten MCW, et al. (2010) Gill-associated virus and recombinant protein vaccination in Penaeus monodon. Aquaculture 308: 82–88. [Google Scholar]
- 20. Uawisetwathana U, Leelatanawit R, Klanchui A, Prommoon J, Klinbunga S, et al. (2011) Insights into eyestalk ablation mechanism to induce ovarian maturation in the black tiger shrimp. PLoS One 6: e24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tharntada S, Ponprateep S, Somboonwiwat K, Liu H, Soderhall I, et al. (2009) Role of anti-lipopolysaccharide factor from the black tiger shrimp, Penaeus monodon, in protection from white spot syndrome virus infection. J Gen Virol 90: 1491–1498. [DOI] [PubMed] [Google Scholar]
- 22. Luo T, Li F, Lei K, Xu X (2007) Genomic organization, promoter characterization and expression profiles of an antiviral gene PmAV from the shrimp Penaeus monodon. Molecular Immunology 44: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 23. Arts JAJ, Cornelissen FHJ, Cijsouw T, Hermsen T, Savelkoul HFJ, et al. (2007) Molecular cloning and expression of a Toll receptor in the giant tiger shrimp, Penaeus monodon. Fish & Shellfish Immunology 23: 504–513. [DOI] [PubMed] [Google Scholar]
- 24. Karoonuthaisiri N, Sittikankeaw K, Preechaphol R, Kalachikov S, Wongsurawat T, et al. (2009) ReproArray(GTS): a cDNA microarray for identification of reproduction-related genes in the giant tiger shrimp Penaeus monodon and characterization of a novel nuclear autoantigenic sperm protein (NASP) gene. Comp Biochem Physiol Part D Genomics Proteomics 4: 90–99. [DOI] [PubMed] [Google Scholar]
- 25. Wongsurawat T, Leelatanawit R, Thamniemdee N, Uawisetwathana U, Karoonuthaisiri N, et al. (2010) Identification of testis-relevant genes using in silico analysis from testis ESTs and cDNA microarray in the black tiger shrimp (Penaeus monodon). BMC Mol Biol 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leelatanawit R, Klinbunga S, Aoki T, Hirono I, Valyasevi R, et al. (2008) Suppression subtractive hybridization (SSH) for isolation and characterization of genes related to testicular development in the giant tiger shrimp Penaeus monodon. BMB Rep 41: 796–802. [DOI] [PubMed] [Google Scholar]
- 27. Leelatanawit R, Sittikankeaw K, Yocawibun P, Klinbunga S, Roytrakul S, et al. (2009) Identification, characterization and expression of sex-related genes in testes of the giant tiger shrimp Penaeus monodon. Comp Biochem Physiol A Mol Integr Physiol 152: 66–76. [DOI] [PubMed] [Google Scholar]
- 28.Buaklin A, Klinbunga S, Mensveta P (2011) Identification and expression analysis of the Broad-Complex core protein isoform 6 (BR-C Z6) gene in the giant tiger shrimp Penaeus monodon (Penaeidae: Decapoda). [DOI] [PubMed]
- 29. Phiriyangkul P, Puengyam P, Jakobsen IB, Utarabhand P (2007) Dynamics of vitellogenin mRNA expression during vitellogenesis in the banana shrimp Penaeus (Fenneropenaeus) merguiensis using real-time PCR. Mol Reprod Dev 74: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 30. Tsutsui N, Kawazoe I, Ohira T, Jasmani S, Yang WJ, et al. (2000) Molecular Characterization of a cDNA Encoding Vitellogenin and Its Expression in the Hepatopancreas and Ovary during Vitellogenesis in the Kuruma Prawn, Penaeus japonicus. Zool Sci 17: 651–660. [DOI] [PubMed] [Google Scholar]
- 31. Dhar AK, Bowers RM, Licon KS, Veazey G, Read B (2009) Validation of reference genes for quantitative measurement of immune gene expression in shrimp. Molecular Immunology 46: 1688–1695. [DOI] [PubMed] [Google Scholar]
- 32. Cale JM, Millican DS, Itoh H, Magness RR, Bird IM (1997) Pregnancy Induces an Increase in the Expression of Glyceraldehyde-3-Phosphate Dehydrogenase in Uterine Artery Endothelial Cells. Journal of the Society for Gynecologic Investigation 4: 284–292. [PubMed] [Google Scholar]
- 33. Puissant C, Bayat-Sarmadi M, Devinoy E, Houdebine L-M (1994) Variation of transferrin mRNA concentration in the rabbit mammary gland during the pregnancy–lactation–weaning cycle and in cultured mammary cells. A comparison with the other major milk protein mRNAs. European Journal of Endocrinology 130: 522–529. [DOI] [PubMed] [Google Scholar]
- 34. Calvo EL, Boucher C, Coulombe Z, Morisset J (1997) Pancreatic GAPDH Gene Expression during Ontogeny and Acute Pancreatitis Induced by Caerulein. Biochemical and Biophysical Research Communications 235: 636–640. [DOI] [PubMed] [Google Scholar]
- 35. Mansur NR, Meyer-Siegler K, Wurzer JC, Sirover MA (1993) Cell cycle regulation of the glyceraldehyde3phosphate dehydrogenaseluracil DNA glycosylase gene in normal human cells. Nucleic Acids Research 21: 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain M, Nijhawan A, Tyagi AK, Khurana JP (2006) Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochemical and Biophysical Research Communications 345: 646–651. [DOI] [PubMed] [Google Scholar]
- 37. Filby A, Tyler C (2007) Appropriate ‘housekeeping’ genes for use in expression profiling the effects of environmental estrogens in fish. BMC Molecular Biology 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6: 279–284. [DOI] [PubMed] [Google Scholar]
- 39. Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, et al. (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochemical and Biophysical Research Communications 313: 856–862. [DOI] [PubMed] [Google Scholar]
- 40. Deindl E, Boengler K, van Royen N, Schaper W (2002) Differential expression of GAPDH and β-actin in growing collateral arteries. Molecular and Cellular Biochemistry 236: 139–146. [DOI] [PubMed] [Google Scholar]
- 41. Spanakis E (1993) Problems related to the interpretation of autoradiographic data on gene expression using common constitutive transcripts as controls. Nucleic Acids Research 21: 3809–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krüssel JS, Huang H-Y, Simón C, Behr B, Pape AR, et al. (1998) Single Blastomeres within Human Preimplantation Embryos Express Different Amounts of Messenger Ribonucleic Acid for β-Actin and Interleukin-1 Receptor Type I. Journal of Clinical Endocrinology & Metabolism. 83: 953–959. [DOI] [PubMed] [Google Scholar]
- 43. Foss DL, Baarsch MJ, Murtaugh MP (1998) Regulation of hypoxanthine phosphoribosyltransferase, glyceraldehyde-3-phosphate dehydrogenase and β-actin mRNA expression in porcine immune cells and tissues. Animal Biotechnology 9: 67–78. [DOI] [PubMed] [Google Scholar]
- 44. Carlyle WC, Toher CA, Vandervelde JR, McDonald KM, Homans DC, et al. (1996) Changes inβ-actin mRNA Expression in Remodeling Canine Myocardium. Journal of Molecular and Cellular Cardiology 28: 53–63. [DOI] [PubMed] [Google Scholar]
- 45. Sellars MJ, Vuocolo T, Leeton LA, Coman GJ, Degnan BM, et al. (2007) Real-time RT-PCR quantification of Kuruma shrimp transcripts: A comparison of relative and absolute quantification procedures. Journal of Biotechnology 129: 391–399. [DOI] [PubMed] [Google Scholar]
- 46. Dirnhofer S, Berger C, Untergasser G, Geley S, Berger P (1995) Human β-actin retropseudogenes interfere with RT-PCR. Trends in Genetics 11: 380–381. [DOI] [PubMed] [Google Scholar]
- 47. Ingerslev H-C, Pettersen EF, Jakobsen RA, Petersen CB, Wergeland HI (2006) Expression profiling and validation of reference gene candidates in immune relevant tissues and cells from Atlantic salmon (Salmo salar L.). Molecular Immunology 43: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 48. Olsvik P, Lie K, Jordal A-E, Nilsen T, Hordvik I (2005) Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Molecular Biology 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Hiel M, Van Wielendaele P, Temmerman L, Van Soest S, Vuerinckx K, et al. (2009) Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Molecular Biology 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Infante C, Matsuoka MP, Asensio E, Canavate JP, Reith M, et al. (2008) Selection of housekeeping genes for gene expression studies in larvae from flatfish using real-time PCR. BMC Mol Biol 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 53. Kortner TM, Valen EC, Kortner H, Marjara IS, Krogdahl Å, et al. (2011) Candidate reference genes for quantitative real-time PCR (qPCR) assays during development of a diet-related enteropathy in Atlantic salmon (Salmo salar L.) and the potential pitfalls of uncritical use of normalization software tools. Aquaculture 318: 355–363. [Google Scholar]
- 54. Mehdi Khanlou K, Van Bockstaele E (2012) A critique of widely used normalization software tools and an alternative method to identify reliable reference genes in red clover (Trifolium pratense; L.). Planta 236: 1381–1393. [DOI] [PubMed] [Google Scholar]
- 55. Kajiura-Kobayashi H, Kobayashi T, Nagahama Y (2005) Cloning of cDNAs and the differential expression of A-type cyclins and Dmc1 during spermatogenesis in the Japanese eel, a teleost fish. Dev Dyn 232: 1115–1123. [DOI] [PubMed] [Google Scholar]
- 56. Habu T, Taki T, West A, Nishimune Y, Morita T (1996) The mouse and human homologs of DMC1, the yeast meiosis-specific homologous recombination gene, have a common unique form of exon-skipped transcript in meiosis. Nucleic Acids Res 24: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, et al. (1998) The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell 1: 707–718. [DOI] [PubMed] [Google Scholar]
- 58. Ozaki Y, Miura C, Miura T (2006) Molecular cloning and gene expression of Spo11 during spermatogenesis in the Japanese eel, Anguilla japonica. Comp Biochem Physiol B Biochem Mol Biol 143: 309–314. [DOI] [PubMed] [Google Scholar]
- 59. Okutsu T, Kang B, Miwa M, Yoshizaki G, Maeno Y, et al. (2010) Molecular cloning and characterization of Dmc1, a gene involved in gametogenesis, from the whiteleg shrimp Litopenaeus vannamei. Fisheries Science 76: 961–969. [Google Scholar]
- 60. Takanami T, Sato S, Ishihara T, Katsura I, Takahashi H, et al. (1998) Characterization of a Caenorhabditis elegans recA-like gene Ce-rdh-1 involved in meiotic recombination. DNA Res 5: 373–377. [DOI] [PubMed] [Google Scholar]
- 61. Ding Z-j, Wang T, Chong K, Bai S (2001) Isolation and characterization of OsDMC1, the rice homologue of the yeast DMC1 gene essential for meiosis. Sexual Plant Reproduction 13: 285–288. [Google Scholar]
- 62. Doutriaux MP, Couteau F, Bergounioux C, White C (1998) Isolation and characterisation of the RAD51 and DMC1 homologs from Arabidopsis thaliana. Mol Gen Genet 257: 283–291. [DOI] [PubMed] [Google Scholar]
- 63. Bishop DK, Park D, Xu L, Kleckner N (1992) DMC1: a meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69: 439–456. [DOI] [PubMed] [Google Scholar]
- 64. Stielow B, Krüger I, Diezko R, Finkernagel F, Gillemans N, et al. (2010) Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3. PLoS Genet 6: e1001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baillet A, Le Bouffant R, Volff JN, Luangpraseuth A, Poumerol E, et al. (2011) TOPAZ1, a Novel Germ Cell-Specific Expressed Gene Conserved during Evolution across Vertebrates. PLoS One 6: e26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pfennig F, Kurth T, Meißner S, Standke A, Hoppe M, et al. (2012) The social status of the male Nile tilapia (Oreochromis niloticus) influences testis structure and gene expression. Reproduction 143: 71–84. [DOI] [PubMed] [Google Scholar]
- 67. Zhang W, Wan H, Jiang H, Zhao Y, Zhang X, et al. (2011) A transcriptome analysis of mitten crab testes (Eriocheir sinensis). Genetics and Molecular Biology 34: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chu KH, Tam YK, Chung CK, Ng WL (1993) Morphometric relationships and reproductive maturation of the shrimp, Metapenaeus ensis, from commercial catches in Hong Kong. Fisheries Research 18: 187–197. [Google Scholar]
- 69. Tan-Fermin JD, Pudadera RA (1989) Ovarian maturation stages of the wild giant tiger prawn, Penaeus monodon Fabricius. Aquaculture 77: 229–242. [Google Scholar]
- 70. Kim YK, Tsutsui N, Kawazoe I, Okumura T, Kaneko T, et al. (2005) Localization and developmental expression of mRNA for cortical rod protein in kuruma prawn Marsupenaeus japonicus. Zoolog Sci 22: 675–680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative expression levels in term of copy numbers of a known testis-relevant marker, Dmc1 , to the expression levels of the housekeeping genes in wild broodstock from Andaman sea black), wild broodstock from Gulf of Thailand (diagonal lines), 18-month-old domesticated shrimp (DS) (gray), 14-month-old DS (horizontal lines), 10-month-old DS (diamond), and 4-month-old DS (gray spots). Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
(TIF)
Relative expression levels in term of copy numbers of a known ovary-relevant marker, Vitellogenin ( Vg ), to the expression levels of the housekeeping genes in three ovary sample groups: (A) Wild broodstock (WB) from four different ovarian maturation stages, (B) Domesticated shrimp (DS) at 18-, 14-, 10-, and 4-month-old (C) Domesticated broodstock before the ablation (D0), and after the ablation for 1 (D1), 4 (D4), and 7 (D7) days. Different letters above the bars of each graph signify statistical differences in gene expression levels within the sample group.
(TIF)
Melting curves of qPCR amplicons in (A) testis samples and (B) ovary samples.
(TIF)