Abstract
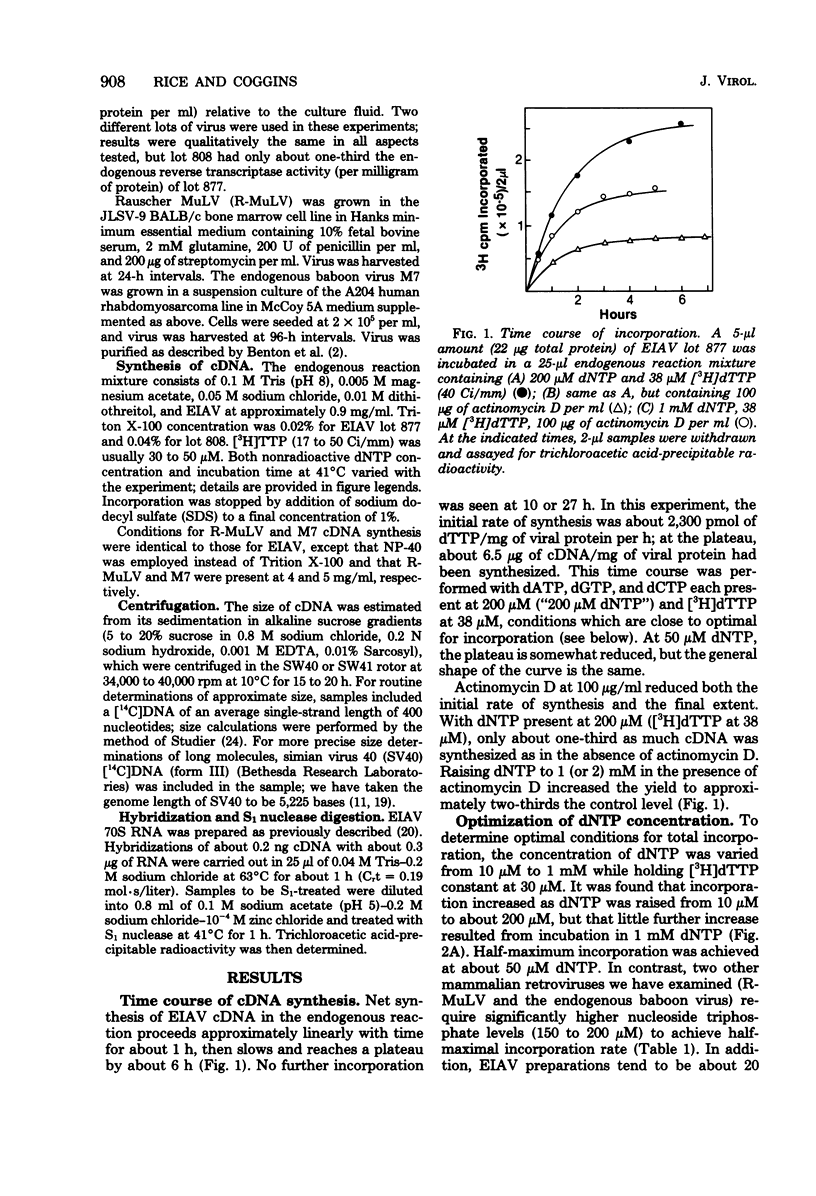
In the endogenous reverse transcriptase reaction, equine infectious anemia virus is able to synthesize complementary DNA (cDNA) of 8,000 nucleotides in high yield. After 2 h in 50 μM dNTP, about 2.8 μg of cDNA per mg of protein is produced, almost 30% of which is long cDNA. The system thus compares favorably with the other two well-characterized endogenous reaction systems, Moloney murine leukemia virus and avian sarcoma virus. Elongation rates of 100 to 150 nucleotides per min have been observed; these rates are comparable to those seen with purified avian myeloblastosis virus reverse transcriptase and significantly higher than those observed in vivo. In the absence of actinomycin D, equine infectious anemia virus does not require high dNTP levels for either optimal incorporation or long cDNA synthesis. The amount of long cDNA synthesized is maximal at 2 h in 50 μM dNTP; neither longer time nor higher dNTP levels (through 1.8 mM) increased this yield. Half-maximum yield in 2 h was achieved at about 15 μM dNTP, which is very similar to the published KM's for isolated avian and murine reverse transcriptases. Total incorporation, on the other hand, continues to rise slowly through 1 mM dNTP; the half-maximum was 30 to 50 μM dNTP. In the presence of 100 μg of actinomycin D per ml, however, higher dNTP levels are required for long cDNA synthesis. We conclude that equine infectious anemia virus is exceptionally well-suited to studies of the physical organization of the retrovirus genome and to investigations of the mechanism of synthesis of the double-standard cDNA endogenous reaction product.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer B. G., Crawford T. B., McGuire T. C., Frazier M. E. RNA-dependent DNA polymerase associated with equine infectious anemia virus. J Virol. 1977 Apr;22(1):16–22. doi: 10.1128/jvi.22.1.16-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton C. V., Hodge H. M., Fine D. L. Comparative large-scale propagation of retroviruses from Old World (Mason-Pfizer monkey virus) and New World (squirrel monkey virus) primates. In Vitro. 1978 Feb;14(2):192–199. doi: 10.1007/BF02618222. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor T. H., Wang C. S. In vitro synthesis of a 2.1 x 10(6) dalton DNA in the endogenous retrovirus reverse transcriptase reaction. Biochem Biophys Res Commun. 1976 Sep 7;72(1):251–257. doi: 10.1016/0006-291x(76)90987-6. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Charman H. P., Bladen S., Gilden R. V., Coggins L. Equine infectious anemia virus: evidence favoring classification as a retravirus. J Virol. 1976 Sep;19(3):1073–1079. doi: 10.1128/jvi.19.3.1073-1079.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Archer B. G., Crawford T. B. Characterization of RNA from equine infectious anemia virus. J Virol. 1977 Nov;24(2):489–497. doi: 10.1128/jvi.24.2.489-497.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. On the association of reverse transcriptase with polynucleotide templates during catalysis. Biochemistry. 1976 Aug 10;15(16):3605–3611. doi: 10.1021/bi00661a031. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor viruses. I. Directing influence of DNA in the reaction. J Virol. 1972 Jan;9(1):116–129. doi: 10.1128/jvi.9.1.116-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S. In vitro synthesis and characterisation of full- and half-genome length complementary DNA from avian oncoviruses. Nature. 1978 Feb 2;271(5644):481–483. doi: 10.1038/271481a0. [DOI] [PubMed] [Google Scholar]
- Leis J., Hurwitz J. RNA-dependent DNA polymerase from avian myeloblastosis virus. Methods Enzymol. 1974;29:143–150. doi: 10.1016/0076-6879(74)29017-7. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Barnett D., Becvar C. S. Production of equine infectious anemia antigen in a persistently infected cell line. Arch Gesamte Virusforsch. 1973;42(4):361–370. doi: 10.1007/BF01250717. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Tanaka S., Ushimi C. Physicochemical studies of equine infectious anemia virus. IV. Determination of the nucleic acid type in the virus. Arch Gesamte Virusforsch. 1970;31(3):273–280. doi: 10.1007/BF01253762. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rice N. R., Simek S., Ryder O. A., Coggins L. Detection of proviral DNA in horse cells infected with equine infectious anemia virus. J Virol. 1978 Jun;26(3):577–583. doi: 10.1128/jvi.26.3.577-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Travaglini E. C., Dube D. K., Surrey S., Loeb L. A. Template recognition and chain elongation in DNA synthesis in vitro. J Mol Biol. 1976 Sep 25;106(3):605–621. doi: 10.1016/0022-2836(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



