Abstract
The effect of claudins on paracellular fluxes has been predominantly studied in either Madin-Darby canine kidney (MDCK) or LLCPK cells. Neither model system has a very low transepithelial resistance (TER) as observed in leaky epithelia. Moreover, results from one model system are not always consistent with another. Opossum kidney (OK) cells form tight junctions yet have a very low TER. We therefore set out to characterize the paracellular transport properties of this cell culture model. Ussing chamber dilution potential measurements revealed that OK cells exhibit a very low TER (11.7 ± 1.4 Ω·cm2), slight cation selectivity (PNa/PCl = 1.10 ± 0.01), and the Eisenman permeability sequence IV; the permeability of monovalent cations ranking K+ > Cs+ > Rb+ > Na+ > Li+. Quantitative real-time PCR studies found that OK cells endogenously express claudin-4 > -1 > -6 > -20 > -9 > -12 > -11 > -15. Overexpression of claudin-4 significantly increased TER, decreased Na+ and Cl− permeability, and increased levels of claudin-1, -6, and -9 mRNA. Knockdown of claudin-4 in the overexpressing cells significantly decreased TER without altering claudin expression; thus claudin-4 forms a barrier in OK cells. Knockdown of endogenous claudin-4 decreased claudin-1, -9, and -12 expression without altering TER. Claudin-2 overexpression decreased TER, significantly increased Na+ and Cl− permeability, and decreased claudin-12 and -6 expression. Together these results demonstrate that claudin expression is tightly coupled in OK cells.
Keywords: claudin-4, claudin-2, paracellular transport, opossum kidney cells
transepithelial ion transport occurs via either a transcellular or paracellular pathway. Although transcellular transport has been extensively studied, comparatively little is known about the driving forces, rates, and regulation of paracellular transport processes. The importance of paracellular transport is becoming increasingly apparent, especially across renal and intestinal epithelia. In the kidney, paracellular transport permits the reabsorption of water along with ions including sodium (Na+), chloride (Cl−), calcium, and magnesium (15, 20). Ion flux across these and other epithelia is dependent on the presence and distribution of a family of tight junction proteins called claudins. At least 27 mammalian claudin isoforms have been identified to date (32). Some are considered to be “barrier forming,” while others are “pore forming,” depending on the effect of their overexpression on transepithelial resistance (TER) (33).
Claudin-4 is of particular interest. It is expressed in intestinal epithelia of the duodenum and colon as well as the thin ascending limb and collecting duct of the renal tubule (3, 26). Its expression has also been reported in human distal convoluted tubule (25) and rabbit proximal convoluted tubule (36). The specific paracellular permeability characteristics conferred by claudin-4 are unclear and in some cases contradictory (18, 21, 40, 41). Claudin-4 is most often considered a barrier forming claudin because its overexpression increases TER in Madin-Darby canine kidney (MDCK) and LLCPK cells (40, 41). Claudin-4 has also been reported to be “anion selective,” most likely due to a lysine residue (K65) in its first extracellular loop (9, 21). This intrinsic preference for anions (permselectivity) could be achieved by either the obstruction of cation flux or promotion of anion flux. Claudin-4 overexpression in MDCK cells decreases Na+ permeability (40, 41). This finding was confirmed by RNA interference, where the knockdown of endogenous claudin-4 in MDCK cells increases Na+ permeability (18). In LLCPK cells, claudin-4 acts as a Cl− pore, its knockdown resulting in a significant drop in Cl− flux (18). Another investigation employing mouse cortical collecting duct cells supports this finding, as the knockdown of claudin-4 decreased Cl− permeability (21). Surprisingly, both these RNA interference studies found that TER increased in the absence of claudin-4 (18, 21). These conflicting results bring into question whether the permselectivity properties measured after overexpressing or knocking down a claudin are conferred directly via that isoform or by a perturbation in the expression of endogenous isoforms present in the model system used.
Most studies examining the effect of claudins on paracellular permeability employ either MDCK or LLCPK cells. Unfortunately, the expression of endogenous claudin isoforms has not been exhaustively characterized in either model system, making the assessment of perturbations in other claudin expression difficult (41). Moreover, neither LLCPK cells nor MDCK cells are representative of very loose epithelia. LLCPK cells have a TER of ∼100 Ω·cm2 and MDCKII cells ∼40–45 Ω·cm2 (41). In contrast, MDCKI cells have a TER in the range of ∼3,000 Ω·cm2 (17). Existing model systems of very loose epithelia include OK cells (opossum), NRK-52E (rat), HK2 (human), and HRPTE (human) cells. The latter two cell systems are not suitable for studying paracellular fluxes because even after 2 wk in culture, HK2 and HRPTE cells fail to form tight junctions (35). Thus either OK or NRK-52E cells are a preferred model of a low resistance epithelia. We chose to use OK cells because the opossum genome has been sequenced and this cell line demonstrates a very low TER (29). OK cells were derived from the proximal tubule, a very loose epithelium. Moreover, they have been frequently used to investigate Na-H exchanger isoform 3 (NHE3) function, the predominant apical proximal tubular isoform, as they express NHE3 (2, 5, 6). However, the type and nature of the tight junction are incompletely characterized in OK cells.
We therefore characterized OK cells and found that they are slightly cation selective and have a very low TER. After identifying the claudins expressed in this cell line, we overexpressed claudin-4, an endogenous isoform and knocked it down. This revealed that it forms a paracellular barrier. Overexpression of claudin-2, which is not endogenously expressed, decreased TER. Manipulation of claudin expression in this model system caused significant alterations in the expression of endogenous claudins, inferring a tight coupling of claudin expression in OK cells.
MATERIALS AND METHODS
Antibodies.
The following primary antibodies were employed: rabbit polyclonal anti-claudin-4 (Thermo Scientific, Waltham, MA), mouse anti-HA (16B12; Covance, Mississauga, ON, Canada), and rabbit anti-zona occludens-1 (anti-ZO-1; Invitrogen, Carlsbad, CA). Secondary antibodies used were as follows: horseradish peroxidase conjugated donkey anti-rabbit and goat anti-mouse (Santa Cruz Biotechnology, Santa Cruz, CA) as well as DyLight 488 conjugated AffiniPure donkey anti-rabbit and DyLight 549 conjugated AffiniPure donkey anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA).
Cell culture and stable cell lines.
All cell lines were originally obtained from ATCC (Rockville, MD). OK and LLC-PK1 cells were maintained in DMEM/F-12 medium, supplemented with 10% FBS and 5% penicillin streptomycin glutamine at 37°C in a 5% CO2 incubator. HEK 293 and MDCKII cells were maintained in DMEM, containing 10% FBS and 5% penicillin streptomycin glutamine.
Polyclonal stable cell lines expressing claudin-4HA, mouse claudin-2HA (see below for construction details), and pcDNA3.1+ (Invitrogen) were created by transfecting plasmid DNA into OK cells with Fugene 6 and then selecting for transfected cells with G418 (Invitrogen). To make monoclonal stable cell lines, the polyclonal stable cells were plated with limiting dilutions so that individual colonies could be selected and grown to confluence in the presence of G418. Expression of claudin-2 and claudin-4 in individual stable cell lines was confirmed by immunofluorescence microscopy, immunoblot, and quantitative RT-PCR.
RNAi knockdown.
Claudin-4 knockdown was performed in monoclonal stable cell lines expressing claudin-4HA and pcDNA 3.1+. Two different small interfering (si)RNA sequences against the opossum claudin-4 sequence cloned (see below and Fig. 6) were designed using Thermo Scientific Dharmacon RNAi Technologies siDESIGN center software and then synthesized (Dharmacon, Lafayette, CO). The sequences were as follows: #1 5′-AUG GUC UUG GCC UUG GAG GUU-3′ and #2 5′-UCA UCC ACA GGC CCU AUU-3′. As a control, each sequence was scrambled using GenScript software (GenScript, Piscataway, NJ): scrambled #1 5′-AGG CUC AUG CGG UUG UGG UUU-3′ and scrambled #2 5′-GCU CUA ACA CCC UCC AGC CUU-3′. The cells were seeded at 6 × 105 cells/snapwell insert for Ussing chamber experiments or into each well of a six-well plate for RT PCR (details below) and transfected with 200 nM scrambled siRNA or claudin-4 siRNA using Oligofectamine (Invitrogen) according to the manufacturer's protocol. Ninety-six hours posttransfection, the cells were collected for RT-PCR or mounted in Ussing Chambers for dilution potential studies.
Fig. 6.
Comparison of claudin-4 sequence between Monodelphis domestica and Didelphis virginiana opossums. The NCBI sequence of Monodelphis domestica opossum claudin-4, top sequences (XM_001366871.1) was compared with the Didelphis virginiana opossum claudin-4, bottom sequence (obtained from the OK cell cDNA library) using Emboss Needle Pairwise Alignment software. Base pair differences coding for a synonymous amino acid are in green and nonsynonymous changes are displayed in red. The coding amino acid is written above. The blue sequence at the 3′-end of the Didelphis virginiana sequence was inserted and codes for the HA tag.
Immunohistochemistry.
For transient transfections, OK cells were seeded on glass coverslips, transfected with pcDNA 3.1+ or claudin-4HA using Fugene 6, and then fixed using 4% paraformaldehyde 24 h later. The stable cell lines were plated and allowed to reach confluence before immunofluorescence studies (>5 days). Before incubation with antibodies, the cells were washed three times with PBS containing 1 mM CaCl2 and 1 mM MgCl2, fixed with 4% paraformaldehyde, quenched with 5% glycine in PBS, and then permeabilized with 0.2% Triton-X100 and blocked with 5% milk in PBS. Antibodies and DAPI were applied at a dilution of 1:500 in 5% milk in PBS for 1 h at room temperature. Finally, the samples were mounted with Dako (Glostrup, Denmark) and analyzed using a custom assembled spinning disc confocal microscope detailed by Jaumouille et al. (23).
Immunoblotting.
HEK 293 cells (plated at 3 × 106 cells/10 cm dish) were transfected with pcDNA 3.1+ or claudin-4HA and then harvested after 48 h using the protocol described below. Cells were resuspended in 400 μl of SDS-PAGE sample buffer containing 4.6% SDS, 0.02% bromphenol blue, 20% glycerol, 2% 2-ME, 130 mM Tris·HCl pH 6.8, and a protease inhibitor cocktail (Calbiochem, Gibbstown, NJ) and then mechanically sheared by passing through a 23-gauge needle. The lysates were subjected to SDS-PAGE under denaturing conditions and then transferred to a nitrocellulose membrane. Before incubation with the antibodies, the membrane was blocked overnight with 5% milk in TBS and 0.1% Tween 20. Primary antibodies (1:1,000) were applied at 4°C overnight, followed by a 2-h incubation with horseradish peroxidase-coupled secondary antibodies (1:5,000) at room temperature. Proteins were detected with Western Lightning Plus ECL reagents (PerkinElmer, Boston, MA) and visualized using a Kodak Image Station 440CF (Kodak, Rochester, NY).
Detection of endogenously expressed claudins by PCR.
To examine claudin expression in OK cell cDNA, two sets of degenerate PCR primers for each claudin gene were designed based on the NCBI sequence of the Monodelphis domestica opossum (31) (Table 1). This approach was necessary as OK cells were derived from Didelphis virginiana (27) and therefore contain intrinsic genetic differences. Three different templates were utilized for PCR: genomic DNA (extracted directly from OK cells), cDNA (generated by reverse transcription of OK cell RNA isolated 5 days after plating), and no reverse transcriptase cDNA (generated as per cDNA but without the addition of reverse transcriptase). A 1.5% agarose gel was used to analyze which claudins are expressed based on the amplification of the appropriate size product from cDNA. The presence of each claudin detected was confirmed by cloning it from this cDNA.
Table 1.
Opossum kidney claudin PCR primers
| Forward (Set 1) | Reverse (Set 1) | Forward (Set 2) | Reverse (Set 2) | |
|---|---|---|---|---|
| Cldn 1 | AGTTGCTGGGCTTCATCCT | GTCGAAGACTTTGCACTGGA | CAGTTGCTGGGCTTCATCCTG | GAGTCGAAGACTTTGCACTGGATC |
| Cldn 2 | TGGCTTCTAGTGCCATCTCC | TCGAACTTCATGCTGTCAGG | GGTGGCTTCTAGTGCCATCTC | CTCGAACTTCATGCTGTCAGGC |
| Cldn 3 | AAGGTGTACGACTCGCTGCT | ACGTAGTCCTTGCGGTCGTA | CAAGGTGTACGACTCGCTGC | CGTAGTCCTTGCGGTCGTAG |
| Cldn 4 | AAGGTGTACGACTCGCTGCT | GGGTTGTAGAAGTCCCGGAT | — | — |
| Cldn 5 | CCTGGAAAGCAACATTGTGA | CCACAGAGCACATAGAGCGA | CGGCCTTCCTGGAAAGCAAC | GCCACAGAGCACATAGAGCGAG |
| Cldn 6 | CCTGTATGCTGGACTGCTCA | TACTCAGAAGGACCTCGGGA | CCCTGTATGCTGGACTGCTC | GTACTCAGAAGGACCTCGGGAG |
| Cldn 7 | CAACTGTTGGGGTTCACCAT | CAGGCAGAGCCAAGACTGA | ACTGTTGGGGTTCACCATGG | AGGCAGAGCCAAGACTGAG |
| Cldn 8 | TCAGGATGCAGTGCAAAATC | TTCTCGTTTCTGAGCCGAAT | CGCCAACATCAGGATGCAGT | GGGCTTCTCCTAGTTCTCGT |
| Cldn 9 | TCTGCTGGTGGCTATCACTG | AGGTGCAGCATAGCAGTCCT | GTCTGCTGGTGGCTATCACTGG | GGTGCAGCATAGCAGTCCTC |
| Cldn 10 | ATCATCGCCTTCATGGTAGC | ATGTAACCGTCCAGAGCCAG | GATCATCGCCTTCATGGTAGCT | CAGCATGGAGGGGAAGTCCT |
| Cldn 11 | GCAACTGGTTGGATTTGTGA | GAGGATGTCCACGAGTGGT | CTGCAACTGGTTGGATTTGTGACG | AGGATGTCCACGAGTGGTTTG |
| Cldn 12 | CGGGATGTCCATGCAGCAAC | CTGGTCCACCGAGGAATACC | — | — |
| Cldn 14 | CTCCTGGGTTTCTTGCTCAG | ATTGGTGGTCCAGGAGACAG | CCTGGGTTTCTTGCTCAGCT | TTGGTGGTCCAGGAGACAGC |
| Cldn 15 | GCTGGGGCTACTAATGCTTG | GCAAGCATGGAAGGAAACTC | CGCTGGGGCTACTAATGCTTGG | GGCAAGCATGGAAGGAAACTCC |
| Cldn 16 | CAGGTGTTCCTGGGATTGTT | CAGCAAGTGAGGACTGCTCC | TCCTGGGATTGTTGGCTCTG | GCAAGTGAGGACTGCTCCAG |
| Cldn 17 | CCTTCATTGGGAGCAACATT | CCCTGATGATGAGATTGGCT | GTCTGCCTTCATTGGGAGCA | ATTGGCTGTCCAGGACACAG |
| Cldn 18 | ACTCTTTGCCAAATGATGGG | AGCTGGAAGACCAAGAATAGTG | CGACTCTTTGCCAAATGATGGGG | ATGGTCGGCACTCAGTTAACCC |
| Cldn 19 | AACTCTGGCTTCCAGCTCCT | GACATCCAGAGCCCTTCGTA | CAACTCTGGCTTCCAGCTCC | GGACATCCAGAGCCCTTCGTAG |
| Cldn 20 | GGTAAATGCAAATGTGGGCT | CCTGGCTTCTTGATGCATTT | AAGGGCTGTGGATGGATTGC | GGAGGGTAGAACCTGGCTTC |
| Cldn 22 | ACTCTGGCAGACTTGCGTTT | AACCCAGGACACTGGAATGA | GGACTCTGGCAGACTTGCGT | CCCAGGACACTGGAATGAGG |
| Cldn 23 | GCTGGGCTACTATGAGGCTG | TTTTGCAGGACATGGGTGTA | GAGCTGGGCTACTATGAGGC | GCGGTTTTGCAGGACATGGG |
Two sets of primers were used for each claudin isoform, generated from the NCBI sequence of Monodelphis domestica. A dashed line instead of the second set of primers indicates that only one set of primers was necessary to identify expression of that particular claudin.
Cloning and plasmid construction.
pcDNA 3.1+ (Invitrogen) and pGEM-T Easy (Promega, Madison WI) vectors were utilized to generate constructs containing the claudin genes we found to be expressed in OK cell cDNA. We also cloned GAPDH. All sequences were cloned by PCR, using homologous primers to the Monodelphis domestica or Mus musculus (claudin-2) sequence found in the NCBI database. For claudins-2, -4, -9 (variant 1), -11, -12, and GAPDH, the PCR product was shuttled directly into pcDNA 3.1+. For these constructs, a Kozak (28) sequence was introduced between the restriction site and the coding sequence in the 5′-primer (except for claudin-11 and GAPDH) and an HA tag was inserted before the stop sequence in the 3′-primer. The genes were amplified by PCR from the OK cell cDNA library or mouse kidney cDNA (for mouse claudin-2) using primers with unique restriction enzyme sites (Table 2). PCR products were then digested with enzymes corresponding to the unique restriction sites and ligated into the pcDNA 3.1+ vector that was previously linearized using the same restriction enzymes. The gene in each construct was sequenced and compared with the Monodelphis domestica sequence using the Emboss Pairwise Alignment tool (http://www.ebi.ac.uk/Tools/psa/emboss_needle/nucleotide.html) (for claudin-4 sequence comparison refer to Fig. 6).
Table 2.
Primers and restriction sites used for cloning the opossum kidney claudins and mouse claudin-2
| Forward Primer | Reverse Primer | |
|---|---|---|
| Claudin-1 | 5′-ATG GCC AAC GCG TTG-3′ | 5′-TCA CAC ATA GTC CTT TCC ACT GGA GG-3′ |
| Claudin-2 | 5′-CGC GGA TCC GCC ACC ATG GCC TCC CTT GGC GTT-3′ (BamHI site) | 5′-CCG GAA TTC TCA CGC ATA GTC AGG AAC ATC GTA TGG GTA CAC ATA CCC AGT CAG GCT G-3′ (EcoRI site) |
| Claudin-4 | 5′-CGC GGA TCC GCC ACC ATG GGG TCC ATG GGG CTCC-3′ (BamHI site) | 5′-CCG GAA TTC TCA CGC ATA GTC AGG AAC ATC GTA TGG GTA CAC GTA GTT GCT GGT AGG GGC-3′ (EcoRI site) |
| Claudin-6 | 5′-ATG GCT TCT GCC GGC CTC C-3′ | 5′-TTA TAC ATA ATT CTT GGC CTG GTA CTC AG-3′ |
| Claudin-9 | 5′-CGG GGT ACC GCC ACC atg GCT TCA GCT GGG CTG G-3′ (KpnI site) | 5′-GGA ATT CTC ACG CAT AGT CAG GAA CAT CGT ATG GGT ACA CAT AAT CCC GTT TGT CCA GG-3′ (EcoRI site) |
| Claudin-11 | 5′-CGC GGA TCC atg GTT GCC ACT TGC CTG C-3′ (BamHI site) | 5′-CCG GAA TTC TCA CGC ATA GTC AGG AAC ATC GTA TGG GTA TAC GTG GGC ACT CTT GGC G-3′ (EcoRI site) |
| Claudin-12 | 5′-CGC GGA TCC GCC ACC ATG GGT TGT CGG GAT GTC cAT GC-3′ (BamHI site) | 5′-CCG GAA TTC TCA CGC ATA GTC AGG AAC ATC GTA TGG GTA GGT GGC GTG GCT CAC CAC AGG-3′ (EcoRI site) |
| Claudin-15 | 5′-ATG TCA GTT GCT GTA GAG ACA TTT GGA-3′ | 5′-CTA TAC ATA GGC ATT TTT CCC ATA TTT GCC-3′ |
| Claudin-20 | 5′-ATG GCA TCA TCA GGT CTA CAG CTC C-3′ | 5′-TCA TAC ATA ATC CTT CAG GTT GTA GCC TGC-3′ |
| GAPDH | 5′-CCC AAG CTT ATG TCC AAG GTG CAC ATT AGT AGA TTT GG-3′ (HindIII site) | 5′-CCG GAA TTC TCA CGC ATA GTC AGG AAC ATC GTA TGG GTA CTC CTT GGT GGC CAT GTA CG-3′ (EcoRI site) |
Primers used for amplification and cloning of each claudin expressed in the Didelphis virginiana opossum cDNA library and claudin-2 from mouse kidney cDNA.
For the cloning of claudin-1, -6, -15, and -20, primers without an HA tag or restriction sites were designed corresponding to the extreme 5′- and 3′-coding sequence (Table 2). PCR was performed using these primers and products were inserted into pGEM-T Easy by ligation. Each gene was sequenced and compared with the corresponding sequence from the Monodelphis domestica opossum. We were only able to clone a truncated version of claudin-15 from the Didelphis virginiana opossum cDNA generated (data not shown). All sequences obtained were deposited into the GenBank Database.
Real-time PCR.
RNA was isolated from OK cells (seeded at 3 × 106 /10 cm dish and harvested at day 5) using a Qiagen RNA isolation kit. For RT-PCR experiments on cells after siRNA knockdown, RNA was isolated by the same means; however, cells were seeded at 6 × 105 and they were collected 96 h after transfection. Random primers, SuperScript II reverse transcriptase (Invitrogen), and 1 μg of RNA were used to generate cDNA that was then employed to quantify the expression of each claudin gene and the GAPDH gene. This experiment was performed in triplicate. IDT software (Integrated DNA Technologies, San Diego, CA) was used to design Taqman real-time PCR primers and probes, based on the cloned sequences of the Didelphis virginiana opossum claudins (Table 3). To determine the absolute claudin expression, plasmid standards of each of the previously described claudin constructs were diluted into concentrations ranging from 0.000002 to 2 pg/μl and used to generate standard curves corresponding to each gene. A linear relationship was established between copies of claudin and fluorescence intensity, which was then used to calculate the copies of each claudin in the experimental OK cDNA samples. To compare the expression levels of claudins between different stable cell lines, standards were generated by dilution of control cDNA and the quantity of claudin expression was normalized to GAPDH for each cDNA sample. Expression levels were quantified using an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA).
Table 3.
Opossum kidney claudin RT-PCR primers and probes
| Claudin-1 | F: GAATTCTATGATCCCCTGACCC |
| Probe: /56-FAM/CTCCTTTGCTGTTCCTGTCCCAAGA/3IABLFQ/ | |
| R: AGGAGTCGGGTAAGAGGTTG | |
| Claudin-4 | F: CTCCAGGTAGTGGGCATTG |
| Probe: /56-FAM/CCTTCATCGGCAGCAACATCGTG/3IABLFQ/ | |
| R: ACACAGTTCATCCACAGGC | |
| Claudin-6 | F: CGTCTTGTACTGACTTCTGGG |
| Probe: /56-FAM/TGCTGGACTGCTCATGCGATCA/3IABkFQ/ | |
| R: CAACTCCCGTTTCTGGACTC | |
| Claudin-9 | F: GTGTGGAAGATGAGGTGGC |
| Probe: /56-FAM/AGGACCAGGATCCCAGAGATGAGG/3IABkFQ/ | |
| R: CACCAGAGGATTGTAGAAGTCC | |
| Claudin-11 | F: AGATTGTGTCATGGCTACGAG |
| Probe: /56-FAM/AGGTTATGTCCAGGCTTGCCGAG/3IABkFQ/ | |
| R: ACAGTCAAGAGCAGGAAGATG | |
| Claudin-12 | F: TGCTGTTCTTGTGGTACTGTG |
| Probe: /56-FAM/TCTGGCCGAGTAGGGCTGAGAATA/3IABLFQ/ | |
| R: ACAGGGATGTCTATCTCGATGG | |
| Claudin-15 | F: CCACCTCGACCATCTTTGAG |
| Probe: /56FAM/TCCTTCCATGCTTGCCCTGTCTG/3IABLFQ/ | |
| R: ATAGCTGTGATCATGAGTGCC | |
| Claudin-20 | F: GGTAAATGCAAATGTGGGCTC |
| Probe: /56FAM/ATGGTACAGCACCGGGATGTTC AG/3IABkFQ/ | |
| R: CACATACACAGGAAGGGCTAG |
Successful probes and primers used in the quantitative real-time PCR experiments with claudins1, -4, -6, -9, -11, -12, -15, and -20 in opossum kidney (OK) cells.
R, reverse; F, forward.
Electrophysiology: Ussing chambers.
Opossum kidney cells and stable cell lines were seeded at 1 × 105 cells on six-well Snapwell inserts (Corning, NY) and grown to confluence (day 5). For claudin-4 knockdown experiments, cells were seeded at 6 × 105 and mounted 96 h after transfection. Ussing Chamber studies were carried out with slight modifications to published procedures (7, 16, 19, 20, 40). Initially, we corrected for baseline conditions of empty Ussing chambers with buffer A (145 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4) at 37°C. The Snapwell inserts with confluent OK monolayers were washed three times using buffer A and then mounted between the two hemichambers, both of which were filled with 10 ml of buffer A. Current clamps were performed using a DVC 1000 I/V clamp (World Precision Instruments, Sarasota, FL) and electrodes containing an agarose bridge with 3 M KCl. Data were acquired as a trace and recorded using PowerLab (ADInstruments, Colorado Springs, CO) running Chart 4.0 software. To determine the TER and permeability properties of the epithelia, a 90-μA current was applied across each monolayer and a dilution potential was induced by replacing buffer A in the apical hemichamber with buffer B (80 mM NaCl, 130 mM mannitol, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4). The osmolality of the buffers, measured using an Advanced Osmometer model 3D3 (Advanced Instruments, Norwood, MA) was 310 ± 10 mmol/kgH20. TER measured was quite small (11 ± 1 Ω·cm2), and the variation in the intrinsic resistance of Snapwell filters was considerable, relative to the low resistance of the confluent monolayer. To eliminate this variability, the dilution potential and resistance of each filter were determined following removal of the cells by trypsinization (30 min at 37°C), and this measurement was subtracted from the values generated by that specific filter with cells grown on it. Dilution potentials were therefore corrected for changes in liquid junction potentials by subtracting the dilution potential of an empty filter, as has commonly been done by others (7, 16, 19, 20). The Goldman-Hodgkin Katz and Kimizuka Koketsu (24) equations were used, as described previously (19), to calculate the absolute permeability of sodium and chloride and to determine the relative permeability of sodium to chloride (PNa/PCl) ratio. The permeability of K+, Li+, Cs+, Rb+, I−, and Br− was obtained by the same protocol, except NaCl was substituted for an equimolar concentration of KCl, LiCl, CsCl, RbCl, NaI, or NaBr in both buffers A and B. Osmolality of these buffers were similar to the Na+ containing buffers.
To estimate the contribution of changes in paracellular and transcellular resistance to the increase in TER after claudin-4 overexpression, we used a modified “membrane permeabilization method” originally developed by Wills et al. (42). Inserts with OK cells stably expressing pcDNA or claudin-4HA were mounted in the Ussing chamber as described above. The chamber was perfused bilaterally with 10 ml of Ringer solution containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4 at 37°C. Inserts were voltage clamped at 0 mV, and the short-circuit current (Isc) was recorded. TER was determined by introducing positive voltage pulses, lasting 3.5 s every 40 s, across the monolayer (the resistance of the blank insert was subtracted from the TER measured). Once Isc and resistance were steady, the apical solution was exchanged for a “high potassium,” Ringer's solution containing: 10 mM NaCl, 135 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4, to depolarize the apical membrane. The voltage clamp was compensated for changes in liquid junction potential according to values obtained by making similar measurements with blank inserts. The magnitude of pulses applied was adjusted based on the TER of each insert so as to maintain the Isc between 25 and 45 μA. Immediately after the Isc and resistance stabilized, the ionophore was added to the apical compartment of the chamber. We chose to use gramicidin to obviate the need of matching our buffer to the intracellular chloride concentration. A stock solution of gramicidin (Sigma, St. Louis, MO) at a concentration of 100 mg/ml was prepared in 70% ethanol shortly before the experiment. Two microliters of stock solution were added to the apical compartment of the chamber every 90 s, four times, increasing the gramicidin concentration in the apical compartment from 0 to 80 μg/ml in 20 μg/ml increments. While equivalent amounts of ethanol had no effect on Isc (data not shown), gramicidin caused an increase in Isc as well as a modest decrease in TER. The first eight TER and Isc measurements after the beginning of the Isc increase as well as the last measurement before the Isc began to increase were used to plot Gep (=1/TER) vs. Isc. These data were then fit with a linear equation Gep = Isc/Eb + Gpar, where Eb is the electromotive force of the basolateral membrane and Gpar is the paracellular conductance (for each insert, R2 > 0.96). Gpar was calculated by solving for the y intercept. Transcellular conductance (Gtrans = Isc/Eb) was then determined by solving the equation Gtrans = Gep − Gpar for the Gep value before the addition of gramicidin. The differences in total (ΔGep), transcellular (ΔGtrans), and paracellular (ΔGpar) conductance caused by overexpression of claudin-4 were calculated as the difference between the conductance obtained for inserts transfected with claudin-4 and those transfected with empty vector.
To confirm that ion flux was moving via a paracellular pathway we reversed the driving force for ion flux. To this end, the dilution potential experiment was performed exactly as described above, except that buffer B was added to the basolateral rather than the apical compartment. The dilution potential recording obtained was then used in our calculation of the PNa/PCl ratio. To ensure ion flux was occurring via a paracellular pathway, we performed a further experiment. We added 100 nM ouabain (Sigma) to the basolateral compartment for 20 min before performing dilution potential measurements to eliminate transcellular flux across the epithelium by blocking the sodium-potassium ATPase. The pretreated cells were then subjected to the same Ussing chamber experiment described above.
Statistical analysis.
Data are reported as means ± SE. Statistical significance was analyzed with Student's t-test and values <0.05 were considered significant.
RESULTS
OK cells form loose, slightly cation selective tight junctions.
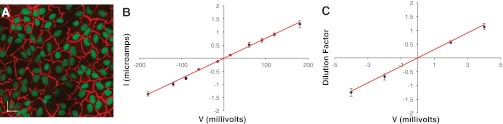
To visualize whether confluent monolayers of OK cells form cell-to-cell contacts, they were immunostained with an anti-ZO-1 antibody that detects a tight junction associated polypeptide (Fig. 1A). We observed linear staining at cell-to-cell contacts, suggesting that OK cells do form tight junctions. We then examined the electrophysiological characteristics of this junction. Confluent monolayers of OK cells were mounted in Ussing Chambers and subjected to increasing current pulses (from −180 to +180 μA). Consistent with OK cells having a low resistance tight junction, we observed a slight linear increase in the recorded voltage (Fig. 1B). Ussing Chambers were also employed to perform dilution potential measurements. Under iso-osmolar conditions induced by adjusting the concentration of NaCl in each hemichamber, we imposed increasing dilution factors across the monolayer and recorded the potential difference (V) generated. We found that the dilution factor imposed correlated linearly with the dilution potential measured across the monolayer (Fig. 1C).
Fig. 1.
Characteristics of the opossum kidney (OK) cell tight junctions. A: confluent monolayers of OK cells grown on a glass coverslip and immunostained with anti-zona occludens-1 (ZO-1; red) and DAPI (green). Scale = 25 μm. B: plot of voltage (V; mV) measured after the imposition of a current (I; μA) across OK cell monolayers. C: plot of potential difference (mV) vs. the dilution factor applied across confluent monolayers of OK cells mounted in Ussing chambers. Data are presented as means ± SE. Red lines represent a linear fit of the experimental data; R2 = 0.996 for B, R2 = 0.995 for C, and n = 3 for both.
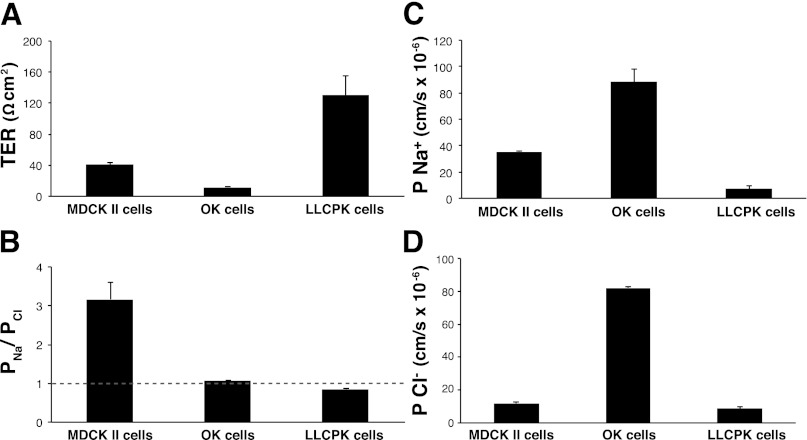
To assess the validity of our results and compare them with other model systems, we measured TER and performed dilution potential studies on MDCKII and LLC-PK1 cells, along with the OK cells (Fig. 2). We measured a TER of 41 ± 2 Ω·cm2 for MDCKII cells, 130 ± 5 Ω·cm2 for LLC-PK1 cells, and 11 ± 1 Ω·cm2 for OK cells. A permeability ratio (PNa/PCl) of 3.2 ± 0.4 was measured for MDCKII cells, 0.85 ± 0.02 for LLC-PK1 cells, and 1.10 ± 0.01 for OK cells. The absolute Na+ permeability was determined to be 35 ± 1 × 10−6 cm/s for MDCKII, 7.4 ± 1.8 × 10−6 cm/s for LLC-PK1, and 88 ± 10 × 10−6 cm/s for OK cells (Fig. 2C). Similarly, we measured an absolute Cl− permeability of 12 ± 2 × 10−6 cm/s for MDCKII, 8.6 ± 1.8 × 10−6 cm/s for LLC-PK1 and 82 ± 10 × 10−6 cm/s for OK cells (Fig. 2D). Thus, compared with the previously described LLC-PK1 and MDCKII cells, OK cells have a low TER (Fig. 2A), are slightly cation selective (Fig. 2B), and have relatively high Cl− and Na+ permeability (Fig. 2, C and D). To ensure that ion flux across OK cell monolayers was occurring via the paracellular pathway, we diluted the basolateral compartment thereby reversing ion flux across the monolayer. We measured an identical PNa/PCl ratio, 1.10 ± 0.01, when this was done. Similarly, the elimination of transcellular flux, by pretreating with 100 nM ouabain, resulted in a PNa/PCl ratio of 1.11 ± 0.01, which was not significantly different from the PNa/PCl ratio of untreated OK cells.
Fig. 2.
Electrophysiology of Madin-Darby canine kidney II (MDCKII), OK, and LLCPK cells. Comparison of transepithelial resistance (TER; A), permeability ratio (PNa/PCl; B), and absolute permeability of sodium (Na+; C) and chloride (Cl−; D) across confluent monolayers of MDCKII cells, OK cells, and LLCPK cells; n ≥ 4 per cell type. Grey dashed line represents the ratio of sodium to chloride permeability (PNa/PCl) ratio of 1. Data are presented as means ± SE.
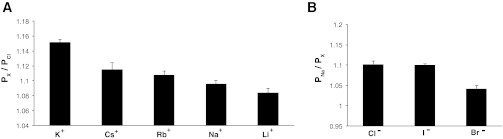
To delineate the permeability profile of OK cell tight junctions, we performed dilution potential measurements with other monovalent ions (Table 4). The permeability sequence of cations (relative to Cl−) across OK cell monolayers ranks K+ >> Cs+ > Rb+ > Na+ > Li+ and for anions, relative to Na+, ranks Cl− ≈ I− > Br− (Fig. 3, A and B). Next, we turned our attention to identifying the molecular determinants of tight junction selectivity properties in this cell model.
Table 4.
Cation and anion transport properties across OK cells
| Buffer | NaCl | LiCl | KCl | RbCl | CsCl | NaI | NaBr |
|---|---|---|---|---|---|---|---|
| DP (cells and blank insert, mV ± SE) | 0.83 ± 0.03 | 0.87 ± 0.04 | 1.30 ± 0.02 | 0.99 ± 0.04 | 1.02 ± 0.06 | 0.89 ± 0.02 | 0.35 ± 0.06 |
| DP (blank insert, mV ± SE) | 0.12 ± 0.03 | 0.25 ± 0.03 | 0.21 ± 0.02 | 0.198 ± 0.002 | 0.19 ± 0.01 | 0.15 ± 0.04 | 0.09 ± 0.04 |
| PX+/PCl− (±SE) | 1.10 ± 0.01 | 1.08 ± 0.01 | 1.15 ± 0.01 | 1.11 ± 0.01 | 1.12 ± 0.01 | 1.10 ± 0.01 | 1.03 ± 0.01 |
Dilution potentials (DP) and calculated permeability ratios of OK cells on semipermeable membranes and of just the semipermeable membranes measured from Ussing chamber DP experiments using NaCl, KCl, CsCl, LiCl, RbCl, NaBr, and NaI buffers; n = 8 for all except CsCl (n = 7) and NaBr (n = 4).
Fig. 3.
Permeability of monovalent cations and anions across monolayers of OK cells. OK cell tight junction permeability to cations (K+, Cs+, Rb+, Na+, and Li+) with respect to Cl− (A) anions (Br−, I− and Cl−) with respect to Na+ (B). Data are presented as means ± SE; n ≥ 4 per ion.
OK cells endogenously express claudins-1, -4, -6, -9, -11, -12, -15, and -20.
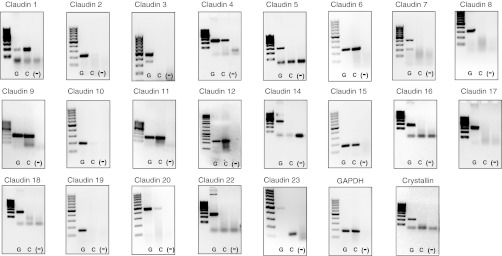
To determine the endogenous expression of claudins in OK cells, we generated a cDNA library from confluent cell monolayers. We then created two sets of degenerate primers, targeting a single exon for each claudin isoform, using the NCBI sequence of the Monodelphis domestica (31) opossum (note OK cells were generated from a different species of opossum, Didelphis virginiana; Ref. 27). Employing these primers, we performed PCR on genomic DNA to test the primer set integrity, cDNA to determine expression of each claudin, and cDNA generated in the absence of reverse transcriptase to ensure that genomic DNA contamination of the cDNA was not providing a false positive result (Fig. 4). We included a positive control, GAPDH, and found its expression in both genomic DNA and cDNA but not in the template lacking reverse transcriptase. In contrast, expression of our negative control, the lens protein crystallin, was found only in genomic DNA, indicating that our cDNA samples were not contaminated with genomic DNA. Using this methodology, we observed expression of claudins-1, -4, -6, -9, -11, -12, -15, and -20 in OK cell cDNA (Fig. 4). It is noteworthy that these results were identical to those observed when cDNA from subconfluent monolayers of OK cells was used (results not shown).
Fig. 4.
Claudin expression in OK cells. PCR was performed to identify the expression of each claudin isoform, GAPDH (as positive control) and crystallin (as a negative control) on 3 templates: genomic DNA (lane one, G), cDNA (lane 2, C), and cDNA prepared without reverse transcriptase [lane 3, (-)]. Final PCR products were electrophoresed on 1.5% agarose DNA gels (with a 100-bp or 1.0-kB ladder), and visualized using ethidium bromide and ultaviolet-based detection.
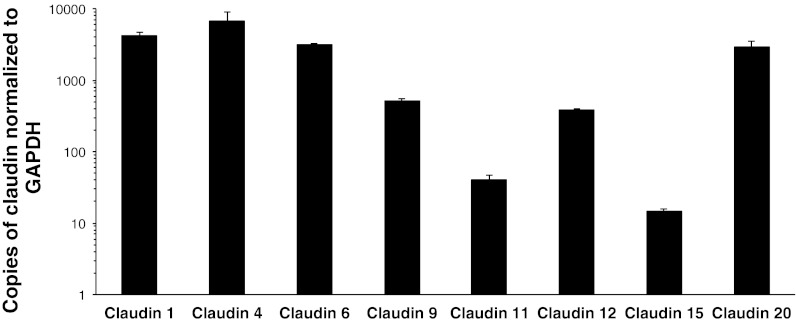
To quantify the relative expression of each claudin, we cloned the identified isoforms from the OK cell cDNA library and then performed quantitative real-time PCR. Surprisingly, the claudin that was most abundant in this loose, cation-selective epithelial cell line was claudin-4, followed by claudin-1 > -6 > -20 > - 9 > -12 > -11 > -15 (Fig. 5). Comparison between the Didelphis virginiana claudin-4 sequence obtained and the published Monodelphis domestica sequence is made in Fig. 6
Fig. 5.
Relative expression of claudins in OK cells. RT-PCR analysis of claudin expression in OK cells. Results are normalized to GAPDH expression; n = 6 per group. Data are presented as means ± SE.
Claudin-4 overexpression increases TER without altering PNa/PCl.
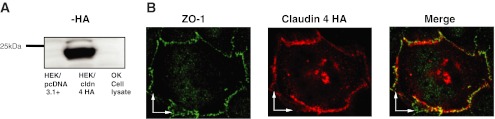
We next enquired what function claudin-4, a barrier forming claudin, might play in this loose epithelial model. To this end claudin-4 was shuttled into the mammalian expression vector pcDNA3.1+ and an HA epitope tag inserted at the carboxy terminus. Immunoblot analysis of HEK 293 cells transiently transfected with claudin-4 revealed a 24-kDa band when probed with an anti-HA antibody (Fig. 7A). This band was not evident when HEK 293 cells were transfected with the empty vector pcDNA 3.1+. To determine the subcellular localization of claudin-4, we transiently transfected the construct back into OK cells and costained with antibodies against HA and ZO-1. This revealed that claudin-4 colocalized with ZO-1 at the tight junction, confirming appropriate subcellular localization of the construct (Fig. 7B).
Fig. 7.
Expression of claudin-4 in HEK 293 and OK cells. A: immunoblot probed with anti-HA of HEK 293 cell lysate that was transiently transfected with pcDNA 3.1+, empty vector, (lane 1), or claudin-4HA (lane 2) and OK cell lysate (lane 3). B: representative immunofluorescence image of an OK cell transiently transfected with claudin-4HA and immunostained with anti-ZO-1 (green) and anti-HA (red). Scale bars = 5 μm.
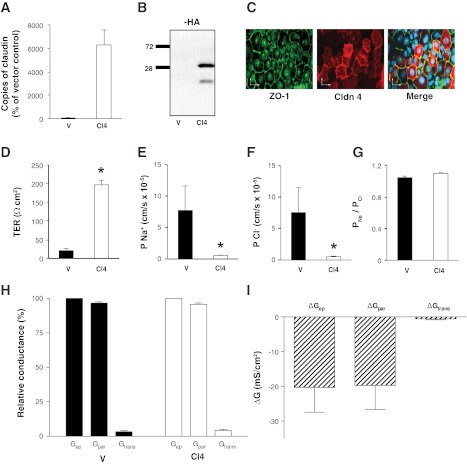
Having confirmed the expression and appropriate subcellular localization, we proceeded to ascertain the effects of claudin-4 overexpression on tight junction electrophysiology. To this end, we generated stable cell lines overexpressing claudin-4 to enable electrophysiological measurements on confluent monolayers. Claudin-4 overexpression was confirmed by real-time PCR, immunoblot, and immunofluorescence microscopy (Fig. 8, A–C). Importantly, claudin-4 was predominantly localized with ZO-1 at the tight junction.
Fig. 8.
Characteristics of OK cell lines stably overexpressing claudin-4. Stable claudin-4 overexpression in OK cell lines was verified by RT-PCR, presented as percentage of vector-transfected control (A), immunoblot probed for HA (B), and by immunostaining (C) a confluent monolayer of OK cells overexpressing claudin-4HA with anti-ZO-1 (green) and anti-HA (red); the scale bars represents 25 μm. Ussing Chamber studies were employed to determine TER (D), absolute permeability to sodium (Na+; E), absolute permeability to chloride (Cl−; F), ratio of sodium to chloride permeability (PNa/PCl; G), relative contribution of paracellular conductance (Gpar), and transcellular conductance (Gtrans) to the total epithelial conductance (Gep; H), and absolute contribution of the changes in paracellular conductance (ΔGpar) and transcellular conductance (ΔGtrans) to the change in total epithelial conductance (ΔGep; I) in OK stable cell lines expressing the empty vector or claudin-4HA. OK stable cell lines overexpressing pcDNA 3.1+ are abbreviated V and represented with black bars, and those overexpressing claudin-4HA are abbreviated Cl4 and represented with white bars. Data are presented as means ± SE; n ≥ 3 cell lines for each group. *P value < 0.05.
To explore the electrophysiological changes induced by claudin-4 overexpression, we performed TER and dilution potential measurements in Ussing chambers. We found that overexpression of claudin-4 in each of the individual stable cell lines caused a significant increase in TER compared with empty vector-transfected OK cells (Fig. 8D). Consistent with this, both Na+ and Cl− permeabilities were significantly decreased in each of the claudin-4 overexpressing lines (Fig. 8, E and F). Moreover, the decrease in flux was reduced proportionately, such that the PNa/PCl ratio was not significantly altered (Fig. 8G).
To determine whether the increase in TER was due to changes in paracellular or transcellular conductance, we first estimated the paracellular and transcellular conductance across monolayers of OK cells transfected with claudin-4 or the empty vector. This was accomplished by permeabilizing the apical membrane with increasing concentrations of gramicidin. Despite the high concentrations used, gramicidin had only a modest effect on TER (we typically observed 5–6% decrease in TER over the experimental period). Paracellular conductance (Gpa) was the predominant conductance across the monolayer of cells transfected with either claudin-4 or empty vector. Paracellular conductance accounted for >96% of total conductance (Gep) in empty vector-transfected and >95% of Gep in claudin-4-transfected cells (Fig. 8H). Thus, although expression of claudin-4 caused a decrease in both paracellular and transcellular conductance, the observed difference in total epithelial conductance was overwhelmingly (>97%) due to a decrease in paracellular conductance (Fig. 8I).
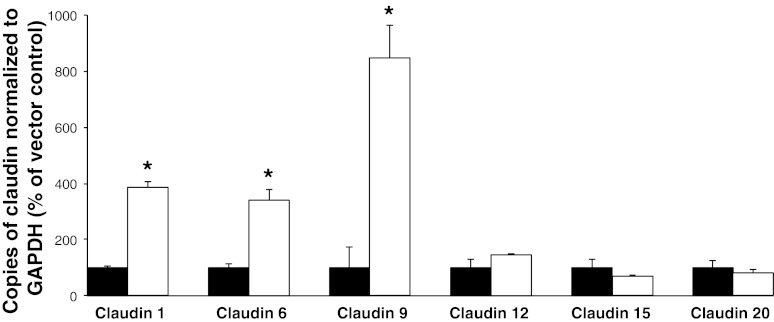
Claudin-4 overexpression increases expression of claudins-1, -6, and -9.
There are instances where overexpression of a single claudin may alter the expression of other claudin isoforms (7, 43). Therefore, to ascertain whether the changes observed were due to the altered expression of claudin-4 or secondary to an effect on other claudins, we quantified the expression of the other claudins identified in the OK cell line by real-time PCR. We found no alteration in the level of expression of claudins-11, -12, -15, or -20 between empty vector expressing and claudin-4 overexpressing cell lines. However, we observed a significant increase in endogenously expressed claudin-1, -6, and -9 mRNA in the stable cell lines overexpressing exogenous claudin-4 (Fig. 9).
Fig. 9.
Effects of claudin-4 overexpression on the level of expression of other claudins. RT-PCR analysis of claudin expression in each of the 3 OK stable cell lines expressing empty vector (black bars) or stable cell lines overexpressing claudin-4HA (n = 4). Gene expression is represented as percentage of control (OK cells stably expressing pcDNA 3.1+) and normalized to the expression of GAPDH. Data are presented as means ± SE; n ≥ 3 for each group. *P value < 0.05.
Claudin-4 knockdown in cells overexpressing claudin-4 causes a significant drop in TER without significantly altering the expression of other claudins.
In an attempt to dissect out the specific role of claudin-4 in OK cells, we employed siRNA to knockdown claudin-4 then performed Ussing Chamber experiments and determined endogenous claudin expression. There were no significant alterations in TER or absolute Na+ or Cl− permeability across confluent monolayers of OK cells stably expressing pcDNA 3.1+ (the empty vector) when endogenous claudin-4 was knocked down by ∼64% (Fig. 10, A, C, and D). There was a slight decrease in the PNa/PCl ratio (P = 0.04) when the empty vector expressing cells were treated with siRNA against claudin-4 compared with scrambled siRNA (Fig. 10B). In contrast, the knockdown of claudin-4 in the cell lines overexpressing claudin-4 caused a significant decrease in TER, as well as a significant, proportional increase in Na+ and Cl− permeability, such that the PNa/PCl ratio remained unaltered (Fig. 10, A–D).
Fig. 10.
Effect of claudin-4 knockdown. OK cells expressing the empty vector (V) or overexpressing claudin-4 were treated for 96 h with scrambled RNA (black bars) or claudin-4 small interfering (si)RNA (grey bars). Ussing chamber experiments were then performed to determine TER (A), PNa/PCl (B) and absolute Na+ (C) and Cl− (D) permeability. RT-PCR analysis of claudin -1 (E), -4 (F), -6 (G), -9 (H), and -12 (I) expression in empty vector expressing cells (V) and cells overexpressing claudin-4 (CL4) after transfection with scrambled siRNA or claudin-4 siRNA. Gene expression is represented as percentage of control (OK cells stably expressing pcDNA 3.1+) and normalized to the expression of GAPDH. Data are presented as means ± SE; n ≥ 3 cell lines. *P value < 0.05.
Figure 10, E–I, outlines the alterations in endogenous claudin expression after transient expression of siRNA directed against claudin-4 or a scrambled siRNA in OK cells expressing the empty vector or those overexpressing claudin-4 (note for these experiments we used siRNA#1, but the results were equivalent when siRNA #2 was used). Endogenous claudin-4 knockdown in vector expressing controls results in significantly decreased claudin -1, -4, -9, and -12 mRNA levels. With the exception of claudin-4 itself, siRNA treatment in the overexpressing cells did not affect the expression of the other claudins (Fig. 10, E–I), making it possible to attribute the electrophysiological effects in the overexpressing cells to claudin-4 knockdown.
Claudin-2 expression in OK cells creates a cation selective pore.
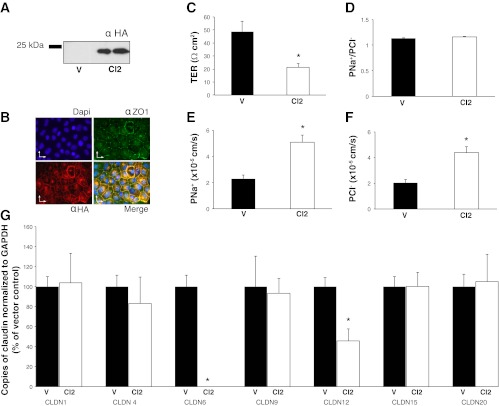
Claudin-2 expression has been previously observed in the proximal tubule (11, 25, 36). Given that we could not detect it in OK cells, a proposed proximal tubule cell culture model, we sought to determine its role in this model system. To this end we generated stable cell lines overexpressing mouse claudin-2 with a carboxy-terminal HA tag. Expression of claudin-2 was confirmed by immunoblot analysis (Fig. 11A) where a 25-kDa band was observed in the stably transfected cells but not the cells expressing the empty vector. Expression was also confirmed by immunofluorescence (Fig. 11B) where colocalization of claudin-2 (HA, red) with the tight junction marker ZO-1 (green) was observed. Ussing chamber experiments utilizing these cells revealed a significant decrease in TER and an increase in both Na+ and Cl− permeability when claudin-2 was overexpressed (Fig. 11, E, and F). The PNa/PCl ratio was not significantly altered (Fig. 11D). Similarly, mRNA levels of claudins-1, -4, -9, -15, and -20 were unaltered in the overexpressing cells, compared with vector-transfected controls (Fig. 11G). However, the mRNA level of claudin-12 significantly decreased and claudin-6 expression is nearly eliminated by the introduction of claudin-2 into OK cells (Fig. 11G).
Fig. 11.
Expression of mouse claudin-2 in OK cells. Overexpression of claudin-2, with an HA epitope tag, in OK cells was demonstrated by Western blot (A) and by immunofluorescence microscopy (B), DAPI staining is blue; ZO-1 green and claudin-2 (HA) is red; scale bars = 25 μm. Ussing chamber dilution potential experiments were used to determine the TER (C), PNa/PCl (D), Na+ (E), and Cl− (F) permeability of cells expressing empty vector (V, black bars) or overexpressing claudin-2 (CL2, white bars). G: RT-PCR analysis of claudin -1, -4, -6, -9, -12, -15, and -20 expression in OK cells expressing the empty vector (V, black bars) or claudin-2 (CL2, white bars). Gene expression is represented as percentage of control (OK cells stably expressing pcDNA 3.1+) and normalized to the expression of GAPDH. Data are presented as means ± SE; n ≥ 3 cell lines. *P value < 0.05.
DISCUSSION
We have characterized a very loose epithelial cell culture model and shown that it can be used to study the effect of claudins on paracellular ion transport. OK cells form tight junctions and exhibit low resistance and slight cation selectivity. We observed a linear relationship between voltage and current, as well as between the dilution factor and resulting dilution potential generated. Hence, Ussing chamber dilution potential experiments can be performed successfully on a loose epithelium. The observed electrophysiological characteristics are likely conferred by the expression of endogenous claudins, which ranks claudin-4 > -1 > -6 > -20 > -9 > -12 > -11 > -15.
The OK cell model system was employed to overexpress claudin-4, a perturbation that led to significantly increased TER (without altering PNa/PCl) and increased claudin-1, -6, and -9 mRNA levels. To dissect out the specific role of claudin-4 in OK cells, we knocked it down in the vector-transfected and the claudin-4 overexpressing cell lines. Knockdown of endogenous claudin-4 decreased claudin -1, -9, and -12 expression without significantly altering the resistance or the absolute permeability of Na+ and Cl−. A 64% knockdown of claudin-4 although significant, compared with scrambled RNA treated control, may not have been sufficient to cause electrophysiological alterations. Obvious alterations in electrophysiology occurred only when claudin-4 was knocked down in the overexpressing stables. This did not induce significant differences in the expression of the other endogenous claudins, allowing us to conclude that the decreased TER and increased Na+ and Cl− permeability are due to claudin-4. Thus claudin-4 acts as a barrier in OK cells.
As we were unable to detect claudin-2 in this common proximal tubular cell culture model, we overexpressed it and found decreased TER as well as claudin-12 and -6 expression. Taken together our results demonstrate that OK cells model the electrophysiological characteristics of the proximal tubule. However, manipulation of claudin expression in this model system significantly alters endogenous claudin expression, suggesting that claudin expression is strongly interdependent in OK cells.
Recent in vivo tubular perfusion studies measured TER across the mouse proximal tubule S2 segment as 11.3 ± 0.4 Ω·cm2 and PNa/PCl to be 1.10 ± 0.02 (34). This is consistent with the TER measured across the proximal tubule of other species (8, 38). We measured OK cell TER and PNa/PCl as 11 ± 1 Ω·cm2 and 1.10 ± 0.01, respectively. These values very closely approximate the electrophysiological properties of the proximal tubule S2 segment in vivo (34). Furthermore, the murine tubular perfusion studies found that K+ was the most permeable cation followed by Rb+ > Na+ > Li+ > choline+ (relative to Cl−). This is identical to the permeability sequence we established for OK cells (K+ > Cs+ > Rb+ > Na+ > Li+). Therefore, both of these epithelia are associated with Eisenman sequence IV, the permeability sequence corresponding to a pore with a weak field strength binding site (10). This infers that there is a weak interaction between the pore and monovalent cations permitting cations to permeate the pore largely in their hydrated state. OK cells may therefore be a reasonable cell culture model to explore the paracellular electrophysiological properties of the proximal tubular S2 segment with.
To be able to manipulate the paracellular permselectivity of this model system, we extensively characterized claudin expression in OK cells. This was accomplished via degenerate PCR, examining the expression of all known claudin isoforms. We found the expression of claudin-4, -1, -6, -20, -9, -12, -11, and -15. This low resistance, cation selective epithelial monolayer contained some supposed barrier forming claudins [-1 (14, 22, 30), -4 (41), -6 (37), and - 9 (37)] as well as pore forming, cation selective claudins [-12 (12) and -15 (9, 39)] and claudins with unknown function (claudin-11 and -20). Claudin expression in the proximal tubule has been incompletely explored. Thus far, claudin-2, -10, -11 (26), and -4 (36) have been identified in this nephron segment. Claudin-6 and -9 expression has also been reported in the proximal tubule of neonatal (but not adult) mice (1). Claudin-12 and -20 expression has not been examined in the proximal tubule to date. The claudins known to be expressed both in the proximal tubule and OK cells are therefore claudins-4, -6, -9, and -11. Regardless of this discrepancy in claudin expression, the identified array of claudins expressed in OK cells form tight junctions with electrophysiological properties very similar to the proximal tubule in vivo.
Claudin-2 has been observed in the proximal tubule of several species (11, 25, 36). We were therefore surprised that we could not identify it in OK cells, a proximal tubular model. However, a previous study also failed to identify claudin-2 in OK cells (35). We therefore overexpressed mouse claudin-2 and observed a decreased TER and significantly increased Na+ and Cl− permeability. There was a slight, but insignificant, increase in the PNa/PCl ratio, suggesting that claudin-2 forms a cation selective pore in OK cells. This has been reported previously in other model systems (4, 12, 13, 41). Claudin-2 overexpression also decreased claudin-12 expression and eliminated claudin-6 expression almost completely, making it impossible to ascribe the effects on permeability to claudin-2 alone and complicating the use of OK cells as a molecular model of the proximal tubule.
It was surprising to find claudin-4 to be the most highly expressed claudin in our model system, as it has been shown to confer high resistance and anion selectivity to other epithelia in culture (9, 21, 40, 41). It is interesting to speculate as to why this loose cation selective epithelium is so rich with claudin-4. Perhaps it is the ratio of claudins, or the complex that they form, that dictates the permselectivity of a monolayer? Hence, the presence of other claudins (for example, claudin-12 and -15) may change, offset, or reduce the effect of claudin-4 on the permselectivity of OK cell tight junctions. Consistent with claudin-4 altering paracellular permeability, the increase in TER after claudin-4 overexpression in OK cells was caused overwhelmingly by a decrease in paracellular conductance. However, when claudin-4 is overexpressed in OK cells, neither Cl− nor Na+ permeability was preferentially reduced, such that the PNa/PCl ratio remains unaltered. This is not the case in MDCK cells, where expression of claudin-4 leads to a selective decrease in Na+ flux yet has no effect on Cl− flux, thereby decreasing the PNa/PCl ratio (40). Our findings are also inconsistent with data from LLCPK and cortical collecting duct cells. In these model systems, claudin-4 appears to create a Cl− permeable pore (18, 21). Such discrepancies regarding claudin function also exist for claudin-11. The expression of this protein decreases TER in LLCPK cells yet increases TER in MDCK cells (41).
Conflicting results such as these suggest an effect conferred by the model system where claudin overexpression or knockdown was performed. Claudin expression or knockdown can have variable effects on paracellular permeability characteristics, depending on the background claudin expression profile and charge selectivity of the model system (18, 21, 40, 41). We therefore examined the effect of claudin-4 overexpression on the expression of endogenous claudins in OK cells and found that claudin-1, -6, and -9 were upregulated. After claudin-4 knockdown in the overexpressing cells, TER was significantly reduced while the expression of claudins-1, -6, and -9 was unaltered. Therefore, in OK cell tight junctions, claudin-4 forms a permeability barrier without altering ion selectivity.
Manipulation of claudin expression in OK cells was complicated by significant changes in endogenous claudin expression. Consistent with this, the knockdown of endogenous claudin-4 significantly altered the expression of claudins-1, -9, and -12. However, there were no significant effects on the resistance and absolute permeability of Na+ and Cl−. Taken together our experiments suggest that claudin expression in this loose epithelial cell culture model is tightly linked, making interpretation of the functional effects of single claudins on paracellular transport in OK cells very difficult.
A limitation of our study is that the protein expression and subcellular localization of endogenous claudins after overexpression or knockdown were not determined, despite trying multiple different antibodies. Further, some of the claudins in the above experiments may interact with each other. This possibility and potential regulatory mechanisms could be explored with our newly characterized model system.
In summary, we measured TER and performed dilution potential measurements across confluent monolayers of OK cells and observed that they were a loose, slightly cation selective epithelium. The measurements made were remarkably similar to those measured across the proximal tubule of a variety of species. We proceeded to identify the claudins providing these functional characteristics in this model system. To our surprise claudin-4, a barrier forming claudin was highly expressed and claudin-2 was absent. To validate the model system and provide information about the role of claudin-4 in a loose epithelium, we overexpressed it in OK cells and found that it increased TER but also endogenous claudin-1, -6, and -9 expression. Knockdown of claudin-4 in the overexpressing cells decreased TER without altering endogenous claudin expression, suggesting that claudin-4 forms a barrier in OK cells. Overexpression of claudin-2 decreased TER but also claudin-6 and -12 expression, while knockdown of endogenous claudin-4 had no significant effects on TER but decreased claudin-1, -9, and -12 expression. Together our results infer that claudin expression in this model system is tightly linked.
GRANTS
This work was funded by grants from the Kidney Foundation of Canada and the Canadian Institutes of Health Research. R. T. Alexander is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research, a KRESCENT New Investigator Award, and an Alberta Innovates Health Solutions Clinical Investigator Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.B., J.R., R.W., and R.T.A. conception and design of research; J.B., R.S.B., J.R., A.R., W.P., and R.T.A. performed experiments; J.B., R.S.B., J.R., A.R., W.P., R.W., and R.T.A. analyzed data; J.B., J.R., R.W., and R.T.A. interpreted results of experiments; J.B., J.R., and R.T.A. prepared figures; J.B. and R.T.A. drafted manuscript; J.B., J.R., R.W., and R.T.A. edited and revised manuscript; J.B., R.S.B., J.R., A.R., W.P., R.W., and R.T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Marek Duszyk for assistance with the Ussing chamber experiments.
REFERENCES
- 1.Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, Lin F, Baum M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol 291: F1132–F1141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhter S, Kovbasnjuk O, Li X, Cavet M, Noel J, Arpin M, Hubbard AL, Donowitz M. Na+/H+ exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol 283: C927–C940, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Amasheh S, Fromm M, Gunzel D. Claudins of intestine and nephron - a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 201: 133–140, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol Cell Physiol 269: C126–C133, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215: 147–159, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bello-Reuss E. Cell membranes and paracellular resistances in isolated renal proximal tubules from rabbit and Ambystoma. J Physiol 370: 25–38, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283: C142–C147, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Eisenman G, Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol 76: 197–225, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Enck AH, Berger UV, Yu ASL. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 281: F966–F974, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H. Tight junction proteins claudin-2 and -12 are critical for vitamin d-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19: 1912–1921, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of Zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156: 1099–1111, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia NH, Ramsey CR, Knox FG. Understanding the role of paracellular transport in the proximal tubule. News Physiol Sci 13: 38–43, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122: 1507–1517, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Hansson GC, Simons K, van Meer G. Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J 5: 483–489, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem 281: 36117–36123, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118: 5109–5118, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci USA 107: 18010–18015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol 78: 849–855, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Jaumouille V, Krishnan D, Alexander RT. The calmodulin antagonist W-7 inhibits the epithelial Na+/H+ exchanger via modulating membrane surface potential. Channels (Austin) 5: 308–313, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kimizuka H, Koketsu K. Ion transport through cell membrane. J Theor Biol 6: 290–305, 1964 [DOI] [PubMed] [Google Scholar]
- 25.Kirk A, Campbell S, Bass P, Mason J, Collins J. Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant 25: 2107–2119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Koyama H, Goodpasture C, Miller MM, Teplitz RL, Riggs AD. Establishment and characterization of a cell line from the American opossum (Didelphys virginiana). In Vitro 14: 239–246, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res 15: 8125–8148, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang M, Ramsey CR, Knox FG. The paracellular permeability of opossum kidney cells, a proximal tubule cell line. Kidney Int 56: 2304–2308, 1999 [DOI] [PubMed] [Google Scholar]
- 30.McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Skare IB, Lynch RD, Schneeberger EE. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J Cell Sci 113: 3387–3398, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, Jurka J, Kamal M, Mauceli E, Searle SM, Sharpe T, Baker ML, Batzer MA, Benos PV, Belov K, Clamp M, Cook A, Cuff J, Das R, Davidow L, Deakin JE, Fazzari MJ, Glass JL, Grabherr M, Greally JM, Gu W, Hore TA, Huttley GA, Kleber M, Jirtle RL, Koina E, Lee JT, Mahony S, Marra MA, Miller RD, Nicholls RD, Oda M, Papenfuss AT, Parra ZE, Pollock DD, Ray DA, Schein JE, Speed TP, Thompson K, VandeBerg JL, Wade CM, Walker JA, Waters PD, Webber C, Weidman JR, Xie X, Zody MC, Graves JA, Ponting CP, Breen M, Samollow PB, Lander ES, Lindblad-Toh K. Genome of the marsupial Monodelphis domestica reveals innovation in noncoding sequences. Nature 447: 167–177, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett 585: 606–612, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions. I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol 279: G250–G254, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 107: 8011–8016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prozialeck WC, Edwards JR, Lamar PC, Smith CS. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol In Vitro 20: 942–953, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L. The renal segmental distribution of claudins changes with development. Kidney Int 62: 476–487, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol 295: R1713–R1719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seely JF. Variation in electrical resistance along length of rat proximal convoluted tubule. Am J Physiol 225: 48–57, 1973 [DOI] [PubMed] [Google Scholar]
- 39.Tamura A, Hayashi H, Imasato M, Yamazaki Y, Hagiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology 140: 913–923, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 285: F1078–F1084, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Wills NK, Lewis SA, Eaton DC. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol 45: 81–108, 1979 [DOI] [PubMed] [Google Scholar]
- 43.Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278: 17350–17359, 2003 [DOI] [PubMed] [Google Scholar]