Abstract
Nephrolithiasis is a major public health problem with a complex and varied etiology. Most stones are composed of calcium oxalate (CaOx), with dietary excess a risk factor. Because of complexity of mammalian system, the details of stone formation remain to be understood. Here we have developed a nephrolithiasis model using the genetic model Drosophila melanogaster, which has a simple, transparent kidney tubule. Drosophilia reliably develops CaOx stones upon dietary oxalate supplementation, and the nucleation and growth of microliths can be viewed in real time. The Slc26 anion transporter dPrestin (Slc26a5/6) is strongly expressed in Drosophilia kidney, and biophysical analysis shows that it is a potent oxalate transporter. When dPrestin is knocked down by RNAi in fly kidney, formation of microliths is reduced, identifying dPrestin as a key player in oxalate excretion. CaOx stone formation is an ancient conserved process across >400 My of divergent evolution (fly and human), and from this study we can conclude that the fly is a good genetic model of nephrolithiasis.
Keywords: oxalate, Cl− transport, prestin, Malpighian tubules, gene knockdown, Slc26
kidney stones are a major healthcare burden (>US$5.3 billion/yr in the US alone), with a complex and varied etiology that is poorly understood at the genetic level (36, 45, 61). Up to 75% of human kidney stones are made of calcium oxalate (CaOx). Some of these CaOx stones are associated with rare genetic disorders (primary hyperoxaluria) or intestinal disorders that cause fat malabsorption and enteric hyperoxaluria. In the remainder, the pathogenesis seems more complex, and multiple environmental and genetic factors that ultimately influence the urinary composition appear to play a role. Urinary oxalate excretion is not the only determinant of CaOx stone disease. Since oxalate is required for the formation of CaOx stones, factors that determine oxalate absorption from the diet, metabolic production, and elimination in the urine are all likely to play some role in stone risk.
Recent work has shown Drosophilia to provide a surprisingly good model for renal function (13), including calcium and now oxalate handling. Although human SLC26A6 is known to transport oxalate, only one study to date has evaluated it in oxalate nephrolithiasis (37). Here we describe the characterization of a Slc26 homolog (prestin, Slc26a5/6) in Drosophilia 1) which mediates tubule oxalate crystal formation, 2) which is an electrogenic Cl−/Ox2− exchanger, and 3) which when knocked down decreases CaOx crystal formation.
MATERIAL AND METHODS
Drosophilia.
Flies were kept on standard medium or dietary salt substitution in vials at 22°C, 12:12-h photoperiod, and 40% relative humidity. CantonS was used as wild-type and line c825, which expresses GAL4 in the principal cells of the initial and main segments of the Malpighian tubule (50).
Cell-type specific knockdown of dPrestin.
An elegant transgenic system, GAL4/UAS (upstream activation sequence), allows tissue- or cell-type specific genetic intervention in Drosophilia (4). The “GAL4 driver” lines contain the gene for the yeast transcription factor GAL4 under control of a Drosophilia promoter of choice. When crossed to a second transgenic fly line in which a genetic construct of choice is placed downstream of the UAS promoter, then the transgene is expressed only in those cells where GAL4 is being expressed. In this case, we used the Uro-GAL4 driver (53), in which the promoter of the utterly tubule principal-cell specific gene urate oxidase drives GAL4 expression, and crossed it to NIGFly line 5845R3 (National Institute of Genetics), which contains a hairpin dsRNA sequence directed against dPrestin.
Dietary salt substitution.
This was performed as described previously (51). Na-oxalate was dissolved in 100 ml of standard growth media (0.1% low; 1% high) just after its preparation and mixed, and the diet was left to set. Diet was freshly prepared to avoid any changes in the concentration of the salts due to evaporation.
Birefringence experiments.
Adult flies (7 days) were allowed to feed in normal food or dietary substitution for 24 h. Malpighian tubules were dissected in Schneider's medium and transferred immediately to poly-l-lysine-coated slides with PBS for visualization using a Zeiss Axiophot microscope. For in vitro experiments, PBS + 0.75 mM Na-oxalate was used.
X-ray diffraction.
Drosophilia Malpighian tubules were drawn into a quartz capillary and dried, and diffraction data were collected on an in-house X-ray source (Δφ = 180° at 1°/min.). Images were background corrected using either pixel-by-pixel subtraction or radial averaging.
Real-time RT-PCR.
Drosophilia Malpighian tubule cDNA was generated as described (5). The cDNA was used as a template for the generation of the complete prestin open reading frame and the PCR fragment used for RNAi.
Quantitative RT-PCR.
Quantitative RT-PCR validation was performed as described elsewhere (49). mRNA was prepared from 7-day-old CantonS or experimental tubules using Qiagen RNAeasy column. Superscript II and an oligo-dT were used for reverse transcription. For each sample, 500 ng of cDNA were added to 12.5 μl of 2 μM SYBR green reaction mix (Finnzymes, GRI, Essex, UK) and 1 μl of 6.6 μM forward and reverse primers. An Opticon 2 thermocycler was set as follows: 95°C for 15 min followed by 35 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. The ribosomal protein 49 (rp49) gene was used as a standard in all experiments. For each condition, we used four independent samples; each PCR was repeated three independent times to verify results.
Animal health and welfare.
Xenopus laevis were housed and cared for in accordance and approval of the Institutional Care and Use Committees of the Mayo Clinic.
Drosophilia Slc26a5 constructs.
The sequence of Drosophilia prestin (Slc26a5; CG5485) has been previously reported (59). We designed PCR primers to amplify the open reading frame plus restriction sites. The Drosophilia prestin sequence was verified.
Oocyte experiments.
Drosophilia prestin was subcloned into the pGEMHE Xenopus expression vector, capped cRNA synthesized; oocytes injected with 50 nl cRNA (0.2 μg/μl, 10 ng/oocyte) or water as previously for other transporters (22, 30, 39, 41); and incubated at 16°C in OR3 media. Oocytes were studied 3–10 days after injection.
Electrophysiology.
Electrophysiology protocols were performed as we previously reported for Slc26a6 (30, 32, 62). All solutions were either ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH 7.5) or iso-osmotic ion replacements (47). Cl− was replaced by gluconate. For HCO3− solutions, we used 5% CO2/33 mM HCO3− (pH 7.5).
Two electrode voltage clamp.
For these experiments, membrane currents were recorded with an OC-725C voltage clamp (Warner Instruments, Hamden, CT), filtered at 2–5 kHz, digitized at 10 kHz. I-V protocols consisted of 40-ms steps from Vh (−60 mV) to −140 mV and +60 mV in 20-mV steps (30, 47).
Ion-selective microelectrodes.
Ion-selective microelectrodes were used to monitor pHi and intracellular Cl− ([Cl−]i) of oocytes (41, 42). Intracellular pH and Cl− microelectrodes had slopes of at least −54 mV/pH unit or decade, respectively.
RESULTS
Dietary loading recapitulates oxalate nephrolithiasis in Drosophilia.
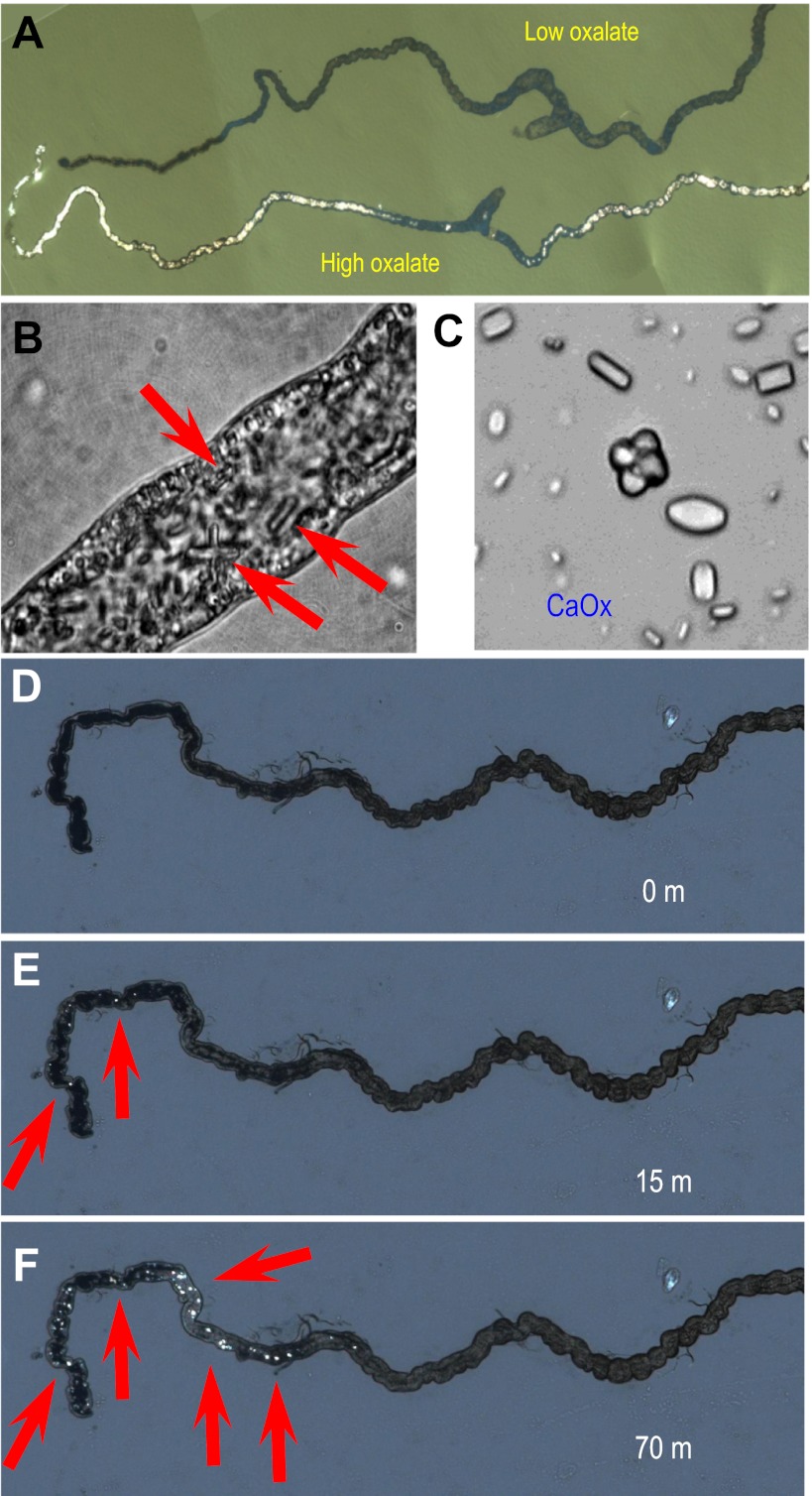
In humans, high dietary oxalate levels are associated with increased incidence of CaOx stones. Experimental food for flies does not normally contain oxalate. To induce increased oxalate elimination via Malpighian tubules, Drosophilia larvae were fed on diet supplemented with sodium oxalate (see materials and methods). Remarkably, CaOx microliths were observed within 2 days (Fig. 1, A–C); CaOx crystals show characteristic birefringence (Fig. 1, A and D–F), allowing a rapid screen for their existence, even in live larvae.
Fig. 1.
Oxalate nephrolithiasis in Drosophilia renal tubules. A: tubules dissected from larvae fed for 2 days on oxalate-enriched diet and viewed under polarizing light to demonstrate brilliant birefringence in only tubules: low oxalate (top) or high oxalate (bottom). B: higher magnification of tubule from an oxalate-fed larva, showing luminal inclusions with hexagonal prism morphology (red arrows) characteristic of calcium oxalate (CaOx) monohydrate crystals. C: magnified view of crystals obtained from a single tubule. D–F: time-lapse observation of dynamics of formation of oxalate crystals in vitro in freshly dissected anterior tubules: 0 (D), 15 (E), and 70 (f) min after addition of Na-oxalate to the bathing solution. A full time-lapse movie is available as Supplemental Movie S1.
To confirm that monolith formation was a direct product of oxalate transport by the renal tubules, dissected tubules were incubated in vitro in culture medium supplemented with oxalate. Rapid nucleation of microliths was observed within minutes (Fig. 1, D–F) and could be followed over several hours (see Supplemental Movie S1; Supplemental Material for this article is available online at the Am J Physiol Renal Physiol website). The Drosophilia tubule is a site of rapid calcium excretion (15), so transport of oxalate into the lumen could result in supersaturation with respect to CaOx (which has a solubility product ∼2.4 × 10−9 at 25°C), exactly mimicking conditions conducive to stone formation in humans.
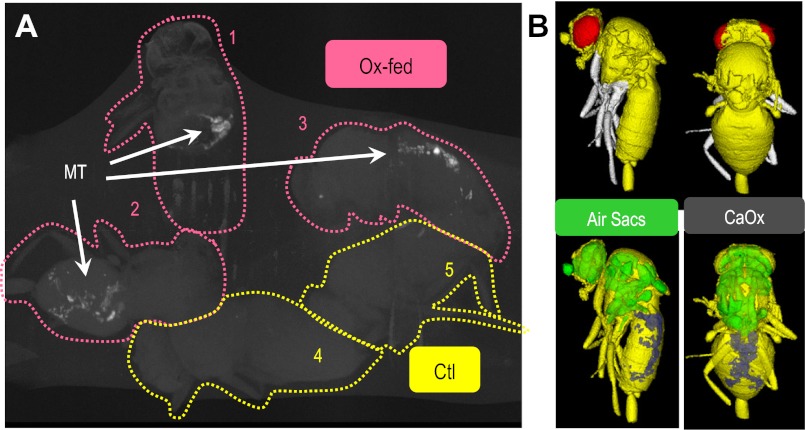
Likewise feeding adult Drosophilia with oxalate supplementation results in rapid (6–12 h) CaOx-microlith formation. These crystals can be observed by tubule microdissection and birefringence (Fig. 1) or alternatively in the intact animal using micro-computed tomography (micro-CT; Fig. 2). As with a human patient, CT allows an in situ assessment of CaOx microlith location and abundance. Figure 2 illustrates that oxalate fed-Drosophilia have CT dense material (CaOx) in the Malpighian tubules (MTs) while control Drosophilia do not.
Fig. 2.
Micro-computed tomography (micro-CT) detects CaOx crystals in Drosophilia (A) CaOx crystals (“stone”) are detected in oxalate-fed Drosophilia (Ox-fed) but not controls (Ctl). As with human CT, micro-CT reveals Ca-opaque spots (CaOx-monohydrate and dehydrate based on analysis in Fig. 5 and Supplemental Fig. S2). After 36 h of oxalate-supplemented food, crystals can be observed by birefringence (as in Fig. 4B). Knowing this, whole flies (3 Ox-fed and 2 Ctl) were fixed with ethanol, air dried, and then attached to polyethylene tubing. The entire assembly was embedded in wax (to prevent dehydration and movement of flies), then scanned over 24 h using micro-CT with a voxel resolution was 6 μm. Image is a maximum intensity display which shows the calcium (white) nicely but suppresses the “soft” tissue information (bodies of the flies). These experiments have been repeated at least three times with similar results. B: surface rendering of micro-CT image for fly 3. Botttom: images include the density extremes: low density (air sacs) and high density (CaOx).
Drosophilia prestin (Slc26a5/a6) is an electrogenic Cl−/ox2− exchanger.
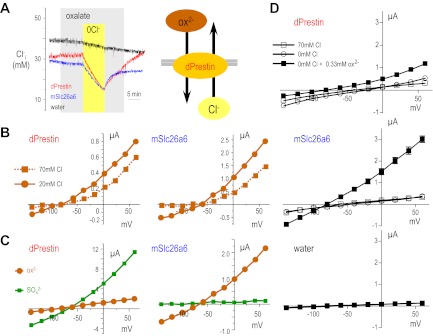
Next, we sought to determine the protein responsible for the CaOx accumulation. Recently, Schaechinger and Oliver (46) showed that nonmammalian prestin (Slc26a5) could function as an electrogenic Cl−/ox2− exchanger. Therefore, we cloned and tested the function of Drosophilia prestin (dPrestin) in Xenopus oocyte expression system. Figure 3 shows an experiment in which oxalate is exchanged for Cl− when dPrestin or mammalian Slc26a6 are expressed in Xenopus oocytes and monitored intracellular Cl− with a Cl− electrode (Fig. 3A). In oocytes expressing dPrestin and mSlc26a6, Cl− removal caused marked reduction of [Cl−]i and a marked hyperpolarization. Readdition of Cl− elicited depolarization and recovery of [Cl−]i. Control (water-injected) oocytes did not show these responses. When a similar solution protocol is repeated while voltage clamping oocytes, voltage- and oxalate-dependent currents were observed (Fig. 3B). Since Cl− removal in the absence of oxalate caused only slight currents in dPresrin and mSlc26a6 expressing oocytes (Fig. 3D), almost all of their transport currents are due to Cl−/ox2− exchange. These data illustrate that dPrestin mediates electrogenic Cl−/ox2− exchange (Fig. 3A, right). This activity is similar to the activity that we and others have reported for Slc26a6 (9, 19, 26, 27, 30, 32, 37, 58, 62).
Fig. 3.
Drosophilia prestin and mouse Slc26a6 have similar transport function. A: representative traces of intracellular chloride ([Cl]i) of oocytes injected with Drosophilia Prestin (dPrestin) cRNAs (red), mouse Slc26a6 (mSlc26a6) cRNAs (blue), or water (black) are shown. Oocytes were perfused with 70 mM Cl− containing 0.33 mM oxalate (light grey) and then Cl− removed (0Cl−, yellow) eliciting Cl− exit and oxalate entrance (right model). B: voltage-clamp experimental results illustrate the voltage-dependence of Cl−/ox2− exchange in 20 mM (solid line) and 70 mM Cl− (dotted line) for dPrestin (red) and mSlc26a6 (blue). C: voltage-clamped oocytes expressing dPrestin (left) or mouse Slc26a6 (right) were exposed to ox2− (brown circles) or SO42− (green squares), and current-voltage (I-V) relationships are plotted. D: effect of 0 Cl− without oxalate on the Cl−/ox2− exchange activities of dPresin (red), mSlc26a6 (blue), and water (black) was examined by voltage-clamp experiments. □, ○, and ■ show the IV relationship in 70 mM Cl−, 0 mM Cl−, and 0 mM Cl− with 0.33 mM oxalate, respectively. Data for B–D are means ± SE (n = 4–5).
dPrestin is functionally analogous to mammalian Slc26a6.
The above experiment can be repeated with sulfate or HCO3− and Cl− removal to illustrate electrogenic Cl−/SO42− (Fig. 3C) or electrogenic Cl−/nHCO3− exchange, respectively (24). These activities have also been reported and characterized for Slc26a6 (Fig. 3C; Refs. 9, 19, 26, 27, 30, 32, 58, 62). Interestingly, the absolute magnitude of Cl−/ox2− exchange is similar in Fig. 3. These new data reveal that dPrestin-mediated Cl−/SO42− exchange is faster than Cl−/ox2− exchange in insects (Fig. 3C, left), while the reverse is true for mouse Slc26a6 (Fig. 3C, right). This difference in substrate preference likely represents a Drosophilia (perhaps even Diptera) adaptation not needed in mammals. Varying [ox2−] and performing similar experiments reveals that dPrestin is close to saturation at 1 mM (Km = 0.64 ± 0.09 mM at 0 mV; Vmax = 0.85 ± 0.06 μA at 0 mV) while mouse Slc26a6 is not (Km = 3.5 ± 1.1 mM at 0 mV; Vmax = 6.00 ± 1.49 μA at 0 mV). Table 1 deltails the voltage dependence of the oxalate Km for both dPrestin and mSlc26a6. For Vm > 0 mV, the Km for dPrestin compared with mSlc26a6 are significantly different (P < 0.02). In contrast for Vm close to resting Vm of epithelial cells (−60 mV), there is no statistical difference.
Table 1.
Km oxalate transport kinetics in oocytes
| Vm, mV | dPrestin, mM | mSlc26a6, mM |
|---|---|---|
| −40 | 1.93 ± 0.58ns | 1.38 ± 0.09 |
| −20 | 1.42 ± 0.09ns | 3.45 ± 2.0 |
| 0 | 0.64 ± 0.09* | 3.51 ± 1.10 |
| +20 | 0.60 ± 0.12† | 2.45 ± 0.26 |
| +40 | 0.37 ± 0.05† | 1.74 ± 0.20 |
| +60 | 0.23 ± 0.04† | 1.44 ± 0.24 |
Values are means ± SE; ns, not significant; *P < 0.02 for dPrestin vs. mSlc26a6 at the same Vm. †P < 0.005 for dPrestin vs. mSlc26a6 at the same Vm (n > 3 for each data point of each clone).
dPrestin knockdown decelerates crystal accumulation.
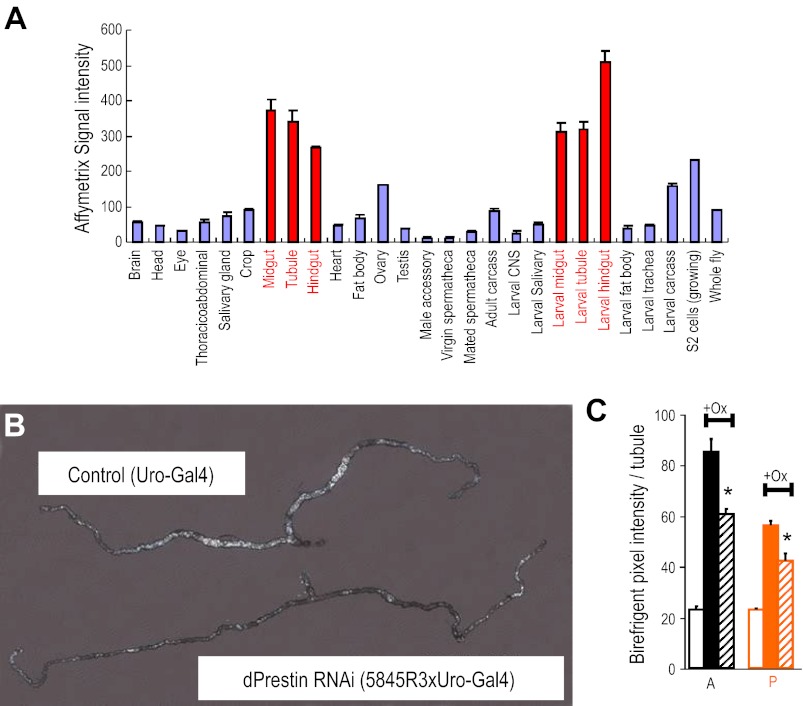
As dPrestin is expressed in epithelia, and functions as a Cl−/ox2− exchanger, it could mediate tubule oxalate transport. As mammalian Slc26a6, dPrestin mRNA is enriched in the tubule and the gut (Fig. 4A). Within the tubule, it is possible to titrate expression of genes cell specifically using the GAL4/UAS system with appropriate GAL4 drivers (51), so dPrestin was taken down in the initial and main segments of the tubule with the Drosophilia GAL4 line c825, which drives expression in tubule principal cells. The different dsRNA lines were found to reduce dPrestin mRNA levels by 50–70% (Supplemental Fig. S1), and this was sufficient to produce a marked phenotype (Fig. 4B). Birefringent luminal concretions were markedly reduced in the tubule (Fig. 4B), confirming that dPrestin mediates rapid oxalate transport. This effect is more pronounced in the anterior Malpighian tubules (Fig. 4C), which have higher overall transport (15).
Fig. 4.
Drosophilia prestin: localization and knockdown. A: dPrestin mRNA expression across different Drosophilia tissues and life-stages. dPrestin is broadly expressed at low level but conspicuously abundant in the epithelia of the alimentary canal (midgut, tubules, and hindgut). Data from FlyAtlas.org (7). B: impact of selective dPrestin knockdown in tubule on oxalate formation in vivo. dPrestin RNAi mutants [dPrestin RNAi (5834R3xUro-Gal4)] show remarkably reduced deposition of oxalate, compared with corresponding parental controls (Uro-Gal4). C: birefringence quantification of pixel intensity for anterior (A; black) and posterior (P; orange) tubules. Open boxes are tubule pixel background; solid boxes are control +oxalate; hatched boxes are RNAi-dPrestin + oxalate. *P < 0.05. Quantitative PCR of dPrestin mRNA in Malpighian tubules is available as Supplemental Fig. 1.
Tubule crystals are CaOx.
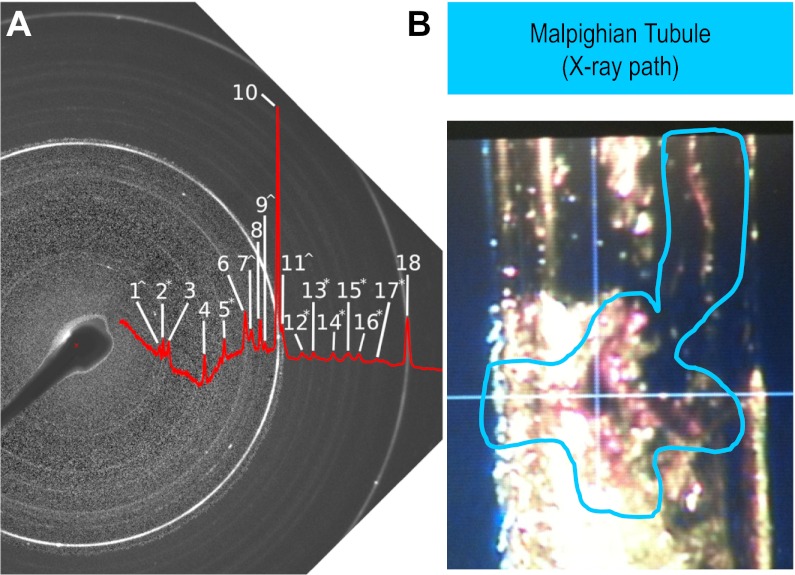
To make sure that crystals formed in Drosophilia Malpighian tubules were indeed CaOx (2), we used X-ray diffraction to definitively identify the crystals that formed in the tubules (Fig. 5). Figure 5B shows a Malpighian tubule with crystals, which was placed in the beam path of an X-ray diffractometer. The resulting diffraction pattern (Fig. 5A, 1–18) was identified by comparison to standards (21, 52, 56). Peaks assigned to CaOx monohydrate and dihydrate were identified (Table 2; Supplemental Fig. S2) as well as NaCl and hexagonal lipid from the partly dried tubule preparation.
Fig. 5.
Crystal identity by X-ray diffraction of tubules. A: negative image and peak profile of X-ray diffraction pattern from Drosophilia Malpighian tubules after exposure to Na-oxalate (see materials and methods). Diffraction rings characteristic of crystal powders are annotated (1–18). B: single Malpighian tubule of 48-h high oxalate fed flies was placed in a quartz pipette for X-ray beam shooting (in blue line circle). Quartz does not disturb X-ray diffraction.
Table 2.
X-ray diffraction ring peaks
| Identification/Ring No. | Peak |
|---|---|
| CaOx dihydrate^ | |
| 1 | 6.19 |
| 7 | 3.17 |
| 9 | 2.96 |
| 11 | 2.78 |
| CaOx monohydrate* | |
| 2 | 5.93 |
| 5 | 3.64 |
| 12 | 2.59 |
| 13 | 2.49 |
| 14 | 2.35 |
| 15 | 2.27 |
| 16 | 2.21 |
| 17 | 2.12 |
| CaCO3 | |
| 8 | 3.04 |
| NaCl | |
| 3 | 5.65 |
| 6 | 3.26 |
| 10 | 2.82 |
| 18 | 1.99 |
| Lipid acyl chains | |
| 4 | 4.12 |
DISCUSSION
Drosophilia model for Ox2− transport and regulation.
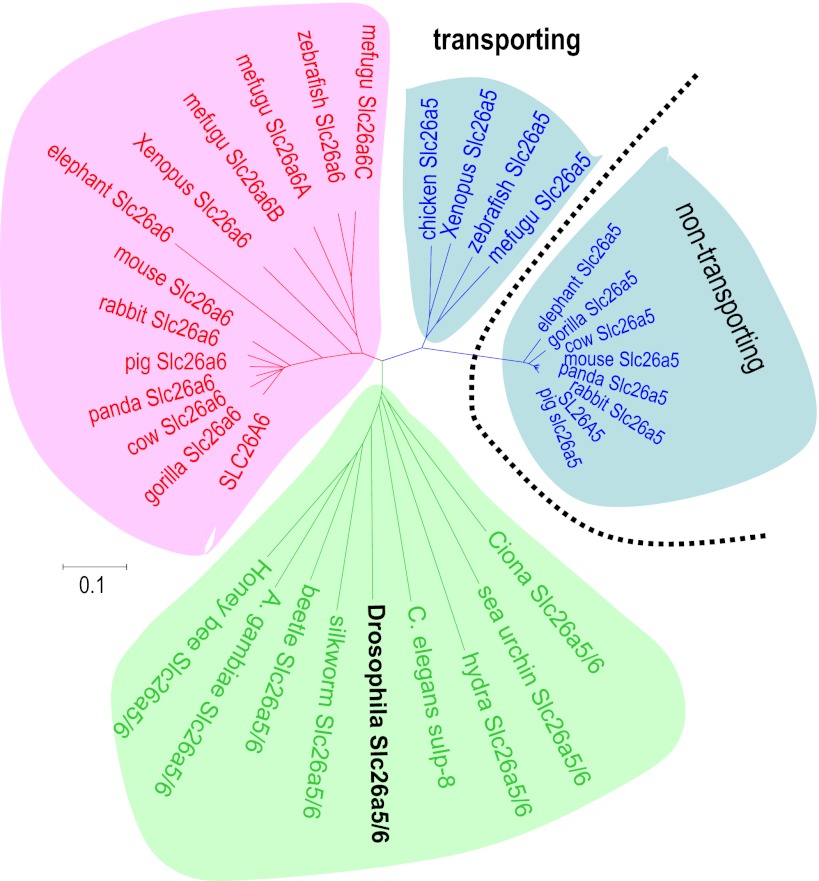
Mutations in Slc26 anion transporter-channel proteins cause a variety of human diseases, e.g., diarrhea, deafness, goiter, and diastrophic dysplasia (1, 18, 23, 25, 33–35, 38, 43, 64). Mouse studies have revealed additional roles for these Slc26 proteins in mammalian physiology: deafness, goiter, and acidosis (Slc26a4 and pendrin; Refs. 17, 44); cochlear motor protein (Slc26a5 and prestin; Refs. 10, 14); proximal tubule NaCl absorption, mouse urolithiasis, and intestinal HCO3− secretion (Slc26a6, Pat-1, and CFEX; Refs. 26, 48, 57, 58); sperm motility (Slc26a8 and Tat-1; Ref. 55); and gastric acid secretion (Slc26a9; Ref. 63). Additionally, several Slc26 proteins are reported to be controlled by WNK kinase phosphorylation (11, 28, 40), potentially linking them to hypertension (8, 29, 60). In the absence of an obvious Slc26a6 orthologue in flies, Drosophilia prestin (Slc26a5) seems to function as a Slc26a6-homologue transporter, perhaps representing ancestral gene function. Figure 6 shows that insect and worm Slc26a5/a6 proteins sit between vertebrate Slc26a5 and Slc26a6 clades, confirming their ancestral origin apparently predating the divergence into vertebrate subfamilies. Although there are other Drosophilia Slc26 genes, these appear to be an adaptive radiation of a Slc26a11 ancestor (Supplemental Fig. S3), a member with poorly understood transport function. dPrestin mediates the transports of not only oxalate and chloride but also bicarbonate, sulfate; and formate and thus likely contributes to the metabolism of these compounds in gut and Malpighian tubules.
Fig. 6.
Phylogenetic tree of animal Slc26a5 and Slc26a6 proteins. Phylogeny of Slc26a5 and Slc26a6 from several species [human (Homo sapiens), pig (Sus scrofa), giant panda (Ailuropoda melanoleuca), rabbit (Oryctolagus cuniculus), cow (Bos taurus), mouse (Mus musculus), gorilla (Gorilla gorilla), elephant (Loxodonta africana), chicken (Gallus gallus), Xenopus (X. tropicalis), zebrafish (D. rerio), mefugu (T. rubripes), sea squirt (Ciona intestinalis), fruit fly (Drosophilia melanogaster), silkworm (Bombyx mori), beetle (Tribolium castaneum), mosquito (Anopheles gambiae), honey bee (Apis mellifera), and roundworm (C. elegans)]. The sequences used are as follows with the accession numbers in parentheses: human SLC26A5 (AF523354); human SLC26A6 (NM_134263); chicken Slc26a5 (NP_001072945); Xenopus Slc26a5; Xenopus Slc26a6; Slc26a5 (NM_201473); zebrafish Slc26a6 (BC155340); Ciona (C. intestinalis) Slc26a5/6 (AAP57206); fly (D. melanogaster) Slc26a5/6 (NM_140767); honey bee (Apis mellifera) Slc26a5/6 (XM_001121249); and C. elegans sulp-8 (NM_073092). Bars indicate 10% replacement per site.
The present work shows that a major renal pathophysiology (CaOx kidney stones) can be recapitulated rather precisely in the potent genetic model, Drosophilia, and identify a key transporter (dPrestin) that participates in the process (Fig. 7). Recently, ethylene glycol-intoxication associated with oxalate stones was reported in Drosophilia (6) and we did further examination including genetic association with oxalate stones for establishing fly stone model. The studies in this report show that Drosophilia can specifically mimic 1) CaOx crystal formation (Figs. 1 and 5), 2) tubule oxalate secretion (Figs. 1 and 4), 3) gut and renal tubule Cl−/ox2− exchange (Fig. 3), and finally 4) whole animal gut oxalate absorption to CaOx crystal formation (Figs. 1 and 2). These studies also illustrate that dPrestin (Slc26a5/a6) is key to this physiological process and mimics the pathophysiology of CaOx renal stone formation.
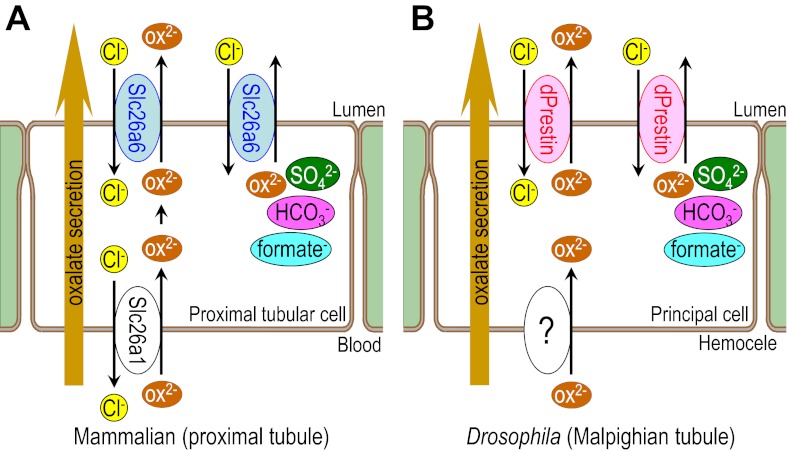
Fig. 7.
Comparative model for oxalate excretion in mammalian and Drosophilia at renal tubules. Model illustrating (A) oxalate transport in mammalian proximal tubule and (B) oxalate transport in the Drosophilia Malpighian tubule. At luminal side, Slc26a6 (mammalian) and dPrestin (Drosophilia) excrete oxalate, which is transported from blood (homocele) side by basolateral oxalate transporter [Slc26a1 (mammalian) and unidentified transporter (Drosophilia)]. Excess oxalate secretion causes Ca-oxalate stone formation in lumen. Both mammalian Slc26a6 and Drosophilia prestin also mediate transport of sulfate, bicarbonate, and formate exchanged for chloride. Thus mammalian and Drosophilia share almost same system of oxalate excretion.
Using Slc26a6 knockout mice, the group of Aronson (31) reported that oxalate secretion into intestinal lumen is important for protecting against stone formation, which means that oxalate transporter (Slc26a6) in gut contributes to keep out oxalate from body fluid by mediating oxalate secretion into the intestinal lumen. This is a first defense system to eliminate oxalate at gut. Once oxalate goes into body fluid, it should be excreted from renal tubules but direct evidence of oxalate secretion at renal tubules was reported in few studies (3). In this study, we used c42- and Uro-drivers for specific expression of dPrestin at Malpighian tubules but not at gut and showed that renal secretion is also a quite important defense factor for oxalate secretion. For preventing oxalate accumulation in body fluid, the transporter-depending oxalate secretion system in both gut and renal tubules is important. This study showed the direct evidence that oxalate transporter (dPrestin) at renal tubules (MT) excrete oxalate to the lumen.
Given that nucleation and growth of CaOx crystals can be followed in real time in an intact renal tubule, and the ease of genetic and physiological intervention in this model, these results confirm that Drosophilia can be used to rapidly study factors that modulate ion transport and crystallization within the tubules. This approach is particularly valuable, because there are presently very limited clinical therapies for this very common kidney disorder. Nonetheless, we have found the pharmacology of transport in Drosophilia to be very similar to that of humans. For example, well-known drugs like ouabain (54), amiloride (12, 20), or the antidiabetic sulfonylureas (16) work well on Drosophilia renal tubule and with similar IC50s to human. Furthermore, the low solubility product of CaOx implies that once formed, stones are unlikely to redissolve rapidly. Thus the ability to view the nucleation event, and screen for therapies that inhibit it, is uniquely valuable.
GRANTS
This work was supported by National Institutes of Health Grants DK-60845 and EY-017732 (M. F. Romero), P50-DK-083007 (to J. C. Lieske, M. F. Romero, and T. Hirata), EB-000305 (E. L. Ritman), and DK092408 to M. F. Romero and J. A. T. Dow).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.H., P.C., D.P.B., J.R.T., J.A.D., and M.F.R. performed experiments; T.H., P.C., D.S.B., D.P.B., E.L.R., J.R.T., J.A.D., and M.F.R. analyzed data; T.H., P.C., D.S.B., E.L.R., J.R.T., J.A.D., and M.F.R. interpreted results of experiments; T.H., P.C., D.S.B., D.P.B., J.R.T., J.A.D., and M.F.R. prepared figures; T.H., P.C., J.A.D., and M.F.R.drafted manuscript; T.H., P.C., D.S.B., E.L.R., J.R.T., J.A.D., and M.F.R. edited and revised manuscript; T.H., P.C., D.S.B., D.P.B., E.L.R., J.R.T., J.A.D., and M.F.R. approved final version of manuscript; J.A.D. and M.F.R. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heather L. Holmes and Elyse M. Scileppi for excellent technical support. We thank Drs. M.-H. Chang, J. C. Lieske, A. E. Krambeck, W. K. Ranatunga, and S.-A. Davies for helpful comments on the manuscript.
REFERENCES
- 1. Aronson PS. Essential roles of CFEX-mediated Cl(-)-oxalate exchange in proximal tubule NaCl transport and prevention of urolithiasis. Kidney Int 70: 1207–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Benzerara K, Miller VM, Barell G, Kumar V, Miot J, Brown GE, Jr, Lieske JC. Search for microbial signatures within human and microbial calcifications using soft x-ray spectromicroscopy. J Investig Med 54: 367–379, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol 300: F311–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, Davies SA, Dow JA. The Dh gene of Drosophilia melanogaster encodes a diuretic peptide that acts through cyclic AMP. J Exp Biol 205: 3799–3807, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Chen YH, Liu HP, Chen HY, Tsai FJ, Chang CH, Lee YJ, Lin WY, Chen WC. Ethylene glycol induces calcium oxalate crystal deposition in Malpighian tubules: a Drosophilia model for nephrolithiasis/urolithiasis. Kidney Int 80: 369–377, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophilia melanogaster models of human disease. Nat Genet 39: 715–720, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl−-transporting epithelia. Proc Natl Acad Sci USA 100: 663–668, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark JS, Vandorpe DH, Chernova MN, Heneghan JF, Stewart AK, Alper SL. Species differences in Cl− affinity and in electrogenicity of SLC26A6-mediated oxalate/Cl− exchange correlate with the distinct human and mouse susceptibilities to nephrolithiasis. J Physiol 586: 1291–1306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl channel regulated by the WNK kinases. J Physiol 584: 333–345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dow JA, Maddrell SH, Gortz A, Skaer NJ, Brogan S, Kaiser K. The Malpighian tubules of Drosophilia melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Dow JT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev 83: 687–729, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Drexl M, Mellado Lagarde MM, Zuo J, Lukashkin A, Russell IJ. The role of prestin in the generation of electrically evoked otoacoustic emissions in mice. J Neurophysiol 99: 1607–1615, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Dube K, McDonald DG, O'Donnell MJ. Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophilia melanogaster: roles of sequestration and secretion. J Insect Physiol 46: 1449–1460, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophilia melanogaster renal function. J Exp Biol 208: 3771–3783, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10: 153–161, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17: 411–422, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Giannakou ME, Dow JA. Characterization of the Drosophilia melanogaster alkali-metal/proton exchanger (NHE) gene family. J Exp Biol 204: 3703–3716, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Graf DL. Crystallographic tables for the rhombohedral carbonates. Am Mineral 46: 1283–1316, 1961 [Google Scholar]
- 22. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Hastbacka J, Superti-Furga A, Wilcox WR, Rimoin DL, Cohn DH, Lander ES. Atelosteogenesis type II is caused by mutations in the diastrophic dysplasia sulfate-transporter gene (DTDST): evidence for a phenotypic series involving three chondrodysplasias. Am J Hum Genet 58: 255–262, 1996 [PMC free article] [PubMed] [Google Scholar]
- 24. Hirata T, Kato A, Cabrero P, Chang MH, Dow JAT, Romero MF. Drosophilia Slc26a5 functions as a Cl-/Oxalate exchanger similar to mammalian Slc26a6. J Am Soc Nephrol 20: 385A, 2009 [Google Scholar]
- 25. Hoglund P, Sormaala M, Haila S, Socha J, Rajaram U, Scheurlen W, Sinaasappel M, de Jonge H, Holmberg C, Yoshikawa H, Kere J. Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum Mutat 18: 233–242, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc Natl Acad Sci USA 101: 2064–2069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurita Y, Nakada T, Kato A, Mistry AC, Chang MH, Romero MF, Hirose S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am J Physiol Regul Integr Comp Physiol 294: R1402–R1412, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, Webb BT, Yan D, Arnos KS, Corey D, Dallos P, Nance WE, Chen ZY. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet 12: 1155–1162, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Makela S, Eklund R, Lahdetie J, Mikkola M, Hovatta O, Kere J. Mutational analysis of the human SLC26A8 gene: exclusion as a candidate for male infertility due to primary spermatogenic failure. Mol Hum Reprod 11: 129–132, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Marengo SR, Romani AM. Oxalate in renal stone disease: the terminal metabolite that just won't go away. Nat Clin Pract Nephrol 4: 368–377, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Monico CG, Weinstein A, Jiang Z, Rohlinger AL, Cogal AG, Bjornson BB, Olson JB, Bergstralh EJ, Milliner DS, Aronson PS. Phenotypic and functional analysis of human SLC26A6 variants in patients with familial hyperoxaluria and calcium oxalate nephrolithiasis. Am J Kidney Dis 52: 1096–1103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Plata C, Sussman CR, Sindic A, Liang JO, Mount DB, Josephs ZM, Chang MH, Romero MF. Zebrafish Slc5a12 encodes an electroneutral sodium monocarboxylate transporter (SMCTn). A comparison with the electrogenic SMCT (SMCTe/Slc5a8). J Biol Chem 282: 11996–12009, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Plata C, Vázquez N, Romero MF, Gamba G. Opposite effects of WNK3 and WNK4 upon the Slc26a9 anion transporter/channel. FASEB J 22: 1202–1209, 2008 [Google Scholar]
- 41. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+ driven anion exchanger (NDAE1): a new bicarbonate transporter. J Biol Chem 275: 24552–24559, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Rossi A, van der Harten HJ, Beemer FA, Kleijer WJ, Gitzelmann R, Steinmann B, Superti-Furga A. Phenotypic and genotypic overlap between atelosteogenesis type 2 and diastrophic dysplasia. Hum Genet 98: 657–661, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 75: 585–595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci USA 104: 7693–7698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney, electrogenic Na+/HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Singh AK, Amlal H, Haas PJ, Dringenberg U, Fussell S, Barone SL, Engelhardt R, Zuo J, Seidler U, Soleimani M. Fructose-induced hypertension: essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney Int 74: 438–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Southall TD, Terhzaz S, Cabrero P, Chintapalli VR, Evans JM, Dow JA, Davies SA. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol Genomics 26: 35–45, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Sözen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophilia epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stergiopoulos K, Cabrero P, Davies SA, Dow JA. Salty dog, an SLC5 symporter, modulates Drosophilia response to salt stress. Physiol Genomics 37: 1–11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tazzoli V, Domeneghetti MC. The crystal structures of whewellite and weddellite: re-examination and comparison. Am Mineral 65: 327–334, 1980 [Google Scholar]
- 53. Terhzaz S, Finlayson AJ, Stirrat L, Yang J, Tricoire H, Woods DJ, Dow JA, Davies SA. Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophilia. Cell Signal 22: 737–748, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JA. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA 101: 13689–13693, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Toure A, Lhuillier P, Gossen JA, Kuil CW, Lhote D, Jegou B, Escalier D, Gacon G. The Testis Anion Transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum Mol Genet 16: 1783–1793, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Walker D, Verma PK, Cranswick LMD, Jones RL, Clark SM, Buhre S. Halite-sylvite thermoelasticity. Am Mineral 89: 204–210, 2004 [Google Scholar]
- 57. Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, Thomas PJ, Muallem S. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3− secretion: relevance to cystic fibrosis. EMBO J 25: 5049–5057, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Weber T, Gopfert MC, Winter H, Zimmermann U, Kohler H, Meier A, Hendrich O, Rohbock K, Robert D, Knipper M. Expression of prestin-homologous solute carrier (SLC26) in auditory organs of nonmammalian vertebrates and insects. Proc Natl Acad Sci USA 100: 7690–7695, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol 28: 120–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular and functional characterization of the Slc26A6 anion exchanger, functional comparison to Slc26a1. Am J Physiol Renal Physiol 283: F826–F838, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Xu J, Song P, Miller ML, Borgese F, Barone S, Riederer B, Wang Z, Alper SL, Forte JG, Shull GE, Ehrenfeld J, Seidler U, Soleimani M. Deletion of the chloride transporter Slc26a9 causes loss of tubulovesicles in parietal cells and impairs acid secretion in the stomach. Proc Natl Acad Sci USA 105: 17955–17960, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature 405: 149–155, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.