Abstract
Increased gpdh-1 transcription is required for accumulation of the organic osmolyte glycerol and survival of Caenorhabditis elegans during hypertonic stress. Our previous work has shown that regulators of gpdh-1 (rgpd) gene knockdown constitutively activates gpdh-1 expression. Fifty-five rgpd genes play essential roles in translation suggesting that inhibition of protein synthesis is an important signal for regulating osmoprotective gene transcription. We demonstrate here that translation is reduced dramatically by hypertonic stress or knockdown of rgpd genes encoding aminoacyl-tRNA synthetases and eukaryotic translation initiation factors (eIFs). Toxin-induced inhibition of translation also activates gpdh-1 expression. Hypertonicity-induced translation inhibition is mediated by general control nonderepressible (GCN)-2 kinase signaling and eIF-2α phosphoryation. Loss of gcn-1 or gcn-2 function prevents eIF-2α phosphorylation, completely blocks reductions in translation, and inhibits gpdh-1 transcription. gpdh-1 expression is regulated by the highly conserved with-no-lysine kinase (WNK) and Ste20 kinases WNK-1 and GCK-3, which function in the GCN-2 signaling pathway downstream from eIF-2α phosphorylation. Our previous work has shown that hypertonic stress causes rapid and dramatic protein damage in C. elegans and that inhibition of translation reduces this damage. The current studies demonstrate that reduced translation also serves as an essential signal for activation of WNK-1/GCK-3 kinase signaling and subsequent transcription of gpdh-1 and possibly other osmoprotective genes.
Keywords: C. elegans, organic osmolytes, hypertonic stress, osmoregulation
maintenance of cellular and extracellular water balance is essential for life. All cells are exposed to osmotic stress from changes in intracellular solute levels that occur during normal fluctuations in metabolism and membrane solute flux. Countless diverse organisms live in osmotically unstable environments and are exposed to extreme changes in extracellular osmolality. Mammalian cells are largely protected from extracellular osmotic perturbations by the kidney, which tightly regulates blood ionic and osmotic concentrations. However, cells in the renal medulla and gut are subjected normally to large osmotic challenges due to the renal concentrating mechanism and changes in fluid and food intake. In addition, several disorders, such as renal failure, diabetes, syndrome of inappropriate antidiuretic hormone secretion, and hypernatremia, disrupt plasma osmolality, and hypertonic solutions are used widely to treat diverse clinical problems such as intracranial hypertension and hemorrhagic shock.
Cellular osmotic homeostasis requires the regulated gain or loss of solutes and activation of mechanisms that repair or remove osmotic stress-induced damage and protect cells from further damage. The mechanisms by which eukaryotic cells detect osmotic perturbations and activate downstream effector responses are poorly understood. Defining these mechanisms represents a central and longstanding problem in cell physiology.
We recently carried out a genome-wide RNA interference (RNAi) screen in Caenorhabditis elegans to identify eukaryotic cellular osmosensing and osmosignaling mechanisms. When exposed to hypertonic environments and associated water loss, C. elegans accumulates the organic osmolyte glycerol (27). Accumulation is mediated in part by increased expression of the glycerol synthesis enzyme glycerol-3-phosphate dehydrogenase-1 (GPDH-1) (27, 28). Using a transgenic worm strain expressing GFP driven by the gpdh-1 promoter, we screened over 16,000 double-stranded RNA (dsRNA) clones to identify genes that normally suppress gpdh-1 expression in the absence of hypertonic stress. This screen identified 122 regulators of gpdh-1 or rgpd genes. Loss of rgpd gene function constitutively activates gpdh-1 expression and glycerol accumulation (28).
The rgpd genes fall into several well-defined functional classes. However, the largest class representing 65 out of 122 genes are genes that function in protein homeostasis or proteostasis. Proteostasis is the maintenance of the complement of properly functioning cellular proteins and involves multiple processes including protein synthesis, folding, assembly, trafficking, and degradation (3, 11).
Fifty-five or eighty percent of the rgpd proteostasis genes perform critical roles in new protein synthesis. For example, 10 rgpd genes encode aminoacyl-tRNA synthetases, enzymes that catalyze the ligation of amino acids to cognate tRNAs and thus function to translate the genetic code and mediate polypeptide elongation. Another 10 rgpd genes encode eukaryotic translation initiation factors (eIFs) that are required for initiation of mRNA translation (28).
The striking enrichment of rgpd genes with genes required for protein synthesis suggested the intriguing hypothesis that changes in the rate of mRNA translation function as part of an osmosensing/signaling pathway. The current studies were undertaken to test this idea directly. We demonstrate that both hypertonic stress and RNAi silencing of rgpd protein synthesis genes inhibit translation and induce gpdh-1 transcription. Inhibition of translation initiation during hypertonic stress occurs via activation of the highly conserved general control nonderepressible (GCN)-2 kinase with subsequent phosphorylation of eIF-2α. gpdh-1 expression is activated downstream from eIF-2α phosphorylation by with-no-lysine kinase (WNK) and Ste20 kinases, which play highly conserved roles in salt and water homeostasis in animals ranging from C. elegans to humans. Our studies are the first to demonstrate a regulatory role for changes in translation and WNK and Ste20 kinases in osmosensitive gene expression. This work together with our previous studies provides the foundation for detailed molecular understanding of the role of changes in protein translation in stress resistance, protein homeostasis and cell signaling.
MATERIAL AND METHODS
C. elegans strains.
The following strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN): wild-type N2 Bristol, RE666 [ire-1(v33)], RB772 [atf-6(ok551)], ST60 [gcn-1(nc40)], and RB980 [gcn-2(ok886)]. Strains MC365 [gcn-2(ok871)] and MC366 [pek-1(ok275)] were generously provided by Drs. Michael Crowder and Xianrong Mao. Unless otherwise stated, worms were cultured at 20°C using standard methods (4). Hypertonic agar plates were generated by adding additional NaCl to standard nematode growth medium.
Quantitative PCR.
Synchronized L4/young adult worms were harvested and frozen in liquid nitrogen. RNA was prepared using RNAqueous-Micro kit (Ambion, Austin, TX). RNA was reverse-transcribed with SuperScriptIII (Invitrogen, Carlsbad, CA) to generate cDNA. Quantitative real-time PCR was conducted using a Strategene Mx-4005P PCR system (La Jolla, CA) with Agilent Brilliant II SYBR Green QPCR Master Mix (Santa Clara, CA). The housekeeping genes rpl-2 and act-1 were used as internal controls.
[35S]methionine labeling of total protein.
Incorporation of [35S]methionine into total protein was used to assess rates of protein synthesis and degradation. Radiolabeling was carried out using methods similar to those described by others (2). Briefly, synchronized L4 worms were fed [35S]methionine-labeled OP50 bacteria for 4 h, washed, incubated with unlabeled OP50 for 1 h to purge radioactive intestinal bacteria, and then washed thoroughly with NGM buffer. Washed worms were flash frozen in liquid nitrogen and stored at −80°C before extraction. Protein was extracted from thawed samples by trichloroacetic acid-ethanol protein precipitation. Total protein concentration and radioactivity incorporation were measured by BCA assay (Pierce Biotechnology) and liquid scintillation counting, respectively.
Western analyses.
Worms were sonicated in lysis buffer (62.5 mM Tris·HCl pH 6.8, 2% wt/vol SDS, 10% glycerol, and complete protease inhibitor cocktail tablet; Roche, Indianapolis, IN) and centrifuged at 16,100 g for 5 min. Total protein concentration was measured in all samples with a BCA assay (Pierce Biotechnology, Rockford, IL). Samples were diluted in NuPage LDS buffer (Invitrogen), and aliquots containing equal amounts of total protein were loaded onto a SDS-polyacrylamide gel and separated by electrophoresis. Proteins were transferred from the gel to nitrocellulose membranes and analyzed by Western blotting using a polyclonal anti-phospho-eIF2α (Ser51) antibody (Cell Signaling Technology, NE BioLabs, Ipswich, MA) and a polyclonal anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Total protein transferred to the nitrocellulose membrane was stained with Ponceau S (Sigma Aldrich, St. Louis, MO) according to the manufacturer's suggested protocol. Relative Western blot band and total protein stain intensities were measured using ImageJ 1.46j software (National Institutes of Health).
RNAi experiments.
RNAi was performed by feeding worms a strain of Escherichia coli engineered to transcribe dsRNA homologous to a target gene. The strains were obtained from commercially available RNAi feeding libraries (Geneservice, Cambridge, England; Open Biosystems, Huntsville, AL). A bacterial strain expressing 202 bases of dsRNA that is not homologous to any predicted C. elegans gene was used as a control for nonspecific RNAi effects. dsRNA feeding was carried out for 2 days by transferring synchronized L1 larvae to agar plates seeded with control or specific RNAi bacteria. Dual RNAi feeding experiments were performed by seeding plates with equal amounts of bacteria expressing wnk-1 and gck-3 dsRNA.
Statistical analysis.
Statistical significance was determined using Student's t-test when two means were compared, a one-way ANOVA (Bonferroni post hoc) when three or more means were compared, and a two-way ANOVA (Bonferroni post hoc) when means were compared across two factors. P values of ≤0.05 were taken to indicate statistical significance.
RESULTS
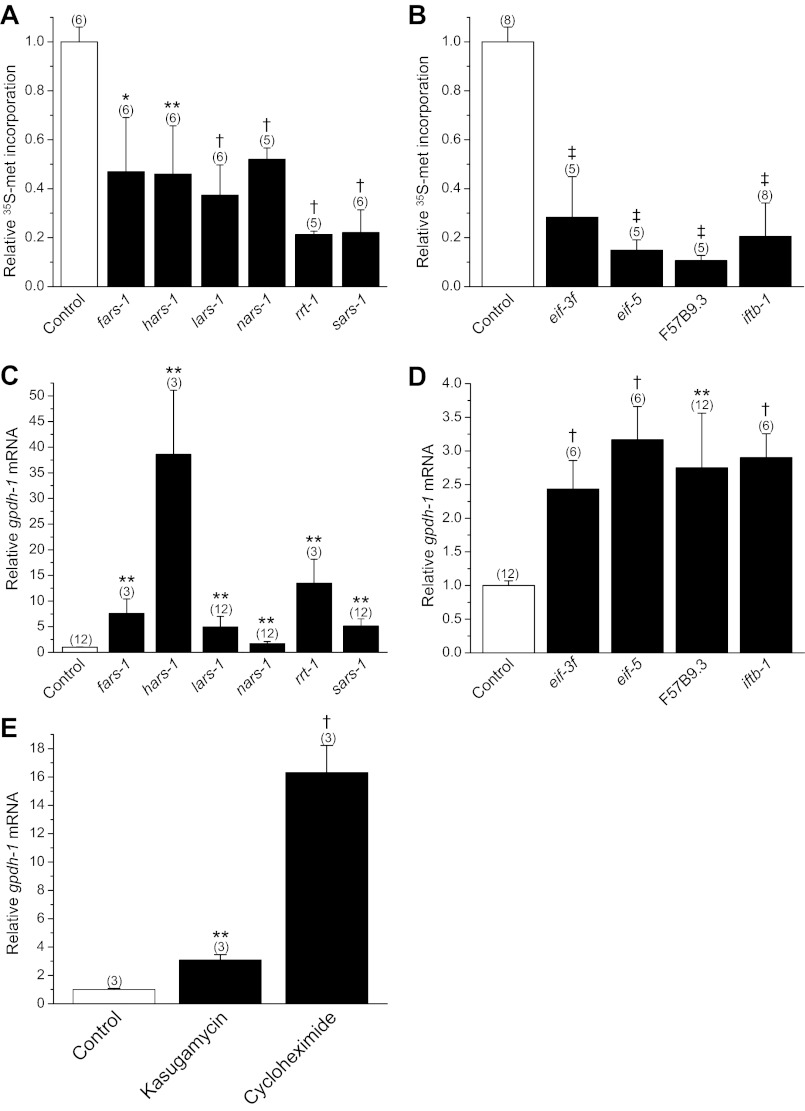
RNAi silencing of rgpd protein synthesis genes activates gpdh-1 expression in the absence of hypertonic stress (28). Given their functional importance, we postulated that knockdown of these genes should also inhibit protein synthesis. We tested this idea by quantifying [35S]methionine incorporation into total protein in worms fed bacteria producing dsRNA homologous to rgpd genes encoding six aminoacyl-tRNA synthetases (fars-1, hars-1, lars-1, nars-1, rrt-1, and sars-1 encode phenylalanyl-, histidyl-, leucyl-, asparaginyl-, arginyl-, and seryl-tRNA synthetases, respectively) and four eIFs (eif-3f, eif-5, F57B9.3, and iftb-1 encode the eIF3 F-subunit, eIF5, eIF4A, and the eIF2-β subunit, respectively). RNAi silencing of all of these genes significantly (P < 0.04) inhibited [35S]methionine incorporation ∼55–85% compared with control worms (Fig. 1, A and B). Knockdown of these genes also significantly (P < 0.01) increased mRNA levels for gpdh-1 ∼1.7- to nearly 40-fold (Fig. 1, C and D).
Fig. 1.
Relationship between inhibition of protein synthesis and gpdh-1 expression. A and B: [35S]methionine incorporation into total protein in worms fed bacteria expressing scrambled double-stranded RNA (dsRNA, control) or dsRNA homologous to regulators of gpdh-1 (rgpd) genes encoding aminoacyl-tRNA synthetases (fars-1, hars-1, lars-1, nars-1, rrt-1, and sars-1 encode phenylalanyl-, histidyl-, leucyl-, asparaginyl-, arginyl-, and seryl-tRNA synthetases, respectively) and eukaryotic translation initiation factors (eif-3f, eif-5, F57B9.3, and iftb-1 encode the eIF3 F-subunit, eIF5, eIF4A, and eIF2 β-subunit, respectively). Values are expressed relative to [35S]methionine incorporation in control worms. *P < 0.04, **P < 0.01, †P < 0.001, ‡P < 0.0001, compared with control. C and D: relative gpdh-1 mRNA levels in worms fed dsRNA expressing bacteria. **P < 0.01; †P < 0.001, compared with control. E: effect of inhibition of protein synthesis for 6 h by 1 mg/ml kasugamycin or 1 mg/ml cycloheximide on gpdh-1 mRNA levels. gpdh-1 mRNA is expressed relative to that observed in control worms with no drug treatment. **P < 0.01, †P < 0.001, compared with control. All values are means ± SE; n is shown in parentheses above each data point.
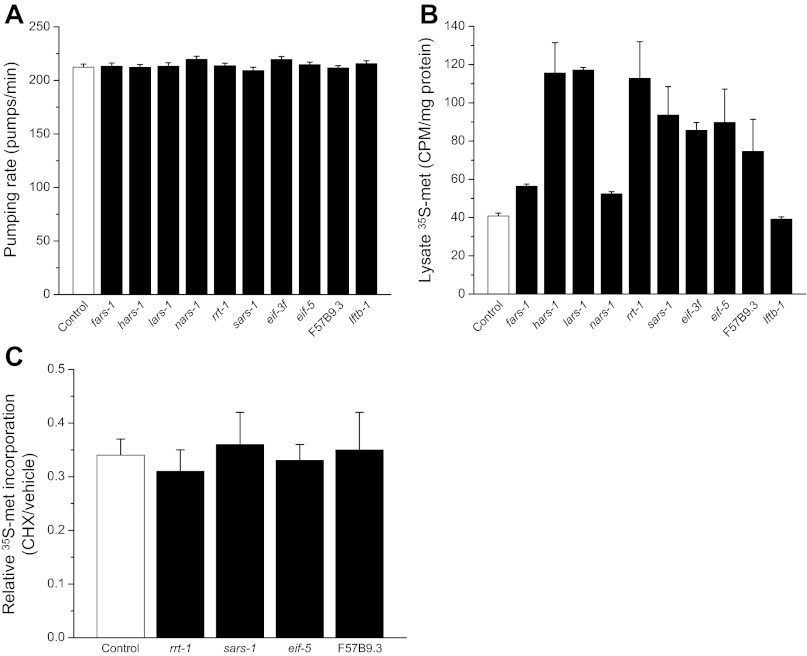
It is conceivable that the reduction of [35S]methionine labeled total protein in RNAi worms is due to reduced ingestion of radioactive bacteria, reduced [35S]methionine uptake in the intestine, and/or increased protein degradation. As shown in Fig. 2A, pharyngeal pumping rates were not significantly (P > 0.08) different in control vs. RNAi worms. In addition, [35S]methionine levels in worm lysates, which contain both free and protein incorporated label, were not significantly (P > 0.4) reduced in worms fed control or RNAi bacteria (Fig. 2B). These results demonstrate that ingestion of bacteria and intestinal uptake of [35S]methionine are not inhibited by silencing of rgpd protein synthesis genes.
Fig. 2.
Effect of RNA interference (RNAi) silencing of rgpd protein synthesis genes on [35S]methionine uptake and protein degradation activity. A and B: rate of pharyngeal pumping and whole lysate [35S]methionine levels in worms fed bacteria producing scrambled dsRNA (control) or dsRNA homologous to aminoacyl-tRNA synthetases (fars-1, hars-1, lars-1, nars-1, rrt-1, and sars-1) and eukaryotic translation initiation factors (eif-3f, eif-5, F57B9.3, and iftb-1). RNAi gene silencing had no significant (P > 0.08) inhibitory effect on ingestion of labeled bacteria (A) or uptake of [35S]methionine from the intestine (B). Values are means ± SE; n = 30 for pharyngeal pumping measurements and n = 6 for lysate 35S levels. C: effect of 500 μg/ml cycloheximide on [35S]methionine incorporation into total protein in worms fed bacteria expressing scrambled (control), rrt-1, sars-1, eif-5, or F57B9.3 dsRNA. Data are expressed as [35S]methionine incorporation in cycloheximide (CHX)-treated worms relative to worms exposed to vehicle (DMSO) only. Cycloheximide blocks protein synthesis and the decline in [35S]methionine incorporation therefore reflects total protein degradation activity. Values are means ± SE; n = 3 for each dsRNA tested.
To assess the effect of RNAi on protein degradation, we fed worms bacteria expressing scrambled dsRNA or one of four rgpd protein synthesis genes (rrt-1, sars-1, eif-5, or F57B9.3). [35S]methionine incorporation into total protein was measured in worms treated for 12 h with drug vehicle only (DMSO) or 500 μg/ml cycloheximide, which completely inhibits protein synthesis in C. elegans (25). In cycloheximide-treated worms, reductions in the levels of [35S]methionine-labeled protein reflect protein degradation activity (6). As shown in Fig. 2C, treatment of control worms with cycloheximide reduces [35S]methionine incorporation 65–70%. The reductions in [35S]methionine incorporation were not significantly (P > 0.5) different in rrt-1, sars-1, eif-5 or F57B9.3 dsRNA-fed worms. Taken together, data in Fig. 2 demonstrate that reductions in the levels of [35S]methionine-labeled total protein in RNAi worms (Fig. 1, A and B) reflect inhibition of protein synthesis rather than increased protein degradation or reduced [35S]methionine uptake.
We also tested whether inhibition of protein synthesis by bacterial toxins could induce gpdh-1 expression. Worms were treated for 6 h on 51 mM NaCl agar plates containing either 1 mg/ml kasugamycin or 1 mg/ml cycloheximide. Kasugamycin inhibits translation initiation while cycloheximide blocks the elongation step of protein synthesis. As shown in Fig. 1E, both compounds elevated gpdh-1 mRNA levels 3–16 fold (P < 0.005).
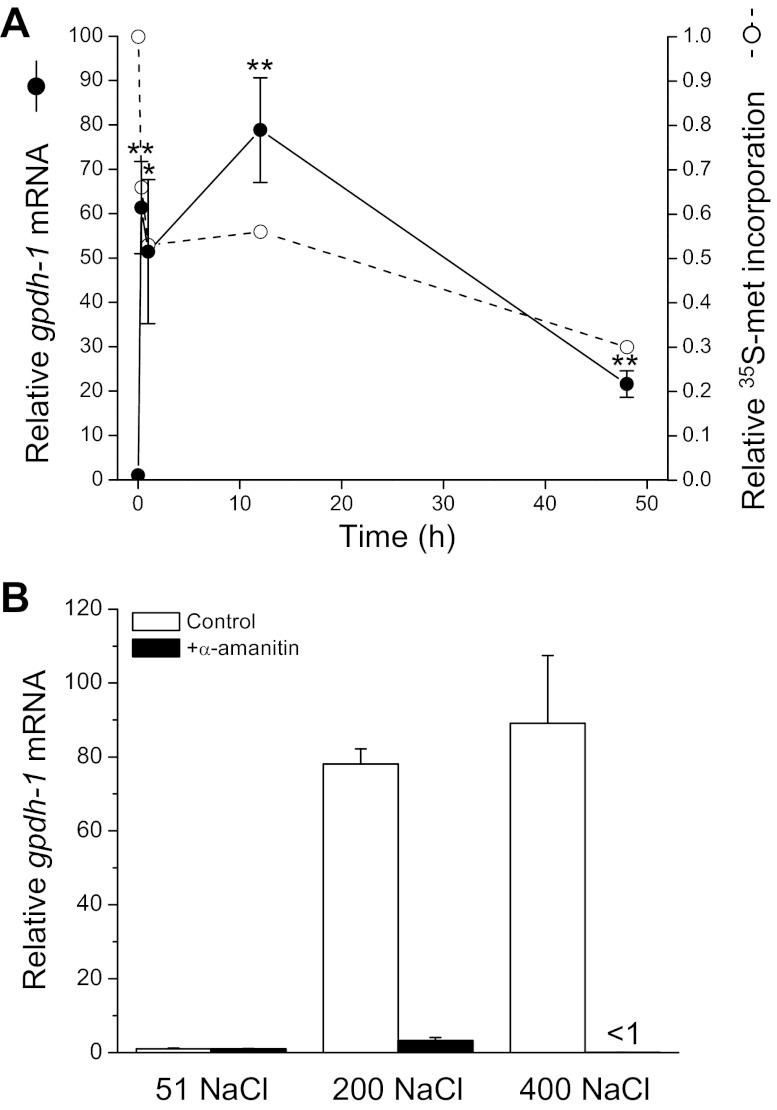
Results shown in Fig. 1 and 2 support the hypothesis that reductions in protein synthesis serve as a signal to activate gpdh-1 expression during hypertonic stress. Consistent with this idea, we have shown previously that hypertonic stress causes a rapid and prolonged inhibition of translation (6). Figure 3A shows the relationship between changes in gpdh-1 expression and [35S]methionine incorporation into total protein. Within 20 min of exposure to hypertonic stress, protein synthesis is inhibited ∼30% and gpdh-1 mRNA levels increase 50–60 fold. gpdh-1 expression remains elevated and translation inhibited for ≥48 h after worms are transferred to hypertonic medium.
Fig. 3.
Effect of hypertonic stress on protein synthesis and gpdh-1 expression. A: time course of changes in gpdh-1 mRNA levels and relative [35S]methionine incorporation into total protein in worms exposed to 200 mM NaCl. Hypertonic stress inhibits [35S]methionine incorporation and increases gpdh-1 expression. [35S]methionine incorporation and mRNA levels are expressed relative to worms at time 0. Values for gpdh-1 mRNA levels are means ± SE (n = 3 for each time point). *P < 0.04, **P < 0.01, compared with time 0. Data on [35S]methionine incorporation are replotted from Burkewitz et al. (6). Values are means ± SE (n = 5–8 for each time point). All values are P < 0.001, compared with time 0. B: effect of α-amanitin on gpdh-1 mRNA levels. Worms were exposed for 1 h to 51, 200, or 400 mM NaCl agar plates with or without 200 μg/ml α-amanitin. mRNA levels are expressed relative to worms exposed to 51 mM NaCl without α-amanitin. gpdh-1 expression in α-amanitin treated 200 and 400 mM NaCl worms was not significantly (P > 0.07) increased above that of untreated worms exposed to 51 mM NaCl. Values are means ± SE (n = 3).
Increased gpdh-1 mRNA levels during hypertonic stress could reflect increased gpdh-1 gene transcription and/or increased mRNA half-life. To determine whether increased transcription is required for the elevated gpdh-1 mRNA levels, we treated worms with the mushroom toxin α-amanitin, which is a potent inhibitor of RNA polymerase II. Worms were exposed for 1 h to 51, 200, or 400 mM NaCl agar plates with or without 200 μg/ml α-amanitin. As shown in Fig. 3B, α-amanitin completely blocked hypertonic stress-induced elevation of gpdh-1 mRNA levels indicating that increased transcription is primarily responsible for the increased expression. Based on the results shown in Figs. 1–3, we conclude 1) that hypertonic stress induces rapid inhibition of protein synthesis and induction of gpdh-1 transcription, and 2) that inhibition of protein synthesis brought about by hypertonic stress, RNAi silencing of essential protein synthesis genes, or toxins represents an important signal for activation of gpdh-1 gene expression.
Two questions arise from the studies described above. First, what are the upstream signaling mechanisms by which hypertonic stress inhibits translation in a manner that specifically activates gpdh-1 transcription? Second, what are the downstream signaling mechanisms that transduce reductions in translation into increased expression of gpdh-1?
Regulation of protein synthesis occurs primarily at the initiation step via changes in the phosphorylation state of eIFs. In mammalian cells, GCN, PKR-like endoplasmic reticulum (ER) kinase (PERK), heme-regulated inhibitor kinase (HRI), protein kinase RNA-activated (PKR) mediate eIF phosphorylation under stress conditions (16, 23, 40). HRI and PKR kinase homologs have not been identified in the C. elegans genome (32). We therefore examined the role of the unfolded protein response (UPR) and signaling through PERK and GCN kinases in mediating transcription of gpdh-1.
The UPR is a highly conserved intracellular signaling and transcriptional/translational program activated by the accumulation of unfolded proteins in the ER lumen. Activation of the UPR reduces ER protein misfolding (i.e., ER stress) by inhibiting protein synthesis and by upregulating the ER's protein folding capacity. Translation is inhibited by PERK-mediated phosphorylation of eIF-2α (18).
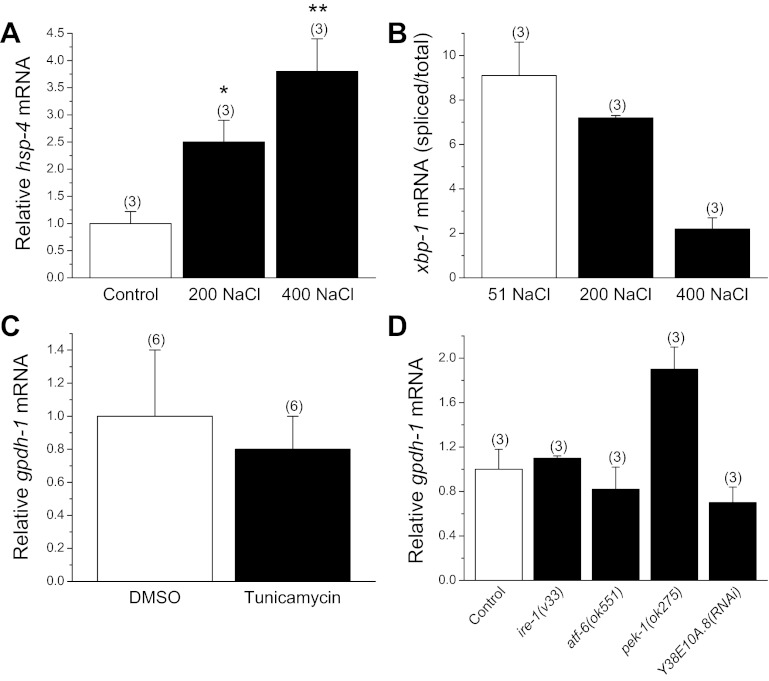
Hypertonic stress causes widespread protein damage in C. elegans (5, 6, 10) suggesting that it might also activate the UPR. To determine whether UPR signaling regulates gpdh-1 expression, we first assessed whether ER stress is induced during exposure to hypertonic conditions. HSP-4 is a conserved ER chaperone protein that is induced by ER stress (8). Using an hsp-4 transcriptional GFP reporter transgenic worm strain, we were unable to detect GFP fluorescence changes in animals exposed to 200 or 400 mM NaCl for up to 12 h. We therefore quantified hsp-4 mRNA levels. Exposure to 200 or 400 mM NaCl increased hsp-4 mRNA levels 2.4–4-fold (P < 0.05; Fig. 4A).
Fig. 4.
Role of unfolded protein response (UPR) signaling in regulating gpdh-1 expression. A: effect of a 2-h exposure to 200 or 400 mM NaCl agar plates on hsp-4 mRNA levels. mRNA is expressed relative to control worms. *P < 0.05, **P < 0.03, compared with control. B: xbp-1 mRNA splicing during hypertonic stress. Spliced to total xbp-1 mRNA ratio should increase if hypertonic stress activates the UPR. mRNA ratio at 200 mM NaCl was not significantly (P > 0.3) different from control. At 400 mM NaCl, mRNA ratio was significantly (P < 0.001) decreased compared with control. C: effect of activation of the UPR by tunicamycin on gpdh-1 expression. Worms were treated with drug or vehicle (DMSO) on 51 mM NaCl agar plates. Tunicamycin had no significant (P > 0.7) effect on gpdh-1 mRNA levels. D: effect of deletion mutations or RNAi silencing of endoplasmic reticulum (ER) membrane sensors on gpdh-1 expression. N2, mutant, and RNAi worms were exposed to 200 mM NaCl agar plates for 2 h. mRNA levels are expressed relative to N2 worms. Inositol requiring enzyme-1 (IRE-1), activating transcription factor-6 (ATF-6), and PEK-1 function to detect misfolded proteins in the ER lumen and activate the UPR. PEK-1, a protein kinase RNA-like ER kinase (PERK) ortholog, inhibits protein synthesis by phosphorylating eIF2α. Y38E10A.8 is a predicted PEK-1 paralog. Loss of function of these genes has no significant (P > 0.1) inhibitory effect on gpdh-1 transcription. All values are means ± SE; n is shown in parentheses above each data point.
While relatively small, the changes in hsp-4 mRNA levels suggested that the UPR might be activated by hypertonic stress. We therefore carried out a number of additional studies to test for the role of UPR signaling in regulating gpdh-1 expression. Inositol requiring enzyme-1 (IRE-1) is an ER membrane kinase/endonuclease that catalyzes the splicing and subsequent activation of the transcription factor X box binding protein-1 (XBP-1) in response to ER stress. If the UPR is activated, the ratio of spliced to total xbp-1 mRNA should increase. To further assess UPR activation, then we quantified the amount of spliced and total xbp-1 mRNA. As shown in Fig. 4B, spliced xbp-1 mRNA levels did not change significantly (P > 0.3) in worms exposed to 200 mM NaCl. In 400 mM NaCl-treated animals, the ratio decreased significantly (P < 0.001; Fig. 4B). These results demonstrate that the UPR is not activated under hypertonic stress conditions.
Tunicamycin blocks N-glycosylation of proteins, which in turn triggers ER stress. If UPR signaling regulates gpdh-1 transcription, tunicamycin should increase gpdh-1 mRNA levels. We treated worms for 6 h with 5 μg/ml tunicamycin or vehicle (DMSO) only and quantified gpdh-1 expression. Tunicamycin induced expression of the hsp-4 GFP reporter (data not shown). However, as shown in Fig. 4C, there were no significant (P > 0.7) differences in gpdh-1 mRNA levels in control vs. tunicamycin-treated worms.
Finally, inhibition of the ER membrane sensors that detect unfolded proteins, IRE-1, PERK, and activating transcription factor-6 (ATF-6; Ref. 18), should inhibit gpdh-1 expression if UPR signaling is involved in regulating gpdh-1 gene transcription via inhibition of protein synthesis. We used RNAi or worm strains carrying deletion mutations to inhibit UPR signaling in animals exposed to 200 mM NaCl for 2 h. As shown in Fig. 4D, hypertonic stress-induced gpdh-1 expression was not inhibited in ire-1(v33), pek-1(ok275) (a PERK ortholog) and atf-6(ok551) deletion mutants. RNAi silencing of the predicted C. elegans PEK-1/PERK paralog Y38E10A.8 also had no inhibitory effect (Fig. 4D). Thus, despite the small increases in hsp-4 mRNA with hypertonic stress, we conclude that the UPR is not substantially activated under these conditions and that UPR signaling does not regulate gpdh-1 expression.
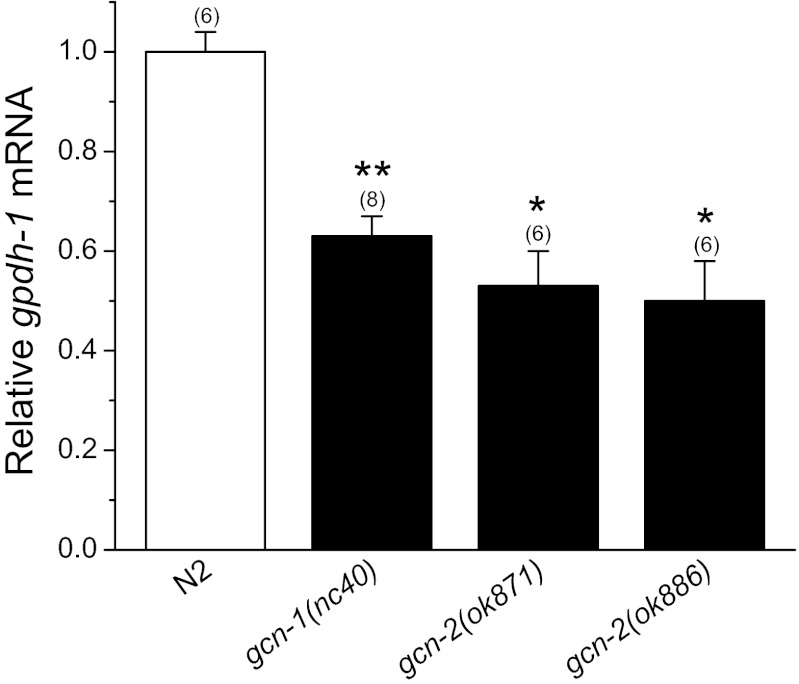
The kinase GCN-2 is activated in eukaryotic cells during starvation (45, 47). Activation requires interaction with the accessory protein GCN-1 (37). We used worm strains harboring loss-of-function alleles of gcn-1 and gcn-2 to assess the role of this signaling pathway in regulating gpdh-1 expression. As shown in Fig. 5, hypertonicity-induced gpdh-1 expression was reduced 40–50% (P < 0.0002) by loss of either GCN-1 or GCN-2 function.
Fig. 5.
Role of by general control nonderepressible (GCN)-2 kinase signaling in regulating gpdh-1 expression. Loss-of-function alleles of gcn-1 or gcn-2 inhibit gpdh-1 expression induced by a 2 h exposure to 200 mM NaCl. *P < 0.0002, compared with N2 animals. Values are means ± SE; n is shown in parentheses above each data point. *P < 0.0002, **P < 0.0001 compared with N2 worms.
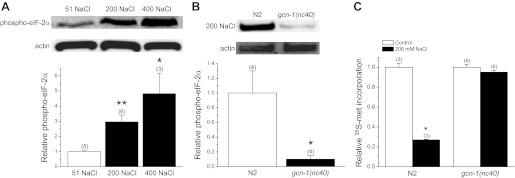
GCN-2 directly phosphorylates eIF2α resulting in turn in inhibition of translation (45, 47). We quantified changes in C. elegans eIF-2α phosphorylation using a phosphospecific antibody. eIF-2α phosphorylation increased 2–5-fold (P < 0.04) in worms exposed to 200 or 400 mM NaCl for 2 h (Fig. 6A). Disruption of GCN-2 signaling by loss-of-function mutation of gcn-1 decreased (P < 0.03) hypertonic stress-induced eIF-2α phosphorylation >90% (Fig. 6B) and prevented the reduction of translation observed under hypertonic conditions (Fig. 6C). Taken together, data in Figs. 5 and 6 indicate that GCN-2 kinase signaling is involved in inhibiting translation during hypertonic stress. Inhibition of translation in turn leads to downstream activation of gpdh-1 transcription (Fig. 1).
Fig. 6.
Role of GCN-2 kinase signaling in regulating eIF-2α phosphorylation and protein synthesis during hypertonic stress. A: effect of hypertonic stress on eIF-2α phosphorylation. eIF-2α phosphorylation was quantified using a phosphospecific antibody. Top: Western blots of phosphorylated eIF-2α in worms exposed to 51 (control), 200, or 400 mM NaCl for 2 h. Blots were also probed with an anti-actin polyclonal antibody to assess protein loading. Bottom: quantification of phosphorylated eIF-2α. *P < 0.04, **P < 0.002, compared with control. B, top: Western blots of phosphorylated eIF-2α and actin in N2 worms or gcn-1(nc40) mutants exposed to 200 mM NaCl for 2 h. Bottom: quantification of phosphorylated eIF-2α. *P < 0.03, compared with control. *P < 0.03, compared with N2. (C) [35S]methionine incorporation into total protein in N2 worms or gcn-1(nc40) mutants exposed to either 51 or 200 mM NaCl for 2 h. *P < 0.0001, compared with N2 worms. All values are means ± SE; n is shown in parentheses above each data point.
We recently carried out a genome-wide RNAi screen focused on identifying genes required for activation of gpdh-1 expression during hypertonic stress (S. Ells and K. Strange, unpublished observations). One of the strongest regulators of gpdh-1 expression identified in our screen was the kinase WNK-1. WNK kinases are highly conserved in metazoa and play important roles in salt and water homeostasis (9, 33). In C. elegans, WNK-1 functions together with the Ste20 kinase GCK-3 to regulate systemic volume homeostasis in hypertonic environments (9).
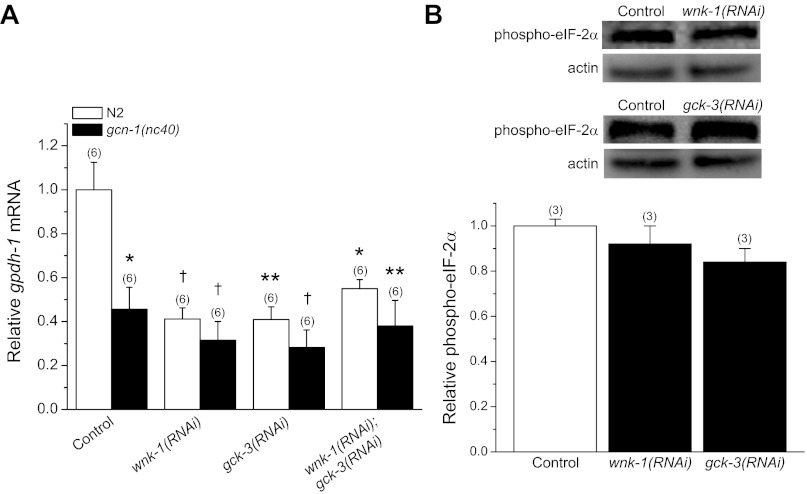
As shown in Fig. 7A, RNAi silencing of either wnk-1 or gck-3 inhibited gpdh-1 expression 60–70% in worms exposed to 200 mM NaCl for 2 h. Combined RNAi knockdown of wnk-1 and gck-3 had no additive effect on gpdh-1 expression (Fig. 7A) suggesting that the two kinases function in a common signaling pathway as we have described for acute volume regulation (9). In addition, there were no additive effects of wnk-1 and/or gck-3 RNAi in gcn-1(nc40) mutant worms (Fig. 7A). These results indicate that WNK-1 and GCK-3 function together with GCN-1/GCN-2 to regulate gpdh-1 transcription.
Fig. 7.
Role of WNK-1 and GCK-3 in regulating gpdh-1 expression. A: effect of RNAi silencing of wnk-1, gck-3, or both wnk-1 and gck-3 on gpdh-1 mRNA levels in N2 worms and gcn-1(nc40) mutants. Worms were exposed to 200 mM NaCl for 2 h. *P < 0.01, **P < 0.005, †P < 0.001, compared with N2 control worms fed bacteria expressing scrambled dsRNA. gpdh-1 expression in N2 worms and gcn-1(nc40) loss-of-function mutants fed wnk-1 and/or gck-3 dsRNA was not significantly (P > 0.2) different suggesting that WNK-1 and GCK-3 function in a common pathway together with GCN-1/GCN-2. B: effect of RNAi silencing of wnk-1 or gck-3 on eIF-2α phosphorylation. Top: Western blots of phosphorylated eIF-2α and actin in wnk-1(RNAi) and gck-3(RNAi) worms exposed to 200 mM NaCl for 2 h. Bottom: quantification of phosphorylated eIF-2α. Data are expressed relative to phosphorylated eIF-2α in N2 worms fed bacteria expressing scrambled dsRNA. All values are means ± SE; n is shown in parentheses above each data point.
WNK-1 and GCK-3 could function upstream or downstream from GCN-1/GCN-2. To determine where they function in the signaling pathway, we quantified the effect of RNAi silencing of wnk-1 or gck-3 on eIF-2α phosphorylation induced by a 2-h exposure to 200 mM NaCl. As shown in Fig. 7B, hypertonic stress-induced eIF-2α phosphorylation was not significantly (P > 0.07) different in wnk-1(RNAi) and gck-3(RNAi) worms compared with N2 animals fed bacteria expressing a scrambled dsRNA. These results demonstrate that WNK-1 and GCK-3 function downstream from inhibition of translation initiation to regulate gpdh-1 expression.
DISCUSSION
Translation initiation is the primary step at which protein synthesis is regulated. Cap-dependent initiation requires the coordinated function of >25 distinct proteins including multiple eIFs. Phosphorylation of eIF2α or dephosphorylation of eIF4 complex binding proteins (4E-BPs) inhibit translation initiation (16, 23). Multiple environmental stressors inhibit protein synthesis via changes in the phosphorylation state of eIF2α and/or 4E-BPs (20, 40, 48). For example, in Chinese hamster ovary cells heat shock inhibits translation and decreases 4E-BP1 phosphorylation (43). Survival of renal medullary cells exposed to high urea levels requires activation of GCN-2 and subsequent eIF2α phosphorylation (7). Oxidative stress in yeast cells inhibits translation in part through activation of GCN-2 kinase signaling and eIF2α phosphorylation (38).
Our results demonstrate that hypertonic stress in C. elegans induces eIF-2α phosphorylation via the GCN-2 kinase signaling cascade (Fig. 6A). Inhibition of this cascade by loss of GCN-1 function blocks eIF-2α phosphorylation (Fig. 6B) and the reduction in translation induced by 200 mM NaCl (Fig. 6C) demonstrating that GCN-1/2 signaling is the primary mechanism for inhibiting protein synthesis during this level of hypertonic stress.
Inhibition of rgpd genes that function in protein synthesis inhibits translation and activates gpdh-1 expression (Figs. 1, A–D) (28). gpdh-1 expression is also activated by the protein synthesis inhibitors cycloheximide and kasugamycin (Fig. 1E). These results demonstrate that reductions in translation serve as a signal for activation of osmosensitive gene expression. Consistent with this conclusion, hypertonic stress-induced gpdh-1 expression is inhibited 40–60% in gcn-1 and gcn-2 loss-of-function mutants (Fig. 5). Given that hypertonicity-induced reductions in translation are completely blocked in the gcn-1 mutant (Fig. 6, B and C), other signals must also regulate the activation of gpdh-1. We suggested previously (28) that hypertonic stress-induced gene expression may also be regulated by a pathway that responds to systemic volume loss induced mechanical signals associated with the nematode cuticle. Rohlfing et al. (36) have subsequently shown that the protein encoded by the rgpd gene osm-8 functions in the cuticle extracellular matrix together with the hypodermal transmembrane protein PTR-23 to regulate constitutive expression of gpdh-1. However, neither of these proteins have clear homologs in other animals suggesting that they are part of a nematode-specific signaling pathway.
GCN-2 kinases are highly conserved in eukaryotes. They are activated by uncharged tRNAs and thus play a central role in regulating protein synthesis during amino acid starvation (17, 46). The mechanism by which hypertonic stress activates GCN-2 in C. elegans is uncertain. However, recent studies in yeast have shown that both hypertonic (49) and acid (21) stresses increase uncharged tRNA levels. As discussed in the Introduction, 10 identified rgpd genes encode aminoacyl-tRNA synthetases, which are tRNA charging enzymes. Knockdown of these enzymes is expected to increase uncharged tRNA levels. We therefore suggest that the proximal signal for activating GCN-2 during hypertonic stress in C. elegans is an increase in uncharged tRNAs. Interestingly, certain aminoacyl-tRNA synthetases are inhibited by small increases in inorganic ion concentrations (1, 30). Thus it is conceivable that elevated intracellular ionic strength or the levels of specific inorganic ions induced by cell shrinkage could increase uncharged tRNA levels simply by inhibiting the charging enzymes.
How inhibition of translation activates downstream signaling and effector mechanisms is unclear but is a topic of considerable biological importance. Inhibition of protein synthesis extends lifespan in multiple diverse species. Extended lifespan is likely due to improved protein homeostasis, increased availability of cellular resources required for prolongevity mechanisms, and/or selective translation of prolongevity mRNAs (34, 42). Importantly, enhanced longevity is widely accepted to be associated with increased resistance to diverse stressors (14, 24, 39). During stressor-induced inhibition of cap-dependent translation, genes required for survival under particular stress conditions are selectively translated (20, 41). The mechanisms responsible for selective mRNA translation are not well understood but are under intense study. Recent work suggests that translation of 10–15% of all mRNAs can occur via cap-independent mechanisms that utilize specialized mRNA structural elements such as internal ribosome entry sites (31, 41). Transcript length may also play an important role in selective mRNA translation under conditions that inhibit protein synthesis (35).
Our results demonstrate that the C. elegans WNK and Ste20 kinases WNK-1 and GCK-3 are required for hypertonic stress-induced gpdh-1 transcription (Fig. 7A), that they likely function in a common pathway with GCN-1/GCN-2 (Fig. 7A), and that they function downstream from eIF-2α phosphorylation and translation inhibition (Fig. 7B). WNK kinases are highly conserved in all metazoa and function in a variety of fundamental and diverse processes including regulation of development, sensory nerve function, insulin and MAPK signaling, and salt and water homeostasis (33). GCK-3 is a member of the GCK-VI subfamily of Ste20 kinases. The mammalian GCK-VI kinases SPAK and OSR1 play key roles in regulating ion transport pathways that are essential for systemic ion and fluid balance (12, 33).
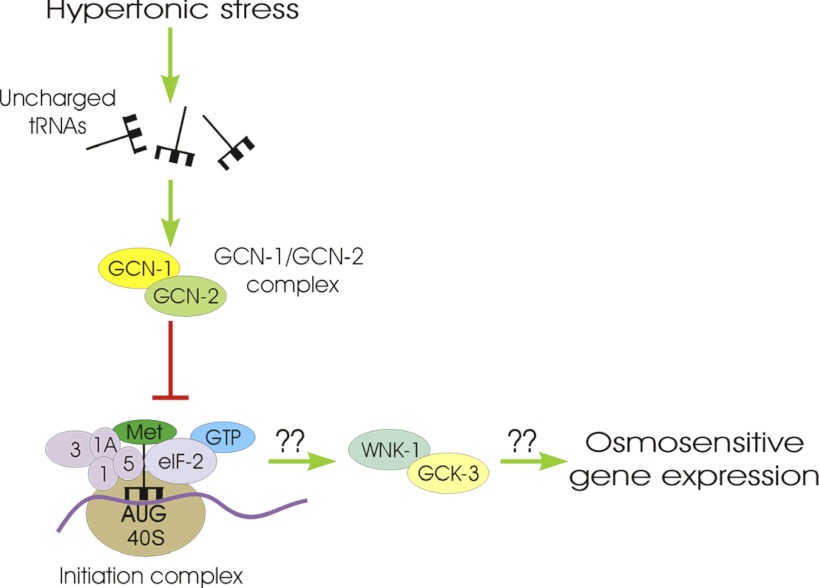
WNK-1 and GCK-3 control important physiological processes in C. elegans including systemic fluid balance during hypertonic stress (9), cell cycle- and cell volume–dependent activity of the CLC anion channel CLH-3b (13, 15), developmental processes (19, 26), and hypertonic stress-induced expression of the antimicrobial peptide NLP-29 (29). Our findings together with those of Lee et al. (29) define a novel role for WNK and GCK-VI kinases in regulating osmosensitive gene expression. We suggest that inhibition of mRNA translation activates WNK-1/GCK-3 signaling, which in turn activates gpdh-1 transcription. The mechanisms that induce gpdh-1 transcription are unclear but may involve phosphorylation-induced changes in the activity of transcription factors similar to what has been proposed for mitogen activated protein kinase-dependent regulation of SKN-1 function during oxidative stress (22, 44) and RNAi silencing of eIFs (44). A model summarizing our results is shown in Fig. 8.
Fig. 8.
Model illustrating the putative signaling mechanisms involved in activating gpdh-1 transcription. We postulate that hypertonic stress increases uncharged tRNA levels resulting in GCN-2 activation. The initiation complex comprises the preinitiation complex (methionine-charged tRNA, eIF-2α/β/γ, eIF1, eIF1A, eIF3, eIF5, a 40S ribosome subunit, and GTP) bound to an mRNA AUG start codon. Activated GCN-2 phosphorylates eIF-2α, which prevents GTP hydrolysis and subsequent binding of a 60S ribosome subunit to the 40S subunit to form an elongation competent 80S ribosome. Inhibition of mRNA translation activates WNK-1 via an unknown mechanism. WNK-1 binds to GCK-3 as we have shown previously (9) and likely activates it via phosphorylation (12, 33). GCK-3 in turn induces transcription of gpdh-1 and most likely other osmosensitive genes via an unknown signaling mechanism.
In conclusion, our studies have demonstrated that inhibition of protein synthesis induced by hypertonic stress, RNAi silencing of genes that play essential roles in translation, or bacterial toxins serves as an important signal for activation of gpdh-1 transcription and most likely the transcription/translation of other osmosensitive genes. As we have shown previously, inhibition of translation also serves the additional critical role of protecting proteins from damage induced by water loss and concomitant cell shrinkage (6). Our studies thus provide the basis for development of a systems level understanding of the stress resistance, signaling, and protein homeostasis roles of translation regulation. Understanding these roles has broad implications for understanding the pathophysiology of aging, cellular stresses associated with disease, and how organisms cope with natural and human-induced environmental stressors.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-61168 (to K. Strange).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.C.L. and K.S. conception and design of research; E.C.L. performed experiments; E.C.L. and K.S. analyzed data; E.C.L. and K.S. interpreted results of experiments; E.C.L. and K.S. prepared figures; E.C.L. and K.S. drafted manuscript; E.C.L. and K.S. edited and revised manuscript; E.C.L. and K.S. approved final version of manuscript.
REFERENCES
- 1.Anderson JW, Fowden L. Properties and substrate specificity of the leucyl-, the threonyl- and the valyl-transfer-ribonucleic acid synthetases from Aesculus species. Biochem J 119: 691–697, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson LL, Mao X, Scott BA, Crowder CM. Survival from hypoxia in C. elegans by inactivation of aminoacyl-tRNA synthetases. Science 323: 630–633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkewitz K, Choe K, Strange K. Hypertonic stress induces rapid and widespread protein damage in C. elegans. Am J Physiol Cell Physiol 301: C566–C576, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkewitz K, Choe KP, Choung-Hee LE, Deonarine A, Strange K. Characterization of the proteostasis roles of glycerol accumulation, protein degradation and protein synthesis during osmotic stress in C. elegans. PLos One 7: e34153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Q, Brooks HL. Phosphorylation of eIF2α via the general control kinase, GCN2, modulates the ability of renal medullary cells to survive high urea stress. Am J Physiol Renal Physiol 301: F1202–F1207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol 293: C915–C927, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Choe KP, Strange K. Genome-wide RNAi screen and in vivo protein aggregation reporters identify degradation of damaged proteins as an essential hypertonic stress response. Am J Physiol Cell Physiol 295: C1488–C1498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci 9: 759–767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J 409: 321–331, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol 125: 113–125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol 190: 719–729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falin RA, Morrison R, Ham A, Strange K. Identification of regulatory phosphorylation sites in a cell volume- and Ste20 kinase-dependent ClC anion channel. J Gen Physiol 133: 29–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5: 827–835, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307: 1776–1778, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev 91: 1219–1243, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep 9: 70–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hueso G, Aparicio-Sanchis R, Montesinos C, Lorenz S, Murguia JR, Serrano R. A novel role for protein kinase Gcn2 in yeast tolerance to intracellular acid stress. Biochem J 441: 255–264, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto CK. The elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19: 2278–2283, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11: 113–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon CJ. The genetics of ageing. Nature 464: 504–512, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kourtis N, Tavernarakis N. Cell-specific monitoring of protein synthesis in vivo. PLos One 4: e4547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupinski AP, Muller-Reichert T, Eckmann CR. The Caenorhabditis elegans Ste20 kinase, GCK-3, is essential for postembryonic developmental timing and regulates meiotic chromosome segregation. Dev Biol 344: 758–771, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Lamitina ST, Morrison R, Moeckel GW, Strange K. Adaptation of the nematode Caenorhabditis elegans to extreme osmotic stress. Am J Physiol Cell Physiol 286: C785–C791, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci USA 103: 12173–12178, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KZ, Kniazeva M, Han M, Pujol N, Ewbank JJ. The fatty acid synthase fasn-1 acts upstream of WNK and Ste20/GCK-VI kinases to modulate antimicrobial peptide expression in C. elegans epidermis. Virulence 1: 113–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loftfield RB, Eigner EA, Pastuszyn A, Lovgren TN, Jakubowski H. Conformational changes during enzyme catalysis: role of water in the transition state. Proc Natl Acad Sci USA 77: 3374–3378, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malys N, McCarthy JE. Translation initiation: variations in the mechanism can be anticipated. Cell Mol Life Sci 68: 991–1003, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning G. Genomic overview of protein kinases. WormBook 1–19, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91: 177–219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta R, Chandler-Brown D, Ramos FJ, Shamieh LS, Kaeberlein M. Regulation of mRNA translation as a conserved mechanism of longevity control. Adv Exp Med Biol 694: 14–29, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Rogers AN, Chen D, McColl G, Czerwieniec G, Felkey K, Gibson BW, Hubbard A, Melov S, Lithgow GJ, Kapahi P. Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab 14: 55–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohlfing AK, Miteva Y, Moronetti L, He L, Lamitina T. The Caenorhabditis elegans mucin-like protein OSM-8 negatively regulates osmosensitive physiology via the transmembrane protein PTR-23. PLoS Genet 7: e1001267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattlegger E, Hinnebusch AG. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J 19: 6622–6633, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, Ashe MP, Grant CM. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem 281: 29011–29021, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Shore DE, Carr CE, Ruvkun G. Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genet 8: e1002792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell 40: 228–237, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Spriggs KA, Stoneley M, Bushell M, Willis AE. Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biol Cell 100: 27–38, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Tavernarakis N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol 18: 228–235, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Vries RG, Flynn A, Patel JC, Wang X, Denton RM, Proud CG. Heat shock increases the association of binding protein-1 with initiation factor 4E. J Biol Chem 272: 32779–32784, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Robida-Stubbs S, Tullet JM, Rual JF, Vidal M, Blackwell TK. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet 6: e1001048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 15: 4497–4506, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson WA, Roach PJ. Nutrient-regulated protein kinases in budding yeast. Cell 111: 155–158, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Curr Opin Cell Biol 20: 222–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaborske JM, Narasimhan J, Jiang L, Wek SA, Dittmar KA, Freimoser F, Pan T, Wek RC. Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J Biol Chem 284: 25254–25267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]