Abstract
In primary culture, the gastric parietal cell's deeply invaginated apical membrane, seen in microscopy by phalloidin binding to F-actin (concentrated in microvilli and a subapical web), is engulfed into the cell, separated from the basolateral membrane (which then becomes the complete plasma membrane), and converted, from a lacy interconnected system of canaliculi, into several separate vacuoles. In this study, vacuolar morphology was achieved by 71% of parietal cells 8 h after typical collagenase digestion of rabbit gastric mucosa, but the tight-junctional protein zonula occludens-1 (ZO-1) was completely delocalized after ∼2 h, when cells were ready for culturing. Use of low-Ca2+ medium (4 mM EGTA) to release cells quickly from gastric glands yielded parietal cells in which ZO-1 was seen in a small spot or ring, a localization quickly lost if these cells were then cultured in normal Ca2+ but remaining up to 20 h if they were cultured in low Ca2+. The cells in low Ca2+ mostly retained, at 20 h, an intermediate morphology of many bulbous canalicular expansions (“prevacuoles”), seemingly with narrow interconnections. Histamine stimulation of 20-h cells with intermediate morphology caused colocalization of proton-pumping H-K-ATPase with canaliculi and prevacuoles but little swelling of those structures, consistent with a remaining apical pore through which secreted acid could escape. Apparent canalicular interconnections, lack of stimulated swelling, and lingering ZO-1 staining indicate inhibition of membrane fission processes that separate apical from basolateral membrane and vacuoles from each other, suggesting an important role for extracellular Ca2+ in these, and possibly other, endocytotic processes.
Keywords: canaliculi, apical/basolateral membrane, tight junction, calcium, membrane fission
isolated gastric glands and isolated parietal cell cultures have provided valuable information on gastric secretory mechanisms as well as important insight to general questions in cell biology. Effective gastric gland preparations were first developed by Berglindh and Obrink (5) including methodology for quantifying responsiveness to secretory stimuli in vitro. Catherine Chew and her colleagues (7) were the first to provide a detailed study of optimizing procedures to establish and maintain functional parietal cells in primary culture. Our laboratory and many others have used these valuable preparations for a myriad of studies.
One phenomenon that has intrigued us for some time is the morphological change in parietal cell structure as the cells transition from the innate epithelial position in the gastric mucosa to the freely separated parietal cell incubated in a culture dish. As in all epithelia, parietal cells in situ are joined by tight junctions (zonula occludens) between themselves and other glandular cells, separating the bulk mucosal fluid from interstitial fluid as well as separating the apical and basolateral membrane domains. Distinct to the parietal cell is a uniquely evolved morphology in which the apical membrane invaginates deeply into the cell in the form of many narrow, microvilli-lined canaliculi, such that no part of the cytoplasmic space is far from some part of the luminal space (13).
Profound changes in parietal cell structure occur when the cell is stimulated by secretagogues to transform from a resting, nonsecreting, state to one of maximal HCl secretion. In the resting state, proton pumps (H-K-ATPase) are sequestered in a cytoplasmic pool of small vesicles called tubulovesicles. Stimulation, mainly via a cAMP pathway initiated by binding of histamine to basal H2 receptors, causes fusion of tubulovesicles with the canalicular membranes, further increasing canalicular membrane area, lengthening the microvilli, and inserting the proton pumps into the apical membrane. The enormous recruitment of tubulovesicles into the apical surface results in a 5- to 10-fold increase in apical surface (13). Newly inserted pumps secrete hydrochloric acid across the apical membrane into the stomach lumen (9, 11, 12). In both resting and stimulated states, the canaliculi can be visualized in microscopy with the binding of labeled phalloidin to F-actin, which is abundant both in the microvillar cores and in a subapical web, or with immunolocalization of the membrane-cytoskeletal linker protein ezrin, which is concentrated at the canalicular membrane (1, 16, 17, 37).
Parietal cells maintained in culture develop a morphology markedly different from that of the cells in situ. Within 24 h in primary culture, individual parietal cells have retracted their narrow apical pole into the cell and separated the apical (canalicular) membrane from basolateral membrane, with the latter becoming the new surrounding plasma membrane. The separated apical membrane converts from narrow, interconnected canaliculi into several separate and roughly spherical intracellular vacuoles. The vacuolar membranes, like the predecessor canaliculi, are associated with abundant ezrin and F-actin (1, 7, 21, 32). These vacuoles maintain functional characteristics of the apical membrane, in that tubulovesicles fuse with them upon histamine stimulation, resulting in secretion of HCl into the vacuolar lumina, swelling them since they no longer have a luminal connection to an apical pore for outflow of the acid.
Both the canaliculi of parietal cells in situ and the apical vacuoles that develop in primary cultures have some interesting parallels in other cell types. Hepatocytes have an apical membrane, facing the bile canaliculus, which is complexly infolded, increasing surface area. Although these folds do not extend nearly as deeply into the cytoplasm as do parietal cell canaliculi, both types of inward extensions of apical membrane have been shown to share involvement of a member of the ezrin/radixin/moesin (ERM) protein family as a membrane/cytoskeleton linker: ezrin in parietal cells and radixin in hepatocytes. Furthermore, a hepatocyte-derived cell line, WIF-B (a hybrid of rat hepatoma and human fibroblast cells), engulfs apical membrane into cytoplasmic vacuoles (34). Madin-Darby canine kidney (MDCK) cells engulf some of their apical membrane into a microvilli-lined vacuolar apical compartment (VAC) when cell-cell contact is disrupted by chelation of extracellular Ca2+ and then restore those membranes to the apical surface when a confluent monolayer is reestablished (36). Mechanisms that pull these apical membranes into the liver and kidney cell lines may share some commonalities with parietal cell sequestration of apical membrane into the cytoplasm. In the parietal cell in situ, the inward pulling forces must be opposed by a mechanism that holds the canalicular membrane connected to the apical pole of the cell, and the canalicular lumen continuous with the gland lumen, for draining of secreted acid into the stomach. Intact tight junctions must be pivotal to this function, adding to their common functions of forming a selective paracellular permeability barrier and separating distinctive apical and basal membrane domains (3, 14).
One goal of the studies presented here was to provide a more complete characterization of the process of conversion from canaliculi to vacuoles (1, 7, 21) by observing the different stages leading up to the vacuolar morphology. Parietal cells were isolated by a previously described (1) method of collagenase digestion of gastric mucosa, which gave a yield of 70–75% stimulation-responsive parietal cells that were cultured for up to 20 h on Matrigel, followed by fixation and preparation for immunocytochemistry at various times. An interesting intermediate, or transitional, morphology was seen, involving many bulbous expansions of canaliculi, more numerous and smaller than the ultimate separated vacuoles.
A secondary goal was to view cellular localization of tight-junctional proteins during this conversion, considering the possibility that clusters of such proteins lingering at the apical pole might provide clues to the timing of the ultimate separation of apical and basolateral membranes during this process. In an attempt to see the tight-junction-associated protein zonula occludens-1 (ZO-1; Ref. 33), very soon after cell separation, we used a well-established procedure of lowering extracellular Ca2+ concentration ([Ca2+]) by chelation with EGTA to disrupt tight junctions (6, 10, 24, 29, 35) and compared these cells with those separated by the usual collagenase digestion procedure.
In addition to characterizing and quantitating the canalicular-to-vacuolar conversion in isolated parietal cells, we investigated the importance to this process of extracellular Ca2+. We found that, in low extracellular [Ca2+] (0.15 μM, calculated), cultured parietal cells linger in a transitional state between canalicular and fully vacuolar. This suggests that normal extracellular [Ca2+] (1.8 mM) facilitates the pinching closed of narrowing membrane pores, or necks, to initiate the fissions separating engulfed apical membrane from basolateral membrane and separating forming vacuoles from each other.
MATERIALS AND METHODS
Isolation of gastric glands and parietal cells.
All procedures and treatments for handling animals were reviewed and approved by the Berkeley Animal Care and Use Committee. New Zealand White rabbits were sedated with ketamine (35 mg/kg) and xylazine (5 mg/kg ip) and then anesthetized with pentobarbital sodium (∼70 mg/kg iv). The gastric vasculature was perfused with PBS (in mM: 146 Na+, 147 Cl−, 3 K+, 3.6 PO43−, 1 Ca2+, and 1 Mg2+, pH 7.3), via retrograde cannulation of the abdominal aorta, to loosen the lamina propria and begin separation of glands before removal of the stomach. The mucosa was scraped off, minced, washed with PBS and then with MEM (Mediatech 50–011-PB, with 20 mM HEPES and 5 μM cimetidine, pH adjusted to 7.3 with NaOH), and then digested with collagenase (Worthington type 4, 1 mg/ml in MEM, with 1 mg/ml BSA) for 30 min at 37°C with constant gentle stirring. The digestion was filtered through nylon mesh (0.2 mm) fabric to remove relatively undigested tissue. Gastric glands and large aggregates of cells settled out in ∼15 min at 1 g, leaving a suspension of individual cells and small aggregates, which was put through a 40-μm cell strainer. Intact cells were recovered by centrifugation twice at 100 g for 10 min, resuspended in Complete Medium [DMEM/F-12 (GIBCO-BRL, Grand Island, NY), 20 mM HEPES, 0.2% BSA, 10 mM glucose, 1× insulin-transferrin-selenium-A (GIBCO), 1 mM glutamine, 100 U/ml penicillin/streptomycin, 400 μg/ml gentamicin sulfate, and 15 g/l geneticin, pH 7.4], centrifuged again, and resuspended a final time (“time zero”) in Complete Medium. These cells were designated “collagenase-isolated cells.” Both Complete Medium and MEM contain 1.8 mM Ca2+ (“normal” [Ca2+]). In previous tests, other methods, including pronase digestion and Nycodenz gradient separation, yielded up to 95% parietal cells, but they were not as healthy or responsive in our hands as were cells from the procedure used here (1).
Parietal cell culture.
Collagenase-isolated cells in Complete Medium were plated onto coverslips coated with Matrigel (BD Biosciences, Bedford, MA) and kept at 37°C in a humidified non-CO2 incubator up to 20 h. Viable cells attached to the substrate during ∼3 h incubation. After a 3-, 6-, and 20-h incubation, cell samples were taken for immunofluorescence microscopy.
Other collagenase-isolated cells were kept suspended in Complete Medium in a 50-ml conical tube with gentle agitation at 37°C. At 0 and 1 h, samples were plated on poly-l-lysine (P1399, Sigma)-coated coverslips and prepared for immunofluorescence microscopy. Because of the 3-h time for adequate attachment to Matrigel, suspended cells sampled at 0 and 1 h were analyzed with the cells cultured for 3, 6, and 20 h on Matrigel for a more complete time course.
EGTA method of cell isolation.
In an attempt to examine tight junctional proteins sooner after separation of cells from the epithelium, we used low [Ca2+] to disrupt tight junctions in gastric glands that settled after the previously described collagenase digestion of mucosal tissue. These glands were resuspended in MEM with added penicillin/streptomycin (100 U/ml) and gentamicin sulfate (400 μg/ml). EGTA was added to 4 mM (“time zero”), and glands were gently agitated in a 50-ml conical tube for 40 min. Free [Ca2+] was calculated as 0.15 μM with Ca-EGTA Calculator v1.3, based on Schoenmakers et al. (28). Samples were taken at t = 0 and 30 min and plated on poly-l-lysine-coated coverslips for microscopy. After 40 min, agitation was stopped. A small portion of this gland suspension was taken at this time for continued agitation at 37°C and additional sampling and plating at 1 and 4 h. These samples from 0, 0.5, 1, and 4 h were scored for glands and released parietal cells, identified by mAb 2G11 against H-K-ATPase β-subunit (8). The 50-ml tube containing most of the suspension was set in a vertical stand for 20-min settling of glands from released cells. The resulting supernatant (“1 h”), considered released cells, was centrifuged at 100 g for 10 min and resuspended in Complete Medium. These cells were designated “EGTA-isolated cells in Complete Medium.” Samples were placed on polylysine-coated coverslips and processed for immunocytochemistry. Number of 1-h cells recognized by 2G11 divided by total cells seen in DIC microscopy yielded percentage of parietal cells obtained by this method. Remaining cells were plated onto several Matrigel-coated coverslips for incubation and then processed for immunocytochemistry at 4 and 20 h.
In additional experiments, to observe continued effect of low extracellular [Ca2+], some EGTA-isolated cells were resuspended in Complete Medium with 4 mM EGTA, designated “EGTA-isolated cells in EGTA,” for plating on Matrigel.
Although some additional cells were maintained in MEM or in suspension for up to 20 h, these appeared relatively unhealthy or were poorly responsive, so for cells at t = 3 h and beyond the experiments reported here involve cells transferred to Complete Medium and plated on Matrigel.
Immunofluorescence and microscopy.
Cells were fixed with either 4% formaldehyde in PBS (for cells on Matrigel) or 2% formaldehyde in PBS (for cells on polylysine) for 10 min, followed by permeabilization in 0.5% Triton X-100 in PBS for 15 min and blocking in 2% BSA in PBS for 1 h. For H-K-ATPase and ZO-1 probing, cells were incubated for 1 h with mouse monoclonal anti-H-K-ATPase β-subunit 2G11 and mouse monoclonal anti-ZO-1 (Invitrogen, Eugene, OR; 1:50 dilution), followed by secondary antibody Alexa Fluor 555-conjugated goat-anti-mouse (Invitrogen; 1:200) for 1 h. For F-actin probing, Alexa Fluor 488-Phalloidin (Invitrogen; 1:200) was used. Images of Alexa Fluor 555 (ex 543 nm and em 590–655 nm) and Alexa Fluor 488 (ex 488 nm and em 505–580 nm) were collected 1) on a Nikon Microphot FXA microscope using a DIC ×40/0.7 air objective with a Photometrics Sensys camera and Inovision software, or 2) on a Zeiss LSM 780 confocal microscope at one Airy unit pinhole with Plan-Neofluar ×40/1.3 oil DIC objective.
Some parietal cell cultures at 20 h were stimulated with 0.4 mM histamine and 30 μM isobutylmethylxanthine (IBMX) for 30 min at 37°C in Complete Medium ±4 mM EGTA, directly on their coverslips in the 37°C incubator. After stimulation, cells were fixed, permeabilized, and probed for F-actin and H-K-ATPase as above. Some other cells were treated as controls with secondary antibody and no primary.
Cells stained for F-actin and H-K-ATPase were scored according to morphological appearance, which was divided into three states: 1) canalicular, containing lacy or stringy intracellular phalloidin staining (F-actin); 2) transitional, with some of the stringy canalicular structures along with numerous small, bulbous expansions of canaliculi; and 3) vacuolar, with only vacuoles as phalloidin-staining internal structures, generally larger and fewer than the bulbous expansions seen in the transitional state. Nonparietal cells (no H-K-ATPase staining) and dead cells (cell shape) were not scored. Score is expressed as percentage of total parietal cells.
Western blotting.
Samples were obtained from cells plated onto Matrigel or cells held in suspension. For cells on Matrigel, a method of cell recovery (with Cell Recovery Solution; BD Biosciences, Bedford, MA) was used to remove the cells from culture wells or Petri dishes. Recovered solution was centrifuged in a 50-ml conical tube, 600 g, 10 min. The pellet was washed in 1 ml cold PBS and transferred to an Eppendorf tube. For suspended cells, a 1-ml sample was transferred to an Eppendorf tube. The Eppendorfs were centrifuged (600 g, 3 min), and the pellets were resuspended in 1× SDS-PAGE sample buffer (1% SDS, 0.4 M urea, 5% 2-mercaptoethanol, 0.25 mM EDTA, 10% glycerol, 0.0025% bromophenol blue, and 30 mM Tris·HCl, pH 6.8), sonicated, heated to 100° for 5 min, centrifuged to pellet DNA, and tested for protein concentration by the filter-paper method of Minamide and Bamburg (25). Samples were then run on Bis-Tris gels, either 4–12% (NuPAGE, Invitrogen) or 10% acrylamide. Proteins were transferred from gels to nitrocellulose membranes in Idea Scientific transfer tanks, blocked with 5% nonfat milk in 0.1% Tween-20 (EMD) in TBS (140 mM NaCl and 20 mM Tris, pH 7.4) for 1 h at room temperature, and then probed with monoclonal antibodies against ZO-1 (1:1,000) and actin (pan Ab-5, 1:5,000, from Thermo-Fisher, Waltham, MA). After washes in TBS-Tween, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, 1:2,000), for 1 h at room temperature, and then washed and developed with chemiluminescence reagent (Western Lightning; Perkin-Elmer Life Sciences, Boston, MA) for exposure to film (Blue X-ray; Phenix Research Products, Candler, NC). Densitometric analysis of results from four separate experiments for each set of conditions was done with Image-J software.
RESULTS
Morphological transformation of collagenase-isolated cells cultured in normal [Ca2+].
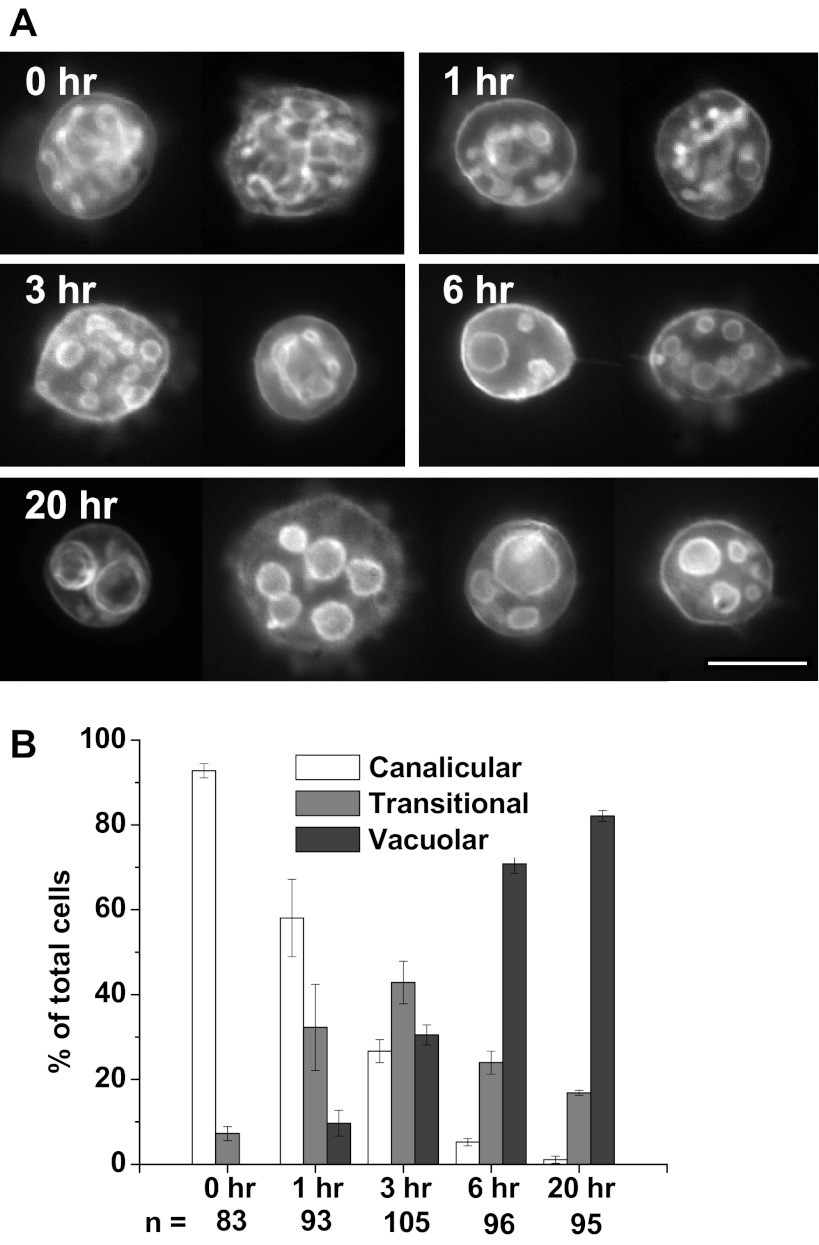
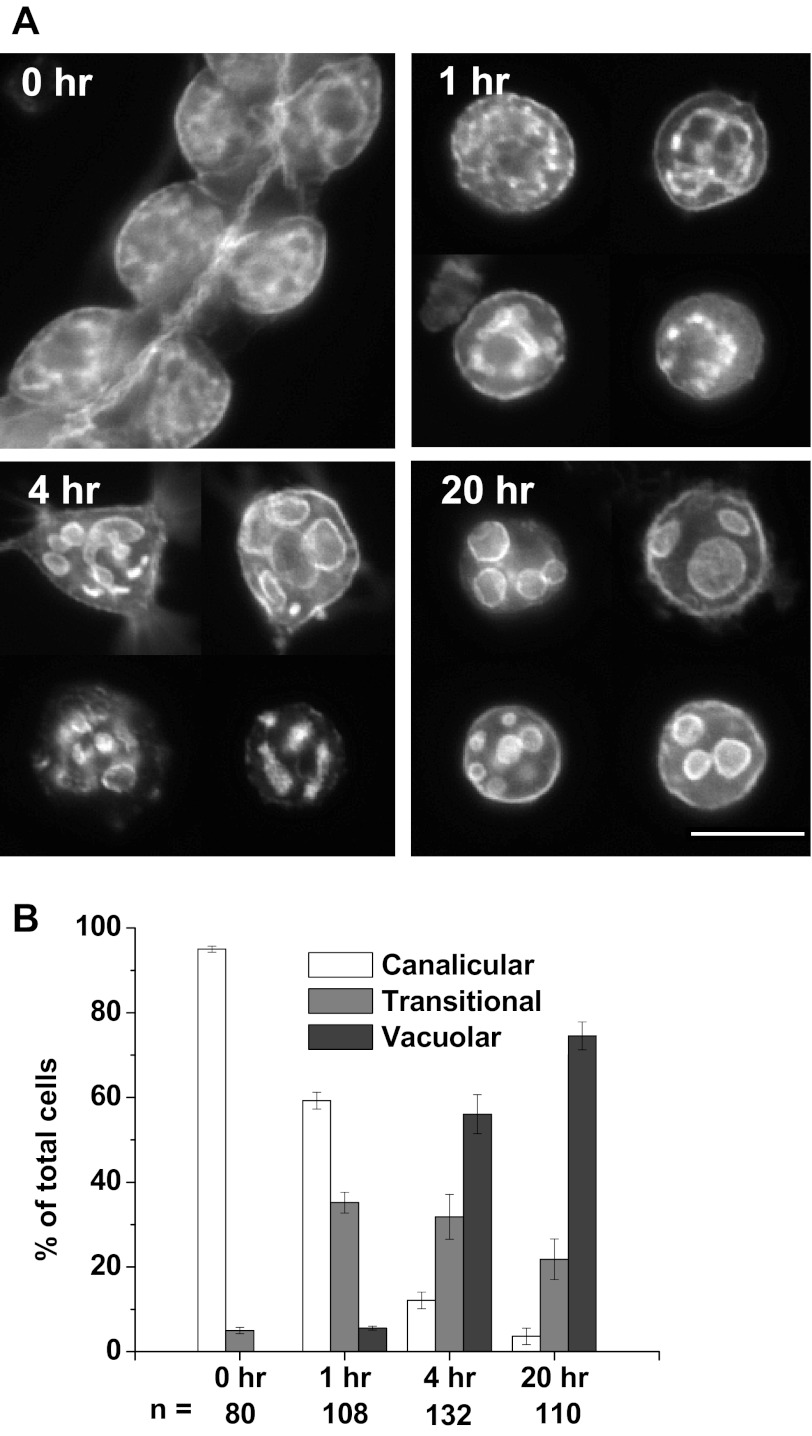
Parietal cells isolated by collagenase digestion and suspended in Complete Medium for culturing on Matrigel were probed for F-actin with fluorescent phalloidin. Figure 1A shows exemplary images of F-actin distribution over a 20-h time course. At time 0 (actually ∼2 h after the cells were separated from the glands), the cells contained an internal lacy network of long tubules seemingly connected to each other, appearing similar to the canalicular membranes seen in gastric glands (16). After an additional hour in suspension (t = 1), many cells (32.3 ± 10.2%) showed canaliculi transitioning into shorter tubules with multiple widening regions (“prevacuoles”). After 3 h on Matrigel, this “transitional” morphology, a combination of canalicular and vacuolar characteristics, predominated (42.8 ± 5.0% of total). However, a “vacuolar” morphology in which the prevacuoles began to seem separated also appeared in many cells at 3 h (30.5 ± 2.4%) and became dominant at 6 h (70.8 ± 2.2%). By 20 h, vacuoles became larger and fewer, varying in number and size [small (0.5–2 μm), medium (2–5 μm), and large (5–10 μm)] with vacuolar cells then 82.1 ± 1.3% of total. F-actin was clearly evident around the vacuoles but also to a lesser degree near the plasma membrane (the basolateral membrane of the cell before its separation from the gland). Many cells were analyzed for their canalicular/vacuolar state, with the percentage in each state at each time shown in Fig. 1B.
Fig. 1.
Appearance of apical membrane vacuoles in isolated cultured parietal cells. A: Cells separated by collagenase digestion and resuspended (t = 0) in Complete Medium with normal Ca2+ concentration ([Ca2+]) were fixed, permeabilized, and probed for F-actin and typical cells are shown for each time. By 6 h, most of the apical canalicular membranes had either developed many bulbous expansions or converted completely to separated spherical vacuoles. Transformation to vacuoles was marked by passage through an intermediate transitional state, which had a combination of canalicular and vacuolar characteristics. Bar = 20 μm. B: rate of vacuolar conversion in collagenase-isolated parietal cells cultured on Matrigel over 20 h. Graph shows the percentages of cells with canalicular, transitional (mixed canalicular/vacuolar features), and fully vacuolar morphology at the times shown, as described in materials and methods. Values are averages ± SE from 3 separate culture preparations, with “n” below each data set representing the total number of parietal cells counted at each time (H-K-ATPase positive). Vacuolar cells predominated by 6 h (70.8 ± 2.2%) and increased to 82.1 ± 1.3% by 20 h.
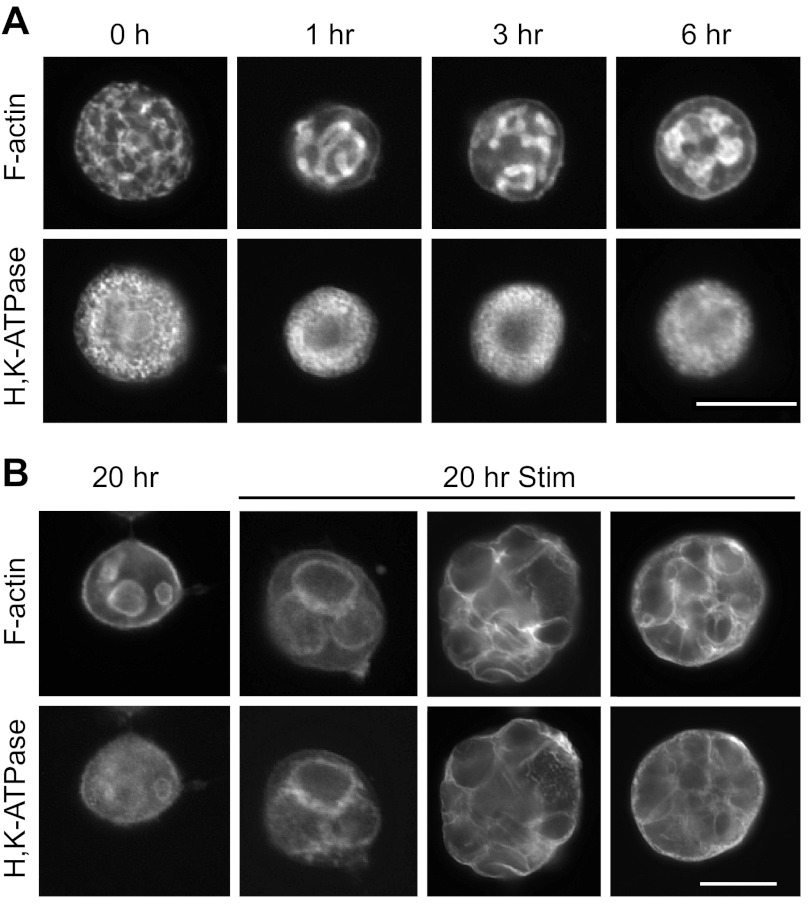
The functional responsiveness of the cells was tested by stimulation with histamine/IBMX and probing for F-actin and H-K-ATPase. In resting, nonstimulated cells (Fig. 2A), F-actin was concentrated at the canalicular membranes, with weaker staining near the plasma membrane. H-K-ATPase, in tubulovesicles, was mainly dispersed in the cytoplasmic spaces between vacuoles, but sometimes became partly localized at vacuolar membranes after the longer times in culture, even without stimulation. After histamine/IBMX stimulation (Fig. 2B), cells responded positively by fusing of tubulovesicles (H-K-ATPase) with vacuolar membranes (F-actin) and considerable swelling of vacuoles, consistent with acid secretion into fully separated vacuoles, as previously shown (1, 7, 21, 32). The overall diameter of stimulated cells also increased concomitant to swelling of vacuoles.
Fig. 2.
Collagenase-isolated parietal cells respond to stimulants of acid secretion. A: resting cells at 0, 1, 3, and 6 h were probed for F-actin (top) and H-K-ATPase (bottom). F-actin was localized to the apical membrane vacuoles and the surrounding plasma membrane, while H-K-ATPase was mostly cytoplasmic. Bar = 20 μm. B: cells cultured for 20 h were stimulated with histamine/isobutylmethylxanthine (IBMX) for 30 min and then stained as in A. When stimulated, cells became enlarged as vacuoles swelled with accumulated acid. H-K-ATPase and F-actin colocalized at the vacuolar periphery. Bar = 20 μm.
Isolating cells from intact glands by EGTA chelation of extracellular Ca2+: effects on dispersal of ZO-1 and formation of apical membrane vacuoles.
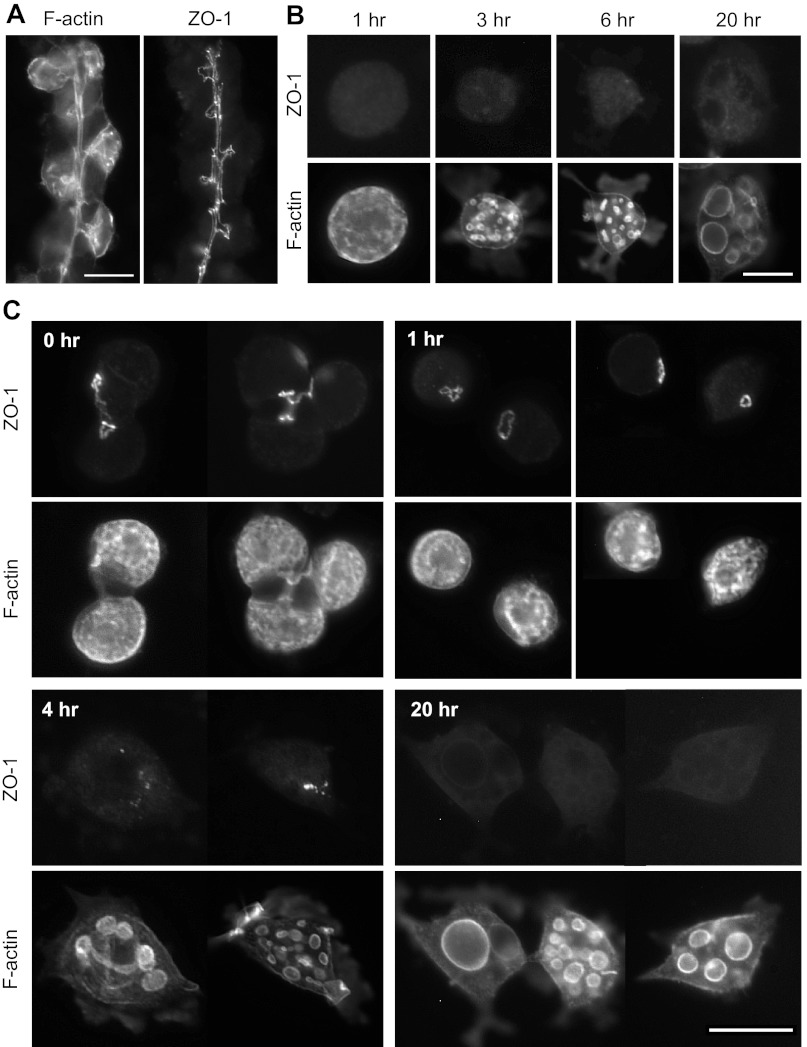
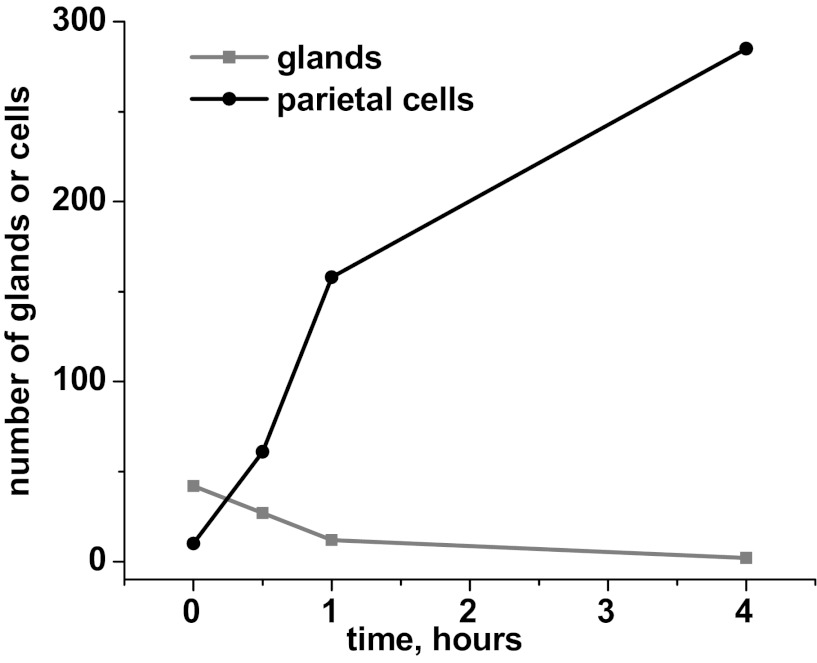
Hypothesizing that tight-junctional connections to neighboring cells play an important role in holding the canalicular system open (and connected to basolateral membrane) at the apical pole of the cell against forces pulling the apical membrane inward, we probed for the tight-junctional protein ZO-1 in gastric glands and in isolated parietal cells. No specific localization of ZO-1 was seen in collagenase-isolated cells after they were ready for observation (see Fig. 6B). Therefore, in an effort to look at ZO-1 very soon after cellular separation, we used Ca2+ chelation with 4 mM EGTA to release individual cells by disruption of tight junctions. After 1 h with EGTA (40-min gentle agitation, 20-min settling of glands), parietal cells were determined to be >80% of total recovered cells. To evaluate the rate of release of cells by this method, we maintained some glands in MEM/EGTA for 4 h, gently agitated, with samples taken at time 0 (addition of EGTA to glands), 30 min, 1 h, and 4 h for counting of parietal cells (H-K-ATPase-positive) and remaining glands. Because there are many parietal cells per gland, the count of cells increases much more steeply than the count of glands decreases (Fig. 3), but the total yield of cells was much lower than the yield from the initial collagenase digestion. Ideally, all the isolated cells would have been released at the same time point, so cells observed at each subsequent time would not be a mixture of cells at very different times after separation from glands. However, because of the slow release of cells, 1 h with EGTA was chosen as a reasonable compromise, producing a cellular yield that was adequate for culturing, immunoprobing, and observation with fluorescence microscopy.
Fig. 6.
Immunolocalization of zonula occludens-1 (ZO-1) in glands and in cells cultured with normal extracellular [Ca2+]. A: gastric glands were probed with antibody for ZO-1 and phalloidin for F-actin. Bar = 20 μm. B: collagenase-isolated cells were cultured as in Fig. 1 and then probed for ZO-1 and F-actin. No specific ZO-1 staining was seen through 20 h. Background is at similar intensity to controls without primary. Bar = 20 μm. C: EGTA-isolated cells in Complete Medium were cultured as in Fig. 4 and then probed for ZO-1 and F-actin. ZO-1 was found at sites of cell-cell contact in small clusters or pairs of cells. In individual cells at 1 h, ZO-1 appeared in a bright spot or ring, presumably at the collapsing apical pole of the cell. By 4 h, ZO-1 appeared in numerous smaller, less intense spots and seemed to disappear by 20 h. Bar = 20 μm.
Fig. 3.
Effect of EGTA (chelating extracellular Ca2+) on the dissociation of gastric glands to cells. Gastric glands were gently agitated in MEM containing 4 mM EGTA to release individual cells by disruption of tight junctions. Samples of the gland/cell mixture were taken at 0 (immediately before addition of EGTA), 0.5, 1, and 4 h, fixed, and probed for H-K-ATPase to identify parietal cells. Within 10 fields at ×20 magnification, glands and individual parietal cells were counted, with the number of released parietal cells increasing most steeply in the first hour.
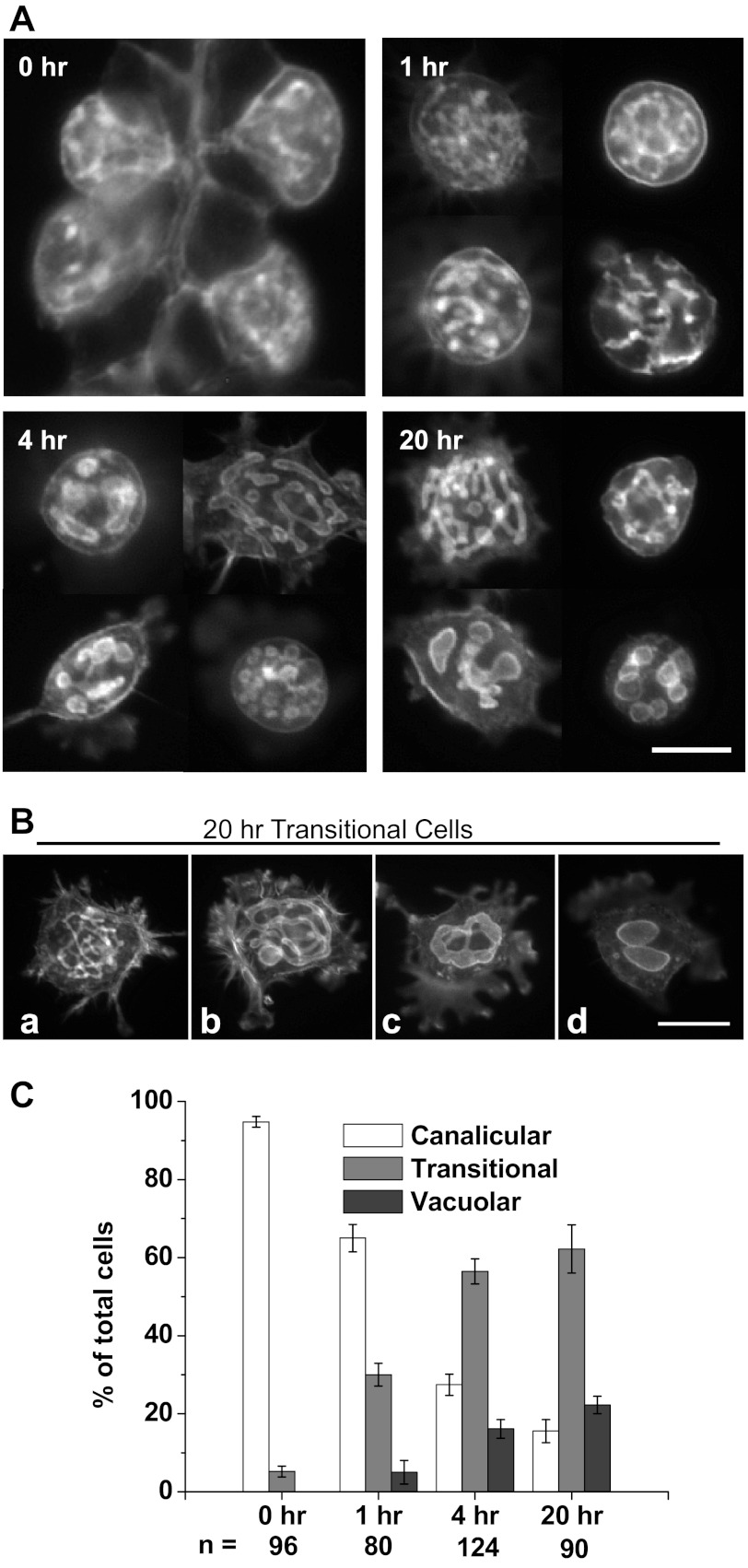
The conversion from canaliculi to vacuoles in EGTA-isolated cells cultured in Complete Medium is similar to that of collagenase-isolated cells, as shown in Fig. 4A, with counting data summarized in Fig. 4B. Following the addition of EGTA to glands at time zero, separated cells after 1 h in EGTA and resuspension in Complete Medium showed mostly canalicular morphology, 59.3 ± 2.0%, with only 5.6 ± 0.5% vacuolar. They appeared healthy and attached to Matrigel strongly, often with lamellipodial extensions. At 4 h 56.1 ± 4.6% of the cells had a vacuolar morphology, increasing to 74.5 ± 3.3% at 20 h. Vacuoles at 20 h were similar in size and number to those seen in the collagenase-isolated cells.
Fig. 4.
EGTA-isolated cells have a rate of vacuolar formation similar to that of collagenase-isolated cells when both are resuspended in normal [Ca2+] (Complete Medium) and cultured on Matrigel. A: at t = 0, a sample of glands was taken for plating and fixation. After 1 h in low [Ca2+], the separated cells were resuspended in Complete Medium, plated onto coverslips, then fixed, permeabilized, and probed for F-actin at 0, 1, 4, and 20 h. Bar = 20 μm. B: rate of vacuolar conversion in EGTA-isolated parietal cells in Complete Medium. Cells were counted and categorized as in Fig. 1B. Time course of conversion to fully vacuolar morphology (74.5 ± 3.3% at 20 h) was similar to that of collagenase-isolated cells (Fig. 1).
EGTA-isolated cells cultured in Complete Medium for 20 h were tested for viability by histamine/IBMX stimulation. As with collagenase-isolated cells, these cells responded well to stimulation by large swelling of vacuoles and coinciding increase in cell size relative to their resting state (Fig. 5). Thus they adhered to Matrigel, engulfed the apical canalicular membranes with conversion to intracellular vacuoles, and responded to stimulation.
Fig. 5.
EGTA-isolated cells cultured in normal [Ca2+] respond to stimulation, behaving like collagenase-isolated cells. As in Fig. 2, cultured cells at 20 h were stimulated with histamine/IBMX for 30 min and prepared for immunostaining. Cells exhibited an increase in diameter and large swelling of vacuoles. Bar = 20 μm.
Figure 6 shows images of ZO-1 in intact glands (Fig. 6A: t = 0 for EGTA-isolated cells), in collagenase-isolated cells after their final resuspension in Complete Medium (Fig. 6B), and in cells after isolation from glands by 1 h with EGTA (Fig. 6C). As expected (38), ZO-1 staining was sharply localized along the lumen of the intact gland with short branches projecting to the apical poles of parietal cells (Fig. 6A). ZO-1 was not clearly localized in any collagenase-isolated cells (Fig. 6B). In contrast, ZO-1 was seen as a small ring or spot in the 1-h EGTA-isolated cells (Fig. 6C) at the remaining apical pole of the cell. Occasionally, small aggregates or pairs of cells were observed with ZO-1 staining at the region of contact between adjacent cells. By 4 h, ZO-1 appeared granular or diffuse and after 20 h, ZO-1 staining was difficult to detect. No colocalization between ZO-1 and F-actin was observed. In general, cells obtained by low-[Ca2+] treatment exhibited an apical spot or granular ZO-1 staining when cells were in the canalicular or transitional state, but there was very diffuse or no ZO-1 staining in cells that had reached the vacuolar state. These results suggested that low extracellular [Ca2+] delayed the separation of the apical/canalicular membrane from the plasma membrane and delayed loss of a distinct apical pole.
Culturing EGTA-isolated cells in low [Ca2+] delays vacuolar conversion.
To study any continuing effect of low extracellular [Ca2+] on the rate at which the engulfed apical membrane is separated from the plasma membrane, as well as the rate of vacuole formation, we cultured EGTA-isolated cells in Complete Medium with 4 mM EGTA. As shown in Fig. 7A with summary data in Fig. 7C, these cells displayed a slower rate of vacuole formation than either the collagenase-isolated or EGTA-isolated cells cultured in normal [Ca2+]. At t = 1 h (just before plating on Matrigel), most cells (65.0 ± 3.5%) contained a lacy canalicular network. At t = 4 h, most cells (56.5 ± 3.2%) appeared transitional, with many small spherical bulges (“prevacuoles”), connected along canaliculi like beads on strings. Many had long lamellipodial extensions. Only 16.1 ± 2.4% of these cells contained vacuoles, which were generally small (0.5–2 μm) in diameter, smaller than those in cells cultured without EGTA. This distribution was maintained through 20 h with a slight increase to 62.2 ± 6.2% transitional and 22.2 ± 2.2% vacuolar. The variety of morphologies at 20 h is shown in Fig. 7B. The fact that only 22% of these cells achieved the vacuolar morphology, vs. 75% in normal [Ca2+], indicates a substantial inhibition of vacuolar conversion in low extracellular [Ca2+].
Fig. 7.
EGTA-isolated parietal cells maintained in low [Ca2+] (4 mM EGTA in Complete Medium) show a slower rate of vacuolar formation than either collagenase- or EGTA-isolated cells cultured in normal [Ca2+]. A: cells were probed for F-actin at 0, 1, 4, and 20 h. At 4 h, the apical membrane remained in the canalicular or transitional state, with the transitional state displaying many small, interconnected, bulbous enlargements of the canaliculi. A transitional morphology predominated through 20 h. B: morphology seen at 20 h ranged from canalicular (a), to transitional (b), to larger canalicular bulges (c), to apparently separate vacuoles (d). Bars = 20 μm. C: rate of vacuolar conversion in EGTA-isolated cells in EGTA was considerably slowed by low extracellular [Ca2+]. Cells were counted and categorized as in Fig. 1B. Transitional cells predominated after 4 h, and comprised 62.2 ± 6.2% of the total at 20 h. Only 22.2 ± 2.2% were vacuolar and 15.6 ± 2.9% were still canalicular at 20 h.
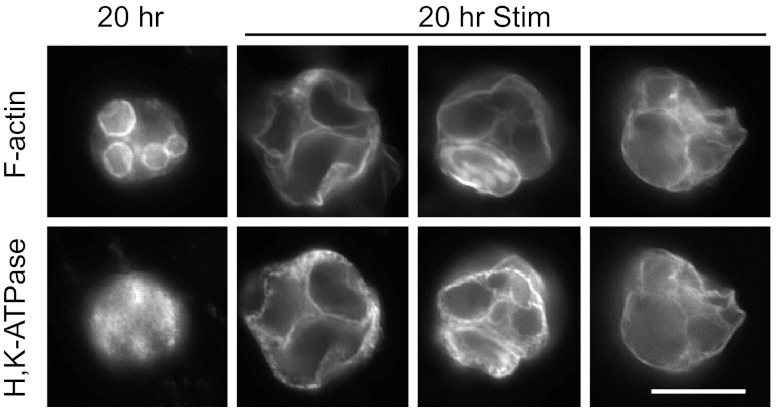
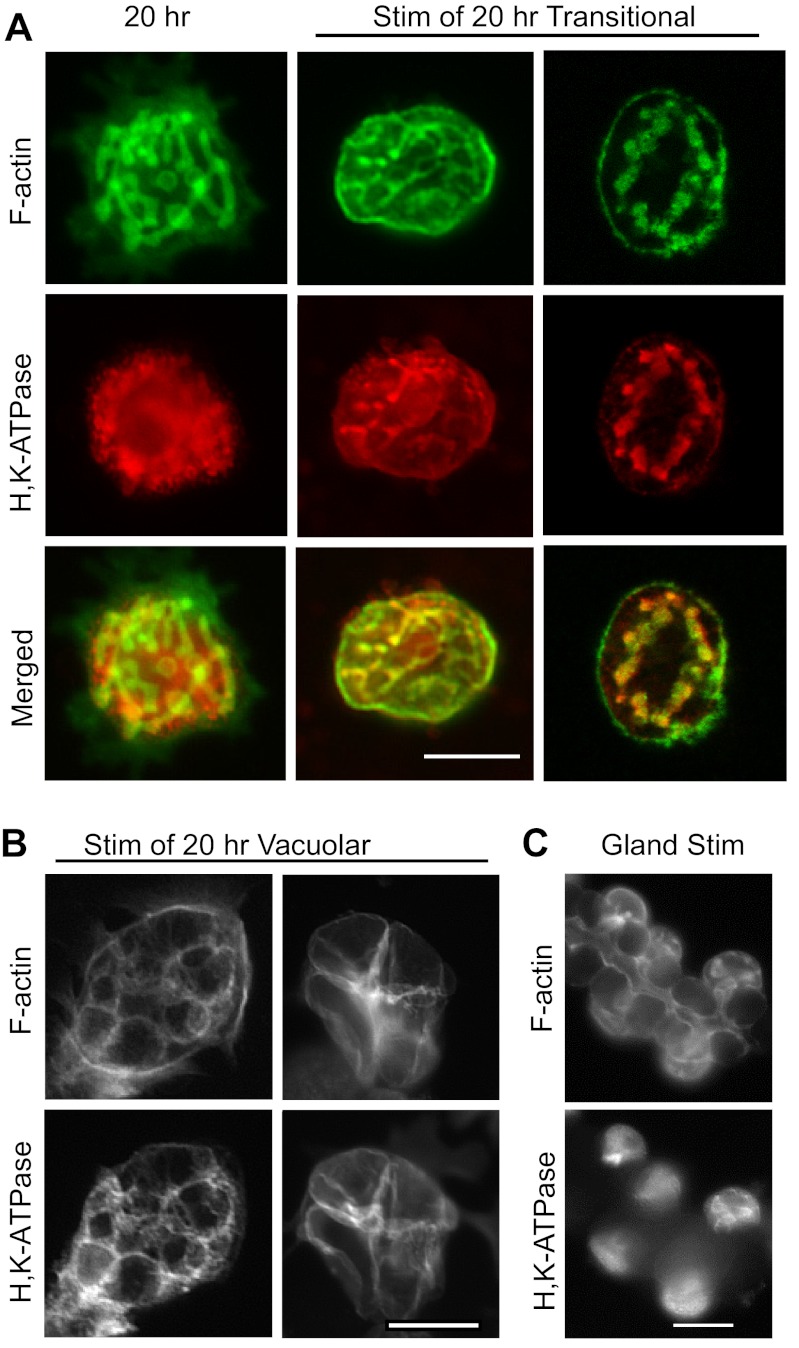
Histamine/IBMX stimulation of cells that had been cultured with EGTA for 20 h led to colocalization of H-K-ATPase with F-actin, indicating fusion of tubulovesicles with apical membrane (Fig. 8A). The majority of stimulated ”transitional“ cells did not show an increase in cell size, in spite of the relocation of H-K-ATPase. However, the few cells that did achieve a more vacuolar morphology after 20 h in EGTA showed vacuolar swelling and a resulting expansion of cell volume (Fig. 8B). Stimulation in terms of H-K-ATPase relocation was seen both with and without EGTA in the stimulating medium. These data suggested that the process of membrane fission that separates the canalicular membranes from the plasma membrane, as well as vacuoles from other vacuoles, had not yet occurred in most of the transitional cells. Narrow connecting tubules seem to remain connected to an apical pore, through which secreted acid could be forced, preventing the prominent swelling of vacuoles typically seen in parietal cells cultured with normal (1.8 mM) extracellular [Ca2+]. When the intact gastric gland is stimulated, parietal cells with the normally open apical pore do not appear to enlarge and canalicular swelling is not evident at this magnification. However, obvious expansion of the gland lumen is seen (Fig. 8C, compared with Fig. 6A), reflecting efflux of secreted acid from the cells.
Fig. 8.
EGTA-isolated cells maintained in EGTA respond to stimulation with colocalization of H-K-ATPase and F-actin. Cells cultured on Matrigel for 20 h were stimulated with histamine/IBMX, as in Figs. 2 and 5. A: very little canalicular swelling in the transitional cells, even though H-K-ATPase is transferred to the canalicular membrane, suggested maintenance of narrow luminal passages connecting enlarged canalicular regions to each other and to an apical pore, allowing secreted acid to escape the cell. B: few cells that achieved full vacuolar conversion showed large cellular and vacuolar swelling. C: gastric glands (t = 0 for EGTA treatment) were stimulated while gently agitated in Complete Medium with added O2, then probed as above. Canalicular swelling was not apparent here, as secreted acid could drain from canaliculi into the gland lumen, but the gland lumen was more dilated than in unstimulated glands also probed for F-actin (see Fig. 6A). Bars = 20 μm.
ZO-1 localization lingers in EGTA-isolated cells cultured in low [Ca2+].
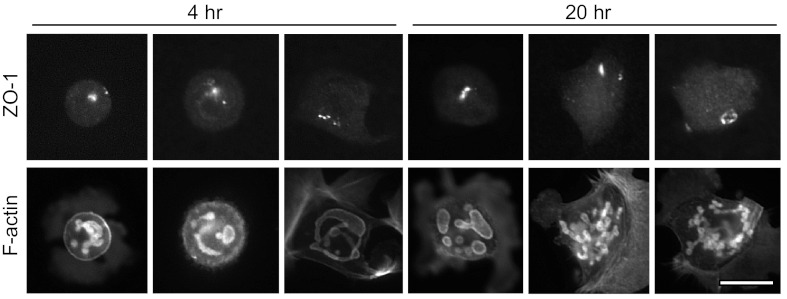
To further examine the results described above, EGTA-isolated cells cultured in EGTA were probed for ZO-1, shown in Fig. 9. Interestingly, ZO-1 appeared in a concentrated spot at 4 h (near the cell surface, as determined by optical sections) and remained at the spot in most of these cells through 20 h. Since this spot was quickly lost from the 1-h EGTA-isolated cells cultured in normal [Ca2+] (Fig. 6), these results indicate that low extracellular [Ca2+] delays the dispersal of tight-junctional proteins from a lingering apical pole after cell-cell junctions are broken.
Fig. 9.
Immunolocalization of ZO-1 in EGTA-isolated cells cultured in low extracellular [Ca2+]. Cells cultured in EGTA for 4 and 20 h were probed for ZO-1 and F-actin. Over 20 h, ZO-1 tended to remain concentrated at a spot or small ring, possibly the lingering apical pole of the cell. Bar = 20 μm.
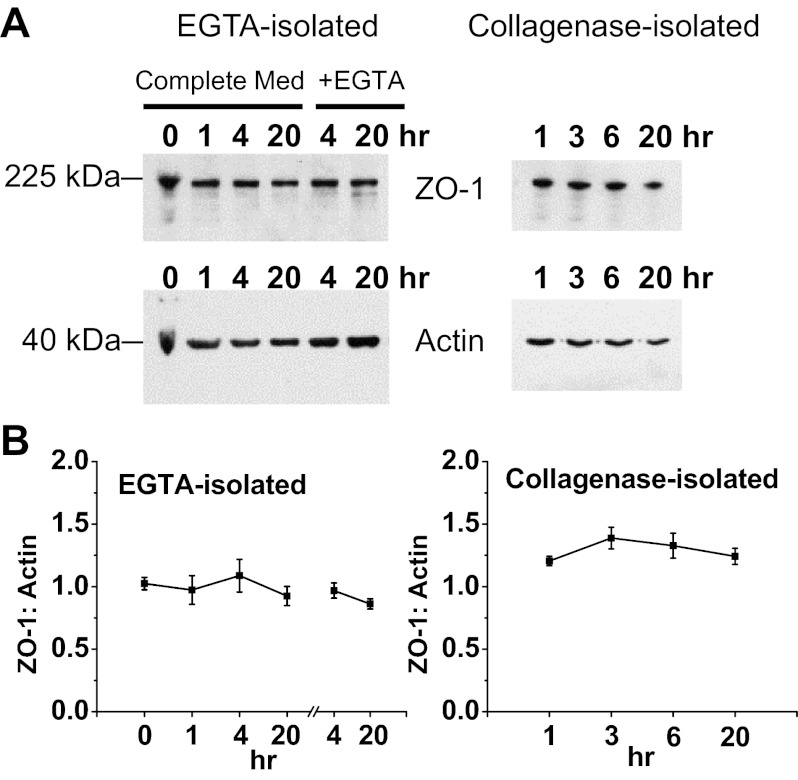
Western blot analysis showed that lack of localized ZO-1 staining in collagenase-isolated cells and in EGTA-isolated cells at later time points was not a result of ZO-1 breakdown. Representative blots in Fig. 10A show that ZO-1 and actin were both present at relatively constant levels through 20 h in all three conditions (collagenase and EGTA-isolated cells in normal or low [Ca2+]). Densitometric analysis shows that the ratio of ZO-1 to actin remained fairly constant over 20 h (Fig. 10B).
Fig. 10.
Detection of ZO-1 and actin in solubilized samples of collagenase-isolated cells, EGTA-isolated cells in normal [Ca2+], and EGTA-isolated cells in low [Ca2+] (EGTA). Samples were taken at indicated times (0 and 1 h in suspension, all later times recovered from Matrigel). A: Western-blotted samples were probed for ZO-1 (225 kDa) and then stripped and reprobed for actin (42 kDa) as a control. Based on protein assay, 20 μg of protein were loaded in all lanes, except for 10 μg for 20-h collagenase. The immunoblots shown are representative of 4 separate experiments. B: analysis of band density and ZO-1-to-actin ratio shows that total ZO-1 levels remained relatively constant from time zero through 20 h in all 3 conditions, indicating little ZO-1 breakdown in spite of loss of localization. For EGTA-isolated cells, culturing ± EGTA applies only to 4 and 20 h, with the 0- and 1-h samples taken before resuspension in Complete Medium and placement on Matrigel. Values are averages ± SE for all experiments (n = 4).
DISCUSSION
This study followed the conversion of gastric parietal cells in primary culture from their in vivo morphology with apical canaliculi to a morphology with intracellular apical membrane vacuoles that are completely separated from the plasma membrane and apparently from each other. When cells are isolated by the traditional method of collagenase digestion, the conversion of the apical canaliculi to apical vacuoles goes through a transitional stage in which many bulbous expansions of the canaliculi initially appear (incipient vacuoles) and is largely complete by ∼6 h when cells are kept on a suitable matrix (Matrigel in this study) in a complete culture medium with normal extracellular [Ca2+] (1.8 mM). After this conversion, histamine stimulation caused vacuolar swelling, consistent with pinching off of canalicular pathways for efflux of acid through an apical pore. Lack of ZO-1 localization could be indicative of complete separation of the engulfed apical membrane from the basolateral membrane.
Parietal cells obtained by an alternate method, disruption of tight junctions in intact gastric glands with 4 mM EGTA for 1 h, yielded cells that initially showed ZO-1 localized in a small ring or spot. This suggested a remaining apical pole with some tight-junctional proteins lingering at a region where there was still an interface between apical and basolateral membrane domains. When subsequently cultured in normal [Ca2+], these cells proceeded through the same vacuolar conversion as collagenase-isolated cells, with vacuolar swelling upon stimulation, and ZO-1 became dispersed (although Western blots indicated continued presence of ZO-1 in these cells as well as in collagenase-isolated cells).
In contrast, culturing cells in low [Ca2+] caused most cells to remain in the transitional stage for 20 h, with some ZO-1 also remaining in a small ring or spot. The canalicular expansions seemed to be interconnected, as suggested by phalloidin staining of remaining narrow regions of canaliculi seen in many cells, and the entire canalicular system seemed to remain connected to the plasma membrane at an apical pore through which secreted acid could drain. Stimulation of these 20-h “transitional” cells with histamine caused colocalization of H-K-ATPase with F-actin at the canalicular membrane but relatively little swelling of the bulbous canalicular regions and relatively little increase in overall cell size, compared with stimulated cells which had been cultured in normal [Ca2+] and had achieved a fully vacuolar morphology. This effect is different from limitation of vacuolar swelling in stimulated cells by various agents that limit net acid secretion across the apical membrane, e.g., NaSCN (26), SCH28080 (1, 4), and omeprazole (1, 27). The appearance is similar to that of a glandular stimulated parietal cell (Fig. 8C), which can drain its acid into the gland lumen, in that both show little swelling of canalicular structures when stimulated. However, the isolated transitional cell, although showing insertion of H-K-ATPase into canalicular membranes, lacks the attached gland lumen to provide visual evidence of acid efflux. Some (∼22%) of the cells cultured in low [Ca2+] did achieve fully vacuolar morphology by 20 h, and these exhibited stimulated vacuolar swelling similar to that of the majority of collagenase isolated cells, indicating that 20-h culture in low [Ca2+] did not, in itself, prevent stimulated acid secretion and adding support for the interpretation that the stimulated transitional cells cultured in the same conditions are secreting acid that must drain through a remaining apical pore.
In the cultured parietal cell, secretion of HCl across the apical (vacuolar) membrane osmotically pulls water both across the vacuolar membrane into its lumen and across the basal membrane (now plasma membrane) into the cytoplasm. Thus both vacuolar volume and total volume of the stimulated cell must increase unless there is an exit path for this secretory volume. The result in most cells cultured with 4 mM EGTA suggests the presence of such a path, requiring canalicular connections between the newly forming vacuoles and a patent apical pore. Although the H-K-ATPase-containing tubulovesicles in the cytoplasm could fuse with remnant apical membrane to initiate acid secretion, the apparently incomplete sealing of canalicular passages and apical pore allowed acid to leak out, limiting expansion of the incipient vacuoles.
Rapid cytoplasmic shrinkage upon stimulation has been reported in cultured parietal cells, requiring K+ and Cl− fluxes (2, 31). The shrinkage occurred within ∼5 min, followed by volume recovery involving Na+/H+ exchange that was complete ∼12 min after the start of stimulation. Our protocol of 30-min stimulation resulted in assessment of cellular response well after this shrinkage/recovery phase, so overall cell swelling would be expected in cells with fully separated vacuoles, while noticeable shrinkage would not be expected in the transitional cells even if all their secreted acid could exit the canalicular space. In addition, the report of rapid cytoplasmic shrinkage suggested possible involvement of a Ca2+-sensitive K+ channel. However, the responses we observed in cells that were stimulated for 30 min after spending 20 h with 4 mM EGTA were similar with or without 4 mM EGTA in the stimulatory medium.
We had long hypothesized that the in vivo canalicular morphology needs to be maintained by opposing forces that pull canaliculi into long, narrow tubular shapes, since membrane-bounded structures tend to seek the lowest-energy spherical shape unless external forces squeeze or pull them into another shape. Continuity of the canalicular membranes with the basolateral membrane at the apical pole and attachment of that apical pole to neighboring cells through tight junctions were thought to oppose inwardly pulling forces that are still poorly characterized and are the subject of ongoing investigation, seeming to involve interactions among membranes, cytoskeletal filaments, and motor proteins. Although nonconfluent MDCK cells only internalize part of their apical membrane as VACs, the inwardly pulling forces may bear some similarity to those constantly at work in the parietal cell. However, the MDCK cell's exocytotic return of VAC membrane to the apical plasmalemma after restoration of confluence is not duplicated by cultured parietal cells. The latter have never been observed to reform tight-junctional connections between cells, probably because the complete internalization of apical membrane makes the now ”basolateral“ plasmalemma appear nonepithelial to any adjacent cells. Thus the parietal cell presents an extreme example of apical membrane being pulled into the cell, opposed only by tight-junctional attachment to neighboring cells in the intact gastric epithelium.
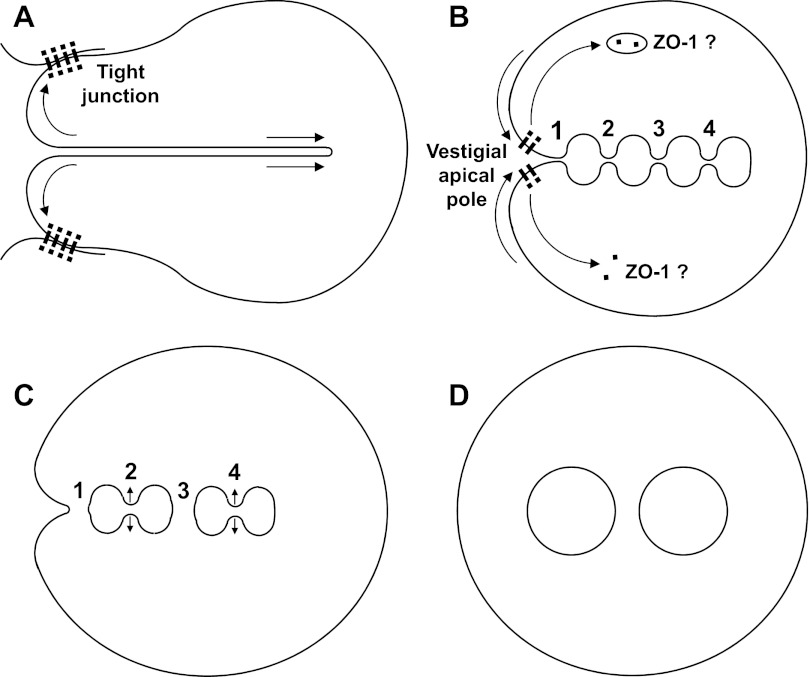
Figure 11 shows a simple model proposing both the membrane fission steps leading to apical vacuoles (Fig. 11D) in an isolated parietal cell cultured in normal extracellular [Ca2+] and also a transitional stage of partial conversion (Fig. 11B) in which the canaliculi of most cells remain for many hours when cells are cultured in low extracellular [Ca2+]. Inward collapse of the apical pole after separation of tight-junctional connections to neighboring cells would relieve tension on canalicular membranes, which could spontaneously develop multiple bulbous expansions (Fig. 11B) as membrane regions seek the lowest-energy spherical shapes. The curvature of these regions could drive intervening canalicular segments to narrow, but the fission of these narrowing membrane necks seems to be greatly delayed in low extracellular [Ca2+], allowing luminal passages to remain for efflux of secreted acid. As long as there is a remaining interface between apical and basolateral membrane domains, some tight-junctional proteins might linger at a vestigial apical pole. In normal extracellular [Ca2+], the fission steps proceed more rapidly, but not in any discernible order, separating vacuoles from each other and the entire apical membrane from the basolateral. Considerable variety among cells in the number and sizes of ultimate vacuoles, compared with the transitional bulbous enlargements, suggests that some of the narrowed canalicular regions randomly expand rather than pinch off (as at points 2 and 4), leading to fewer, larger vacuoles. In normal extracellular [Ca2+], ZO-1 rapidly disappears from the apical pole and may be taken into a diffuse endosomal compartment (18, 30).
Fig. 11.
Modeled hypothesis of apical membrane conversion from canaliculi to vacuoles. In intact gastric gland (A), apical pole of parietal cell is thought to be held open by tight-junctional attachment to neighboring cells, while canaliculi are pulled into cytoplasm as long narrow tubes. After separation of cells by disruption of tight junctions, apical pole collapses inward and tension on canalicular membranes decreases, allowing multiple canalicular regions to expand toward lowest-energy spherical shapes (B) but still with narrow connections (points 1, 2, 3, and 4). With EGTA chelating Ca2+ in extracellular medium (which extends into canaliculi), conversion stalls at stage B and some ZO-1 remains at apical pole. In contrast, with normal extracellular [Ca2+] (1.8 mM), narrowing necks of membrane between forming vacuoles pinch closed at several points, leading to fissions (C). Fission at point 1 separates canalicular system from plasma membrane. Fission at point 3 separates forming vacuoles from each other. Expansion may sometimes prevail over fission, as at points 2 and 4, resulting in fewer and larger separate vacuoles (D).
Most studies of membrane fusion/fission have led to a view that several kinds of proteins interact with membranes in a variety of ways to drive tight curvature of small areas of membrane, enabling energetically favorable merging or separation that leads to relaxed curvature (19). The membrane fissions leading to vacuole formation in isolated parietal cells involve extracytoplasmic membrane surfaces pinching closed in an extracellular medium, as with all types of endocytosis. Although endocytosis by clathrin-coated pits also involves the extracytoplasimic leaflet closing on itself, it is driven by intracellular molecular scaffolding that causes tightly curved invagination of the plasma membrane, eventually pinching off a narrowing membrane neck (20). No such intracellular mechanism is known to drive the membrane fissions in the isolated parietal cell's vacuolar conversion. However, recent findings that interaction of locally elevated cytosolic [Ca2+] with certain membrane-bound proteins generates tight membrane curvature in some kinds of exocytosis (15, 22, 23) raises the possibility that extracellular Ca2+ might play a role in generating the membrane curvatures needed to initiate fissions in parietal cell vacuole formation. Our finding that very low extracellular [Ca2+] inhibits these fission steps in the endocytotic conversion of the apical membrane of cultured parietal cells points to an important area of further study.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant AM-10141-34.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L.N. and J.M.C.Jr. performed experiments; S.L.N. and J.M.C.Jr. analyzed data; S.L.N. and J.M.C.Jr. interpreted results of experiments; S.L.N. and J.M.C.Jr. prepared figures; S.L.N. and J.M.C.Jr. drafted manuscript; S.L.N., J.M.C.Jr., T.E.M., and J.G.F. edited and revised manuscript; J.M.C.Jr., T.E.M., and J.G.F. approved final version of manuscript; J.G.F. conception and design of research.
REFERENCES
- 1.Agnew BJ, Duman JG, Watson CL, Coling DE, Forte JG. Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J Cell Sci 112: 2639–2646, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bachmann O, Heinzmann A, Mack A, Manns MP, Seidler U. Mechanisms of secretion-associated shrinkage and volume recovery in cultured rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 292: G711–G717, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Balda MS, Fallon MB, Van Itallie CM, Anderson JM. Structure, regulation, and pathophysiology of tight junctions in the gastrointestinal tract. Yale J Biol Med 65: 725–735; discussion 737–740, 1992 [PMC free article] [PubMed] [Google Scholar]
- 4.Beil W, Hackbarth I, Sewing KF. Mechanism of gastric antisecretory effect of SCH 28080. Br J Pharmacol 88: 19–23, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglindh T, Obrink KJ. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol (Oxf) 96: 150–159, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol 77: 853–880, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew CS, Ljungstrom M, Smolka A, Brown MR. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol Gastrointest Liver Physiol 256: G254–G263, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Chow DC, Forte JG. Characterization of the beta-subunit of the H+-K+-ATPase using an inhibitory monoclonal antibody. Am J Physiol Cell Physiol 265: C1562–C1570, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Forte JG, Black JA, Forte TM, Machen TE, Wolosin JM. Ultrastructural changes related to functional activity in gastric oxyntic cells. Am J Physiol Gastrointest Liver Physiol 241: G349–G358, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Forte JG, Nauss AH. Effects of calcium removal on bullfrog gastric mucosa. Am J Physiol 205: 631–637, 1963 [DOI] [PubMed] [Google Scholar]
- 11.Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol 6: 45–48, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol 72: 273–296, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Forte TM, Machen TE, Forte JG. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology 73: 941–955, 1977 [PubMed] [Google Scholar]
- 14.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2: a002907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327: 1614–1618, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J 10: 2363–2373, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanzel DK, Urushidani T, Usinger WR, Smolka A, Forte JG. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 256: G1082–G1089, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15: 176–188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci 35: 699–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundmark R, Carlsson SR. Driving membrane curvature in clathrin-dependent and clathrin-independent endocytosis. Semin Cell Dev Biol 21: 363–370, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Mangeat P, Gusdinar T, Sahuquet A, Hanzel DK, Forte JG, Magous R. Acid secretion and membrane reorganization in single gastric parietal cell in primary culture. Biol Cell 69: 223–231, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science 316: 1205–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 23.McMahon HT, Kozlov MM, Martens S. Membrane curvature in synaptic vesicle fusion and beyond. Cell 140: 601–605, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Meldolesi J, Castiglioni G, Parma R, Nassivera N, De Camilli P. Ca2+-dependent disassembly and reassembly of occluding junctions in guinea pig pancreatic acinar cells. Effect of drugs. J Cell Biol 79: 156–172, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minamide LS, Bamburg JR. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem 190: 66–70, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Reenstra WW, Forte JG. Action of thiocyanate on pH gradient formation by gastric microsomal vesicles. Am J Physiol Gastrointest Liver Physiol 244: G308–G313, 1983 [DOI] [PubMed] [Google Scholar]
- 27.Sachs G, Shin, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol 35: 277–305, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques 12: 870–874, 876–879, 1992 [PubMed] [Google Scholar]
- 29.Sedar AW, Forte JG. Effects of calcium depletion on the junctional complex between oxyntic cells of gastric glands. J Cell Biol 22: 173–188, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siliciano JD, Goodenough DA. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol 107: 2389–2399, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnentag T, Siegel WK, Bachmann O, Rossmann H, Mack A, Wagner HJ, Gregor M, Seidler U. Agonist-induced cytoplasmic volume changes in cultured rabbit parietal cells. Am J Physiol Gastrointest Liver Physiol 279: G40–G48, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Soroka CJ, Chew CS, Hanzel DK, Smolka A, Modlin IM, Goldenring JR. Characterization of membrane and cytoskeletal compartments in cultured parietal cells: immunofluorescence and confocal microscopic examination. Eur J Cell Biol 60: 76–87, 1993 [PubMed] [Google Scholar]
- 33.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda J, Zhu L, Karvar S. Phosphorylation of radixin regulates cell polarity and Mrp-2 distribution in hepatocytes. Am J Physiol Cell Physiol 300: C416–C424, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Tidball CS. Magnesium and Calcium as Regulators of Intestinal Permeability. Am J Physiol 206: 243–246, 1964 [DOI] [PubMed] [Google Scholar]
- 36.Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol 107: 1717–1728, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolosin JM, Okamoto C, Forte TM, Forte JG. Actin and associated proteins in gastric epithelial cells. Biochim Biophys Acta 761: 171–182, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Zhu L, Hatakeyama J, Zhang B, Makdisi J, Ender C, Forte JG. Novel insights of the gastric gland organization revealed by chief cell specific expression of moesin. Am J Physiol Gastrointest Liver Physiol 296: G185–G195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]