Abstract
Onset of metabolic acidosis leads to a pronounced increase in renal expression of phosphoenolpyruvate carboxykinase (PEPCK). This response, which is mediated in part by stabilization of PEPCK mRNA, is effectively modeled by treating LLC-PK1-F+-9C cells with an acidic medium. siRNA knockdown of HuR prevented the pH-responsive increase in PEPCK mRNA half-life suggesting that HuR is necessary for this response. A recruitment assay, using a reporter mRNA in which the pH response elements of the PEPCK 3′-UTR were replaced with six MS2 stem-loop sequences, was developed to test this hypothesis. The individual recruitment of a chimeric protein containing the MS2 coat protein and either HuR or p40AUF1 failed to produce a pH-responsive stabilization. However, the concurrent expression of both chimeric proteins was sufficient to produce a pH-responsive increase in the half-life of the reporter mRNA. siRNA knockdown of AUF1 produced slight increases in basal levels of PEPCK mRNA and protein, but partially inhibited the pH-responsive increases. Complete inhibition of the latter response was achieved by knockdown of both RNA-binding proteins. The results suggest that binding of HuR and AUF1 has opposite effects on basal expression, but may interact to mediate the pH-responsive increase in PEPCK mRNA. Two-dimensional gel electrophoresis indicated that treatment with acidic medium caused a decrease in phosphorylation of HuR, but may increase phosphorylation of the multiple AUF1 isoforms. Thus, the pH-responsive stabilization of PEPCK mRNA requires the concurrent binding of HuR and AUF1 and may be mediated by changes in their extent of covalent modification.
Keywords: metabolic acidosis, mRNA stabilization, siRNA knockdown, MS2 recruitment, 2-D gels
the maintenance of acid-base balance is essential for survival. However, metabolic acidosis is a common clinical condition that is characterized by a significant decrease in plasma pH and bicarbonate concentration (45, 48). This disturbance is caused by genetic or acquired defects in metabolism, in renal handling of bicarbonate, and in the excretion of acids. In addition, patients with cachexia, trauma, uremia, end-stage renal disease, and HIV infection frequently develop acidosis as a secondary complication that adversely affects their outcome. Chronic acidosis causes osteomalacia, nephrocalcinosis, and urolithiasis in adults. Increased renal catabolism of plasma glutamine constitutes an essential physiological response to metabolic acidosis (11, 24, 46). This adaptation generates ammonium ions that facilitate the urinary excretion of acid and bicarbonate ions that partially restore acid-base balance. An essential component of this adaptive response is the rapid and pronounced increase in the cytosolic isoform of phosphoenolpyruvate carboxykinase (PEPCK) that occurs within the renal proximal convoluted tubule (12). Previous transcription run-off experiments established that the rapid increase in PEPCK mRNA during acute onset of metabolic acidosis correlates with an increased transcription of the PCK1 gene (25, 26). However, the data also suggested that the sustained increase in PEPCK expression during chronic acidosis is due, at least in part, to stabilization of the PEPCK mRNA. The pH-responsive increases in PEPCK mRNA and protein are reproduced when LLC-PK1-F+-9C cells (39), a clonal line of porcine proximal tubule-like kidney cells, are treated with an acidic medium (pH 6.9, 9 mM HCO3−) for 24 h. The resulting fourfold increase in PEPCK protein is derived from increased transcription and a twofold stabilization of the PEPCK mRNA. Furthermore, the pH-responsive stabilization was recapitulated using a chimeric β-globin mRNA containing the full-length 3′-UTR of PEPCK, which was stably expressed in the LLC-PK1-F+-9C cells from a Tet-regulated promoter. Previous studies also identified the combined PCK6 and PCK7 segments within the 3′-UTR of PEPCK mRNA as the elements that contribute to its rapid turnover (23) and mediate the pH-responsive stabilization (39). The two segments contain highly conserved AU-rich elements (ARE) that bind two well-characterized RNA-binding proteins, human antigen R (HuR) and the 40-kDa isoform of AU-factor-1 (p40AUF1), with high affinity and specificity.
HuR is a 36-kDa protein that normally is localized predominantly in the nucleus (28). It contains three conserved RNA recognition motifs (8) and a 33-amino acid hinge region that functions as a nucleocytoplasmic shuttling sequence (18). In response to various stress conditions, HuR affects the translation and enhances the stability of many mRNAs that contain an ARE (19). However, the mechanism by which HuR mediates the stabilization of mRNAs is poorly understood (7, 28). The shuttling of HuR to the cytoplasm in response to stress conditions, such as heat shock (21), UV irradiation (49), amino acid starvation (51), chronic ethanol exposure (38), hypoxia (33), and ATP depletion (27), correlates with its ability to stabilize mRNAs (35, 49). Alternatively, posttranslational modifications of HuR may affect its RNA-binding affinity or its ability to associate with additional RNA stabilizing factors (2, 16, 29, 34).
The AUF1 or hnRNP D family of proteins contains four isoforms (p37, p40, p42, and p45) that are produced by alternative splicing of a single pre-mRNA (14, 17, 47, 52). Previous studies have established that AUF1 can either stabilize or destabilize various mRNAs by binding to specific ARE-containing segments. The specific effect of AUF1 binding has been postulated to be cell specific, due to changes in the relative abundance of the AUF1 isoforms, or result from various posttranslational modifications (31, 43, 50). HuR and AUF1 share a number of common ARE-binding sites and multiple studies have demonstrated that the two RNA-binding proteins can interact and form a physical association (30, 37, 41). Thus, the dynamic interaction between HuR and AUF1 may determine whether the complex mediates the rapid turnover or facilitates the stabilization of a specific mRNA (3).
Previous studies established that siRNA knockdown of HuR decreased both the basal level and the increased expression of PEPCK mRNA that occurs when LLC-PK1-F+-9C cells are treated with acidic medium (39). By contrast, the partial knockdown of AUF1 failed to affect either the basal or the pH-responsive expression of PEPCK mRNA. Therefore, the focus of the current study was to further assess the individual roles and the potential combinatorial effects of HuR and AUF1 in mediating the pH-responsive increase in PEPCK mRNA in LLC-PK1-F+-9C cells. Interestingly, the resulting data established that expression of HuR is necessary, but not sufficient, to produce a pH-responsive stabilization of PEPCK mRNA. However, the co-recruitment of chimeric constructs of the MS2 coat protein (MS2cp) and of HuR and of p40AUF1 is sufficient to impart a pH-responsive stabilization to a β-globin-PCK2 (βG-PCK2) reporter mRNA that contains six MS2 stem-loop elements. The concomitant knockdown of HuR and AUF1 completely abolished the pH-responsive increases in PEPCK mRNA and protein. Furthermore, two-dimensional Western blot analyses indicated that the extent of phosphorylation of HuR and possibly AUF1 is altered in response to treatment of LLC-PK1-F+-9C cells with an acidic medium. Therefore, a remodeling of the HuR/AUF1 complex associated with the 3′-UTR may mediate the stabilization of PEPCK mRNA when kidney cells are challenged with an acidotic stress.
MATERIALS AND METHODS
Cell culture and siRNA transfections.
LLC-PK1-F+-9C cells (39) were cultured in DMEM-base medium (Sigma) supplemented with penicillin/streptomycin (Sigma), 10% fetal bovine serum, 5 mM glucose, 26 mM NaHCO3, 17 mM NaCl, 2 mM glutamine, 5 mM pyruvate, 5 μM phenol red, and 10 mM HEPES, pH 7.4 at 37°C in a 5% CO2 atmosphere. A physiologic mimic of metabolic acidosis was recapitulated using an acidic (pH 6.9) medium, which was prepared as above, except that 9 mM NaHCO3 and 34 mM NaCl were added to reduce the pH while maintaining equivalent osmolarity (22). A preannealed double-strand stealth siRNA (Invitrogen) targeting the coding region of porcine HuR (HuR97 forward strand -CAGGAGGAGUUACGAAGUCUGUUCA) and a control siRNA that is not encoded in the human, rat, or mouse genome (ctrl378 forward strand -UGUAGGUAGAAGCUAUCAUUACGUG) were used at a final concentration of 30 and 50 nM, respectively. To silence pig AUF1, two separate siRNA oligos that target exon 3, which is common to all four isoforms of AUF1 (378AUF forward strand -CACUCUGAAGUUAGAUCCUAUCACA and 429AUF forward strand -UUUAGGAUCAAUCACCUUCCCAUUC), were used in a combination of 50 nM each. The siRNAs were transfected into 70–80% confluent cells in 12-well plates using Lipofectamine RNAiMAX (Invitrogen) as described previously (39). After 48 h, cells were treated with either pH 7.4 or pH 6.9 medium for 24 h before harvesting in 100-μl lysis buffer for Western blots (40) or in 250-μl TRIzol reagent for RNA isolation.
RNA extraction and real-time quantitative PCR.
Total RNA was isolated using TRIzol (Invitrogen) as per the manufacturer's protocol. An Oligo-dT18 primer (IDT) and the avian myeloblastosis virus-reverse transcriptase (Promega) were used to reverse transcribe 1 μg of RNA. Gene-specific primers and Taqman probes for the detection of endogenous porcine PEPCK mRNA and the control GAPDH mRNA were used for the real-time quantitative PCR (RT-qPCR) analysis as previously reported (39).
Chimeric MS2 expression plasmids.
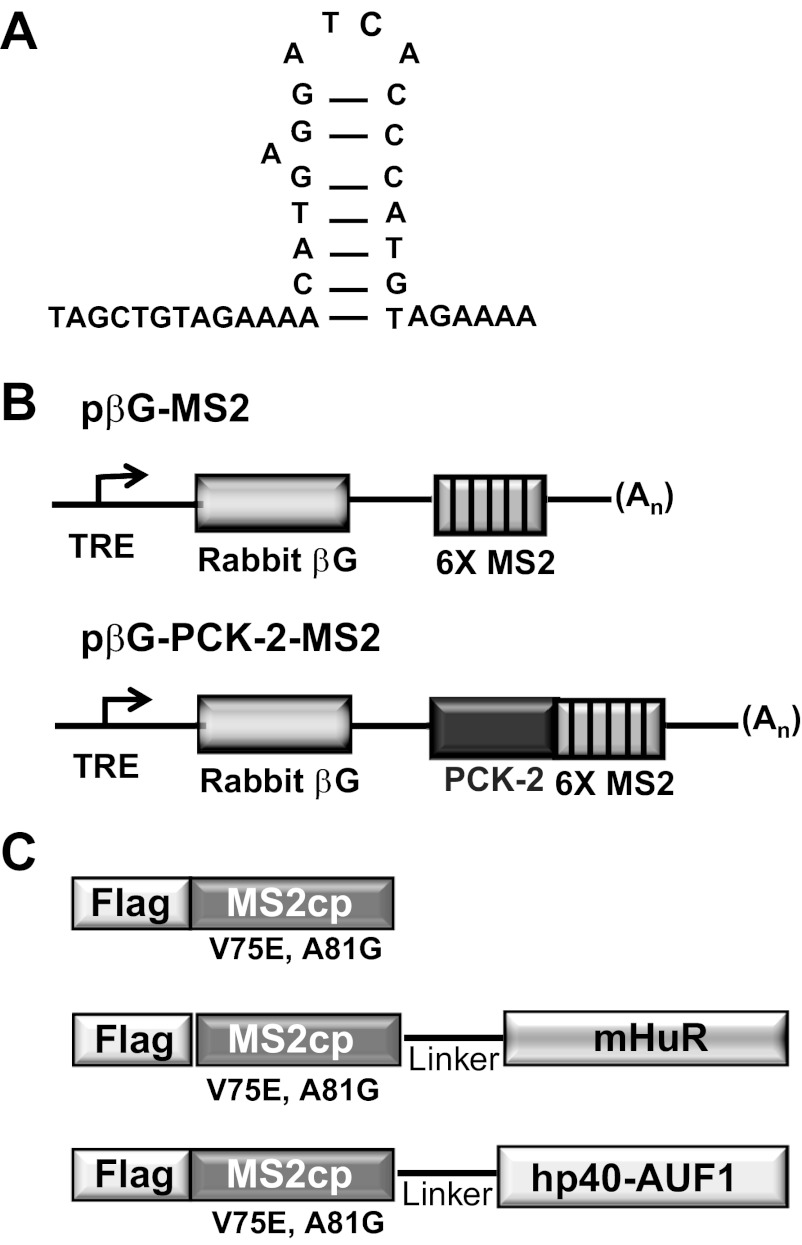
A chimeric reporter construct (pβG-PCK2) that expresses a β-globin-PCK2 mRNA from a tetracycline-responsive promoter was created and maintained as reported earlier (23). This construct contains a tetracycline-responsive promoter, the coding region of the rabbit β-globin (βG) gene, the 5′-fragment (381 bp) of the 3′-UTR of rat PEPCK cDNA (PCK2), and the polyadenylation site of bovine growth hormone (bGH) cDNA. The pPC-β6bs plasmid (36), which is a derivative of pcDNA3.0 (Invitrogen) that encodes six copies of MS2 coat protein-binding sites, was obtained as a kind gift from Jeffrey Wilusz (Department of Microbiology, Immunology and Pathology, Colorado State University). Each of the MS2 stem-loops differs from the wild type by an A→C high-affinity mutation in the loop sequence (Fig. 1A). A NotI/XbaI digestion of pPC-β6bs released a 314-bp fragment containing six repeats of the MS2 coat protein-binding sites. This fragment was inserted into the NotI and XbaI sites of pβG-PCK2 to create the MS2 reporter construct, pβG-PCK2-MS2. This construct retains a single AUF-1-binding site within the PCK2 segment (Fig. 1B). A control reporter construct that lacks the AUF1-binding site, pβG-MS2, was also created using the same strategy, except that the starting plasmid lacked the PCK-2 sequence.
Fig. 1.
Reporter mRNAs and MS2 fusion proteins used in the recruitment assay. A: nucleotide sequence and secondary structure of an MS2 stem-loop. B: pβG-MS2 plasmid contains a tetracycline-responsive promoter element (TRE), the complete coding region for rabbit β-globin (βG), and a 3′-UTR that contains 6 copies of the MS2 stem-loop sequence (6 × MS2). The pβG-PCK2-MS2 plasmid also encodes the PCK-2 sequence that constitutes the 5′-half of the 3′-UTR of rat phosphoenolpyruvate carboxykinase (PEPCK) mRNA. C: MS2 coat protein (MS2cp) fusion contains an NH2-terminal FLAG epitope that is in-frame with the MS2cp sequence. The remaining fusion proteins also contain a hydrophilic linker sequence followed by the mouse human antigen R (HuR) or human p40-AUF1 sequence.
MS2 fusion proteins.
The pcNMS2-Flag (36) plasmid was also obtained from the Wilusz lab. pcNMS2-Flag encodes an NH2-terminal flag peptide sequence that is in-frame with MS2 coat protein (MS2cp). The inserted MS2cp sequence encodes a mutated form of the MS2 bacteriophage coat protein that contains two point mutations (V75E; A81G). The two mutations prevent protein multimerization but retain high-binding affinity to the MS2-stem loop binding sites (32). The open reading frame of mouse HuR was PCR amplified from pGEMTeasy-mHuR (39) with primers containing 5′-BglII (5′-GACTAGATCTAGCG CCATGTCTAATGG-3′) and 3′-XbaI (5′-CGA TTTCTAGATTAAACTTTGTGGGACTTG-3′) sequences and cloned in frame with the COOH terminus of the MS2cp sequence (Fig. 1C). Similarly, the p40AUF1 coding sequence was PCR amplified from pGEMTeasy-p40AUF1 (23) with forward (5′-AATTGGATCCAAAGCCATGTCGGAGG-3′) and reverse (5′-GTCCGATGCTAGCTTAAA CGTATGGTTTGTAGC-3′) primers and inserted in-frame at the BamHI and NheI sites that are downstream of the MS2cp-sequence.
mRNA half-life analysis.
To assess mRNA half-lives, the β-globin reporter RNA plasmids were stably cotransfected with pcDNA3.1/Hygro (Invitrogen) into LLC-PK1-F+-9C cells that stably express the tTA protein (44). The stable transformants were grown in six-well plates in normal medium containing 100 ng/ml doxycycline (Dox) until 70% confluent. The cells were then transiently transformed with pFlag-MS2cp-HuR (2 μg) or pFlag-MS2cp-p40AUF1 (2 μg) plasmid and maintained in normal (pH 7.4) medium minus Dox for 2 days, before being treated with acidic medium (pH 6.9) for 24 h. RNA was isolated at various times after the addition of 1 μg/ml Dox to completely arrest transcription. The relative levels of β-globin and GAPDH mRNAs were quantified by RT-qPCR and normalized to GAPDH mRNA. Since the kidney cells do not express β-globin, the RT-PCR assay detects only the reporter RNA and serves as a common read-out for all the β-globin-based reporters. The RT-PCR assay for βG-cDNAs used forward (5′-TCAGTGAGGGTCTGAATCACC-3′) and reverse (5′-CTGCACCTGAGGAGTGA ATTC-3′) primers and a Taqman probe (5′-FAM-CACCTTTGCTAAGCTGAGTGAACTG CAC-BHQ1–3′).
Western blot analysis.
Bradford assays (6) were performed to determine the concentration of the protein lysates. Samples containing 15 μg of protein were resolved by 10% SDS-PAGE and transferred to Immobilon-F membranes (Millipore). The blots were probed with a mouse monoclonal antibody to HuR (Santa Cruz Biotechnology) and rabbit polyclonal antibodies to AUF1 (Millipore) and PEPCK (Abgent). The blots were reprobed for β-tubulin (Sigma) as a loading control. Subsequently, the blots were developed with goat anti-rabbit 800 and goat anti-mouse 680 (LiCor) secondary antibodies and the resulting fluorescence was quantified using an Odyssey Infra-red Imager.
Two-dimensional Western blot analyses.
Total cell extracts were prepared from 10-cm plates of confluent LLC-PK1-F+-9C cells that were either maintained in pH 7.4 medium or treated with pH 6.9 medium for 24 h before lysis. The freshly prepared lysis buffer contained 7 M urea, 2 M thiourea, 4% wt/vol CHAPS, and 30 mM Tris, pH 8.8 (13) supplemented with 0.1 mg/ml PMSF, 10% protease inhibitor cocktail (Sigma), and 3% Halt phosphatase inhibitor (Pierce). The cell extracts were incubated on ice for 10 min followed by centrifugation for 5 min at 4,000 g at 4°C. A sample containing 30 μg of the supernatant of the total cell lysate was diluted to 450 μl with rehydration buffer and applied to a 7-cm precast IPG Immobiline DryStrip (pH 6–11) purchased from GE Healthcare. Strips were covered with mineral oil and actively rehydrated for 16 h on IPGphor apparatus (GE Healthcare) at 50 μA/strip at 20°C followed by first dimension focusing using 500 V for 1 h, 1,000 V for 1 h, and 8,000 V for 6 h. After being focused, the strips were rinsed, reduced with 1% DTT in equilibration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 1% bromophenol blue), and then treated with 2.5% iodoacetamide. The proteins were resolved in a second dimension on a 12% SDS-polyacrylamide gel, blotted to an Immobilon-F membrane at 100 V for 60 min, and then probed with the appropriate antibodies.
RESULTS
HuR is necessary for the pH-responsive stabilization of PEPCK mRNA.
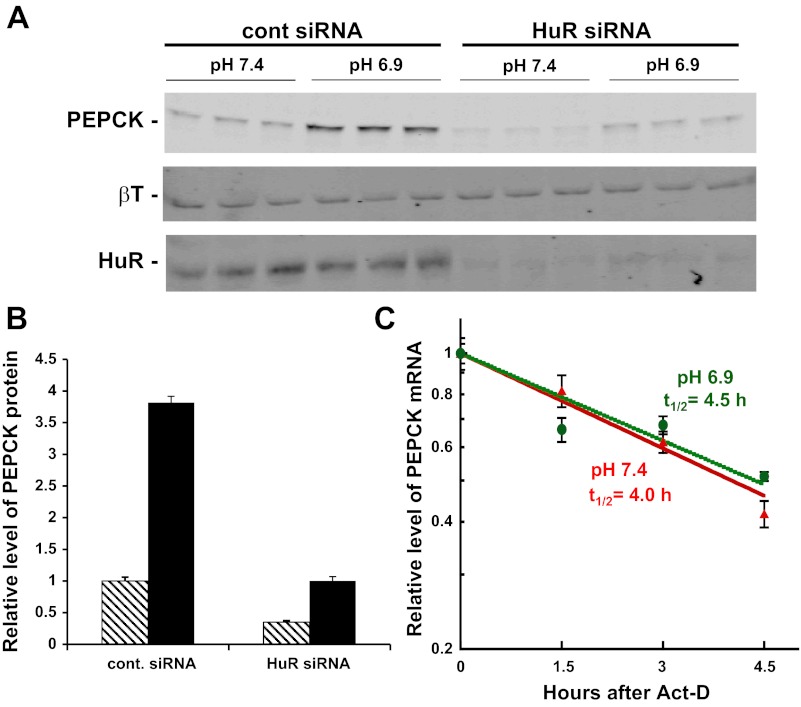
Previous studies (39) established that the half-life of the endogenous PEPCK mRNA is increased from 3.2 to 6.1 h when LLC-PK1-F+-9C cells are transferred from normal (pH 7.4, 26 mM HCO3−) to acidic (pH 6.9, 9 mM HCO3−) medium. The twofold stabilization was reproduced using a chimeric reporter mRNA (βG-PCK1), which contains the 5′-UTR and coding region for β-globin (βG) and the full-length 3′-UTR of PEPCK mRNA (PCK1). However, this response was lost when a binding site for HuR within the highly conserved AU sequence of the PCK6 segment was mutated. To further investigate the possible role of HuR in the stabilization of PEPCK mRNA, a siRNA was used to knockdown the level of HuR in LLC-PK1-F+-9C cells. Western blot analysis demonstrated that this treatment decreased HuR expression by 90% (Fig. 2A). As reported previously (39), the decreased expression of HuR caused a pronounced decrease in the basal level of PEPCK protein and reduced the pH-responsive increase in expression from four- to threefold (Fig. 2B). With the decreased expression of HuR, the measured half-lives of the PEPCK mRNA in normal (t1/2 = 4.0 h) or acidic medium (t1/2 = 4.5 h) were not significantly different (Fig. 2C). Therefore, a decrease in HuR expression inhibits basal expression and prevents the pH-responsive stabilization of PEPCK mRNA, but apparently does not inhibit the enhanced transcription of the PCK1 gene. Thus, HuR is a necessary component of the mechanism that mediates the stabilization of PEPCK mRNA.
Fig. 2.
siRNA knockdown of HuR prevents the pH-responsive stabilization of PEPCK mRNA. A: LLC-PK1-F+-9C cells were transfected with 50 nM control siRNA or 30 nM HuR siRNA and treated with either normal (pH 7.4) or acidic (pH 6.9) medium for 24 h before being harvested. A Western blot of the cell lysates was probed for PEPCK, β-tubulin (βT), and HuR. B: siRNA knockdown of HuR led to a significant reduction in the basal level and the pH-responsive increase of PEPCK protein. C: knockdown of HuR prevents the pH-responsive stabilization of PEPCK mRNA. LLC-PK1-F+-9C cells were cultured in 6-well plates and transfected with 30 nM HuR siRNA. The cells were treated for 24 h in either normal (red) or acidic (green) medium and then exposed to 8 μg/ml actinomycin D (Act-D). RNA was isolated at the indicated times and the levels of PEPCK and GAPDH mRNAs were quantified by RT-qPCR. The log of the relative level of PEPCK mRNA was plotted vs. time after addition of Act-D to assess the half-life. The reported data are means ± SE of triplicate samples for each time point.
Effect of recruitment of HuR and AUF1 to an MS2-based reporter mRNA.
A recruitment assay was developed to further test the role of HuR and AUF1 in the pH-responsive stabilization of PEPCK mRNA. This assay makes use of the high-affinity interaction between the MS2cp and a unique RNA-binding site within the MS2 phage that forms a stem-loop structure (32). Initially, a chimeric βG-MS2 mRNA was stably expressed in LLC-PK1-F+-9C cells from a tetracycline-responsive promoter. However, preliminary experiments indicated that this mRNA has a half-life of 17 h in cells grown in normal medium (data not shown). Therefore, an alternate reporter mRNA (βG-PCK2-MS2) was developed. The latter mRNA contains the standard rabbit β-globin coding sequence followed by the PCK2 segment of the 3′-UTR of PEPCK mRNA and six MS2 stem-loops. The PCK2 segment contains an instability element that binds AUF1, but it does not contribute to the pH-responsive stabilization of PEPCK mRNA (23). Inclusion of the PCK2 segment produced a reporter mRNA that decays with a half-life (t1/2 = 2.9 h) that can be more accurately quantified and that facilitates the identification of stabilizing interactions. Most importantly, the half-life of the βG-PCK2-MS2 reporter mRNA is not affected by treating the cells with an acidic medium (data not shown).
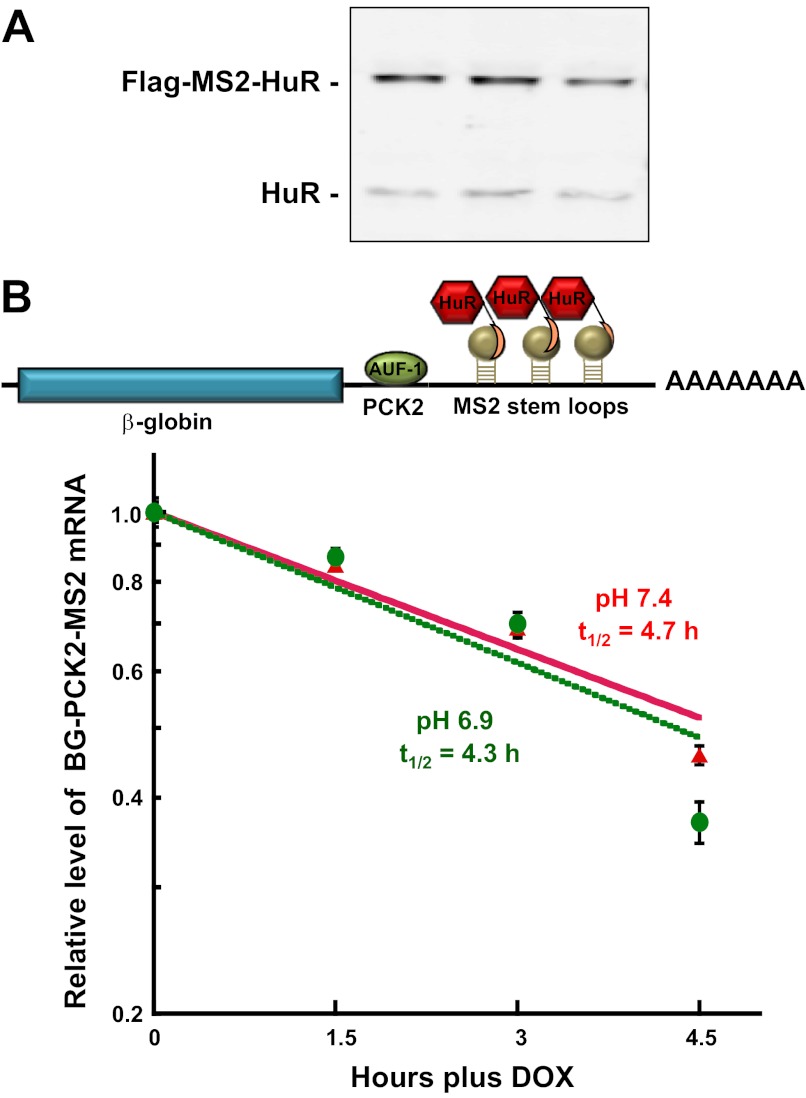
To determine the effect of HuR recruitment, cells that stably express the βG-PCK2-MS2 mRNA were transiently transfected with a plasmid that expresses the chimeric MS2cp-HuR-binding protein. Western blot analyses confirmed that the chimeric MS2cp-HuR was expressed at a level slightly greater than the level of endogenous HuR (Fig. 3A). Expression of the chimeric MS2cp-HuR-binding protein increased the half-life of the βG-PCK2-MS2 mRNA ∼1.5-fold (t1/2 = 4.7 h) in cells grown in normal medium (Fig. 3B). However, the reporter mRNA did not exhibit a significant change in stability when cells were treated with acidic medium (t1/2 = 4.3 h). Therefore, the recruitment of HuR was not sufficient to produce a pH-responsive stabilization of the reporter mRNA.
Fig. 3.
Recruitment of MS2cp-HuR is not sufficient to impart a pH-responsive stabilization of βG-PCK2-MS2 mRNA. A: LLC-PK1-F+-9C cells that stably express the βG-PCK2-MS2 reporter RNA were transiently transfected with 2 μg of pFlag-MS2cp-HuR and then maintained in normal (pH 7.4) medium before being harvested with lysis buffer. Western blot analysis was performed to assess the expression of the endogenous and chimeric forms of HuR. B: half-life analysis of the βG-PCK2-MS2 reporter RNA in LLC-PK1-F+-9C cells that transiently express MS2cp-HuR. Cells were maintained in minimal doxycycline (Dox; 100 ng/ml) until 70% confluent. Following transient transfection with 2 μg of expression plasmid, the cells were maintained in normal (pH 7.4) medium minus Dox for 48 h. The cells were then treated with normal (red) or acidic (green) medium for 24 h. RNA was isolated at various times after addition of 1 μg/ml Dox to arrest transcription. The relative levels of β-globin and GAPDH mRNAs were quantified by RT-qPCR. The log of the normalized data was then plotted against the time after Dox addition. The reported data are means ± SE of triplicate samples.
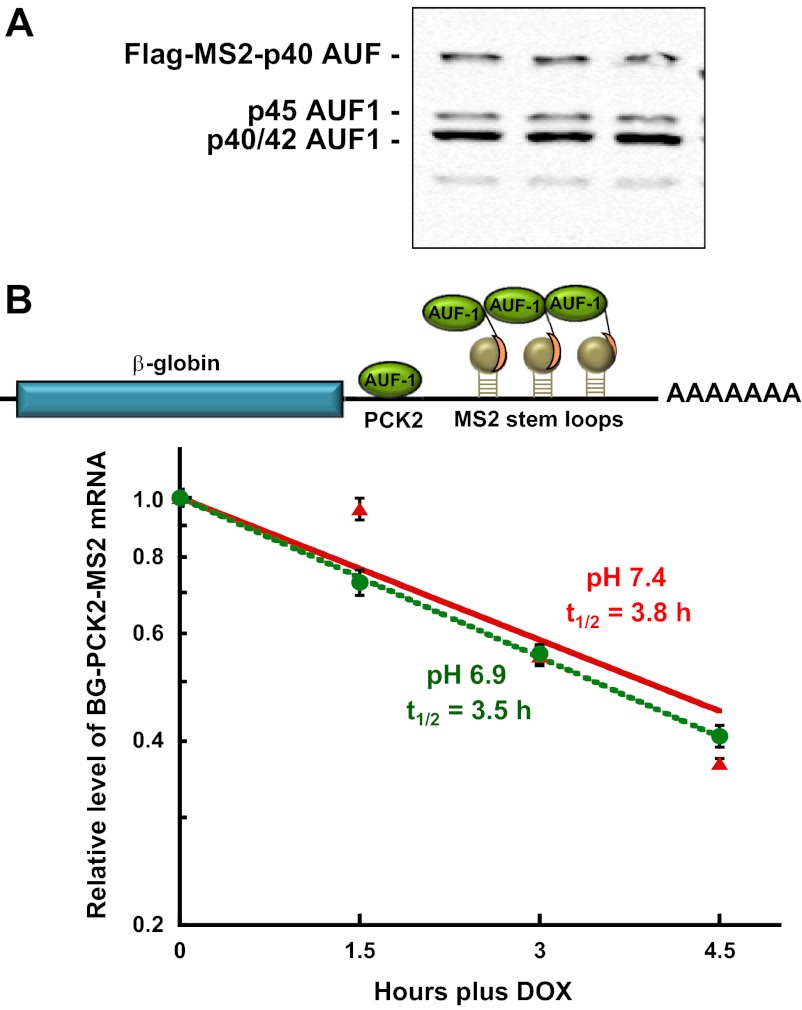
Previous studies (23) established that p40AUF1 binds to many of the same segments within the 3′-UTR of PEPCK mRNA that bind HuR (39). Therefore, the recruitment assay was also used to determine the effect of transient expression of the chimeric MS2-p40AUF1-binding protein. Western blot analysis again demonstrated MS2-p40AUF1 was expressed at a level similar to the sum of the endogenous AUF1 isoforms (Fig. 4A). The transient expression of the chimeric MS2-p40AUF1-binding protein had little effect on the half-life of the βG-PCK2-MS2 mRNA (t1/2 = 3.8 h) in cells grown in normal medium (Fig. 4B). The recruitment of MS2-p40AUF1 also failed to produce a significant change in half-life of the reporter mRNA when the cells were treated with an acidic medium (t1/2 = 3.5 h). Thus, the recruitment of p40AUF1 was also not sufficient to produce a pH-responsive stabilization of the reporter mRNA.
Fig. 4.
Recruitment of MS2cp-p40AUF1 is also not sufficient to impart a pH-responsive stabilization of βG-PCK2-MS2 mRNA. A: LLC-PK1-F+-9C cells that stably express the βG-PCK2-MS2 reporter RNA were transiently transfected with 2 μg of pFlag-MS2cp-p40AUF1 and maintained in normal (pH 7.4) medium before being harvested with lysis buffer. Western blot analysis was performed to assess the expression of the endogenous and chimeric forms of AUF1. B: half-life analysis of the βG-PCK2-MS2 reporter RNA in LLC-PK1-F+-9C cells that transiently express MS2cp-p40AUF1. Cells were maintained in minimal Dox (100 ng/ml) until 70% confluent. Following transient transfection with 2 μg of expression plasmid, the cells were maintained in normal (pH 7.4) medium minus Dox for 48 h. The cells were then treated with normal (red) or acidic (green) medium for 24 h. RNA was isolated at various times after addition of 1 μg/ml Dox to arrest transcription. The relative levels of β-globin and GAPDH mRNAs were quantified by RT-qPCR. The log of normalized data was then plotted against the time after Dox addition. The reported data are means ± SE of triplicate samples.
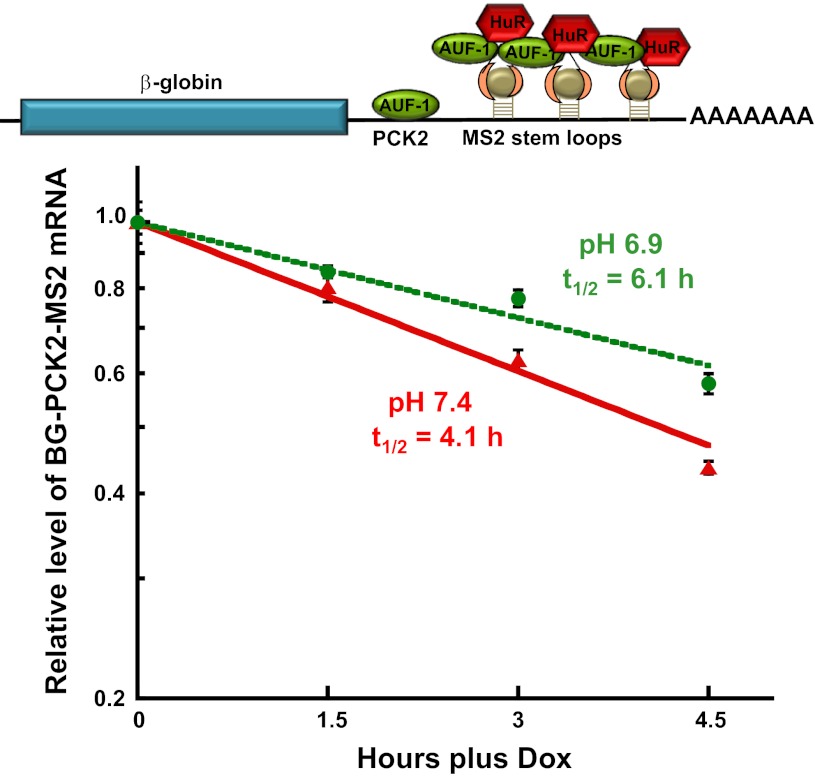
Next, the effect of coexpressing the MS2cp-HuR and MS2cp-p40AUF1 proteins was determined. Since the two chimeric proteins are recruited through the same RNA/protein interaction, coexpression should result in concomitant binding of the MS2cp-HuR and MS2cp-p40AUF1 proteins to one or more of the six adjacent MS2 stem-loops. In contrast to the preceding experiments, coexpression of the two chimeric proteins produced a significant stabilization of the βG-PCK2-MS2 mRNA when cells were switched from normal (t1/2 = 4.1 h) to acidic medium (t1/2 = 6.1 h; Fig. 5). This finding indicates that the pH-responsive stabilization requires the concurrent binding of HuR and p40AUF1 to the terminal segment of the 3′-UTR of the PEPCK mRNA. This finding also implies that HuR and AUF1 must associate with adjacent binding sites in the PEPCK mRNA.
Fig. 5.
Recruitment of both MS2cp-HuR and MS2cp-p40AUF1 imparts a pH-responsive stabilization of βG-PCK2-MS2 mRNA. Half-life analysis of the βG-PCK2-MS2 reporter RNA in LLC-PK1-F+-9C cells that transiently express MS2cp-HuR and MS2cp-p40AUF1. Cells were maintained in minimal Dox (100 ng/ml) until 70% confluent. Following transient transfection with 2 μg of both expression plasmids, the cells were maintained in normal (pH 7.4) medium minus Dox for 48 h. The cells were then treated with normal (red) or acidic (green) medium for 24 h. RNA was isolated at various times after addition of 1 μg/ml Dox to arrest transcription. The relative levels of β-globin and GAPDH mRNAs were quantified by RT-qPCR. The log of normalized data was then plotted against the time after Dox addition. The reported data are means ± SE of triplicate samples.
Co-knockdown of HuR and AUF1 abolishes the pH-responsive increase in PEPCK mRNA and protein.
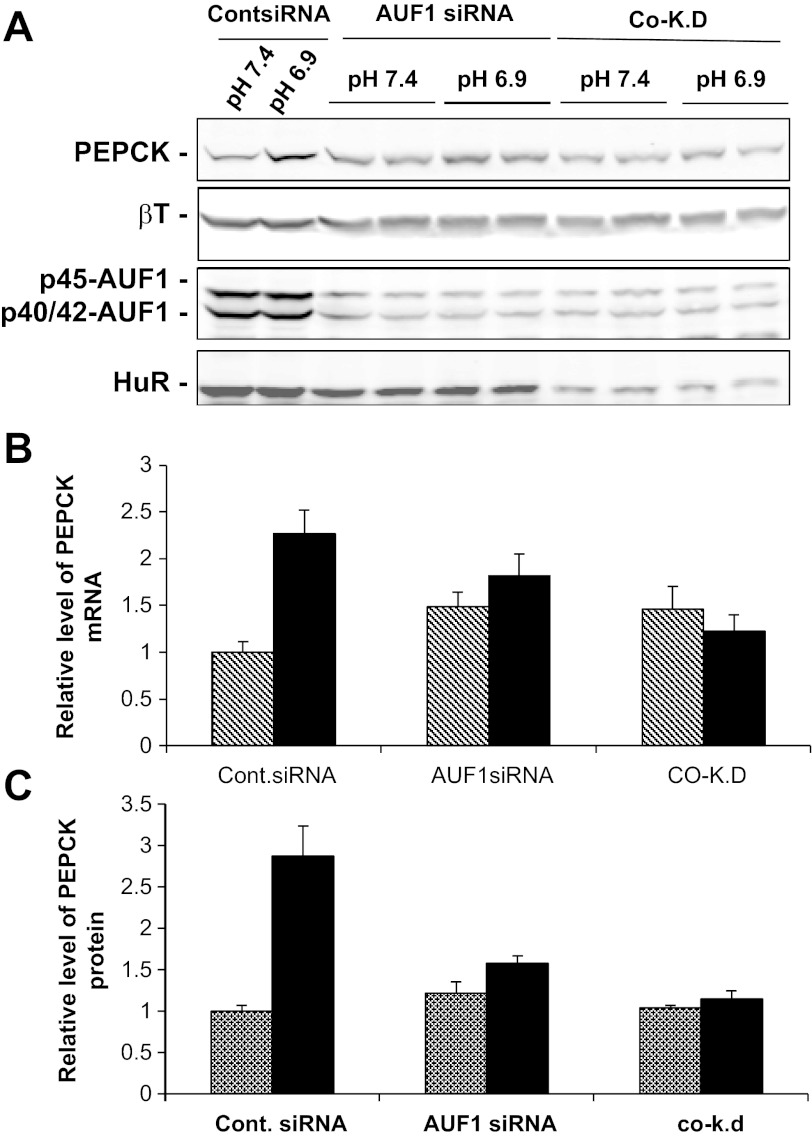
Previous attempts to knockdown AUF1 expression using a single siRNA resulted in only a modest decrease in the three isoforms (39). However, by using a combination of two new siRNAs that are complementary to sequences contained in all of the AUF1 mRNAs, an 80% reduction in total AUF1 protein expression was achieved (Fig. 6A). This level of knockdown produced a slight increase in basal levels of PEPCK mRNA (Fig. 6B) and protein (Fig. 6C) but decreased the pH-responsive increase in PEPCK. Thus, AUF1 may contribute to the rapid degradation of PEPCK mRNA under normal conditions, but it may contribute to the increased expression that occurs in acidic medium. Most importantly, the silencing of both HuR and AUF1 had little effect on the expression in normal medium, but completely blocked the pH-responsive increases of PEPCK mRNA (Fig. 6B) and protein (Fig. 6C). These results support the view that HuR may enhance expression of PEPCK in normal medium, while AUF1 may have an inhibitory effect. However, a cooperative interaction between the two RNA-binding proteins is required to mediate the pH-responsive increase in PEPCK mRNA.
Fig. 6.
Co-knockdown of HuR and AUF1 abolishes the pH-responsive increases in PEPCK mRNA and protein. LLC-PK1-F+-9C cells were transfected with 50 nM control siRNA or 100 nM AUF1 siRNAs in the absence or presence of 30 nM HuR siRNA. After 2 days, the cells were maintained in either normal (pH 7.4) or acidic medium (pH 6.9) for 24 h and then harvested with lysis buffer or TRIzol. A: Western blot analysis was performed to monitor the expression of PEPCK, HuR, AUF1, and βT proteins. B: levels of PEPCK and GAPDH mRNAs were determined by RT-qPCR. C: levels of PEPCK and βT proteins were quantified from the Western blots. The relative levels of PEPCK mRNA (B) and protein (C) in the normal (hatched bars) and acidic samples (filled bars) are means ± SE of triplicate samples.
Two-dimensional gel analyses of potential posttranslational modifications of HuR and AUF1.
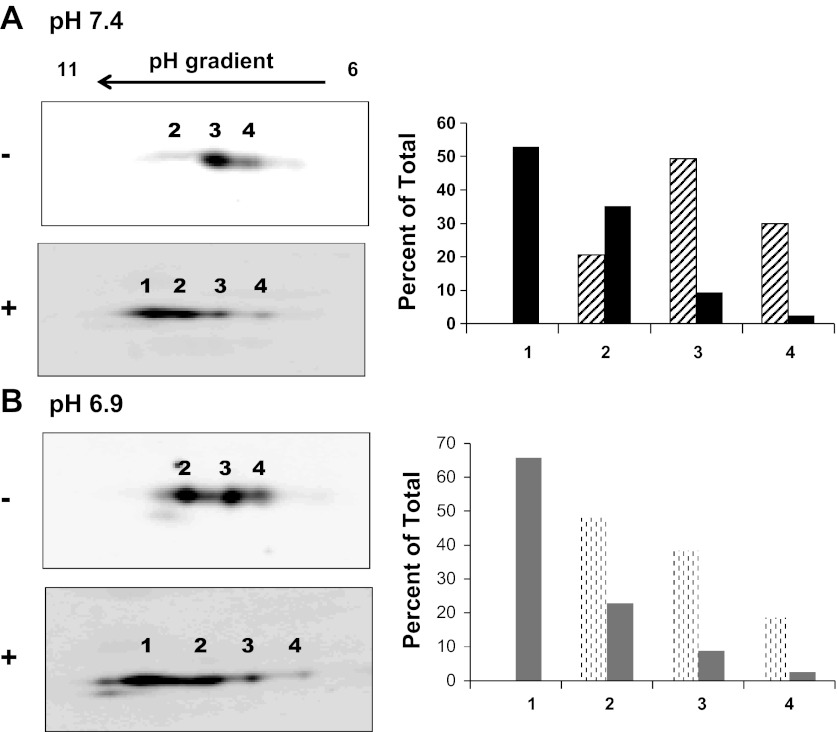
Both the MS2-recruitment studies and the co-knockdown experiments suggest that the pH-responsive stabilization of PEPCK mRNA requires a remodeling of the concurrent binding of HuR and AUF1 to the AREs within the PCK6 and PCK7 segments of the 3′-UTR. However, neither HuR nor AUF1 exhibits a significant increase in expression or cytoplasmic localization when LLC-PK1-F+-9C cells were treated with an acidic medium (39). Therefore, two-dimensional gel electrophoresis and Western blotting were used to assess whether changes in posttranslational modifications may contribute to the stabilizing effect of the bound HuR/AUF1 complex. HuR has a molecular mass of ∼36 kDa and a predicted pI of 9.9. In extracts of LLC-PK1-F+-9C cells that were maintained in normal medium, HuR migrates with the expected molecular mass, but contains three distinct species that differ slightly in isoelectric points (Fig. 7A). The predominant species in normal lysates is the form with the intermediate isoelectric point. When the cell lysate was pretreated with Lambda protein phosphatase, the three species were predominately shifted to a new form that has a more basic pI, consistent with the removal of phosphate groups. When the lysate was prepared from LLC-PK1-F+-9C cells that were treated with acidic medium, the same three species were observed (Fig. 7B). However, the pattern was shifted compared with normal extracts in that the most basic isoform was now the predominant species. Treatment of this lysate with the protein phosphatase again shifted the three charged isoforms to the single more basic form as observed following phosphatase treatment of normal lysates. Therefore, HuR may be phosphorylated at multiple sites in LLC-PK1-F+-9C cells and the treatment of cells with acidic medium may reduce the overall level of phosphorylation of HuR.
Fig. 7.
Two-dimensional gel analysis of the effect of pH on phosphorylation of HuR. Confluent cultures of LLC-PK1-F+-9C cells were treated with normal or acidic medium for 24 h and then lysed with urea and thiourea. The extracts were resolved by 2-dimensional gel electrophoresis using isoelectric focusing strips that ranged from pH 6 to 11. The resulting blots were probed with anti-HuR antibody. Resolution of HuR isoforms in cells treated with normal (A) or acidic (B) medium before (−) or after (+) treatment with protein phosphatase. The intensities of the individual spots in the samples before (hatched bars) or after (filled bars) treatment with protein phosphatase were quantified using an Odyssey Infra-red Imager and graphed as a percent of the total intensity.
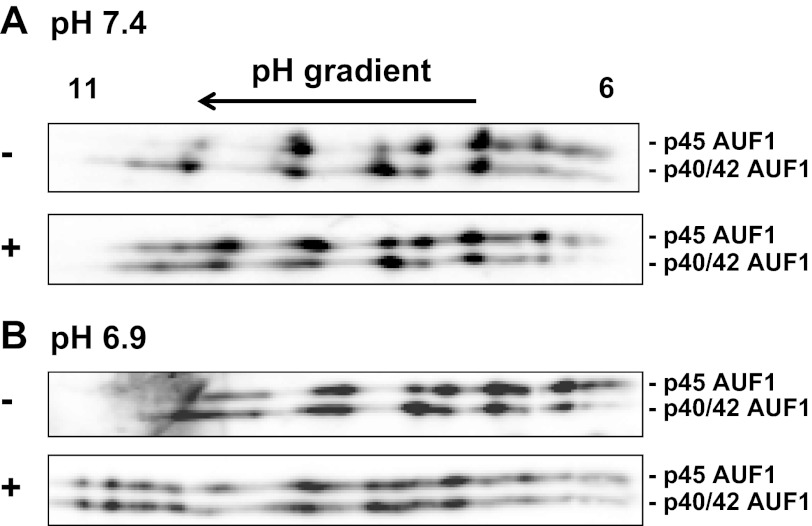
LLC-PK1-F+-9C cells express high levels of p45-, p42-, and p40-AUF1, but very low levels of the p37-isoform. However, the p42- and p40-isoforms comigrate on an SDS gel. Thus, only two apparent molecular weight species were identified when the two-dimensional gels were probed with AUF1 antibodies (Fig. 8). However, each isoform produced multiple variants that differ in apparent pI and that may reflect differences in covalent modifications. Pretreatment of the normal lysate with Lambda protein phosphatase produced only a slight increase in the more basic species of the multiple AUF1 variants. Thus, the complex pattern of AUF1 variants may result primarily from covalent modifications other than phosphorylation. By contrast, a lysate prepared from cells treated with acidic medium exhibits a pattern in which the more acidic variants of both the p45- and p42/p40-AUF1 isoforms are more abundant. When this lysate is pretreated with protein phosphatase, the pattern becomes more similar to that observed for the nonphosphatase- and phosphatase-treated normal lysates. However, additional variants, which migrate with a more basic pI, are also formed (Fig. 8). These data indicate that acidic treatment of LLC-PK1-F+-9C cells may lead to an increase in phosphorylation of the various isoforms of AUF1.
Fig. 8.
Two-dimensional gel analysis of the effect of pH on phosphorylation of AUF1. Confluent cultures of LLC-PK1-F+-9C cells were treated with normal or acidic medium for 24 h and then lysed with urea and thiourea. The extracts were resolved by 2-dimensional gel electrophoresis using isoelectric focusing strips that ranged from pH 6 to 11. The resulting blots were probed with anti-AUF1 antibody. Resolution of AUF1 isoforms in cells treated with normal (A) or acidic (B) medium before (−) or after (+) treatment with protein phosphatase.
DISCUSSION
Previous experiments demonstrated that siRNA silencing of the RNA-binding protein HuR decreased the basal and the pH-stimulated levels of PEPCK mRNA and protein in LLC-PK1-F+-9C cells (39). In the current study, the effect of siRNA knockdown of HuR on the half-life of the endogenous PEPCK mRNA was assessed directly. Transfer of LLC-PK1-F+-9C cells from normal to acidic medium produces a twofold increase in the half-life of PEPCK mRNA (39). However, following the knockdown of HuR, the endogenous PEPCK mRNA decayed with same half-life (t1/2 = 4.0 and 4.5 h) in cells treated with normal or acidic medium, respectively (Fig. 2). This finding indicates that HuR is at least one of the trans-acting factors that are required for the pH-responsive stabilization of PEPCK mRNA. The loss of the pH-responsive stabilization caused only a slight decrease in the fold increase in PEPCK mRNA and protein that occur when cells are transferred from normal to acidic medium. The residual response is probably due to an increased transcription of the PCK1 gene (20, 40). The combined analyses also indicated that the observed decrease in basal expression of PEPCK mRNA and protein was not due to a more rapid decay of the PEPCK mRNA. Thus, the binding of HuR may also enhance the processing, nuclear export, and/or translation of the PEPCK mRNA. These observations are consistent with the accepted roles of HuR in enhancing the translation and stability of its cognate mRNAs (7, 34).
By contrast, siRNA-mediated silencing of AUF1 caused a slight increase in the basal levels of PEPCK mRNA and protein, but partially inhibited the pH-responsive increases that normally occur when the cells are transferred to an acidic medium (Fig. 6). Not surprisingly, the co-knockdown of HuR and AUF1 has offsetting effects on basal expression of PEPCK mRNA and protein. However, the concurrent reduction of both RNA-binding proteins completely blocked the pH-responsive increases in the levels of PEPCK mRNA and protein. Therefore, HuR and AUF1 may impart opposing effects on the basal expression of PEPCK mRNA, but a cooperative interaction between the two RNA-binding proteins may be required to mediate the pH-responsive increase in renal PEPCK. However, the observation that the knockdown of AUF1 alone reduced the pH-responsive increase in PEPCK expression was surprising and is difficult to explain. A possible explanation is that AUF1 may also contribute to the increased transcription of the PCK1 gene. This hypothesis is supported by recent studies, which demonstrated that the nuclear isoforms of AUF1, p42 and p45, bind with high affinity to the promoter and strongly activate transcription of the catalytic subunit of telomerase (42).
An MS2 recruitment assay was developed to further assess the prerequisite for coordinate binding of HuR and AUF1. Previous studies used reporter mRNAs containing multiple MS2-binding elements to characterize the function of proteins involved in mRNA processing, trafficking, localization, and decay (4, 10). Various RNA-binding proteins affect mRNA turnover by either recruiting or blocking the recruitment of a deadenylase and the cellular complexes that mediate the degradation of the mRNA. Thus, the function of the MS2-MS2cp recruitment is simply to experimentally position the identified binding proteins on the 3′-UTR of a reporter mRNA that is not pH-responsive so they can form the protein/protein interactions that are necessary for stabilization. To study mRNA stabilization, it was necessary to design a reporter mRNA that decayed with a half-life that was easily quantified and could still be used to detect a significant stabilization. This required the inclusion of the PCK2 segment that constitutes the 5′-half of the 3′-UTR of the PEPCK mRNA and contains an instability element (23). The resulting reporter mRNA, βG-PCK2-MS2, was identical to the βG-PCK-1 reporter mRNA, which exhibits a pH-responsive stabilization, except that the portion of the 3′-UTR of PEPCK mRNA that contains the pH response elements was replaced with six copies of the MS2-binding element. The two reporter mRNAs decayed with similar half-lives when the cells were grown in normal medium. However, by contrast to the βG-PCK1 mRNA, the βG-PCK2-MS2 mRNA was not stabilized when the cells were transferred to acidic medium. In addition, the individual recruitment of MS2cp-HuR or MS2cp-p40AUF1 failed to produce a pH-responsive stabilization of the βG-PCK2-MS2 mRNA. However, the coexpression of the two fusion proteins produced a 1.5-fold increase in the half-life of the reporter mRNA when cells were transferred to acidic medium. The observed stabilization was both reproducible and significant. Thus, the observed pH-responsive stabilization of the βG-PCK2-MS2 mRNA strongly supports the hypothesis that the concurrent association and probable interaction of HuR and p40AUF1 within the terminal segment of the 3′-UTR are necessary to mediate the pH-responsive stabilization of PEPCK mRNA in LLC-PK1-F+-9C cells. The observed stabilization is slightly less than the twofold stabilization observed with the endogenous PEPCK mRNA or the βG-PCK1 reporter mRNA. There are numerous possibilities as to why the recruitment of the tethered RNA-binding proteins may be less than optimal compared with the direct binding of HuR and AUF1 to the normal pH response element. For example, the RNA-binding affinities of HuR and AUF1 are affected by changes in phosphorylation (2, 15, 50). This effect would not be reproduced in the recruitment assay.
The expression and the nucleocytoplasmic shuttling of HuR or AUF1 are unaltered when LLC-PK1-F+-9C cells are transferred from normal to acidic medium (39). Therefore, two-dimensional gel electrophoresis was used to determine whether HuR or AUF1 underwent pH-responsive changes in covalent modification. Isoelectric focusing of HuR identified three variants, each of which has a pI that is more acidic than the unmodified protein. Treatment of the cell lysates with a protein phosphatase produced a pronounced shift to a new variant that had a more basic pI, consistent with the removal of multiple phosphates. Comparison of the relative abundance of the three variants indicated that treatment of cells with acidic medium caused a decrease in phosphorylation of HuR. Previous studies identified 14 sites of phosphorylation in HuR that occur in various types of cells in response to multiple stimuli that activate different signaling pathways (1, 15). The various modifications have been reported to affect the binding affinity of HuR with various mRNAs, its nucleocytoplasmic distribution, and its ability to stabilize specific mRNAs. For example, oxidative stress promotes the dissociation of HuR from the SIRT1 mRNA causing its more rapid degradation (2). Analysis of the effects of expressing various nonphosphorylatable mimetics of HuR demonstrated that phosphorylation on T118 and S88 promoted HuR binding, while phosphorylation of S100 inhibited its interaction with the SIRT1 mRNA. Thus, a change in phosphorylation of HuR may also affect its interaction with the PEPCK mRNA. Unfortunately, neither the sites nor the kinases that mediate the phosphorylation of HuR in LLC-PK1-F+-9C cells are currently known.
Previous studies used two-dimensional gel electrophoresis to assess changes in covalent modifications of AUF1 in RAW 264.7 cells by lipopolysaccharide stimulation (9) and in parathyroid cells upon changes in serum Ca2+ and phosphate (5). The previous analyses also concluded that each isoform of AUF1, produced by alternative splicing, undergoes extensive and variable covalent modifications to produce multiple variants. Thus, the complex pattern observed with lysates of LLC-PK1-F+-9C cells is consistent with the previous reports. However, the lysates from the cells treated with acidic medium exhibit a significant increase in the variants that have a more acidic pI. This shift was reversed by treatment with protein phosphatase, consistent with the removal of phosphate groups. By contrast, protein phosphatase treatment of the normal lysates had little effect on the observed pattern of variants. Thus, multiple variants of AUF1 may be produced by covalent modification other than phosphorylation. However, treatment with an acidic medium may result in an increase in phosphorylation of AUF1.
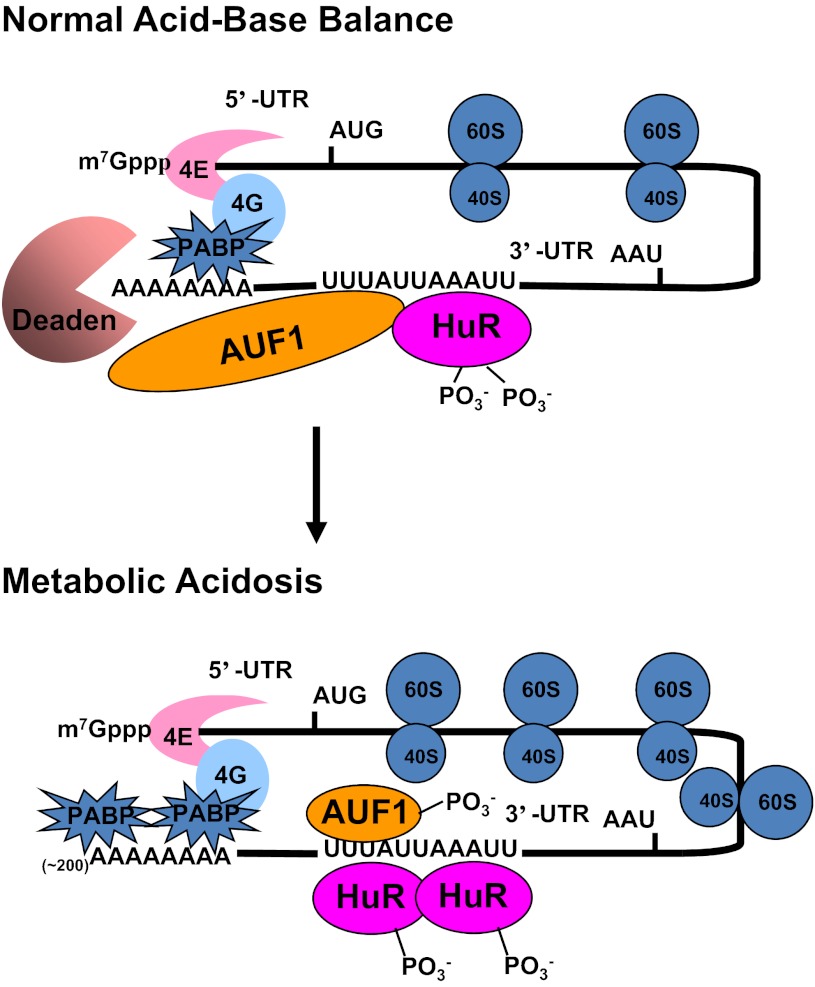
The combined analyses suggest that opposite changes in phosphorylation of HuR and AUF1 may promote the stabilization of the PEPCK mRNA in response to conditions that model a metabolic acidosis. We recently developed a novel RNA pull-down assay that utilizes the expression of chimeric Halo-tag constructs of HuR and AUF1. This analysis established that HuR and AUF1 form a complex in intact LLC-PK1-F+-9C cells and that the binding of HuR to the 3′-UTR of PEPCK mRNA is increased when the cells are treated with acidic medium (unpublished data of Gummadi L, Taylor L, and Curthoys NP). Thus, the following hypothesis is proposed to model our current understanding of the pH-responsive stabilization of PEPCK mRNA (Fig. 9). During normal acid-base balance, both HuR and AUF1 bind to the 3′-UTR of PEPCK mRNA. The associated AUF1 recruits a deadenylase that promotes deadenylation and initiates the more rapid degradation of the PEPCK mRNA. The onset of acidosis leads to changes in phosphorylation of the two RNA-binding proteins and an increased binding of HuR. The remodeled complex is less effective in recruitment of the deadenylase and thus leads to stabilization of the PEPCK mRNA. Further documentation of this hypothesis will require identification of the sites of covalent modification of HuR and AUF1 that occur in LLC-PK1-F+-9C cells.
Fig. 9.
Current model for the pH-responsive stabilization of PEPCK mRNA. Interactions between the cap binding proteins (4E and 4G) and the polyA binding protein (PABP) cause a mRNA to form a circular structure that enhances translation. During normal acid-base balance, both HuR and AUF1 bind to the AU-rich sequences within the 3′-UTR of the PEPCK mRNA that function as a pH response element. The resulting complex recruits a deadenylase (Deaden) that removes the polyA tail and causes dissociation of the PABPs. The deadenylated mRNA is rapidly degraded from the either the 3′-end by the exosome or the 5′-end by decapping and degradation in processing bodies. In response to metabolic acidosis, the levels of phosphorylation of HuR and AUF1 are decreased and increased, respectively. These changes lead to increased binding of HuR and a remodeling of the HuR/AUF1 complex that is bound to the 3′-UTR of PEPCK mRNA. The new complex is less effective at recruiting the Deaden and thereby promotes stabilization of the PEPCK mRNA.
GRANTS
This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-37124 and DK-75517 awarded to N. P. Curthoys.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.G., L.T., and N.P.C. performed experiments; L.G. and L.T. analyzed data; L.G., L.T., and N.P.C. interpreted results of experiments; L.G. and N.P.C. prepared figures; L.G. drafted manuscript; L.G., L.T., and N.P.C. approved final version of manuscript; L.T. and N.P.C. edited and revised manuscript; N.P.C. conception and design of research.
ACKNOWLEDGMENTS
We thank Alisa Shaw for assistance with the two-dimensional gel electrophoresis experiments.
REFERENCES
- 1. Abdelmohsen K, Gorospe M. Posttranscriptional regulation of cancer traits by HuR. WIRE RNA 1: 214–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker A, Epis MR, Porter CJ, Hopkins BR, Wilce MC, Wilce JA, Giles KM, Leedman PJ. Sequence requirements for RNA binding by HuR and AUF1. J Biochem 151: 423–437, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Baron-Benhamou J, Gehring NH, Kulozik AE, Hentze MW. Using the lambdaN peptide to tether proteins to RNAs. Methods Mol Biol 257: 135–154, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bell O, Gaberman E, Kilav R, Levi R, Cox KB, Molkentin JD, Silver J, Naveh-Many T. The protein phosphatase calcineurin determines basal parathyroid hormone gene expression. Mol Endocrinol 19: 516–526, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7. Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 58: 266–277, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science 265: 615–621, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Cok SJ, Acton SJ, Sexton AE, Morrison AR. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J Biol Chem 279: 8196–8205, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Coller J, Wickens M. Tethered function assays using 3′ untranslated regions. Methods 26: 142–150, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Curthoys NP. Renal ammonium ion production and excretion. In: The Kidney: Physiology and Pathophysiology, 4th Edition, edited by Alpern RJ, Hebert SC. New York: Elsevier, 2007, p. 1601–1619 [Google Scholar]
- 12. Curthoys NP, Gstraunthaler G. Mechanism of increased renal gene expression during metabolic acidosis. Am J Physiol Renal Physiol 281: F381–F390, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Curthoys NP, Taylor L, Hoffert JD, Knepper MA. Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. Am J Physiol Renal Physiol 292: F140–F147, 2007 [DOI] [PubMed] [Google Scholar]
- 14. DeMaria CT, Brewer G. AUF1 binding affinity to A+U-rich elements correlates with rapid mRNA degradation. J Biol Chem 271: 12179–12184, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Doller A, Akool el S, Huwiler A, Muller R, Radeke HH, Pfeilschifter J, Eberhardt W. Posttranslational modification of the AU-rich element binding protein HuR by protein kinase Cdelta elicits angiotensin II-induced stabilization and nuclear export of cyclooxygenase 2 mRNA. Mol Cell Biol 28: 2608–2625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doller A, Huwiler A, Muller R, Radeke HH, Pfeilschifter J, Eberhardt W. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2. Mol Biol Cell 18: 2137–2148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ehrenman K, Long L, Wagner BJ, Brewer G. Characterization of cDNAs encoding the murine A+U-rich RNA-binding protein AUF1. Gene 149: 315–319, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Fan XC, Steitz JA. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA 95: 15293–15298, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17: 3448–3460, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feifel E, Obexer P, Andratsch M, Euler S, Taylor L, Tang A, Wei Y, Schramek H, Curthoys NP, Gstraunthaler G. p38 MAPK mediates acid-induced transcription of PEPCK in LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 283: F678–F688, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci USA 97: 3073–3078, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gstraunthaler G, Holcomb T, Feifel E, Liu W, Spitaler N, Curthoys NP. Differential expression and acid-base regulation of glutaminase mRNAs in gluconeogenic LLC-PK1-FBPase+ cells. Am J Physiol Renal Physiol 278: F227–F237, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Hajarnis S, Schroeder JM, Curthoys NP. 3′-Untranslated region of phosphoenolpyruvate carboxykinase mRNA contains multiple instability elements that bind AUF1. J Biol Chem 280: 28272–28280, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Halperin ML. Metabolic aspects of metabolic acidosis. Clin Invest Med 16: 294–305, 1993 [PubMed] [Google Scholar]
- 25. Hwang JJ, Curthoys NP. Effect of acute alterations in acid-base balance on rat renal glutaminase and phosphoenolpyruvate carboxykinase gene expression. J Biol Chem 266: 9392–9396, 1991 [PubMed] [Google Scholar]
- 26. Hwang JJ, Perera S, Shapiro RA, Curthoys NP. Mechanism of altered renal glutaminase gene expression in response to chronic acidosis. Biochemistry 30: 7522–7526, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Renal Physiol 291: F1255–F1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keene JD. Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc Natl Acad Sci USA 96: 5–7, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol 29: 4341–4351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23: 3092–3102, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laroia G, Schneider RJ. Alternate exon insertion controls selective ubiquitination and degradation of different AUF1 protein isoforms. Nucleic Acids Res 30: 3052–3058, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LeCuyer KA, Behlen LS, Uhlenbeck OC. Mutants of the bacteriophage MS2 coat protein that alter its cooperative binding to RNA. Biochemistry 34: 10600–10606, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 273: 6417–6423, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Lopez de Silanes I, Gorospe M, Taniguchi H, Abdelmohsen K, Srikantan S, Alaminos M, Berdasco M, Urdinguio RG, Fraga MF, Jacinto FV, Esteller M. The RNA-binding protein HuR regulates DNA methylation through stabilization of DNMT3b mRNA. Nucleic Acids Res 37: 2658–2671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101: 2987–2992, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lykke-Andersen J, Shu MD, Steitz JA. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103: 1121–1131, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Masuda K, Abdelmohsen K, Kim MM, Srikantan S, Lee EK, Tominaga K, Selimyan R, Martindale JL, Yang X, Lehrmann E, Zhang Y, Becker KG, Wang JY, Kim HH, Gorospe M. Global dissociation of HuR-mRNA complexes promotes cell survival after ionizing radiation. EMBO J 30: 1040–1053, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McMullen MR, Cocuzzi E, Hatzoglou M, Nagy LE. Chronic ethanol exposure increases the binding of HuR to the TNFalpha 3′-untranslated region in macrophages. J Biol Chem 278: 38333–38341, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mufti J, Hajarnis S, Shepardson K, Gummadi L, Taylor L, Curthoys NP. Role of AUF1 and HuR in the pH-responsive stabilization of phosphoenolpyruvate carboxykinase mRNA in LLC-PK-F cells. Am J Physiol Renal Physiol 301: F1066–F1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O′Hayre M, Taylor L, Andratsch M, Feifel E, Gstraunthaler G, Curthoys NP. Effects of constitutively active and dominant negative MAPK kinase (MKK) 3 and MKK6 on the pH-responsive increase in phosphoenolpyruvate carboxykinase mRNA. J Biol Chem 281: 2982–2988, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Palanisamy V, Park NJ, Wang J, Wong DT. AUF1 and HuR proteins stabilize interleukin-8 mRNA in human saliva. J Dental Res 87: 772–776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pont AR, Sadri N, Hsiao SJ, Smith S, Schneider RJ. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Mol Cell 47: 5–15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raineri I, Wegmueller D, Gross B, Certa U, Moroni C. Roles of AUF1 isoforms, HuR and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res 32: 1279–1288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schroeder JM, Ibrahim H, Taylor L, Curthoys NP. Role of deadenylation and AUF1 binding in the pH-responsive stabilization of glutaminase mRNA. Am J Physiol Renal Physiol 290: F733–F740, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Stern R. Fluid, electrolytes and acid-base disturbances. NephSAPTM 3: 192–231, 2004 [Google Scholar]
- 46. Tannen RL, Sahai A. Biochemical pathways and modulators of renal ammoniagenesis. Miner Electrolyte Metab 16: 249–258, 1990 [PubMed] [Google Scholar]
- 47. Wagner BJ, DeMaria CT, Sun Y, Wilson GM, Brewer G. Structure and genomic organization of the human AUF1 gene: alternative pre-mRNA splicing generates four protein isoforms. Genomics 48: 195–202, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Wagner CA. Metabolic acidosis: new insights from mouse models. Curr Opin Nephrol Hypertens 16: 471–476, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol 20: 760–769, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson GM, Lu J, Sutphen K, Suarez Y, Sinha S, Brewer B, Villanueva-Feliciano EC, Ysla RM, Charles S, Brewer G. Phosphorylation of p40AUF1 regulates binding to A + U-rich mRNA-destabilizing elements and protein-induced changes in ribonucleoprotein structure. J Biol Chem 278: 33039–33048, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Yaman I, Fernandez J, Sarkar B, Schneider RJ, Snider MD, Nagy LE, Hatzoglou M. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem 277: 41539–41546, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang W, Wagner BJ, Ehrenman K, Schaefer AW, DeMaria CT, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol 13: 7652–7665, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]