Abstract
Renal ischemia-reperfusion leads to acute kidney injury (AKI), a major kidney disease associated with an increasing prevalence and high mortality rates. A variety of experimental models, both in vitro and in vivo, have been used to study the pathogenic mechanisms of ischemic AKI and to test renoprotective strategies. Among them, the mouse model of renal clamping is popular, mainly due to the availability of transgenic models and the relatively small animal size for drug testing. However, the mouse model is generally less stable, resulting in notable variations in results. Here, we describe a detailed protocol of the mouse model of bilateral renal ischemia-reperfusion. We share the lessons and experiences gained from our laboratory in the past decade. We further discuss the technical issues that account for the variability of this model and offer relevant solutions, which may help other investigators to establish a well-controlled, reliable animal model of ischemic AKI.
Keywords: renal ischemia-reperfusion, mouse, experimental model
acute kidney injury (aki) is a major kidney disease associated with high mortality in human patients. Recent basic science and epidemiologic studies have further suggested a causal role of AKI in the development and progression of chronic kidney disease. Clinically, ischemia is a leading cause of AKI, which may result from a variety of conditions, such as decreased cardiac output, renal vascular occlusion or obstruction, and kidney transplantation. In vitro models, including renal cell cultures, isolated renal tubules, and isolated perfused kidneys, are valuable for the research of the pathophysiological mechanisms of ischemic AKI. Nevertheless, in vivo whole animal models are indispensable, because of the limitation of the in vitro models to mimic the complexity of human body (45). Since the 1960s, various animal models of ischemic AKI have been developed and tested, and currently, two kinds of warm renal ischemia-reperfusion (IR) models are mainly used: 1) bilateral renal ischemic reperfusion (IR) (2–6, 8, 13, 14, 17, 19, 22, 23, 25–27, 30, 31, 35, 39, 41–43, 46, 51, 53–57, 59, 61, 63, 67, 68, 75–80) and 2) unilateral renal IR (1, 9, 15, 18, 20, 21, 24, 29, 33, 34, 37, 38, 40, 44, 50, 58, 60, 62, 64, 66). Depending on whether the contralateral kidney is removed, the unilateral model can be further divided into two subtypes: unilateral IR with contralateral nephrectomy (15, 18, 20, 29, 38, 40, 44, 50) or without contralateral nephrectomy (1, 9, 21, 24, 45, 60). The bilateral ischemic AKI model is commonly used, because it is considered more relevant to human pathological conditions where blood supply is normally affected in both kidneys (2, 10, 11, 16, 30, 32, 37, 47, 49, 52, 70–74, 79). In the bilateral model, some studies performed decapsulation prior to renal ischemia (42, 51) that may have renoprotective effects, as reported earlier (69). However, decapsulation was not conducted in the majority of published studies.
The initial models of ischemic AKI were developed with experimental animals of relatively large size, such as dogs and rabbits (7, 28, 39). Rat models then became the most popular animal model, as among the ∼1,300 publications of ischemic AKI animal studies since 1960s, half of them were conducted in rats. In 1990, the mouse was first introduced into the research field of ischemic AKI (65). The studies with mice were markedly promoted by the availability of various transgenic mice. In the past decade, there have been more studies using mice than those using rats. In addition, the size of a mouse is about 1/10 that of a rat, which means less drug consumption for experimental testing. Despite these notable advantages, the mouse model is known to have bigger variations, causing inconsistency in results. In recent years, we have optimized the mouse model of bilateral renal ischemic AKI. In this review, we share the lessons and experiences that we have learned and have gained in our laboratory. Specifically, we present a detailed experimental protocol and discuss the technical issues that we think may benefit our fellow researchers in this field to establish more reliable, consistent mouse models for ischemic AKI research.
Experimental Procedures for Bilateral Ischemic AKI in Mice
Preparation for experiment.
The equipment, surgical tools and other materials (number needed in parentheses) are a homeothermic monitor system (1), animal hair clipper (1), tissue forceps with blunt points (2), tweezers with ultra-sharp points (1), dissecting and operating scissors with sharp points (2), micro-aneurysm clips (2), micro-aneurysm clip applying forceps (1), 4–0 Vicryl suture with 1/2-circle needle of 17-mm length; needle holder (1), Michel wound clips, Michel wound clip-applying forceps (1), 1-ml syringes, 30 G needles, alcohol swab, cotton swab, gauze sponges, and surgical gloves. The solutions used are saline (0.9% sodium chloride), 5 mg/ml pentobarbital in saline, 0.03 mg/ml buprenorphine in saline. Finally, all of the surgical tools, materials, and solutions are sterilized.
Surgical procedure.
The mouse is anesthetized with 50–60 mg/kg of pentobarbital sodium by intraperitoneal injection. Pentobarbital solution is diluted with sterile saline to have a concentration of 5 mg/ml for injection. Shortly after pentobarbital injection, 50 μg/kg of buprenorphine is administered subcutaneously for relief from pain and distress. After pentobarbital and buprenorphine injections, the hair on both sides of the mouse is removed with the hair clipper. The skin in the surgical area is then wiped clean with 70% alcohol swab.
Surgery.
Immediately after the skin preparation, the mouse is placed on the homeothermic blanket of a homeothermic monitor system and covered by sterile gauze. The body temperature is monitored through a rectal probe and controlled in the range of 36.5–37°C (our routine setpoint is 36.7°C and temperature varies in 0.1°C range). Surgery will not be started until 1) the body temperature is stabilized at the set-point, and 2) the mouse is in deep anesthesia and thus does not respond to pain induced by toe pinch. It usually takes ∼30 min after pentobarbital injection to achieve deep anesthesia.
The mouse is placed on the thermostatic station laying on the right side (Fig. 1A). The skin and muscle on the left flank side are cut open along the back to expose the left kidney (Fig. 1A). The incision is positioned at 1/3 of the body from the back of the mouse and the incision size is 1–1.5 cm along the back. The kidney is then pushed out from the cut with sterile cotton swabs to expose the renal pedicle. Dissection of the pedicle tissue is done with ultra-fine-point tweezers to remove the tissue around the renal pedicle to expose the blood vessels for renal pedicle clamping. After the preparation, the left kidney is returned to the abdomen cavity. The right renal pedicle is prepared by a similar surgical procedure, but the incision is closer to the rib due to the different position of the right kidney (Fig. 1B). After the pedicle preparation, both kidneys are returned back to their original positions in the abdomen cavity. The mouse is then covered with sterile gauze on the thermostatic station for its body temperature to stabilize again, which usually takes 5–10 min.
Fig. 1.
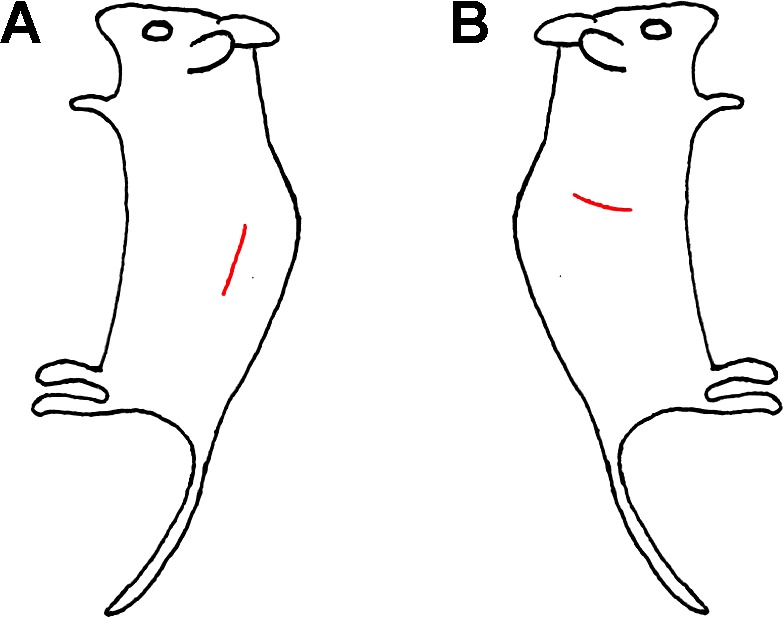
Sites of flank incision of bilateral mouse model of ischemic acute kidney injury (AKI). The incision sites on the left (A) and right (B) sides are labeled with red line.
Renal ischemia.
The right kidney is gently pushed out of body cavity with cotton swabs to expose the pedicle. A micro-aneurysm is used to clamp the pedicle to block the blood flow to the kidney to induce renal ischemia. The duration of right kidney ischemia starts from the time of clamping. Complete ischemia is indicated by color change of the kidney from red to dark purple in a few seconds. After verification of the kidney color changes, the kidney is returned to the abdomen cavity. The mouse is then laid on its right side for the left renal pedicle clamping and ischemia. There is around 1–1.5 min time latency between the right and left kidney clamping. However, the ischemic time of each side is recorded separately to ensure both kidneys receive the same durations of ischemia.
After the ischemia, the micro-aneurysm clips are released at desired times for each kidney to start the reperfusion, which is indicated by the change of kidney color to red. A Vicryl suture is used to close the muscle layer of the incision followed by the closure of the skin wound with Michel wound clips. Immediately after the wound closure, 0.5 ml warm sterile saline is given intraperitoneally to each mouse. The animal is then kept on a heating pad until it gains full consciousness before being returned to its housing cage.
Monitoring the Success of Renal Ischemia-Reperfusion
The success of renal IR is monitored at several levels. First, after clamping, the kidney color should change from red to dark purple, indicative of a successful renal ischemia. The immediate color change at the very beginning requires careful observation to notice. However, the kidney will be in deep dark purple color several minutes later. After removing the clips, kidney color should change back to red to indicate the reperfusion. Usually blood flow is restored immediately after removing the clips, thus special anti-coagulation procedure is not applied here in our experiments.
Second, depending on the severity of kidney injury, there may be a decline of renal function that can be detected by the increases in blood urea nitrogen (BUN) and serum creatinine. A few microliters of blood samples are sufficient for the BUN measurement using the urea nitrogen test kit from StanBio, while 20 μl are needed for the serum creatinine measurement using a kit (based on Jaffe Method) from StanBio. Therefore, we routinely measure the BUN to monitor the renal function before the renal ischemia and at different reperfusion time points. The BUN value in normal control C57BL/6 mice is around 20–40 mg/dl. After 30 min of bilateral renal ischemia, there are slight yet detectable increases in BUN between 6 and 12 h of reperfusion and marked increases between 24 and 48 h (Fig. 2). The serum creatinine is usually determined at the endpoint of the experiment, when larger volumes of blood can be collected when the kidney is removed. As expected, the BUN and serum creatinine increases depend on the severity of kidney injury. For example, in one of our previous tests, after 22 min of renal ischemia, the BUN value increased to 120–150 mg/dl at 48 h of reperfusion, after which BUN decreased toward basal levels as a result of kidney repair and functional recovery. After 25 min of ischemia, BUN reached ∼300 mg/dl at 72 h of reperfusion. In C57BL/6 mice, 22 min and 25 min of ischemia will induce mild to moderate injury with the recovery of renal function in ∼1 wk. Thirty minutes of ischemia induced very severe kidney injury, and a significant proportion of the severely injured mice died at 72 h of reperfusion. The serum creatinine level usually increases to 1–1.5, 2, and 2.5 mg/dl after 48 h of reperfusion following 22, 25, and 30 min of ischemia, respectively.
Fig. 2.
Blood urea nitrogen (BUN) level of C57BL/6 mice after different ischemia-reperfusion periods. Male mice of 8 wk were subjected to 22, 25, and 30 min of bilateral renal ischemia or sham operation. Serum samples were collected at indicated reperfusion times for BUN assay.
Finally, the histological examination of kidney tissues by methods such as hematoxylin-and-eosin (H&E) staining, PAS staining, and TUNEL assay is the direct way to verify and localize the kidney injury. Fig. 3 shows representative images of H&E staining of kidney tissues with or without ischemic AKI. The typical renal tubular damage includes severe tubular lysis, loss of brush border, and sloughed debris in tubular lumen space. In ischemic AKI, the most severely injured site is the S3 segment of proximal tubules located at the outer stripe of outer medulla. We routinely conduct H&E staining to grade tubular damage (0, no damage; 1, 0–25% damaged tubules; 2, 25–50% damaged tubules; 3, 50–75% damaged tubules; 4, >75% damaged tubules) (10, 72). We also analyze apoptosis by TUNEL staining and immunofluorescence of active caspase 3; apoptosis can be quantified by counting positively stained cells (72–74).
Fig. 3.
Renal histology after ischemic AKI. Top: kidney tissues from C57BL/6 mice with 30 min of bilateral renal ischemia and 48 h of reperfusion or sham operation were stained by hematoxylin and eosin. IM, inner medulla. CT, cortex. Bottom: enlarged images of boxed area in the upper panels.
Key Factors for a Consistent Mouse Model of Ischemic AKI
Mouse surgeon.
A well-trained, skillful surgeon is the key to the establishment of a consistent, reliable mouse model of ischemic AKI. A good mouse surgeon not only can reduce surgical trauma but also can complete the whole procedure within the anesthesia time in a smooth, organized manner. As discussed below, 50 mg/kg pentobarbital sodium is normally used in our study for mouse anesthesia. This anesthesia only provides a little more than 1 h of time for the whole experimental procedure, which includes the surgery to expose renal pedicles, the waiting period for body temperature stabilization, 20–30 min of ischemic duration, and finally the closure of the wound. In our experience, a higher dosage of pentobarbital sodium (e.g., >60 mg/kg) prolongs anesthesia, but it significantly increases animal loss during surgery. Less experienced surgeons need longer operation time and, thus, may need to supplement anesthetics for the surgery to be completed, which can affect the final result and lead to animal loss. We emphasize that the surgery has to be conducted in a well-prepared and organized manner by a skillful surgeon. To this end, new surgeons have to fully understand each of the steps, watch the whole procedure, and practice until the whole experiment can be completed within the anesthesia time to yield comparable kidney injury results. Notable variations are introduced during high turnover or rotation of mouse surgeons. Thus, it is advised, unless unavoidable, not to change the mouse surgeon in a study.
Animals.
There are marked differences in the susceptibility to ischemic AKI among different mouse strains and even different colonies of the same strain. National Institutes of Health Swiss mice were shown to be resistant or less sensitive to ischemic AKI than C57BL/6 and BALB/c mice (12). A recent study further showed that 129/Sv mice are also less susceptible to ischemic AKI (48). The mouse strain or colony-related differences in injury susceptibility is particularly relevant in studies using transgenic and gene knockout mouse models. Although most of the transgenic mouse models are described to have comparable genetic background with wild-type strains (e.g., C57BL/6) after more than five generations of backcross, the wild-type mice from the same transgenic models may be significantly different in their ischemic injury sensitivity than the regular C57BL/6 mice. For example, our recent study established a Dicer-knockout mouse model in which Dicer was specifically deleted from the kidney proximal tubules (PT-Dicer-KO). This model had a C57BL/6 background, but the wild-type mice from this model were significantly more resistant to ischemic kidney injury than the regular C57BL/6 mice. As a result, longer (32 vs. 30 min for regular C57BL/6) ischemic time was needed to induce AKI with BUN of ∼200 mg/dl and serum creatinine of ∼2 mg/dl after 48 h of reperfusion (72). We routinely conduct pilot tests to determine the appropriate ischemic duration for a new mouse line to be studied.
Even within the same strain, different mouse colonies may show different susceptibility to ischemia AKI. Our laboratory maintains an in-house C57BL/6J colony established with breeders from The Jackson Laboratory. Mice from this colony are significantly more resistant to ischemic kidney injury than the aged matched male mice directly purchased from The Jackson Laboratory (Fig. 4). The cause of the difference is unclear, but it may be related to colony maintenance, which includes feeding, health, and stress of the animal. In this regard, if mice are shipped from a vendor or other outside sources, they need to have at least 1 wk of rest/stabilization before the experiment. To alleviate the strain and colony differences, we strongly recommend that littermate mice are tested in the same experiment. This is particularly important for Omics studies that analyze hundreds to thousands of genes, proteins, or metabolites and thus require very stringent controls to reduce false positives to narrow down the targets for further in-depth investigation. In studies using a transgenic model, the wild-type littermates from the model, rather than the mice from a matching strain, should be used as the controls. Such controls were included in recent studies to generate convincing evidence for the involvement of specific genes in the pathogenesis of ischemic AKI (5, 27, 51, 72, 80).
Fig. 4.
Ischemic AKI-associated serum creatinine increases in C57BL/6 mice of different colonies. Male C57BL/6J mice of 8 wk obtained directly from the Jackson Laboratory (JAX) and our in-house-bred colony (in-house) were subjected to 28 min of bilateral renal ischemia. Serum samples were collected after 48 h of reperfusion for serum creatinine measurement. *Statistically significant difference comparing to JAX group (P < 0.05).
Ischemic AKI is also affected by the animal age. For example, Kusaka et al. (36) recently showed that aged rats (60–65 wk old) are more susceptible to ischemic AKI than young rats of 6–7 wk. In young adult mice of ∼8–12 wk that are commonly used for ischemic AKI study, age differences of over 1 wk may cause detectable differences in kidney injury. Our suggestion is to use animals of the same or very similar (<1 wk difference) age in each experiment.
In addition, ischemic AKI is known to be affected by sex. Interestingly, while the female mice are generally more resistant to ischemic AKI than males, they are more sensitive to cisplatin-induced nephrotoxic AKI (57, 74). Male mice are commonly used for ischemic AKI research due to their better consistency and sensitivity, which is caused mainly by testosterone (57). The health condition of the animals is another factor that should be considered. Some transgenic or gene knockout models may develop disease conditions that affect AKI.
Key Equipment
In addition to the general surgical tools, the key equipment, especially the microaneurysm clips that are used to induce renal ischemia and the thermostatic system for the body temperature control, are important to the success of the experiment. Appropriate tools and equipment should be purchased and designated for mouse experiment. The microaneurysm clips for mouse renal pedicle clamping are quite fragile and should be handled carefully with specially designed applying forceps to avoid any damage. We use the clips from George Tiemann (item no. 160–863), and similar microaneurysm clips are available from Biomedical Research Instruments and Roboz Surgical Instrument Company. Those clips can be reused multiple times if they are handled properly and with care. Normally, we replace the clips after ∼100 operations or if the surgeon detects damages to the clip, which can cause uneven kidney color changes with nonischemic spots after clamping. The thermostatic station with a rectal probe to monitor and control the mouse body temperature in the accuracy of 0.1°C is necessary because, as discussed below, the variation in body temperature is probably the single most critical factor affecting the severity of ischemic kidney injury. Our thermostatic stations were purchased from Harvard Apparatus (item no. 507222F), and similar equipment is available from other manufacturers.
Surgery
Dehydration status and surgery time.
Kidney injury is greatly affected by the dehydration status of the body. Normally, experimental mice are kept in animal facilities with 12:12-h light-dark cycle. Mice are nocturnal, meaning they are more active in the night time. Thus, the mice housed in dark are more active and drink more water, whereas they sleep more and drink less during the light period. In general, the mice housed under 12:12-h light-dark cycle are more dehydrated in the afternoon than in the morning. Thus, it is highly recommended to conduct the ischemia experiment at a certain time of the day for all experiments in one study. We normally induce renal ischemia at 2:00 PM–5:00 PM.
Anesthesia
We choose to use pentobarbital sodium for anesthesia after we tried a few other anesthetics. Ketamine/xylazine was initially recommended by the veterinarian of our animal facility. We had to give it up because the dosage of ketamine/xylazine to ensure ∼1 h of anesthesia for operation frequently caused animal death. Isoflurane is another drug that we tested for anesthesia that can be switched on and off easily with the gas anesthesia machine, and it is not a controlled substance. However, isoflurane itself has renoprotective effects during renal ischemia-reperfusion injury (79) and consistently, obvious kidney injury could not be induced in mice even after 30 min of bilateral renal ischemia in our pilot tests. In our hands, pentobarbital works best. There may be occasional animal loss following pentobarbital anesthesia, but this is rare if the dosage is well controlled (start from 50 mg/kg and supplement with 5 mg/kg during surgery when necessary). Combined with buprenorphine, this dosage of pentobarbital provides a sufficient level of anesthesia and sedation for the completion of the surgery.
Body Temperature Maintenance
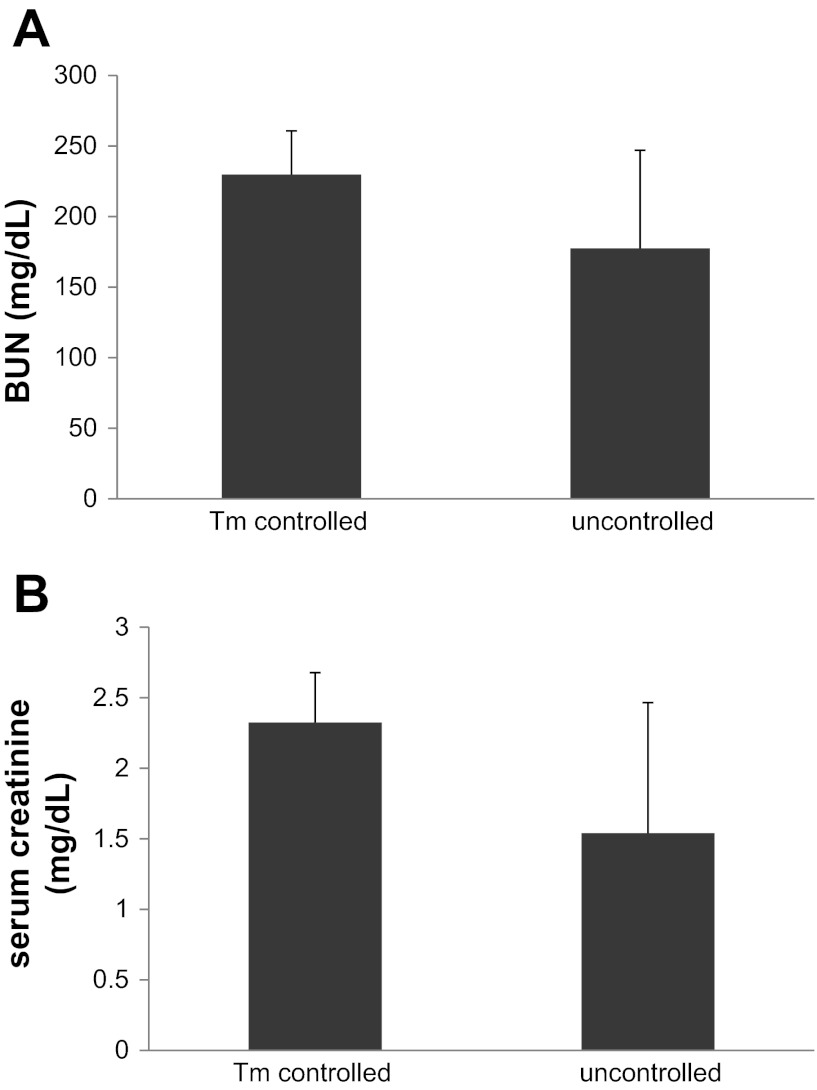
The body temperature of the experimental animal during ischemia is one of the most important factors that affect the severity of AKI. We used to do surgery with only a heating pad to keep mice warm during surgery. However, when our laboratory moved to a new space, there was a notably bigger variation of kidney function after ischemic injury (Fig. 5). Over a period of time troubleshooting, we finally figured out that the fluctuation of room temperature (and thus the mouse body temperature during surgery) was the cause of the variation. To solve this problem, we purchased a homeothermic monitor system, which has a rectal probe to accurately control the body temperature of the mouse under surgery and renal ischemia, which greatly reduced the variation of the outcome (Fig. 5).
Fig. 5.
Renal function after ischemic AKI with and without body temperature control. Male C57BL/6J mice of 8 wk were subjected to 30 min of bilateral renal ischemic injury with or without body temperature control at 36.7°C using a thermostatic station. Serum samples were collected after 48 h of reperfusion. A: blood urea nitrogen. B: serum creatinine.
Operation Time
To minimize variation, both total operation time and the ischemic time latency between right and left kidneys need to be consistent between the animals and experiments. The timeline of our typical experiment is depicted in Table 1.
Table 1.
Timeline of surgical procedure for bilateral ischemic AKI in mice
| Time, min | Procedure |
|---|---|
| 5 | Injection of pentobarbital and buprenorphine, skin preparation |
| 25–30 | Body temperature stabilization |
| 2 | Renal pedicle preparation |
| 5 | Body temperature recovery |
| 2–3 | Renal pedicle clamping |
| 20–30 | Renal ischemia |
| 5 | Wound closure |
| 60–75 | Total |
AKI, acute kidney injury.
1. Anesthesia and preparation for surgery (30 min) involves injection of pentobarbital sodium and buprenorphine, skin preparation, and stabilization of the body temperature on the heating pad of the homeothermic monitor system.
2. Surgery and clamping (10 min) involve the preparation of the renal pedicles, body temperature recovery, and clamping the renal pedicle. In this step, the time latency between the clamping of the right pedicle and the left pedicle is controlled within 1 to 1.5 min. Although it may not matter which side to start, we suggest to clamp the right side first because the right pedicle is relatively short and more difficult to clamp. After the right clamping, it is easy to clamp the left pedicle in 1–1.5 min.
3. The duration of renal ischemia (20–30 min) is determined by the experimental design.
4. Wound closure (5 min) involves suturing of muscle layer and clipping closure of the wound.
Minimization of Surgical Trauma
The purpose of the surgery is to induce ischemic AKI. It is important to minimize other trauma associated with the surgery. Currently, both midline incision (laparotomy) and flank incision are being used by different investigators to expose renal pedicle for clamping. We recommend flank incision, which is much less traumatic. It is also important to limit the incision size. In addition, we recommend the use of Vicryl suture to close the muscle layer of the wound as it is absorbable. Closure of the wound with clips without suturing the muscle layer may lead to incomplete wound healing and infection.
Postsurgery Care
Good postsurgery care is not only humane, but it is also a way to reduce experimental variation and prevent the loss of animals. Regularly, we give saline supplementation immediately after the surgery to prevent dehydration of the mouse. After returning the mouse to its cage, easily accessible water and food supplies are also necessary. The surgery may cause mobility difficulty of the animal. If the water and food are at high positions, it is important to keep some gel food on the floor of the cage. In general, pain reliever is not needed since the mouse behavior does not show significant signs of distress after the initial dose of buprenorphine.
Although the variation of the mouse model of ischemic AKI is generally larger than other (rat) models, relatively consistent results can be achieved with attention to the above-described factors. As shown in Fig. 5, in one of our experiments, after 30 min of ischemia and 48 h of reperfusion, BUN increased to an average of 230 mg/dl with a standard deviation of 31, while serum creatinine was 2.32 ± 0.35. In such severe AKI conditions, we usually detect a <50 mg/dl variation in BUN and <0.5 mg/dl in serum creatinine. For statistical analysis, 5 or 6 pairs of mice are necessary to show the difference between the two groups. Animals are excluded from a study if any of the following occurs: 1) there is abnormal kidney morphology and kidney function before surgery; 2) there are noticeable abnormalities in other organs; 3) surgery is not well performed or accidents occur during surgery; or 4) test substance delivery fails.
Conclusions
Bilateral renal I/R in mice is a commonly used model for AKI research. Nevertheless, the mouse model is less stable than others (e.g., the rat model). However, a well-trained, skillful surgeon who follows the afore-described procedure and pays particular attention to the technical issues, should be able to establish a relatively consistent mouse model for the investigation of the pathogenic mechanism and identification of therapeutic approaches.
GRANTS
The work was partly supported by the American Heart Association (to Q. Wei), National Institutes of Health (to Z. Dong), and the U.S. Department of Veterans Affairs (to Z. Dong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Q.W. and Z.D. conception and design of research; Q.W. performed experiments; Q.W. and Z.D. analyzed data; Q.W. and Z.D. interpreted results of experiments; Q.W. prepared figures; Q.W. drafted manuscript; Q.W. and Z.D. edited and revised manuscript; Q.W. and Z.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Manjeri Venkatachalam at the University of Texas Health Science Center at San Antonio for discussion.
REFERENCES
- 1. Alikhan MA, Jones CV, Williams TM, Beckhouse AG, Fletcher AL, Kett MM, Sakkal S, Samuel CS, Ramsay RG, Deane JA, Wells CA, Little MH, Hume DA, Ricardo SD. Colony-stimulating factor-1 promotes kidney growth and repair via alteration of macrophage responses. Am J Pathol 179: 1243–1256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altmann C, Andres-Hernando A, McMahan RH, Ahuja N, He Z, Rivard CJ, Edelstein CL, Barthel L, Janssen WJ, Faubel S. Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 302: F421–F432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amura CR, Renner B, Lyubchenko T, Faubel S, Simonian PL, Thurman JM. Complement activation and toll-like receptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol Immunol 52: 249–257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arany I, Grifoni S, Clark JS, Csongradi E, Maric C, Juncos LA. Chronic nicotine exposure exacerbates acute renal ischemic injury. Am J Physiol Renal Physiol 301: F125–F133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arfian N, Emoto N, Vignon-Zellweger N, Nakayama K, Yagi K, Hirata KI. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem Biophys Res Commun 425: 443–439, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Balasubramanian S, Jansen M, Valerius MT, Humphreys BD, Strom TB. Orphan nuclear receptor Nur77 promotes acute kidney injury and renal epithelial apoptosis. J Am Soc Nephrol 23: 674–686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balint P, Chatel R, Fekete A, Forgacs I. Haemodynamics and oxygen consumption of the kidney in post-ischaemic renal failure. Clin Sci 26: 471–477, 1964 [PubMed] [Google Scholar]
- 8. Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23: 1467–1473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown WC, Brown JJ, Gavras H, Jackson A, Lever AF, McGregor J, MacAdam RF, Robertson JI. Renin and acute circulatory renal failure in the rabbit. Circ Res 30: 114–122, 1972 [DOI] [PubMed] [Google Scholar]
- 12. Burne MJ, Haq M, Matsuse H, Mohapatra S, Rabb H. Genetic susceptibility to renal ischemia reperfusion injury revealed in a murine model. Transplantation 69: 1023–1025, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Celie JW, Katta KK, Adepu S, Melenhorst WB, Reijmers RM, Slot EM, Beelen RH, Spaargaren M, Ploeg RJ, Navis G, van der Heide JJ, van Dijk MC, van Goor H, van den Born J. Tubular epithelial syndecan-1 maintains renal function in murine ischemia/reperfusion and human transplantation. Kidney Int 81: 651–661, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Hartono JR, John R, Bennett M, Zhou XJ, Wang Y, Wu Q, Winterberg PD, Nagami GT, Lu CY. Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int 80: 504–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int 68: 1956–1961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82: 928–933, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302: F853–F864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gall JM, Wong V, Pimental DR, Havasi A, Wang Z, Pastorino JG, Bonegio RG, Schwartz JH, Borkan SC. Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int 79: 1207–1216, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA 107: 14339–14344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang L, Belousova T, Chen M, Dimattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 82: 867–877, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang N, Tan L, Xue Z, Cang J, Wang H. Reduction of DNA hydroxymethylation in the mouse kidney insulted by ischemia reperfusion. Biochem Biophys Res Commun 422: 697–702, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayakumar C, Mohamed R, Ranganathan PV, Ramesh G. Intracellular kinases mediate increased translation and secretion of netrin-1 from renal tubular epithelial cells. PLos One 6: e26776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaboth U. [Comparative Functional and Morphological Studies on the Ischemia-Damaged Rat Kidney]. Z Gesamte Exp Med 138: 561–580, 1965 [PubMed] [Google Scholar]
- 29. Kawakami T, Park SW, Kaku R, Yang J. Extracellular-regulated-kinase 5-mediated renal protection against ischemia-reperfusion injury. Biochem Biophys Res Commun 418: 603–608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, Lee SC, Kwon BS, Mittler RS, Cho HR, Kwon B. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc Natl Acad Sci USA 109: E13–E22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirov A, Duarte M, Guay J, Karolak M, Yan C, Oxburgh L, Prudovsky I. Transgenic expression of nonclassically secreted FGF suppresses kidney repair. PLos One 7: e36485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko GJ, Linfert D, Jang HR, Higbee E, Watkins T, Cheadle C, Liu M, Racusen L, Grigoryev DN, Rabb H. Transcriptional analysis of infiltrating T cells in kidney ischemia-reperfusion injury reveals a pathophysiological role for CCR5. Am J Physiol Renal Physiol 302: F762–F773, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krishnamoorthy A, Ajay AK, Hoffmann D, Kim TM, Ramirez V, Campanholle G, Bobadilla NA, Waikar SS, Vaidya VS. Fibrinogen beta-derived Bbeta(15–42) peptide protects against kidney ischemia/reperfusion injury. Blood 118: 1934–1942, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kusaka J, Koga H, Hagiwara S, Hasegawa A, Kudo K, Noguchi T. Age-dependent responses to renal ischemia-reperfusion injury. J Surg Res 172: 153–158, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK, Kriz W, Venkatachalam MA. PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol 302: F1210–F1223, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee DH, Wolstein JM, Pudasaini B, Plotkin M. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol 302: F183–F191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D'Agati VD, Cox GN. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol 302: 1166–1175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X, Liu M, Bedja D, Thoburn C, Gabrielson K, Racusen L, Rabb H. Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: implication for murine models of acute kidney injury. Am J Physiol Renal Physiol 302: F519–F525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y, Tong X, Maimaitiyiming H, Clemons K, Cao JM, Wang S. Overexpression of cGMP-dependent protein kinase I (PKG-I) attenuates ischemia-reperfusion-induced kidney injury. Am J Physiol Renal Physiol 302: F561–F570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lieberthal W, Nigam SK. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol 278: F1–F12, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76: 277–285, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Lu X, Li N, Shushakova N, Schmitt R, Menne J, Susnik N, Meier M, Leitges M, Haller H, Gueler F, Rong S. C57BL/6 and 129/Sv mice: genetic difference to renal ischemia-reperfusion. J Nephrol 25: 738–743, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Ma Q, Devarajan P. Induction of proapoptotic Daxx following ischemic acute kidney injury. Kidney Int 74: 310–318, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Matsuda H, Lavoie JL, Gaboury L, Hamet P, Tremblay J. HCaRG accelerates tubular repair after ischemic kidney injury. J Am Soc Nephrol 22: 2077–2089, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Coordination of the cell cycle is an important determinant of the syndrome of acute renal failure. Am J Physiol Renal Physiol 283: F810–F816, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int 60: 2164–2172, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol 301: F436–F442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ongeri EM, Anyanwu O, Reeves WB, Bond JS. Villin and actin in the mouse kidney brush-border membrane bind to and are degraded by meprins, an interaction that contributes to injury in ischemia-reperfusion. Am J Physiol Renal Physiol 301: F871–F882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol 301: F134–F150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park KM, Fogelgren B, Zuo X, Kim J, Chung DC, Lipschutz JH. Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am J Physiol Renal Physiol 298: F818–F826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Patschan D, Hildebrandt A, Rinneburger J, Wessels JT, Patschan S, Becker JU, Henze E, Kruger A, Muller GA. The hormone melatonin stimulates renoprotective effects of “early outgrowth” endothelial progenitor cells in acute ischemic kidney injury. Am J Physiol Renal Physiol 302: F1305–F1312, 2012 [DOI] [PubMed] [Google Scholar]
- 59. Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, Lu B, Sacks SH, Zhou W. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 1474–1485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Qin Y, Alderliesten MC, Stokman G, Pennekamp P, Bonventre JV, de Heer E, Ichimura T, de Graauw M, Price LS, van de Water B. Focal adhesion kinase signaling mediates acute renal injury induced by ischemia/reperfusion. Am J Pathol 179: 2766–2778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Renner B, Ferreira VP, Cortes C, Goldberg R, Ljubanovic D, Pangburn MK, Pickering MC, Tomlinson S, Holland-Neidermyer A, Strassheim D, Holers VM, Thurman JM. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int 80: 165–173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rong S, Park JK, Kirsch T, Yagita H, Akiba H, Boenisch O, Haller H, Najafian N, Habicht A. The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 22: 484–495, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schley G, Klanke B, Schodel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22: 2004–2015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schneider MP, Sullivan JC, Wach PF, Boesen EI, Yamamoto T, Fukai T, Harrison DG, Pollock DM, Pollock JS. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int 78: 374–381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shoskes DA, Parfrey NA, Halloran PF. Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation 49: 201–207, 1990 [DOI] [PubMed] [Google Scholar]
- 66. Siedlecki AM, Jin X, Thomas W, Hruska KA, Muslin AJ. RGS4, a GTPase activator, improves renal function in ischemia-reperfusion injury. Kidney Int 80: 263–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singaravelu K, Padanilam BJ. p53 target Siva regulates apoptosis in ischemic kidneys. Am J Physiol Renal Physiol 300: F1130–F1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song J, Czerniak S, Wang T, Ying W, Carlone DL, Breault DT, Humphreys BD. Characterization and fate of telomerase-expressing epithelia during kidney repair. J Am Soc Nephrol 22: 2256–2265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stone HH, Fulenwider JT. Renal decapsulation in the prevention of post-ischemic oliguria. Ann Surg 186: 343–355, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wei Q, Dong Z. Regulation and pathological role of bid in ischemic acute kidney injury. Ren Fail 29: 935–940, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol 25: 491–499, 2005 [DOI] [PubMed] [Google Scholar]
- 75. White LE, Cui Y, Feltes Shelak CM, Lie ML, Hassoun HT. Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock 38: 320–327, 2012 [DOI] [PubMed] [Google Scholar]
- 76. Xu X, Kriegel AJ, Liu Y, Usa K, Mladinov D, Liu H, Fang Y, Ding X, Liang M. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yasuda K, Vasko R, Hayek P, Ratliff B, Bicer H, Mares J, Maruyama S, Bertuglia S, Mascagni P, Goligorsky MS. Functional consequences of inhibiting exocytosis of Weibel-Palade bodies in acute renal ischemia. Am J Physiol Renal Physiol 302: F713–F721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu W, Beaudry S, Negoro H, Boucher I, Tran M, Kong T, Denker BM. H2O2 activates G protein, alpha 12 to disrupt the junctional complex and enhance ischemia reperfusion injury. Proc Natl Acad Sci USA 109: 6680–6685, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am J Physiol Renal Physiol 303: F139–F148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]