Abstract
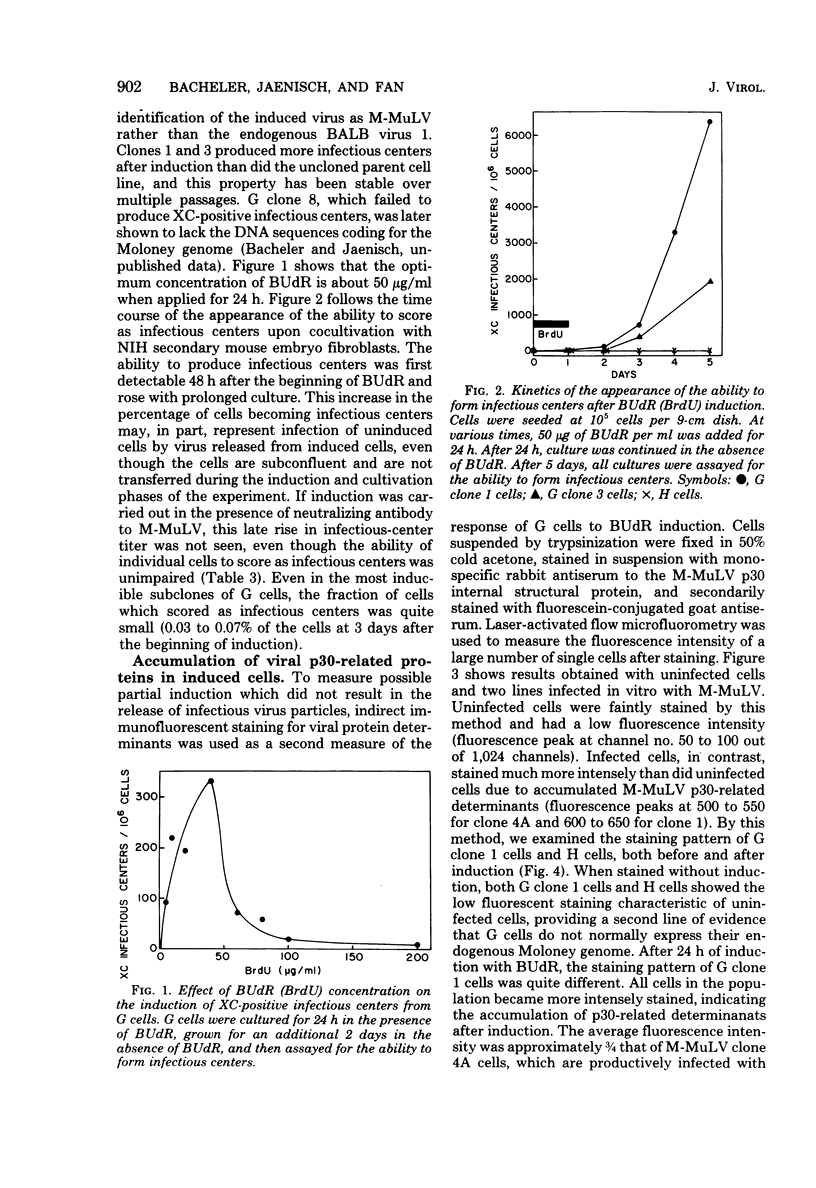
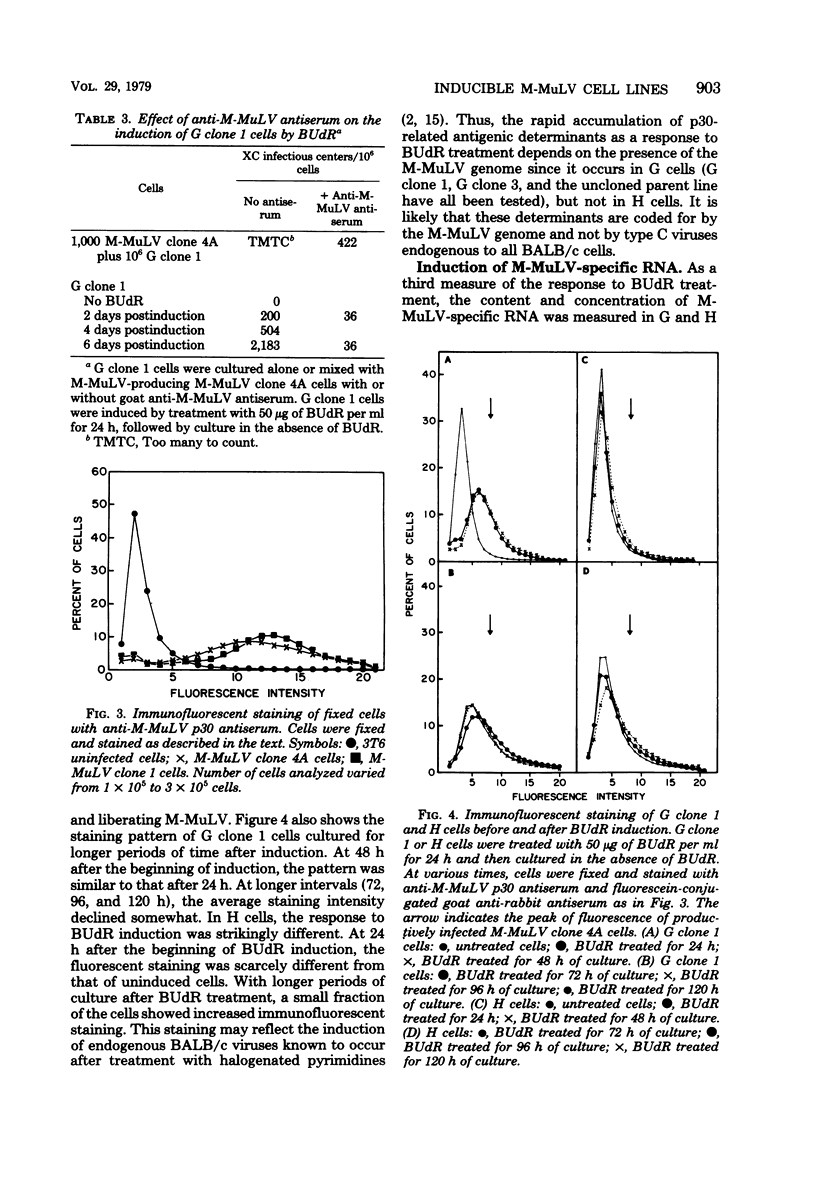
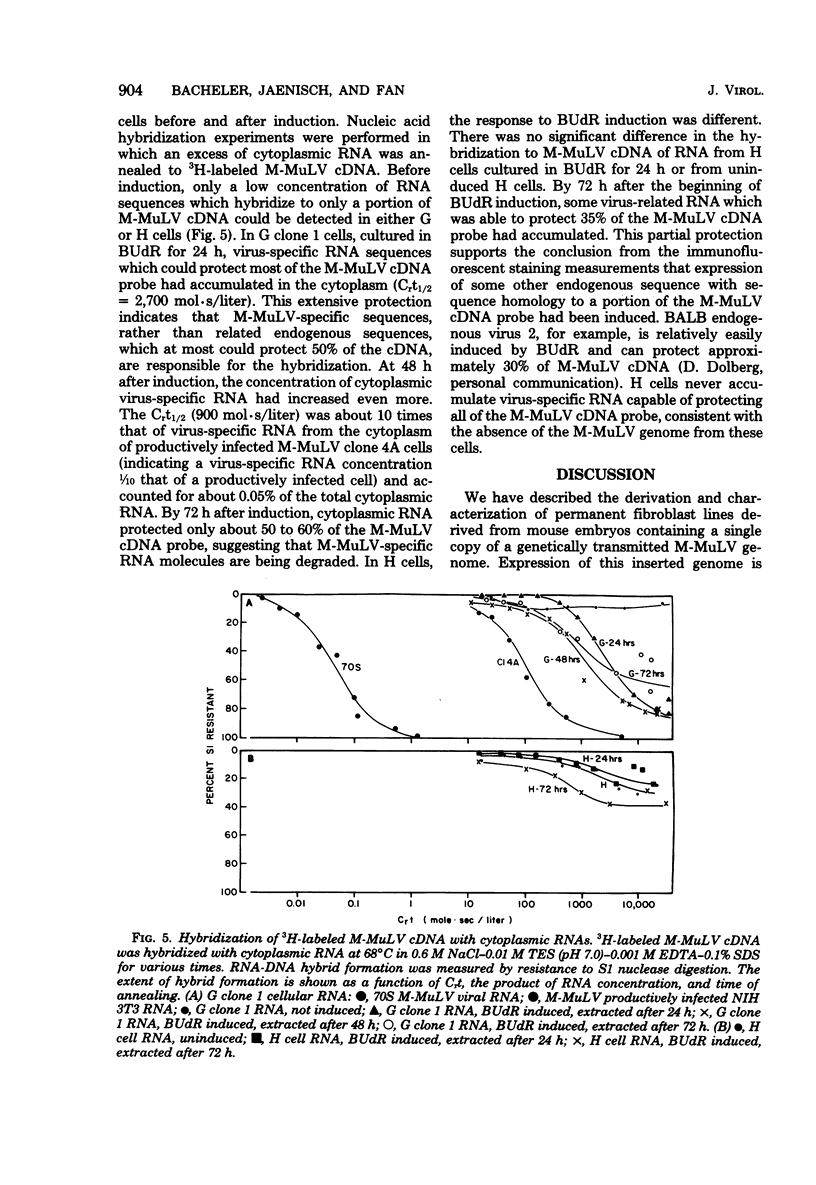
Permanent, non-virus-producing cell lines have been established from a mouse embryo carrying an endogenous, genetically transmitted Moloney murine leukemia virus (M-MuLV) genome. These cells carry the M-MuLV genome, as demonstrated by hybridization of cellular DNA to M-MuLV complementary DNA, but do not express it at the levels of virus production, accumulation of intracellular viral p30, or M-MuLV-specific RNA. Treatment with bromodeoxyuridine (50 microgram/ml for 24 h) resulted in induction of XC-positive NB-tropic virus, although only a small fraction of the cells released virus (less than 0.1% after 48 h). Immunofluorescent staining and flow microfluorometry indicated that a wave of p30 accumulation occurs in the induced cells, with a maximum at 24 to 48 h after the addition of bromodeoxyuridine. Furthermore, most, if not all, cells were induced to produce p30 protein. Similar kinetics were found for the accumulation of M-MuLV-specific RNA in the cytoplasm of induced cells. This rapid induction of virus expression in a majority of cells was dependent on the presence of the M-MuLV genome and probably represents primarily the expression of this endogenous virus since induction was not observed in cells similarly derived from a sibling embryo lacking the M-MuLV genome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Differential cellular regulation of three distinct classes of type C RNA viruses endogenous to mouse cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1129–1137. doi: 10.1101/sqb.1974.039.01.129. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bacheler L. T. Virus-secific transcription in 3T3 cells transformed by the ts-a mutant of polyoma virus. J Virol. 1977 Apr;22(1):54–64. doi: 10.1128/jvi.22.1.54-64.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Smotkin D., Haseltine W., Fan H., Wilson A. T., Paskind M., Weinberg R., Baltimore D. Mechanism of induction of RNA tumor viruses by halogenated pyrimidines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1103–1107. doi: 10.1101/sqb.1974.039.01.125. [DOI] [PubMed] [Google Scholar]
- Cabradilla C. D., Robbins K. C., Aaronson S. A. Induction of mouse type-C virus by translational inhibitors: evidence for transcriptional derepression of a specific class of endogenous virus. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4541–4545. doi: 10.1073/pnas.73.12.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P., Lofgren J. Induction of endogenous guinea pig retrovirus by 5-bromodeoxyuridine: amplification of virus-specific RNA. J Virol. 1978 Jun;26(3):603–614. doi: 10.1128/jvi.26.3.603-614.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. Y., Aaronson S. A., Stephenson J. R. Interactions of chemical inducers and steroid enhancers of endogenous mouse type-C RNA viruses. Virology. 1975 Aug;66(2):579–588. doi: 10.1016/0042-6822(75)90230-5. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Steinkamp J. A., Fulwyler M. J., Coulter J. R., Hiebert R. D., Horney J. L., Mullancy P. F. A new multiparameter separator for microscopic particles and biological cells. Rev Sci Instrum. 1973 Sep;44(9):1301–1310. doi: 10.1063/1.1686375. [DOI] [PubMed] [Google Scholar]
- Vogt M., Bacheler L. T., Boice L. Proposed structure of two defective viral DNA oligomers produced in 3T3 cells transformed by the ts-a mutant of polyoma virus. J Virol. 1976 Mar;17(3):1009–1026. doi: 10.1128/jvi.17.3.1009-1026.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



