Abstract
Ca-activated K channels (BK), which are stimulated by high distal nephron flow, are utilized during high-K conditions to remove excess K. Because BK predominantly reside with BK-β4 in acid/base-transporting intercalated cells (IC), we determined whether BK-β4 knockout mice (β4KO) exhibit deficient K excretion when consuming a high-K alkaline diet (HK-alk) vs. high-K chloride diet (HK-Cl). When wild type (WT) were placed on HK-alk, but not HK-Cl, renal BK-β4 expression increased (Western blot). When WT and β4KO were placed on HK-Cl, plasma K concentration ([K]) was elevated compared with control K diets; however, K excretion was not different between WT and β4KO. When HK-alk was consumed, the plasma [K] was lower and K clearance was greater in WT compared with β4KO. The urine was alkaline in mice on HK-alk; however, urinary pH was not different between WT and β4KO. Immunohistochemical analysis of pendrin and V-ATPase revealed the same increases in β-IC, comparing WT and β4KO on HK-alk. We found an amiloride-sensitive reduction in Na excretion in β4KO, compared with WT, on HK-alk, indicating enhanced Na reabsorption as a compensatory mechanism to secrete K. Treating mice with an alkaline, Na-deficient, high-K diet (LNaHK) to minimize Na reabsorption exaggerated the defective K handling of β4KO. When WT on LNaHK were given NH4Cl in the drinking water, K excretion was reduced to the magnitude of β4KO on LNaHK. These results show that WT, but not β4KO, efficiently excretes K on HK-alk but not on HK-Cl and suggest that BK-α/β4-mediated K secretion is promoted by bicarbonaturia.
Keywords: maxi K, collecting duct, intercalated cells, acidosis, alkalosis, potassium secretion, distal nephron
modern day humans ingest a diet of high-Na, low-K, and high-acid content; however, some remote populations, such as the Yanomami of South America, consume a low-Na, high-K, alkaline diet, similar to ancient man. The low-Na and high-alkaline content of the Yanomami diet indicates that the elimination of K may not depend on distal Na reabsorption but rather on HCO3 secretion, which could also generate a lumen negative potential that could drive K transport in the distal nephron.
Connecting tubule cells and principal cells (PC) of the cortical collecting ducts contain the assembly of epithelial Na channels (ENaC) and renal outer medullary K channels (ROMK) in the apical membrane in series with the Na-K-ATPase in the basolateral membrane; an electrophysiological arrangement that explains Na-dependent K secretion. However, in conditions of high-K intake distal flow is stimulated (19, 23, 24) primarily because of K recycling and inhibition of Na and Cl reabsorption in the thick ascending limb (50). High flow stimulates large, Ca-activated K channels (BK) to secrete K, as demonstrated ex vivo with isolated tubule perfusion (51, 58) and in vivo by volume expansion (38), genetically eliminating ROMK (2), pharmacologic blockade of vasopressin (V2) receptors (41), or treating with a high-K diet (24). Moreover, the arrangement of only ENaC and ROMK in series with the Na-K-ATPase would substantially limit the amount of distal K secretion to a ratio of only two secreted K per three absorbed Na, which is the ratio of the Na-K pump. Isolated perfused tubule studies (rabbit cortical collecting ducts) using electrophysiological techniques have indicated that the ratio of K secreted to Na absorbed can far exceed the pump ratio in high-aldosterone (DOCA-treated) conditions (45); however, the mechanism has never been resolved.
ROMK secrete K under the aldosterone-regulated Na for K exchange mechanism in the distal nephron (11, 16, 59). BK secrete K in the distal nephron during conditions of high-K intake and may also secrete K during conditions of low-Na delivery in a “Na-independent” manner. A recent study showed that blockers of luminal BK or basolateral Na-H exchange inhibited Na-independent K secretion (31). After cell entry, Na would stimulate Na-K-ATPase and drive K into the cell. However, most BK reside in the Na-K-ATPase-deficient intercalated cells (IC) of the distal nephron (24, 34). Moreover, if Na-independent K secretion involves Na-H exchange, the generated HCO3 would require a cellular exit pathway, such as the Cl/HCO3 exchangers of IC.
Although the β1-subunit (BK-β1) is localized in PC (17, 37, 39), IC contain BK-α with the BK-β4 subunit as the BK-α/β4 (17). When placed on a high-K diet, mice with a knockout of the BK-β4 (β4KO) exhibit attenuated K excretion (23). Moreover, the BK-α/β4 of IC secrete K as a counter cation to negative ATP ions extruded from IC (22). BK-α/β4 also reside in MDCK-C11 cells, which have many properties consistent with IC of the distal nephron (22). However, in vivo evidence has been elusive for a role for BK-α/β4 in the steady-state secretion of K under conditions of high-K intake.
We have shown previously that the pore-forming BK-α is expressed in the IC of the distal nephron, and its expression is enhanced by a high-K alkaline diet (24). However, it was not determined whether the associated BK-β4 subunit, also found predominantly in the IC, was enhanced by a high-K alkaline vs. high-K acidic diet. The present studies were performed to determine whether the associated alkaline anionic content of the Yanomami diet played a role in eliminating a high-K load with nominally free Na content and whether the BK-α/β4 have a role in eliminating the high-K alkaline diet, as opposed to the high-K chloride diet. To achieve this objective, we determined the effects of high-K alkaline vs. a high-K acidic diet on expression of BK-β4, and we studied the K and Na balance of WT and β4KO mice on diets with varied Na, K, and alkaline contents. These results contribute to our understanding between the interaction between K secretion and acid/base transport in the distal nephron.
METHODS
Animal studies.
We maintained the mice in accordance with the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Mice had full access to water at all times. For all experiments, we fed 12- to 20-wk-old WT (C57Bl/6, Charles River, Wilmington, MA) and β4KO (generously provided by R. Brenner) mice either regular mouse chow (control; 0.6% K+, 0.32% Na+) or one of several special diets (Harlan Teklad, Madison, WI) for 7–10 days before death. Special diets were either high K with alkaline anions (no. TD.07278; HK-alk; 5.0% K+ with 5% of equal carbonate/citrate/Cl and 0.32% Na+), high K with Cl as the counter anion (no. TD.09075; HK-Cl; 5.0% K with 5% Cl, 0.32% Na), or a low-Na, high-K diet with alkaline anions (no. TD.08240; LNaHK; 5% K with 5% equal molar carbonate/citrate/Cl and 0.01% Na). A subset of mice on LNaHK was supplied with NH4Cl (280 mM) plus sucrose (2%) in their drinking water to induce an acid load. We collected urine samples using metabolic cages (Nalgene), as previously described (23). After treatment, we collected fresh urine samples for analysis of Na and K with a flame photometer (Jenway Clinical PFP7) as previously described (19) and for pH and osmolality measurements using a model 215 pH meter (Denver Instruments) and model 3250 osmometer (Advanced Instruments). At death, we extracted blood from the carotid artery, measured hematocrit, and centrifuged for measurement of plasma K, Na, and osmolality.
The transtubular K gradient (TTKG) was calculated as: (U[K]/P[K])(Posm/Uosm), where UK and PK are the urinary K concentration ([K]) and plasma [K], respectively, and Uosm and Posm are the urinary and plasma osmolalities, respectively.
Some WT and β4KO were treated with HK-alk + amiloride (5 mg·kg−1·day−1) or HK-alk + vehicle dissolved in water via osmotic pumps (ALZET, DURECT), implanted subcutaneously in the back, for 48 h until death. The vehicle values (n >3) were combined with control values when they were not significantly different from controls.
Western blotting.
Western blotting was performed as described previously (22, 23) following manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA) except RIPA buffer was replaced with PBS containing 0.5% SDS. Primary antibodies included anti-BK-β4 at 24 kDa (rabbit polyclonal, diluted 1:500; Alomone Labs) and anti-β-actin at 43 kDa (mouse monoclonal, diluted 1:5,000; Santa Cruz) with either goat anti-rabbit IgG or donkey anti-mouse IgG conjugated horseradish peroxidase secondary antibody (diluted 1:20,000–1:40,000; Santa Cruz). Expression of primary antibodies was quantified by densitometry using Quantity One (Bio-Rad).
Immunohistochemical staining and quantification.
For fluorescent immunohistochemical (IHC) staining of kidney sections, the kidneys were harvested, immediately fixed in Histochoice MB (Electron Microscopy Sciences, Hatfield, PA), embedded in paraffin, and sectioned onto slides for IHC as previously performed in our laboratory (18). Antibodies were used as follows: anti-V-ATPase (goat polyclonal, diluted 1:100; Santa Cruz), anti-aquaporin 3 (goat polyclonal, diluted 1:100; Santa Cruz), and anti-pendrin (mouse monoclonal, diluted 1:200; MBL). After tissue was washed, we incubated it for 1 h (23°C) in the dark with the secondary antibody (donkey anti-rabbit IgG conjugated Alexa Fluor 488 and donkey anti-goat IgG conjugated Alexa Fluor 594, diluted 1:200). The coverslips were mounted onto slides overnight with Prolong Gold (Invitrogen), and sealed with nail polish. These were viewed on a Leica HC fluorescence microscope with a ×40/0.75NAHCXPL Fluotar objective. Images were captured with an QImaging Retiga EXi CCD camera (Surrey, BBC, Canada) and analyzed with ImageJ software (version 1.42; National Institutes of Health, Bethesda, MD). Quantification of V-ATPase signal intensity in IC apical and basolateral membranes was determined following online instructions in single-channel, gray scale images after background correction as performed previously in this laboratory (23).
RESULTS
BK expression with HK-alk vs. HK-Cl.
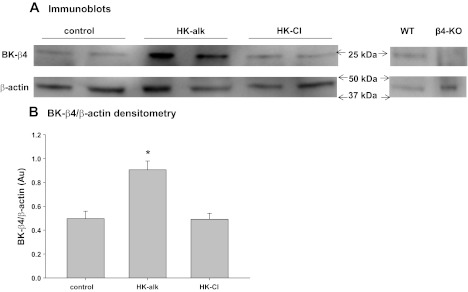
It was shown previously that the pore-forming BK-α subunit, predominantly expressed in the IC of the distal nephron, was intensified in mice on HK-alk by Western blot (24). We performed experiments to determine whether the BK-β4, expressed with the BK-α in IC (17), was enhanced in the renal cortex of mice on a HK-alk as opposed to HK-Cl. As shown in the representative blot of Fig. 1A, The BK-β4 antibody did not detect protein at the size of BK-β4 in β4KO mice. As shown in the summary bar graph of Fig. 1B the expression of BK-β4/β-actin is 0.91 ± 0.07 (n = 5) in HK-alk mice, which was significantly greater (by 82%) than mice treated with control diet (0.50 ± 0.06; n = 5) and mice treated with HK-Cl (0.49 ± 0.05; n = 5). These results indicate that the BK-α/β4 channel has a role to excrete K when the high-K diet is alkaline but not when the diet is acidic.
Fig. 1.
A: Western blots of Ca-activated K channels (BK)-β4 (24 kDA) and β-actin (43 kDA) from mice on a control, high-K alkaline (HK-alk), and high-K chloride (HK-Cl) diet. Negative control shows absence of BK-β4 in β4-knockout (β4KO) kidneys. B: summary bar plot of BK-β4/β-actin expression in control and HK-alk- and HK-Cl-treated mice. WT, wild type. *P < 0.05, using one-way ANOVA plus Student-Newman-Keuls (SNK) test.
Potassium excretion with HK-alk vs. HK-Cl.
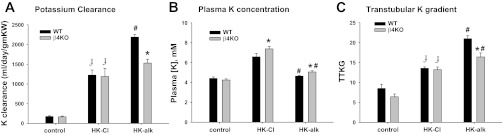
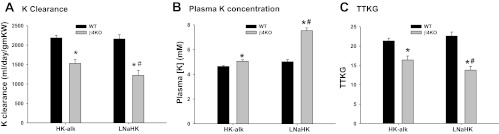
We determined the ability of WT and β4KO to excrete K when consuming a high-K diet with HCO3 (HK-alk) as the major counter anion as opposed to Cl (HK-Cl) for 7–10 days. Figure 2 shows the K clearance (Fig. 2A), plasma K concentration (Fig. 2B), and TTKG (Fig. 2C) for WT and β4KO on control diet, HK-Cl and HK-alk. As shown, the K clearances (Fig. 2A) of 176 ± 18 ml·day−1·g−1 kidney wt (KW) (n = 7) for WT was not significantly different from that of β4KO (167 ± 19 ml·day−1·g−1 KW; n = 10) on a control diet. When placed on HK-Cl for 7–10 days, the K clearances for WT and β4KO were significantly elevated, compared with control, to similar values of 1,045 ± 60 (n = 5) and 1,188 ± 208 ml·day−1·g−1 KW (n = 5), respectively. However, when placed on HK-alk, the K clearance of WT was 2,187 ± 116 ml/day/gm KW (n = 34), which was significantly greater than the K clearance of WT on HK-Cl and β4KO on HK-alk (1,530 ± 97 ml·day−1·g−1 KW; n = 22).
Fig. 2.
Bar plots illustrating differences in K clearance (A), plasma K concentration ([K]; B), and transtubular K gradient (TTKG; C) between wild-type (WT) and (β4KO) mice on control, HK-Cl, and HK-alk diets. ɟP < 0.001, compared with control diet; *P < 0.001, compared with WT; #P < 0.001, compared with HK-Cl using one-way ANOVA plus SNK test.
The plasma K concentrations of WT and β4KO on HK-Cl and HK-alk are shown in Fig. 2B. As shown, the plasma K concentrations of both WT and β4KO on HK-Cl were similar, with values of 7.17 ± 0.26 mM (n = 6) and 7.38 ± 0.27 mM (n = 5), respectively, which were significantly greater than the plasma K concentrations of mice on control diets. When placed on HK-alk. The plasma K concentration of β4KO was 5.06 ± 0.12 mM (n = 22), a value significantly greater than 4.64 ± 0.07 mM (n = 33); both values were significantly less than the respective values on HK-Cl.
The TTKG is a calculation of the luminal to plasma K concentration ratio after secretion/reabsorption of K and before water extraction in the collecting ducts. As shown is Fig. 2C, there was no significant difference in TTKG between WT (8.5 ± 1.1; n = 9) and β4KO (6.4 ± 0.7; n = 9) on a control diet. When placed on HK-Cl, the TTKGs of WT and β4KO were significantly increased to 12.9 ± 0.7 (n = 6) and 13.2 ± 0.7 (n = 5), respectively. However, when placed on HK-alk, the TTKG of WT increased to a significantly greater value of 21.0 ± 0.7 (n = 24), compared with WT on HK-Cl, and β4KO on HK-alk, which was 16.4 ± 1.0 (n = 9).
Effects of HK-alk and HK-Cl on IC profile of WT and β4KO.
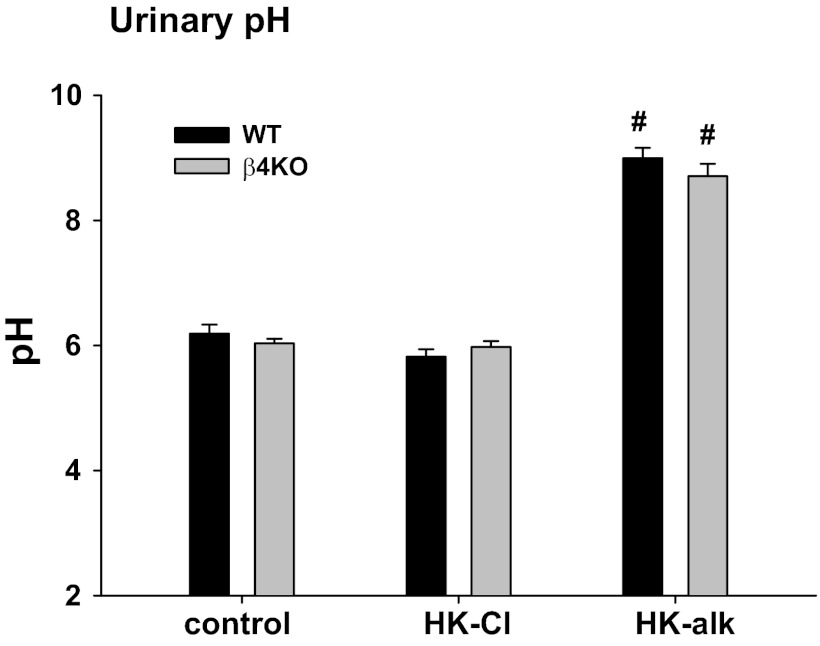
Previous studies revealed that the number of cells identified as α-IC increase in acidosis (46). Moreover, the Cl/HCO3 exchanger (pendrin), identified on the apical membrane of β-IC, exhibits increased expression in the distal nephron when mice consume HCO3 orally (21, 55). We first determined the ability of HK-alk to alkalinize the urine. As shown in Fig. 3, the urinary pH on a control diet was similar in WT and β4KO with values of 6.19 ± 0.14 (n = 6) and 6.04 ± 0.07 (n = 7), respectively. The urinary pH values for WT and β4KO on HK-Cl were 5.97 ± 0.14 (n = 9) and 5.98 ± 0.09 (n = 6), respectively, which were not different from the respective pH values for WT and β4KO on the control diet. When placed on HK-alk, the urinary pH values of WT and β4KO were significantly elevated, compared with HK-Cl; however, the value for WT (9.00 ± 0.16; n = 7) was not different than that of β4KO (8.71 ± 0.20; n = 13).
Fig. 3.
Bar plots illustrating the urinary pH values of WT and β4KO mice on control, HK-Cl and HK-alk diets. Symbols are same as Fig. 2.
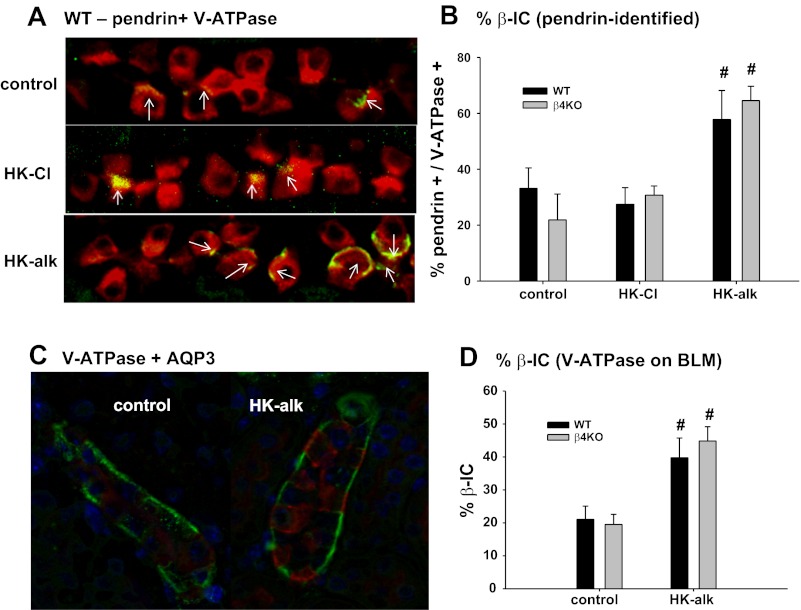
We used double IHC staining with anti-pendrin and anti-V-ATPase to determine whether mice on HK-alk, compared with HK-Cl, elicited an increase in the number of pendrin-marked cells. Pendrin-identified cells mark β-IC plus non-A-non-B cells (26). We used kidneys from four different mice in each group (control, HK-Cl, and HK-alk), with five sections of each kidney for an n of 20 in each group. The means of each group represent the percentage of pendrin-positive cells per V-ATPase positive cells, which mark all IC cells. As shown by the representative kidney sections (Fig. 4A; WT only shown) and the summary bar plots (Fig. 4B), the proportion of pendrin-positive cells for control diet WT and β4KO mice was 34.0 ± 7.3% (n = 18) and 21.0 ± 9.3%, respectively, of the total cells with positive staining for V-ATPase. When mice were placed on HK-Cl, the proportion of β-IC was 27.4 ± 5.9% for WT and 30.7 ± 3.3% for β4KO, values not different from those of the control diet groups. The proportion of pendrin-positive cells in the HK-alk-treated mice increased to 57.8 ± 10.4% for WT and 64.6 ± 5.1% for β4KO, values significantly greater than the respective values for HK-Cl.
Fig. 4.
A: double immunohistochemical (IHC) staining with anti-pendrin (arrows; green) and anti-V-ATPase (red) of renal sections from control, HK-alk, and HK-Cl diet mice. B: bar graph summarizing the %pendrin-positive cells/V-ATPase-positive cells. #P < 0.01 compared with control diet. C: double IHC staining with anti-aquaporin 3 (AQP3; green) and anti-V-ATPase (red) of renal sections from WT on a control and HK-alk diet. D: summary bar plots of %basolateral V-ATPase per total V-ATPase-marked cells, comparing WT and β4KO on control and HK-alk diets.
Figure 4C shows results of quantitative fluorescence analysis of the anti-V-ATPase staining of renal sections from WT mice on control diet and HK-alk. As shown, double IHC staining with anti-aquaporin 3 and anti-V-ATPase reveals distinct PC and IC, respectively, of cortical collecting ducts. Increased basolateral V-ATPase staining is observed in the section from WT on HK-alk. Figure 4D is a bar plot summarizing the percent β-IC in sections from WT and β4KO on control and HK-alk. On a control diet, β-IC WT and β4KO comprised 21.0 ± 4.0% (n = 17) and 19.5 ± 3.1% (n = 10), respectively. On HK-alk, the proportion of β-IC increased similarly and significantly in both WT and β4KO to values of 39.8 ± 6.0% (n = 9) and 44.8 ± 4.3% (n = 12), respectively.
These results showed that HK-alk changed the phenotypic profile of the distal nephron to a high proportion of cells that secrete HCO3 and alkalinize the urine. However, the increase in β-IC and the urine alkalinization with HK-alk is the same for WT and β4KO, suggesting that the BK-α/β4 is not required for secreting HCO3 via pendrin and that the reduced K excretion of β4KO on HK-alk cannot be the result of reduced quantities of β-IC or urine alkalinization.
Compensatory ENaC-mediated Na reabsorption in β4KO.
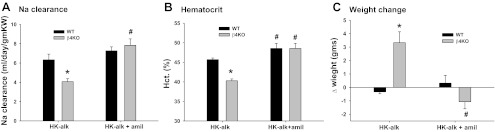
It was a concern that β4KO have deficient K secretion on HK-alk because of defective Na reabsorption, not enabling Na-K exchange. However, as shown in Fig. 5, the data indicate that β4KO on HK-alk is compensating for reduced K secretion by enhancing the ENaC-mediated Na reabsorption in the distal nephron. As shown by the Na clearance values in Fig. 5A, the Na excretion is decreased in β4KO on HK-alk. The Na clearance of 4.1 ± 0.3 ml·day−1·g−1 KW (n = 12) for β4KO was significantly less than the WT value of 6.3 ± 0.6 ml·day−1·g−1 KW (n = 15). However, when treated with amiloride to block ENaC-mediated Na reabsorption, the Na clearance of β4KO increased to 7.8 ± 0.7 ml·day−1·g−1 KW (n = 10), which was not significantly greater than the Na clearance value for WT (7.3 ± 0.4 ml·day−1·g−1 KW; n = 7).
Fig. 5.
Bar plots illustrating effects of amiloride on Na clearance (A), hematocrit (Hct; B), and weight change (C) of WT and β4KO mice on HK-alk diets. *P < 0.001, compared with WT; #P < 0.001, compared with HK-alk, using ANOVA plus SNK test.
The Na clearance for WT on HK-alk increased slightly, but insignificantly, when given amiloride, indicating minimal ENaC-mediated Na reabsorption. We used the hematocrit readings as evidence for Na retention and volume expansion in β4KO on HK-alk. As shown in Fig. 5B, the hematocrit of β4KO was 40.3 ± 0.5% (n = 14), a value significantly less than WT (45.7 ± 0.4%; n = 27). When treated with amiloride, the hematocrit of β4KO was 47.9 ± 1.3% (n = 9), a value not different from the WT value of 48.6 ± 1.3% (n = 9). These data indicate that β4KO are retaining fluid as well as Na when placed on HK-alk. The effect of amiloride, to normalize the hematocrit, indicates that the fluid retention was the result of ENaC-mediated Na reabsorption.
Weight gain or loss is also an indicator of ENaC-mediated Na retention. As shown in Fig. 5C, the weight of WT on HK-alk did not change significantly throughout the course of the diet (Δwt = −0.33 ± 0.15 g; n = 10). However, weight of β4KO on HK-alk increased by 3.3 ± 0.8 g (n = 10). When placed on HK-alk plus amiloride for 2 days, the weights of WT and β4KO were not significantly changed.
The enhanced fluid retention of β4KO and increased Na excretion when placed on amiloride shows that ENaC is overactive in β4KO. β4KO may be attempting to compensate for the decreased K secretion by increasing Na reabsorptive stimulated K secretion.
K handling with low-Na, high-K diet.
The results from Fig. 5 suggest that the β4KO are attempting to compensate for the lack of HCO3 promoted K secretion by enhancing the Na-dependent K secretion. If so, then the ability to excrete a high-K load should be further compromised when the mice are placed on LNaHK.
As shown in Fig. 6A, the K clearance of WT on LNaHK was not different from WT on HK-alk. However, β4KO on LNaHK exhibited significantly less K clearance, with a value of 1,221 ± 129 ml·day−1·g−1 KW (n = 8), compared with the WT value of 2,239 ± 153 ml·day−1·g−1 KW (n = 9). As shown in Fig. 6B, the plasma [K] of WT on LNaHK was 4.71 ± 0.24 mM (n = 9), a value not different from WT on HK-alk. However, the plasma K concentration of β4KO on LNaHK was a significantly greater value of 7.53 ± 0.23 mM (n = 7). The TTKG for WT on LNaHK was 22.9 ± 1.5 (n = 10), a value not different from WT on HK-alk; however, the TTKG for β4KO on LNaHK was only 13.8 ± 1.0 (n = 8), a value significantly less than WT on LNaHK (Fig. 6C).
Fig. 6.
Summary bar plots depicting differences in K clearance (A), plasma [K] (B), and TTKG (C) between WT and β4KO given HK-alk and LNaHK diets. *P < 0.001, compared with WT; #P < 0.001, compared with HK-alk using one-way ANOVA plus SNK test.
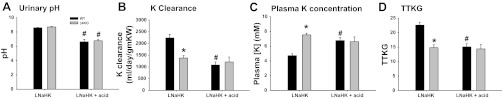
WT should maintain K balance when placed on a low-Na, high-K diet as well as a normal Na, high-K diet as long as the diet is also alkaline. Our LNaHK diet was designed to mimic that of the Yanomami, based on their urinary outputs (33). However, WT may have an exaggerated defect with low Na if the animal is made acidic. The results of acidifying the mice on LNaHK, by giving NH4Cl in the drinking water, are shown in Fig. 7. Figure 7A shows that the urinary pH of WT on LNaHK was 8.59 ± 0.04 (n = 3) with regular water, but significantly lower, at 6.63 ± 0.04 (n = 3) when drinking acid water. For β4KO on LNaHK, the urinary pH was 8.70 ± 0.07 (n = 2) when drinking regular water and 6.77 ± 0.22 (n = 5) when drinking acid water. As shown in Fig. 7B, WT exhibited a significant decrease in K clearance to a value of 1,083 ± 132 ml·day−1·g−1 KW (n = 5) when consuming LNaHK with acid water. This value was ∼50% the value for WT consuming LNaHK with regular drinking water. However, the value of 1,215 ± 208 ml·day−1·g−1 KW (n = 5) for β4KO on LNaHK plus acid water was not different from the value for β4KO on LNaHK with regular drinking water. When mice on LNaHK were treated with acid water, the plasma concentrations of WT and β4KO were significantly elevated, compared with LNaHK, to values of 6.8 ± 0.4 mM (n = 6) and 6.6 ± 0.7 mM (n = 5), respectively (Fig. 7C). The TTKGs for WT and β4KO on LNaHK plus acid water were significantly decreased, compared with LNaHK, to similar values of 15.1 ± 1.1 (n = 5) and 14.4 ± 1.5 (n = 5), respectively (Fig. 7D). These results show that WT maintained K balance when placed on a low-Na, high-K diet, as long as it is also alkaline; however, if made acidic, the ability of WT to maintain K balance on LNaHK was diminished to the capacity of β4KO.
Fig. 7.
Summary bar plots depicting differences in urinary pH (A), K clearance (B), plasma [K] (C), and TTKG (D) between WT and β4KO mice on LNaHK plus normal or acid (NH4Cl) drinking water. *P < 0.001, compared with WT; #P < 0.001, compared with LNaHK using one-way ANOVA plus SNK test.
The amounts of food consumption and urine output for WT and β4KO on each diet are shown in Table 1. All groups of mice on high-K diets consumed nearly twice as much chow as mice on the control diets. There was no significant difference in the quantity of chow consumed by WT and β4KO on any diet. Urinary excretion rates of WT on HK-alk, HK-Cl, and LNaHK were significantly greater than control. Significance was not detected in urinary excretion rates among all groups other than groups on a control diet, which excreted urine at about 20% the rate of mice on high-K diets.
Table 1.
Food intake and urinary volumes
| Mouse | Diet | Food In | SE | P vs. WT | P vs. Con | UV | SE | P vs. WT | P vs. Con | Hct | SE | P vs. WT | P vs. Con |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | Control | 1.5 | 0.2 | 3.5 | 0.3 | 45.6 | 0.4 | ||||||
| β4ΚΟ | Control | 1.7 | 0.3 | NS | 3.8 | 0.4 | NS | 45.9 | 0.8 | NS | |||
| WT | HK-alk | 3.5 | 0.2 | <0.001 | 19.3 | 1.1 | <0.001 | 44.3 | 0.3 | 0.066 | |||
| β4ΚΟ | HK-alk | 3.5 | 0.3 | NS | 0.001 | 19.7 | 1.4 | NS | <0.001 | 40.2 | 0.5 | <0.001 | <0.001 |
| WT | HK-Cl | 3.3 | 0.2 | 0.002 | 16.1 | 1.0 | <0.001 | 47.0 | 0.7 | 0.485 | |||
| β4ΚΟ | HK-Cl | 2.9 | 0.4 | NS | 0.145 | 14.9 | 3.3 | NS | <0.001 | 40.0 | 0.9 | <0.001 | <0.001 |
| WT | HK-alk + amil | 3.0 | 0.4 | 0.004 | 14.9 | 1.8 | <0.001 | 48.6 | 1.3 | 0.020 | |||
| β4ΚΟ | HK-alk + amil | 2.6 | 0.4 | NS | 0.069 | 14.8 | 0.6 | NS | <0.001 | 47.2 | 0.7 | NS | 0.518 |
| WT | LNaHK | 3.6 | 0.3 | <0.001 | 18.3 | 1.3 | <0.001 | 46.2 | 0.8 | 0.771 | |||
| β4ΚΟ | LNaHK | 3.5 | 0.5 | NS | 0.006 | 16.0 | 1.2 | NS | <0.001 | 44.1 | 0.7 | NS | 0.314 |
| WT | LNaHK + acid | 4.0 | 0.1 | <0.001 | 13.4 | 1.2 | <0.001 | 43.5 | 0.9 | 0.212 | |||
| β4ΚΟ | LNaHK + acid | 2.9 | 0.2 | NS | 0.086 | 9.3 | 1.1 | NS | 0.034 | 42.8 | 0.6 | NS | 0.123 |
Values (SE) represent means for food consumption (food: g/day) and urinary excretion rate (UV: mg·kg−1·day−1 kidney wt). HK-alk, high-K alkaline diet; HK-Cl, high-K chloride diet; amil, amiloride; LNaHK, alkaline, Na-deficient, high-K diet; Con, control; Hct, hematocrit P vs. wild type (WT) is the probability that β4-knockout (β4KO) is different from WT on same diet/drug using ANOVA plus SNK test. Not significant (NS) is P > 0.05. P vs. con is the probability that WT or β4KO is different from respective genotypes on a control diet. Number of samples (n) is >5 in all groups. Differences between groups were determined by the ANOVA plus Student-Newman-Keuls test.
DISCUSSION
Our results show that WT C57Bl/6 mice handle HK-alk, regardless of the Na content, with a minimal increase in plasma [K]. The expression of BK-β4 subunit, found in IC of the distal nephron, is enhanced in kidneys of mice on HK-alk but not HK-Cl. When given HK-Cl, or when NH4Cl is added to the drinking water of mice on LNaHK, the plasma [K] increases significantly in WT, demonstrating that mice consuming high K maintain K balance more easily when the diet is alkaline. However, the stimulatory effect of renal HCO3 does not enhance K secretion in β4KO. We found that the defective K secretion of β4KO is not a result of less β-IC or deficient Na reabsorption-K secretion exchange. Rather, Na reabsorption-K secretion exchange is enhanced in β4KO on HK-alk, probably as a compensatory mechanism.
BK-β4 expression with high-K diets.
A previous study showed that mRNA for BK-α and BK-β4 was increased in isolated cortical collecting ducts of rabbit on a high-K diet (32). The anionic content of this diet was not revealed, but it is presumed alkaline since rabbits are vegetarians. We have found by Western blot that HK-alk enhances the BK-α in the renal cortex of mice (24). We show here that the BK-β4, the subunit identified in IC, is upregulated by HK-alk but not by HK-Cl. This is consistent with the notion that the BK-α/β4 of intercalated cells, which are endowed with acid/base transporters, is involved in HCO3-promoted K secretion.
Potassium excretion with alkaline vs. acidic high-K diets.
Our results confirm a previous study showing an impairment of K excretion in β4KO (23). The high-K diet of that study was the same as HK-alk of the present study. The anionic content of HK-alk is a mixture of carbonate, citrate, and Cl. The alkaline anions are ultimately converted to HCO3, where they are secreted by the β-IC of the distal nephron to produce alkaline urine.
We have not determined the role of alkalinity on K handling in WT vs. β4KO on a normal K diet. However, the BK-α/β4 should be only relevant with a high-K load, which induces a flow rate of more than fourfold. Therefore, we would not expect that the alkalinity of the diet would make a difference between WT and β4KO in handling a normal K load. In support of this notion, studies showed no change in K handling when rats on a normal K diet were treated with NaHCO3 in their drinking water (12, 40).
The TTKG, which estimates the K gradient across the final cortical collecting duct, before the K is concentrated in the medullary collecting ducts with water reabsorption, is an indicator of distal K secretion in response to an electro-negative lumen potential generated by an active driving force (54, 57). The TTKGs of mice on a control diet were close to the value of 6.3 found for humans (4). The TTKG was greater when mice consumed HK-alk, compared with HK-Cl (20.9 vs. 13.6; Fig. 2). This finding supports an earlier study showing that acetazolamide-induced bicarbonaturia enhanced the TTKG of humans (4).
The competition between H and K secretion in the distal nephron is well recognized. Metabolic acidosis with excess H secretion tends to cause hyperkalemia (1, 52), and metabolic alkalosis with excess HCO3 secretion tends to cause hypokalemia when consuming normal K diets (36). Decreased intracellular pH can inhibit ROMK (30, 53); however, it is unclear how an alkaline diet affects the intracellular pH of ROMK-residing PC, which are not specialized to transport acid-base. An increase in K secretion may occur with merely a neutralization of the active H transport by the distal nephron, rather than by HCO3-driven K secretion. However, the urinary pH of the LNaHK mice given acid water was 6.6–6.8, which was consistent with no acidification by the α-IC because the pH of the urine entering the distal nephron is ∼6.7 (7). Therefore, the high pH of 8.3 in the final urine of LNaHK could only be derived from HCO3 secreted by the β-IC, which we found equally enhanced, with respect to total IC, in WT and β4KO on HK-alk.
How does an alkaline diet promote K excretion independent of Na reabsorption? One scenario is that HCO3 is secreted from β-IC via pendrin, a Cl/HCO3 exchanger shown in this study and others to be upregulated in animals given an alkaline diet (42, 47, 56). In other cells known to secrete high amounts of HCO3, such as the pancreas (35) and airway serous cells (14), the Cl entering the cell in exchange for HCO3 is recycled back across the apical membrane via a Cl channel such as CFTR, thereby generating an electro-negative lumen potential. However, the presence of CFTR or another Cl channel in the apical membrane of β-IC has not been established.
Defective β4KO secretion is disassociated from IC phenotypic change.
Using either apical pendrin or basolateral V-ATPase staining, we found an approximate increase in the proportion of β-IC from comprising 20 to 35% of total IC in WT and β4KO on a control diet to 40 to 60% of total IC when mice were on HK-alk. That IC convert from β-IC to α-IC according to acid/base status was shown previously (13, 46), with a recent study showing that preventing the conversion of β-IC to α-IC causes acidosis (13). That pendrin estimated a higher percentage of β-IC with HK-alk than basolateral V-ATPase is consistent with the view that pendrin also labels non-A-non-B cells (26), whereas basolateral V-ATPase should only label β-IC. Both antibodies show that β-IC are increased equally in WT and β4KO, indicating that the phenotypic switch is not dependent on BK-α/β4 function and decreased proportion of β-IC cannot be responsible for the diminished TTKG and K excretion of β4KO on HK-alk.
Compensatory role of Na reabsorption in β4KO.
Two recent studies supported the notion that there are two types of K secretion: the well-studied exchange of Na reabsorption for K secretion in the distal nephron and another type of K stimulated transport that is independent of Na reabsorption. Rats treated a high-K diet for several days exhibited a mild increase in plasma [K] with amiloride treatment; however, when treated with high K overnight, amiloride increased plasma [K] to an extremely high value of 8.9 mM (10). Because these overnight-treated rats were not K adapted, they did not develop a Na-independent mechanism for secreting K. In isolated perfused rat collecting ducts, iberiotoxin, a specific BK channel blocker, inhibited K secretion in the absence of luminal Na (31) demonstrating that BK channels mediate Na-independent K secretion.
It might be expected that the Na-dependent component of K secretion would be enhanced if the Na-independent component is compromised. This was evident for β4KO as there was less Na excretion in the β4KO than the WT on HK-alk (Fig. 4). We showed previously that β4KO exhibited reduced Na excretion on HK-alk, along with a reduced hematocrit and increased blood pressure, which was evidence for Na retention and volume expansion.(24). However, we have shown here that amiloride treatment returned the Na excretion to WT levels. Thus, in the absence of HCO3-stimulated K secretion, β4KO compensate by increasing ENaC-mediated Na reabsorption to stimulate K secretion. Further evidence of compensatory Na reabsorption was shown in the β4KO on LNaHK. In the absence of substantial dietary Na, the Na reabsorptive compensatory mechanism was also compromised in β4KO as indicated by a further reduction in TTKG and an increase in plasma [K] to near lethal levels.
The finding that plasma [K] increased to extremely high levels in β4KO on LNaHK indicates that BK-α/β4 is used for Na-independent K secretion. The LNaHK diet, which is alkaline and has a K-to-Na ratio of near 500, might mimic the diet of the Yanomami. Similar to previous studies by our laboratory (19, 23), the plasma [K] of WT increased only slightly, by ∼300 μM, in mice consuming HK-alk, compared with a normal diet (Fig. 1) (19). Moreover, the plasma [K] was not greater in WT mice consuming LNaHK compared with HK-alk. We did not determine the ability to excrete K with a low-Na, HK-Cl diet; however, consuming acid water considerably reduced their ability to handle a Na-deficient high-K load. This result shows that mice can excrete a high-K load absent of Na as long as the diet is alkaline. The naturally high-alkaline content associated with the high-K diet may be an important component of Na independent K secretion by the Yanomami.
K balance.
There is a decreased output of urinary K per K consumed in the WT mice on HK-Cl vs. HK-alk. However, the kidneys are not the only avenue for excreting K and the WT on HK-Cl are likely utilizing several extrarenal epithelia to maintain K balance. Several studies have described K-adapted (44) and aldosterone-regulated K secretion in the colon (6, 8), which handles 10% of K excretion under normal conditions. Even the mice on HK-alk excrete 10% less urinary K than they consume. Although water intake is a highly variable measurement, we observe that mice excrete no greater than 80% of the volume of water consumed, showing that they are utilizing extrarenal avenues to eliminate the volume as well as the K. WNK4 and WNK1 kinases are present in a variety of extrarenal epithelial cells, including colonic crypts, pancreatic ducts, bile ducts, and sweat glands (5, 25), indicating adjustments in Na, Cl, and K homeostasis by several extrarenal tissues. We have also noted that the mice on HK-Cl were very wet, indicating profuse sweating as an avenue to eliminate K after achieving a high plasma [K] level.
Role of IC cells in K secretion.
Although we have shown that the BK-α/β4 of IC have a role in reducing the size of IC during high-flow conditions (23), it has been controversial whether the BK-α/β4 can secrete K because of the paucity of Na-K-ATPase to deliver K across the basolateral membrane of IC. However, a recent study with the isolated rat collecting duct has shown that K secretion is reduced by inhibiting NKCC1 (28), localized in the basolateral membrane of IC (15). In the colon, K uptake via basolateral NKCC1 is the putative source of K that is secreted via apical BK (48, 49). The question remains concerning the mechanism for cellular Na extrusion after Na enters via NKCC1.
It is not unprecedented that IC transport electrolytes other than acid/base. Renal IC contain a pathway for neutral Na-Cl absorption via a Na-dependent Cl/HCO3 exchanger (NDCBE; Refs. 9, 27). It will be interesting to determine how specific transport pathways in IC sustain basolateral K entry and Na extrusion in a cell that is nearly devoid of Na-K-ATPase (43).
Significance.
We found that an alkalinizing diet not only enhanced TTKG, but a high-K diet with Cl as the counter ion is detrimental, causing a large increase in plasma [K] due to a failure to excrete the high-K load. Our results explain why sustained-release KCl tablets can elevate plasma [K] to dangerously high levels and why the antidote to “KCl poisoning” is NaHCO3 (20). On the other hand, KCl is the best treatment for the condition of hypokalemic alkalosis (3, 29).
GRANTS
This project was funded by National Institutes of Diabetes and Digestive and Kidney Diseases Grants RO1-DK-071014 and RO1-DK-73070 (to S. C. Sansom) and a fellowship (no. 11PRE7530018) from the American Heart Association MWA Affiliate (to R. J. Cornelius).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.J.C., D.W., and L.I.H. conception and design of research; R.J.C., D.W., and L.I.H. performed experiments; R.J.C., D.W., and L.I.H. analyzed data; R.J.C., D.W., and L.I.H. interpreted results of experiments; R.J.C., D.W., and L.I.H. prepared figures; R.J.C., D.W., and L.I.H. drafted manuscript; S.C.S. edited and revised manuscript; S.C.S. approved final version of manuscript.
REFERENCES
- 1. Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol 22: 1981–1989, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Berl T, Linas SL, Aisenbrey GA, Anderson RJ. On the mechanism of polyuria in potassium depletion. The role of polydipsia. J Clin Invest 60: 620–625, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carlisle EJF, Donnelly SM, Ethier JH, Quaggin SE, Kaiser UB, Vasuvattakul S, Kamel KS, Halperin ML. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int 39: 1206–1212, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl- -transporting epithelia. Proc Natl Acad Sci USA 100: 663–668, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dolman D, Edmonds CJ. The effect of aldosterone and the renin-angiotensin system on sodium, potassium and chloride transport by proximal and distal rat colon in vivo. J Physiol 250: 597–611, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DuBose TD, Jr, Pucacco LR, Lucci MS, Carter NW. Micropuncture determination of pH, PCO2, and total CO2 concentration in accessible structures of the rat renal cortex. J Clin Invest 64: 476–482, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edmonds CJ, Willis CL. Aldosterone in colonic potassium adaptation in rats. J Endocrinol 117: 379–386, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frindt G, Shah A, Edvinsson JM, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3− loading. Am J Physiol Renal Physiol 284: F584–F593, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gao X, Eladari D, Leviel F, Tew BY, Miro-Julia C, Cheema F, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q. Deletion of hensin/DMBT1 blocks conversion of β- to α-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci USA 107: 21872–21877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, Fong P, Gray MA. Novel role for pendrin in orchestrating bicarbonate secretion in CFTR-expressing airway serous cells. J Biol Chem 286: 41069–41082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gunja N. Decontamination and enhanced elimination in sustained-release potassium chloride poisoning. Emerg Med Australas 23: 769–772, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl− excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295: C1658–C1667, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC. Coupled ATP and potassium efflux from intercalated cells. Am J Physiol Renal Physiol 300: F1319–F1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens 20: 512–517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl- flux in extrarenal epithelia. Proc Natl Acad Sci USA 101: 2064–2069, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Leviel F, Hubner CA, Houillier P, Morla L, El MS, Brideau G, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marples D, Frokiaer J, Dorup J, Knepper MA, Nielsen S. Hypokalemia-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest 97: 1960–1968, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol Renal Physiol 275: F972–F981, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang W, Seldin D, Giebisch G. Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int 75: 25–30, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 52: 146–151, 1975 [DOI] [PubMed] [Google Scholar]
- 34. Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology 139: 620–631, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Peterson LN, Sztorc D, Jamshaid A, Kucharczyk J, Bichet D, Levine DZ. Plasma AVP and renal concentrating defect in chloride depletion metabolic alkalosis. Am J Physiol Renal Fluid Electrolyte Physiol 254: F15–F24, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-beta1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M, Chambrey R. Regulation of the Cl−. J Am Soc Nephrol 15: 2988–2997, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabolic I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Sandle GI, Butterfield I. Potassium secretion in rat distal colon during dietary potassium loading: role of pH regulated apical potassium channels. Gut 44: 40–46, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sansom SC, O'Neil RG. Effects of mineralocorticoids on transport properties of cortical collecting duct basolateral membrane. Am J Physiol Renal Fluid Electrolyte Physiol 251: F743–F757, 1986 [DOI] [PubMed] [Google Scholar]
- 46. Schwartz GJ, Barasch J, Al-Awqati Q. Plasticity of functional epithelial polarity. Nature 318: 368–371, 1985 [DOI] [PubMed] [Google Scholar]
- 47. Sever MS, Erek E, Vanholder R, Kantarci G, Yavuz M, Turkmen A, Ergin H, Tulbek MY, Duranay M, Manga G, Sevinir S, Lameire N. Serum potassium in the crush syndrome victims of the Marmara disaster. Clin Nephrol 59: 326–333, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflügers Arch 459: 645–656, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Sorensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle's loop. J Clin Invest 70: 219–229, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Tannen RL. Effect of potassium on renal acidification and acid-base homeostasis. Semin Nephrol 7: 263–273, 1987 [PubMed] [Google Scholar]
- 53. Tsai TD, Shuck ME, Thompson DP, Bienkowski MJ, Lee KS. Intracellular H+ inhibits a cloned rat kidney outer medulla K+ channel expressed in Xenopus oocytes. Am J Physiol Cell Physiol 268: C1173–C1178, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Vasuvattakul S, Quaggin SE, Scheich AM, Bayoumi A, Goguen JM, Cheema-Dhadli S, Halperin ML. Kaliuretic response to aldosterone: influence of the content of potassium in the diet. Am J Kidney Dis 21: 152–160, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP. Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]
- 57. West ML, Sonnenberg H, Veress A, Halperin ML. The relationship between the plasma potassium concentration and renal potassium excretion in the adrenalectomized rat. Clin Sci (Lond) 72: 577–583, 1987 [DOI] [PubMed] [Google Scholar]
- 58. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]