Abstract
The optimal expansion, trafficking, and function of adoptively transferred CD8+ T cells are parameters that currently limit the effectiveness of antitumor immunity to established tumors. In this study, we addressed the mechanisms by which priming of self tumor-associated Ag-specific CD8+ T cells influenced antitumor functionality in the presence of the inflammatory cytokine IL-12. In vitro priming of mouse tumor-specific CD8+ T cells in the presence of IL-12 induced a diverse and rapid antitumor effector activity while still promoting the generation of memory cells. Importantly, IL-12–primed effector T cells dramatically reduced the growth of well-established s.c. tumors and significantly increased survival to highly immune resistant, established intracranial tumors. Control of tumor growth by CD8+ T cells was dependent on IL-12–mediated upregulation of the high-affinity IL-2R (CD25) and a subsequent increase in the sensitivity to IL-2 stimulation. Finally, IL-12–primed human PBMCs generated tumorspecific T cells both phenotypically and functionally similar to IL-12–primed mouse tumor-specific T cells. These results highlight the ability of IL-12 to obviate the strict requirement for administering high levels of IL-2 during adoptive cell transfer-mediated antitumor responses. Furthermore, acquisition of a potent effector phenotype independent of cytokine support suggests that IL-12 could be added to adoptive cell transfer clinical strategies in cancer patients.
The adoptive transfer of high numbers of tumor-reactive T cells has matured into a feasible and promising antitumor therapy in patients with cancer (1). However, generation of T cells with the ability to persist, traffic, and maintain effector function after adoptive transfer has limited the effectiveness of this treatment strategy. In vitro cytokine priming allows for the programming of differentiated CD8+ T cells with distinct phenotypes and in vivo characteristics (2–5). Thus, understanding the programs imparted to T cells by diverse cytokine families has become an integral part in understanding and using successful T cell-based therapies. IL-2 was the first cytokine used to generate activated effector cells capable of lysing tumor cells in vitro and mediating tumor regression in vivo (6). However, such terminally differentiated effector cells failed to persist in vivo and control tumor growth in mouse models (7). Recent work in our own group demonstrated the ability of a TLR7 agonist, imiquimod, in combination with a dendritic cell vaccine to enhance priming of tumor-specific CD8+ T cells (8). We hypothesized that imiquimod augmented antitumor immunity by inducing proinflammatory cytokines such as type I IFNs, TNF-α, and IL-12 (9–12). However, the mechanism by which these proinflammatory cytokines induced superior priming of tumor-specific CD8+ T cells remained unclear.
Cytokines released by appropriately activated dendritic cells serve as the final requirement for acquisition of CD8+ T cell functionality. In particular, the cytokine IL-12 has been shown to be a potent third signal (13–16). CD8+ T cells lacking this third signal become tolerant and fail to attain cytolytic effector function (17). This becomes an important consideration in designing tumor immunotherapy protocols, if one accounts for a tumor environment that fosters a lack of proinflammatory signals, poor Ag presentation, and an overwhelmingly anti-inflammatory milieu (18–21). IL-12 has been shown to be a potent mediator of antitumor immunity by influencing the function of innate and adaptive immune responses and demonstrating anti-angiogenic properties (22–24). However, high systemic doses of IL-12 with the ability to reach therapeutic levels in the tumor site are not very well tolerated (25, 26). To circumvent this obstacle, we directly primed tumor Ag-specific CD8+ T cells with IL-12 in vitro. This allowed us to dissect the phenotypic changes by which IL-12 could augment antitumor immunity induced by CD8+ T cells.
Use of the Pmel-1 TCR transgenic mouse has previously allowed us to model adoptive transfer immunotherapy in an established s.c. and intracranial tumor model (27, 28). Pmel-1 T cells express a single transgenic TCR that recognizes the gp10025–33 H-2Db– restricted epitope of gp100 (29). This endogenous tumor-associated Ag is expressed by murine B16 melanomas, making this a clinically relevant model to study novel adoptive transfer therapies (30). Malignant melanoma is one of the most common solid tumor types to metastasize to the brain and results in significantly decreased survival and exclusion from most clinical trials (31). Clinically, little advancement has been made in effective treatment courses for tumors developing within the confines of the immune-privileged CNS. Although the standard of care consists of surgical resection, chemotherapy, and radiation, these options often do very little to prolong survival. However, recent work has demonstrated the efficacy of immune-based therapies as a new option for clinicians (32–35). The specificity and durability of tumor immunotherapy can theoretically provide a new avenue to target tumor cells while sparing normal brain tissue. Even more promising, many preclinical models and successful clinical trials have demonstrated meaningful antitumor immune responses (36–39).
Given that immune-based therapies are beginning to grow into a feasible treatment modality for metastatic melanoma, combined with the importance of refining therapeutic strategies in humans, we have addressed the functional importance of priming CD8+ T cells with IL-12 prior to adoptive cell transfer. We used the Pmel-1 TCR transgenic model to establish the in vitro and in vivo effects of priming tumor-specific CD8+ T cells with IL-12. However, we expanded our findings to show similar phenotypic and functional changes in human PBMCs transduced with a tumor-specific TCR and primed with IL-12. Our studies reveal what we believe are the critical determinants of therapeutic efficacy for enhanced antitumor T cell responses and directly apply to the design of future clinical immunotherapy strategies.
Materials and Methods
Animals and cell lines
All mice were bred and kept under defined-flora pathogen-free conditions at the Association for Assessment and Accreditation of Laboratory Animal Care-approved Animal Facility of the Division of Experimental Radiation Oncology at the University of California Los Angeles. Mice were handled in accordance with the University of California Los Angeles animal care policy and approved animal protocols. The B16-F10 murine melanoma cell line was obtained from American Type Culture Collection (Rockville, MD) and the Phoenix-ampho cell line was kindly provided by Caius Radu (Department of Molecular and Medical Pharmacology, University of California Los Angeles).
Tumor implantation and irradiation
C57BL/6 mice (6–12 wk of age) were implanted s.c. in the lower left flank with 1 × 105 to 2.5 × 105 B16-F10 melanoma cells per mouse. Intracranial implantations were done as previously described (30). Mice received 1 × 103 B16-F10 melanoma cells injected in a total volume of 2 µl PBS.
Prior to adoptive transfer, lymphopenia was induced by total body irradiation given at a nonmyeloablative dose (500 cGy).
In vitro activation of Pmel-1 T cells
Lymph nodes and/or spleens were harvested from Pmel-1 mice and cultured with human IL-2 (100 U/ml; National Cancer Institute Preclinical Repository, Developmental Therapeutics Program) or IL-12 (10 ng/ml; Peprotech, Rocky Hill, NJ) and with human gp10025–33 peptide (NH2-KVPRNQDWL-OH, 1µg/ml; Biosynthesis, Lewisville, TX) in X-VIVO 15 (Lonza, Walkersville, MD) supplemented with 2% FBS. After 72 h, cells were washed twice with PBS and recultured in IL-2 (100 U/ml) or IL-12 (10 ng/ml) at a concentration of 1 × 105 cells/ml for an additional 48 h. Cells were washed twice with PBS and resuspended at 10 × 106 or 50 × 106 cells/ml and immediately injected i.v. via tail vein in 0.1 ml PBS per mouse. All mice, with the exception of studies using 5 × 106 CFSE-labeled T cells per mouse, were adoptively transferred with 1 × 106 T cells each. T cells were labeled with 5 µM CFSE according to the manufacturer’s specifications (Invitrogen). Prior to adoptive transfer, cells were analyzed by FACS to ensure that the median fluorescence intensity (MFI) of each group was approximately equal and around 6000–8000 MFI.
Microarray analysis
Pmel-1 T cells were stimulated as described above. Naive Pmel-1 T cells were obtained from CD8+ Thy1.1+ sorted Pmel-1 splenocytes. Shortly after the final 48-h cytokine stimulation for IL-2– and IL-12–primed Pmel-1 CD8+ T cells, RNA was obtained using the RNeasy Mini kit (Qiagen). RNA from two replicate experiments from each group were labeled and hybridized to Affymetrix Mouse Gene 1.0 ST arrays (University of California Los Angeles DNA Microarray Core). Analysis was completed by calculating average intensities for each cytokine condition and determining differential gene expression by the Bio conductor R package, One Channel GUI (40). Genes represented as differentially expressed show a fold change greater than 2 and a p value < 0.05. All array data has been deposited in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE22443.
Retroviral transduction of Pmel-1 T cells and human PBMCs
The Phoenix-ampho cell line was co-transfected with the retroviral packaging vector pCL-Eco and a retroviral vector containing firefly luciferase as previously published (27, 41, 42). Retroviral supernatants were obtained over a 12-h period. After an initial 24 h of activation, Pmel-1 T cells were transduced by spin-fection with 1 ml retroviral supernatant, 1.6 µg/ml polybrene (Sigma-Aldrich), and 2 µg/ml Lipofectamine (Invitrogen) at 850 × g for 2 h at 32°C. Spin-fection was repeated 24 h later under the same conditions. Normal priming conditions were followed after transduction of Pmel-1 T cells.
Human PBMCs were stimulated with anti-CD3 Ab (clone OKT-3) and either IL-2 or IL-12 for 2 d. Activated PBMCs were then transferred to plates containing supernatant from a PG13-based gp100 TCR stable retroviral packaging cell line obtained from Dr. Paul Robbins (Surgery Branch, National Cancer Institute/National Institutes of Health) (43). On the following day, cells were transferred to a fresh plate containing viral supernatant. Cells were then removed from viral supernatant on day 5 and then allowed to rest until they were analyzed on day 8.
Bone marrow-derived dendritic cells and vaccination
The generation of dendritic cells (DCs) from murine bone marrow progenitor cells was performed as previously published (30, 44). Briefly, bone marrow cells were initially cultured overnight in a Petri dish. On day 1, nonadherent cells were collected and plated in 24-well plates with murine IL-4 (500 U/ml; R&D Systems, Minneapolis, MN) and murine GM-CSF (100 ng/ml; R&D Systems). On day 4, 50% of the media was removed, and adherent cells were re-fed with an addition of 1 ml per well of RPMI 1640 plus 10% FBS plus the same concentration of cytokines. DCs were harvested as the loosely adherent cells from the day-8 cultures. DCs were resuspended at 2 × 106 cells/ml in PBS and pulsed with human gp10025–33 (hgp10025–33) peptide at a concentration of 10 µM for 90 min at room temperature. After two washes in PBS, hgp10025–33 peptide-pulsed DCs were immediately prepared for injection in 0.2 ml PBS per mouse. Injections were given s.c. at four sites on the back.
Bioluminescent imaging
Bioluminescent imaging was performed as previously published (27, 45). Mice were initially anesthetized with isoflurane or ketamine/xylazine. To minimize light absorption by black fur, mice were shaved prior to imaging. Seven minutes after i.p. injection of d-luciferin (30 mg/ml), mice were imaged in a Xenogen IVIS imaging system coupled to a CCD camera. Mice were imaged for 7–20 min with an acquisition time of 8–10 s. Analysis of bioluminescent image data was completed with Living Image software. Regions of interest were drawn around discrete anatomical areas and used to calculate bioluminescent signal. This signal was expressed and graphed as total flux (photos/second).
Pmel-1 T cell stimulation and intracellular FACS staining
Approximately 1 × 106 Pmel-1 T cells were stimulated at a 1:10 ratio with naive C57BL/6 splenocytes pulsed with hgp10025–33 peptide. Golgi Plug protein transport inhibitor (BD Biosciences) and allophycocyanin-conjugated anti-CD107a Ab (2 µg, clone 1D4B; BD Biosciences) were added to each well containing T cells and/or pulsed splenocytes. Cells were stimulated at 37°C for 0–300 min. After the appropriate incubation time, cells were placed on ice in the dark until all cells could be stained at the same time. Cells were washed with PBS containing 2% FBS and subsequently stained with surface markers. After extracellular staining, cells were fixed with Fixation Buffer (eBioscience) and permeabilized with 1×Permeabilization Buffer (eBioscience). Intracellular staining was completed in 1×Permeabilization Buffer on ice in the dark. Cells were stored at 4°C until analysis.
Flow cytometry and Abs
Spleens, lymph nodes, and tumors were harvested from mice after adoptive transfer. Spleens and lymph nodes were passed through 70-µm cell strainers to generate single-cell suspensions. Lymphocytes were obtained after hypotonic lysis and enumerated using trypan blue exclusion. A total of 1 × 106 to 2 × 106 cells in PBS with 2% FBS were used for the staining procedure. To determine the number of tumor-infiltrating lymphocytes (TILs), tumors were carefully weighed and subsequently minced with a scalpel. The tumor was then placed on a rotator in collagenase with DNase for 2–3 h. Small mononuclear cells within the tumor were enumerated by trypan blue exclusion. Approximately 1 × 106 lymphocytes were used for staining. TILs were calculated by determining the total number of CD8+ Thy1.1+ cells per milligram of tumor.
Fluorochrome-conjugated Abs to CD4 (clone RM4-5), CD8 (clones 5H10 and 53-6.7), CD25 (clone PC61), CD44 (clone IM7), CD62L, CD107a, IFN-γ (clone XMG1.2), and TNF- α (clone MP6-XT22) were obtained from BD Biosciences. PE-Cy7–conjugated Thy1.1 (clone HIS51) was obtained from eBioscience. The purified blocking anti-CD25 Ab (clone PC61.5.3) was obtained from Bio X Cell. All FACS analysis was performed with the use of an LSRII (BD Biosciences). Gates were set based on isotype-specific control Abs (data not shown). Data were analyzed using FlowJo software.
Western blot
Total cell lysates from IL-2– and IL-12–primed Pmel-1 T cells were obtained by using cell extraction buffer (Invitrogen) supplemented with 1 mM PMSF (Sigma-Aldrich) and protease inhibitor mixture (Sigma-Aldrich) in accordance with the manufacturer’s instructions. SDS gel and immunoblot analyses were performed according to standard protocols. Abs used were STAT5 (9363; Cell Signaling Technology) and β-tubulin (2146; Cell Signaling Technology).
Statistics
Data are represented as the mean ± SE. Significance was determined using a paired Student t test. Generated p values are two-tailed, and p < 0.05 was considered statistically significant. Survival curves were plotted using the product limit estimation test of Kaplan–Meier. Statistical differences in survival were calculated using the Wilcoxon log-rank test. All statistical analysis and graphs were constructed using Graph Pad software.
Results
Priming with Ag and IL-12 imparts a distinct lymph node homing phenotype and functionally enhances CD8+ T cells
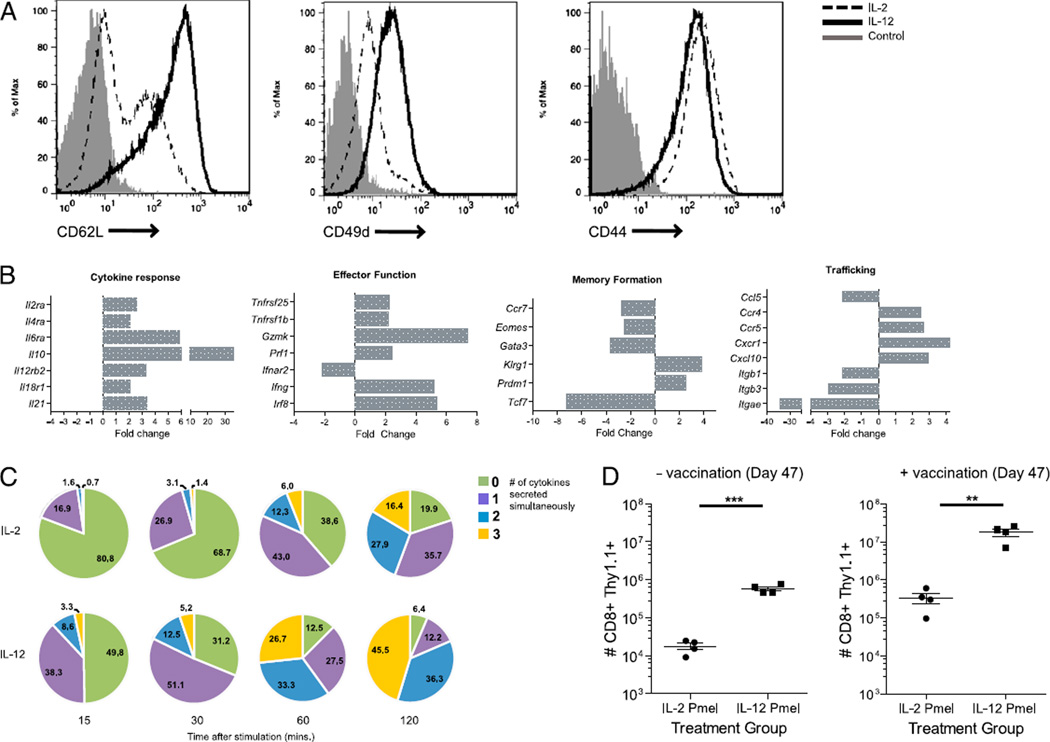
Administration of the TLR7 agonist imiquimod as an adjuvant for immunotherapy in the CNS tumor setting previously demonstrated enhanced DC trafficking and priming of adoptively transferred CD8+ T cells (8).We hypothesized that cytokine secretion induced by TLR activation played a significant role in functionally enhancing tumor-specific CD8+ T cell priming. To investigate these issues in greater depth, we primed naive, gp100-specific splenocytes in vitro with hgp100 peptide in the presence of either IL-2 or IL-12. In comparison with priming CD8+ T cells in the presence of IL-2, priming with IL-12 significantly increased expression of L-selectin (CD62L) and the integrin α4 chain (Fig. 1A). No change was observed in the expression levels of the memory marker CD44.
FIGURE 1.
Phenotypic and functional changes conferred by priming in the presence of IL-12. A, Pmel-1 CD8+ T cells were cultured in the presence of IL-2 or IL-12 and stained after 6 d of in vitro restimulation with the indicated Ab or an isotype control. Cells were gated on the CD8+ Thy1.1+ population and analyzed by FACS. B, Microarray gene analysis was performed on Pmel-1 CD8+ T cells primed in the presence of IL-2 and IL-12. The plots indicated show expression of selected genes associated with cytokine response, effector and memory function of CD8+ T cells, as well as lymphocyte trafficking. Expression levels of IL-12–primed T cells relative to IL-2–primed T cells denote fold change. All genes shown have at least a 2-fold change in expression with a p value < 0.05. C, IL-2– or IL-12–primed Pmel-1 CD8+ T cells were stimulated with hgp100 peptide-pulsed Bl/6 splenocytes for the indicated times. Percentages indicate the number of cytokines (IFN-γ, TNF-α, and CD107a) Pmel-1 T cells were secreting simultaneously. D, Lymphopenic mice were adoptively transferred with 1 × 106 IL-2– or IL-12–primed Pmel-1 T cells. All groups were revaccinated on day 7 with DCs pulsed with gp100. The label “+ vaccination” indicates groups that were revaccinated on day 47. These findings are representative of one experiment that has been conducted at least three times with similar findings. **p < 0.01, ***p < 0.001.
To characterize more fully the gene expression patterns of IL-12– and IL-2–primed T cells, we obtained RNA from IL-2 and IL-12 Ag-primed Pmel-1 T cells rested in their respective cytokine and examined global gene expression levels by cDNA microarray analysis (Fig. 1B). This analysis revealed a striking difference between cells primed in the presence of IL-2 or IL-12. Primarily, IL-12 imparts a strong effector program by upregulating the high-affinity IL-2R α-chain (Il2ra). Furthermore, genes associated with cytotoxic effector function such as IFN-γ (Ifng), Perforin (Prf1), and Granzyme K (Gzmk) were upregulated at least 2-fold (p < 0.05) in relation to IL-2–primed T cells. IL-12 priming also increased the expression of Killer cell lectin-like receptor G1 (Klrg1) and the transcriptional repressor B-lymphocyte induced maturation protein-1 (Blimp-1, Prdm1) while downregulating the expression of the chemokine CCR7 (Ccr7) and the transcription factor Eomesodermin (Eomes) (p < 0.05). This differential regulation of KLRG1, Blimp-1, CCR7, and Eomes suggests that IL-12 priming induces a distinct effector program that has been shown to be characteristic of short-lived effector T cells (46). Furthermore, loss of the Wnt pathway transcription factor T cell factor 1 (Tcf7) is highly indicative of Ag-experienced mature CD8+ T cells (47). This gene expression pattern would classically align IL-12–primed T cells to a more highly differentiated effector phenotype (effector memory T cells) than those primed with IL-2. However, this differentiation program is surprisingly in disagreement with the high expression of L-selectin and CD49d (Fig. 1A), markers commonly reserved for naive or quiescent central memory T cells.
In addition to phenotypic changes conferred by priming in the presence of IL-12, we also examined functional characteristics in IL-12–primed CD8+ T cells. We assessed in vitro effector function by characterizing the ability of IL-2– and IL-12–primed T cells to retain a “polyfunctional” phenotype. Recent publications have demonstrated a positive correlation between clinical outcome and the presence of polyfunctional T cells (48, 49). These cells retain the ability to simultaneously secrete a diverse number of cytokines and retain cytolytic activities in response to Ag encounter. To test this, we restimulated T cells for varying lengths of time in the presence of hgp100 peptide-pulsed APCs. CD8+ T cells primed with IL-12 mobilized a greater number of cells to simultaneously express the cytokines IFN-γ, TNF-α, and the degranulation marker LAMP-1 (CD107a) (Fig. 1C, Supplemental Fig. 1). Furthermore, IL-12–primed cells also mobilized this polyfunctional trait sooner than their IL-2–primed counterparts.
The ability to mediate potent effector responses is one hallmark of CD8+ T cells. However, the ability to mediate long-term protective immunity also governs the efficacy of a T cell response. Previous studies have shown the role of IL-12 responsiveness to be critical in the maintenance of Ag-specific T cells (50). In this study, we investigated the role of IL-12 priming in the formation of memory self-Ag–specific CD8+ T cells. Equal numbers of IL- 2– or IL-12–primed CD8+ T cells were adoptively transferred into mice that were irradiated the day before. One week later, groups were revaccinated with DCs pulsed with gp100 peptide. Recall responses were measured in mice receiving a DC vaccination 40 d later and compared with those of mice not receiving a revaccination. Groups that received IL-12–primed T cells showed a significantly larger number of T cells remaining (Fig. 1D). Groups that received IL-12–primed T cells in addition to a DC vaccination on day 47 also showed significantly higher numbers of Ag-specific memory T cells remaining. This clearly demonstrates the ability of IL-12 priming to confer a significant survival advantage in vivo by maintaining a large population of Ag-specific cells long after initial expansion. More importantly, IL-12 priming plays a dual role in the differentiation of CD8+ T cells. In comparison with IL-2, IL-12 signaling not only induces a strong recall to Ag but also mediates the ability to acquire a protective and diverse effector response.
IL-12 potentiates the IL-2 signaling pathway in CD8+ T cells
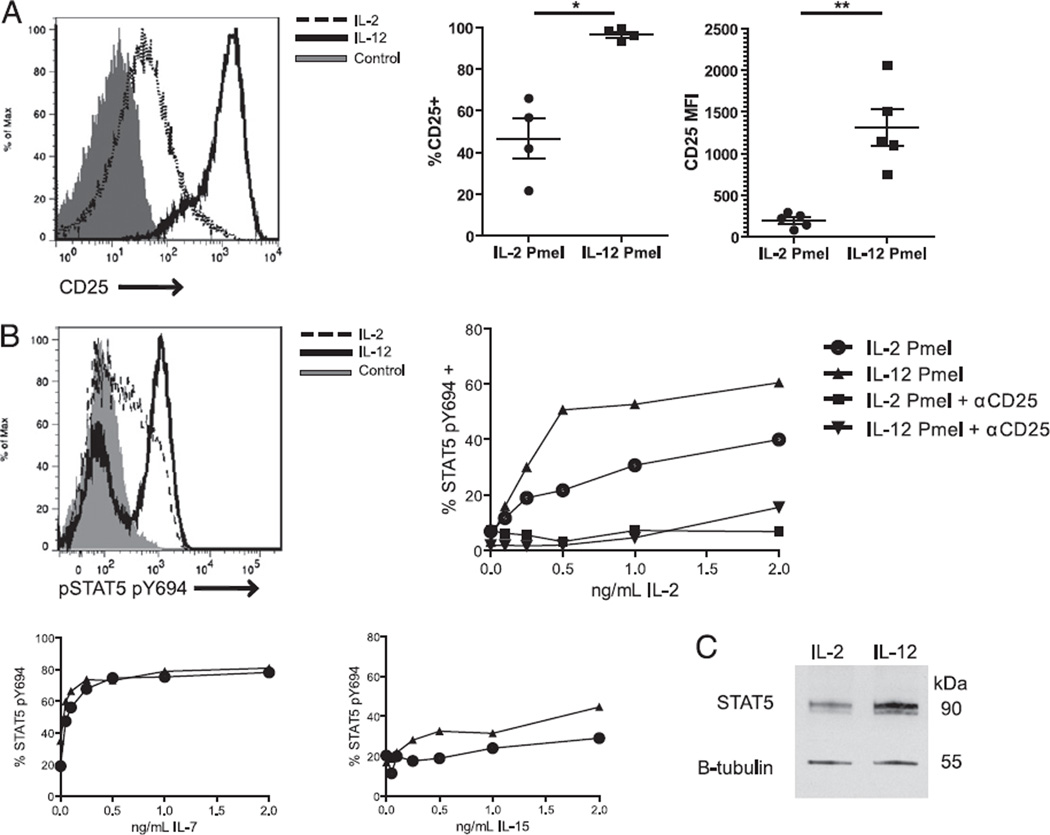
In addition to having a distinct memory phenotype, it is known that IL-12–primed OVA-specific CD8+ T cells demonstrate an upregulation of CD25 during homeostatic proliferation (51). Similarly, in the case of activated effector cells, we have found that IL-12 priming of gp100 tumor-specific Pmel-1 T cells results in an immediate upregulation of the high-affinity IL-2R (CD25) (mean MFI = 1309, SEM 221.2) in comparison with IL-2–primed cells (mean MFI = 195, SEM 38.4) (Fig. 2A). The presence of CD25 within the IL-2R complex drastically lowers the concentration of IL-2 required to illicit downstream signaling events, which can be monitored by measuring the levels of p-STAT5 in response to stimulation with exogenous IL-2. IL-12–primed T cells stimulated with IL-2 show high levels of p-STAT5 when stimulated with as little as 500 pg/ml IL-2 (Fig. 2B). These high levels of p-STAT5 were not observed in IL-2–primed T cells at any concentration level of IL-2. As such, IL-12–primed Pmel-1 T cells show a higher level of STAT5 phosphorylation events in response to IL-2 stimulation across all concentration levels tested. To assess concretely whether the increased expression of CD25 confers enhanced responsiveness to exogenous IL-2, cells were treated with anti- CD25 blocking Ab (clone PC61.6.3) prior to IL-2 stimulation. CD25 Ab blockade of IL-12–primed Pmel-1 T cells significantly dampened IL-2–dependent STAT5 phosphorylation events at all concentration levels (Fig. 2B). In addition to testing responsiveness to IL-2, we also tested the ability of IL-12–primed CD8+ T cells to respond to other common γ-chain cytokines using STAT5 as a downstream transducer. Both IL-2– and IL-12–primed T cells showed similar levels of p-STAT5 in response to IL-7 and IL-15 (Fig. 2B). Thus, IL-12 priming specifically enhances perception of the IL-2 signal and not that of other closely related cytokines. However, the expression of CD25 may not be the only important factor in determining enhanced responsiveness to IL-2. We also checked for total protein levels of STAT5 present in IL- 2– and IL-12–primed T cells by Western blotting. IL-12–primed T cells show a 1.6-fold change in the overall expression of unphosphorylated STAT5 (Fig. 2C). This indicates an overwhelming enhancement in both the perception and transduction of IL-2 by IL-12–primed CD8+ T cells.
FIGURE 2.
IL-12 priming enhances the IL-2 signaling pathway. A, Pmel-1 CD8+ T cells primed in IL-2 or IL-12 were stained for CD25 6 d after in vitro restimulation. B, IL-2– and IL-12–primed CD8+ T cells were stimulated with varying concentrations of IL-2, IL-7, or IL-15 at 37°C and immediately fixed with paraformaldehyde and then stained intracellularly for the expression of activated STAT5 (pSTAT5) by FACS. Blockade of CD25 was performed by incubating cells with anti-CD25 Ab for 30 min on ice, stimulating with IL-2, and then immediately fixing prior to pSTAT5 mAb staining. A representative flow plot shows pSTAT5 expression when cells were unstimulated or stimulated with 2 ng/ml IL-2. C, STAT5a/b and β-tubulin expression were detected by Western blotting using whole-cell lysates from IL-2– and IL-12–primed CD8+ T cells. Results are shown from one of three identical experiments. *p < 0.05, **p < 0.01.
IL-12 priming increases in vivo proliferation at low systemic levels of IL-2
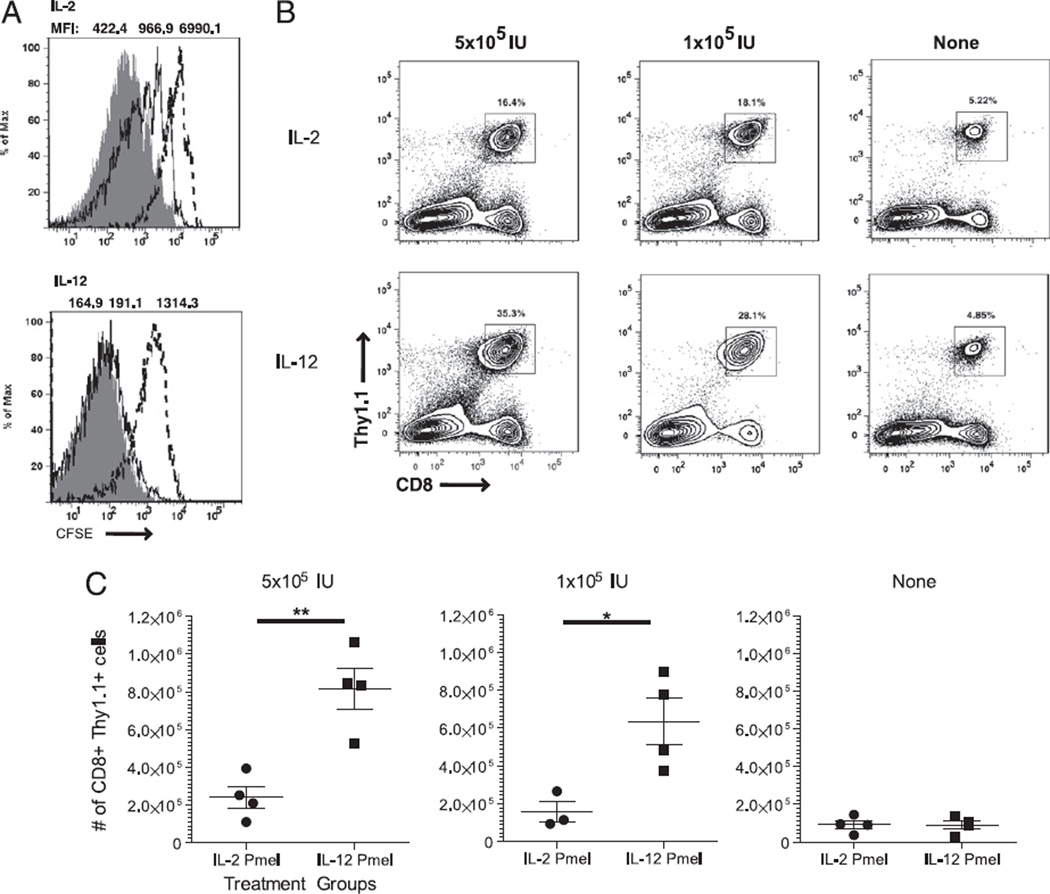
IL-2 is a potent stimulator of T cell clonal expansion in vivo. Because in vitro results demonstrated enhanced IL-2 signaling, we asked whether IL-12–primed CD8+ T cells showed greater proliferation at lower concentrations of IL-2 after adoptive transfer in vivo. To test this question, we labeled IL-2– or IL-12–primed T cells with CFSE and adoptively transferred an equal number of these cells into lymphodepleted mice. Lymphopenia was induced by 500 cGy whole-body irradiation. The recipient mice were then vaccinated with hgp100 peptide-pulsed DCs and either supported with i.p. injections of IL-2 at a high dose (5 × 105 IU), low dose (1 × 105 IU), or without IL-2. Three days after adoptive transfer, spleens were analyzed ex vivo for CFSE dilution. At the high dose, low dose, and absence of IL-2, IL-2–primed T cells exhibited lower CFSE dilution (MFI = 422.4 ± 20.9, 966.9 ± 52.7, 6991 ± 278.1, respectively) compared with that of IL-12–primed Pmel-1 T cells (MFI = 164.9 ± 3.3, 191.4 ± 5.2, 1314 ± 52.3, respectively). IL-12–primed cells displayed complete dilution of CFSE at high and low doses of systemic IL-2 and a moderate level of CFSE dilution without IL-2 support (Fig. 3A). When we evaluated the absolute number of T cells that could be obtained 10 d after adoptive transfer, the number of CD8+ T cells was significantly increased in treatment groups receiving IL-12– primed cells compared with that in groups receiving IL-2–primed cells. Mice that received IL-12–primed T cells supported with high-dose IL-2 possessed, on average, a 4-fold higher number of adoptively transferred cells compared with that of groups receiving IL-2–primed T cells (p < 0.01) (Fig. 3B). Groups of mice that received IL-12–primed T cells, supported with a low dose of systemic IL-2, had a 5-fold higher number of cells than that of groups receiving IL-2–primed T cells (p < 0.05) (Fig. 3C). These results demonstrate the stark differences underlying use of exogenous IL-2 in the two priming conditions. Clearly, IL-12–primed T cells have an enhanced ability to persist and respond to IL-2 in a lymphopenic environment.
FIGURE 3.
IL-12 priming enhances the proliferation and sensitivity to low levels of IL-2. A, Nonlethally irradiated Bl/6 mice were adoptively transferred with 5 × 106 CFSE-labeled IL-2– or IL-12–primed CD8+ T cells. Recipient mice were then vaccinated with hgp10025–33 peptide-pulsed DCs and supported with 5 × 105 IU (gray), 1 × 105 IU (solid), or no (dashed) IL-2. Three days after adoptive transfer, spleens were harvested and stained. MFIs are averages of four mice in each group (p < 0.0001). B and C, Adoptively transferred cells were recovered from spleens and stained for CD8 and Thy1.1. Plots show representative samples from each group of at least four mice. The scatter plot indicates mean ± SEM. This experiment was conducted three times with similar results. *p < 0.05, **p < 0.01.
IL-12 cytokine priming stimulates proliferation and trafficking into diverse anatomical regions
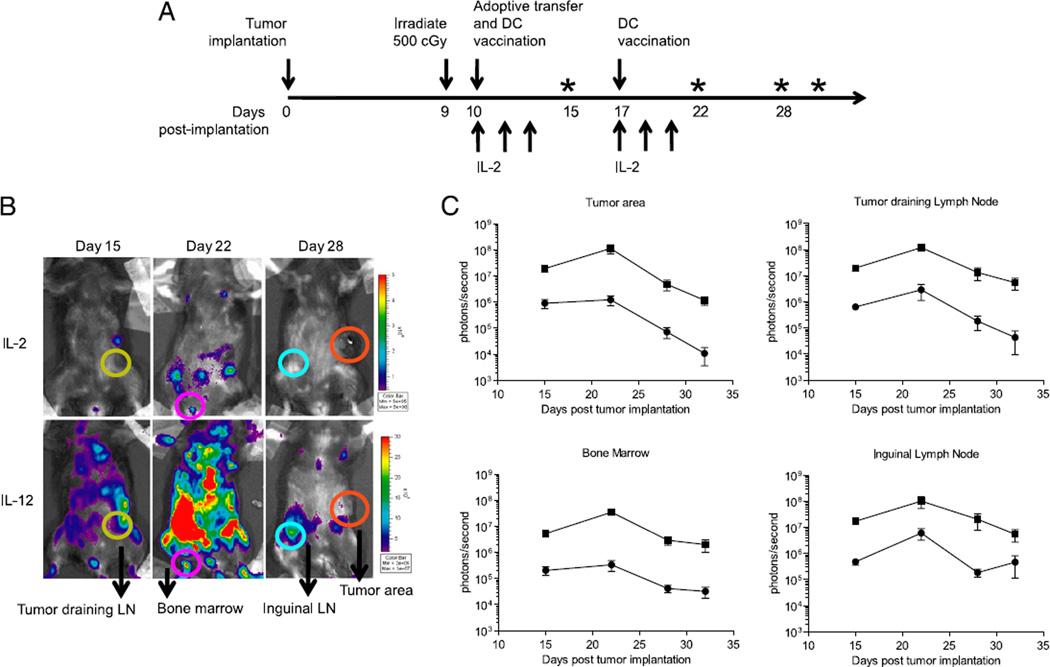
Our data suggested that treatment with IL-12–primed T cells showed increased T cell proliferation in vivo. However, we also wanted to explore the possibility of T cell trafficking through other relevant anatomical locations at different times. To accomplish this, we engineered T cells, primed in the presence of either IL-2 or IL-12, to express firefly luciferase (Supplemental Fig. 2). This gave us the ability to noninvasively monitor trafficking of adoptively transferred T cells in vivo in real time. Furthermore, in vivo bioluminescent imaging allowed us to quantify the relative number of adoptively transferred T cells at discrete anatomical locations. Transduced T cells were adoptively transferred into mice with established s.c. B16-F10 tumors in the lower left flank (Fig. 4A). The results became striking when comparing groups receiving IL-2– and IL-12–primed T cells. When IL-12–primed T cells were transferred, total flux (photons/second) was nearly 1 log-fold higher in the inguinal lymph nodes and at the tumor site when compared with groups receiving IL-2–primed T cells (Fig. 4B). Furthermore, we observed the characteristic proliferative burst of T cells shortly after a DC vaccination 1 wk following adoptive transfer (27, 52). This secondary expansion of T cells after vaccination was significantly more pronounced in groups receiving IL-12–primed T cells compared with that in groups receiving IL-2–primed T cells. Shortly thereafter, imaging showed the eventual contraction of these cells to the tumor-draining lymph node, the neighboring inguinal lymph node, and the bone marrow. Ultimately, real-time imaging demonstrated that although IL- 2– and IL-12–primed T cells displayed nearly the same kinetics of expansion and contraction, IL-12 significantly increased T cell proliferation and trafficking.
FIGURE 4.
Bioluminescent imaging reveals extensive trafficking patterns of IL-12–primed T cells. A, Pmel-1 T cells were transduced with a retroviral vector encoding firefly luciferase, and 1 × 106 cells were adoptively transferred into mice harboring s.c. B16-F10 tumors. For in vivo bioluminescent imaging, anesthetized mice were injected i.p. with 30 mg/ml d-luciferin, and 10-s acquisitions were taken with Living Image software. Asterisks on the timeline indicate days in which images were acquired. B, Regions of interest were drawn around the tumor area (orange), tumor-draining lymph node (yellow), the bone marrow (pink), the inguinal lymph node (blue), and the surrounding tumor area. C, Total flux was analyzed, and average ± SEM was plotted. Results shown are representative of one of two identical experiments including at least four mice per group.
IL-12–primed CD8+ T cells show superior antitumor activity in intracranial and s.c. tumor models
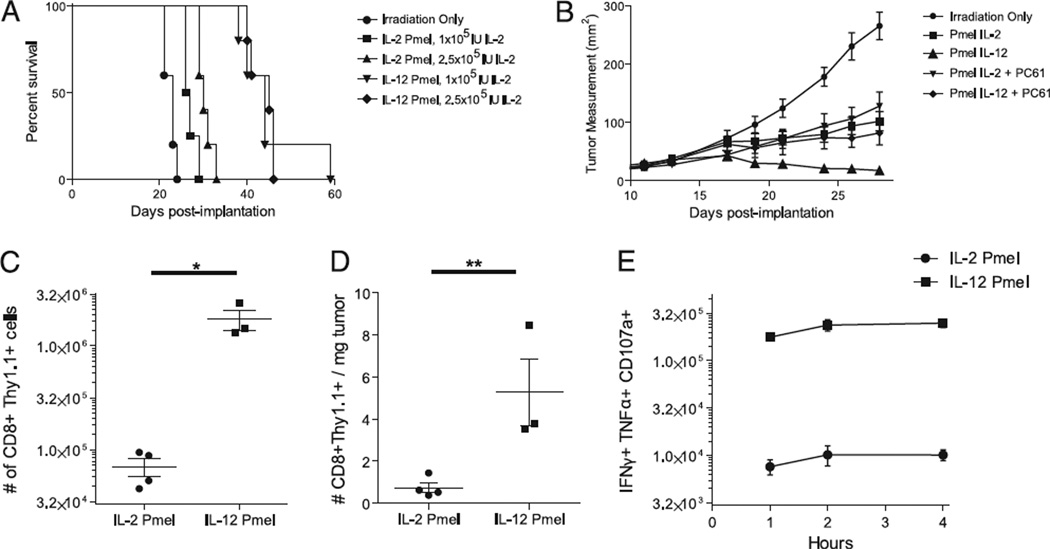
The ability of tumor-associated Ag-specific CD8+ T cells to proliferate in vivo and retain effector function after adoptive transfer still remains a challenge in immunotherapy. Previously, we demonstrated that a tripartite treatment regimen, consisting of IL-2– primed T cells, gp100 peptide-pulsed DC vaccination, and a high dose of systemic IL-2, significantly prolonged survival in an intracranial tumor model (27). To evaluate whether IL-12–primed gp100-specific CD8+ T cells were capable of exhibiting antitumor activity, we adoptively transferred IL-2– or IL-12–primed CD8+ T cells into nonlethally irradiated mice bearing 7-d established intracranial B16-F10 melanoma tumors. When 2.5 × 105 IU of systemic IL-2 was used with adoptive transfer and DC vaccination, the median survival was 30 and 45 d with IL-2– and IL-12– primed cells, respectively (p = 0.0017, Mantel–Cox). With a systemic dose of 1.0 × 105 IU IL-2, the median survival of groups was 26.5 and 44 d with IL-2– and IL-12–primed T cells, respectively (p = 0.0025, Mantel–Cox) (Fig. 5A). In addition, we also used this tripartite treatment with 10-d established s.c. B16-F10 melanoma tumors. Groups that received IL-12–primed T cells showed a significantly decreased tumor size in comparison with that of groups receiving IL-2–primed cells or radiation alone (Fig. 5B). Administration of an anti-CD25 blocking Ab after adoptive transfer did not significantly alter the growth of tumors treated with IL-2–primed T cells. However, the same anti-CD25 blocking Ab significantly reduced the antitumor activity of IL-12–primed T cells (Fig. 5B).
FIGURE 5.
Treatment with IL-12–primed T cells increases intratumor trafficking and decreases tumor burden. A, B16-F10 melanoma cells were allowed to establish intracranially for 7 d. Mice were irradiated 1 d prior to adoptive transfer and treated with 1 × 106 T cells, DC vaccine, and the indicated amount of systemic IL-2. Mice were monitored for survival. B, B16-F10 melanoma cells were allowed to establish s.c. for 10 d. Mice were irradiated 1 d prior to adoptive transfer. Groups received 1 × 106 IL-2– or IL-12–primed T cells. Groups receiving anti-CD25 Ab (clone PC61) received i.p. injections of PC61 in addition to the indicated amount of systemically administered IL-2. C, Spleens were harvested 19 d post-adoptive transfer and analyzed for CD8+ Thy1.1+ cells. D, Tumors were also recovered 19 d post-adoptive transfer. Tumors were weighed and digested with collagenase. Small lymphocytes within the tumor were enumerated and used to calculate the total number of CD8+ Thy1.1+ cells within the tumor. E, Splenocytes were stimulated with hgp100 for the indicated number of hours, and IFN-γ, TNF-α, and CD107a expression is depicted in the CD8+ Thy1.1+ gated population. Each experiment described has been conducted at least three times with similar results and included at least four mice per group. *p < 0.05, **p < 0.01.
As demonstrated previously in non-tumor-bearing mice, groups receiving IL-12–primed T cells possessed significantly increased absolute numbers of T cells (1.8 × 106 cells) 2 wk post-adoptive transfer in comparison with those of groups receiving IL-2– primed T cells (6.8 × 104 cells, p < 0.01) (Fig. 5C). Thus, IL-12 priming of CD8+ T cells appears to impart an instructional program that allows for efficient use of IL-2. However, expansion of adoptively transferred cells does not necessarily correlate with the ability of these cells to display antitumor activity. To ascertain that these cells retained the ability to respond functionally to their cognate Ag, splenocytes from adoptively transferred mice were restimulated ex vivo with gp100 peptide and stained for the polyfunctional markers IFN-γ, TNF-α, and CD107a. Groups treated with IL-12–primed T cells possessed a greater number of polyfunctional T cells (1.8 × 106) compared with that of groups treated with IL-2–primed T cells (6.8 × 104, p < 0.01) (Fig. 5E). Thus, this significant expansion of tumor-specific T cells expands cells with diverse function. More importantly, we were interested to test whether an increase in functional IL-12–primed cells could show enhanced accumulation within tumors. Thus, TILs were stained and analyzed. The density of adoptively transferred TILs (Thy1.1+CD8+ TIL/milligram of tumor) was significantly greater in groups that received IL-12–primed T cells (5.3 cells/mg of tumor) in comparison with that in groups that received IL-2–primed T cells (0.7 cells/mg of tumor, p < 0.05) (Fig. 5D). This lends further support to the idea that the expansion and trafficking of highly functional T cells is critical for producing effective antitumor immunity.
Priming of tumor-specific human PBMCs with IL-12 produces potent effector cells
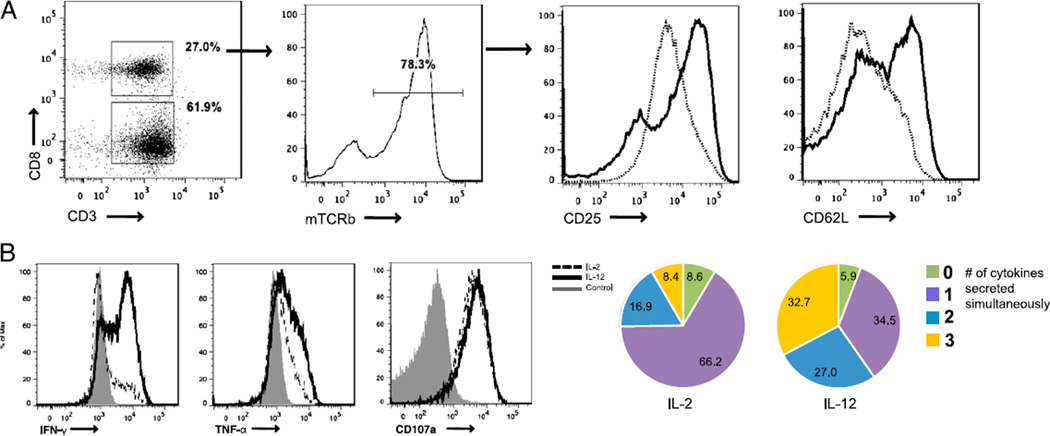
The efficacy of adoptive cell transfer relies upon the maintenance of T cells with the ability to both rapidly proliferate in vivo and retain effector function. Although we have demonstrated these enhanced effector functions in a murine model, we wished to demonstrate the ability of IL-12 to mediate its protective effects in human tumor-specific T cells. Using a clinically relevant retroviral transduction system, we expressed a gp100-specific TCR in normal human PBMCs (43, 53). Although this chimeric TCR contains constant regions of murine origin, the variable regions are of human origin and specifically recognize human gp100154–162 in the context of HLA.A*0201 (54). PBMCs were initially activated using a stimulatory anti-CD3 Ab (clone OKT3) in the presence of either IL-2 or IL-12. We then examined the resulting IL-2– and IL-12–primed TCR-transduced PBMCs for differences in their phenotype and their similarity to IL-12–primed Ag-specific murine T cells. Within the CD8+ T cell population, ~80% of cells expressed the chimeric gp100 TCR as indicated by positive staining for mouse TCR β-chain (Fig. 6A). Similar to results seen in the priming of mouse CD8+ T cells, transduced human CD8+ T cells primed in the presence of IL-12 showed an upregulation of both CD62L and CD25 (Fig. 6A). Furthermore, upon restimulation with Ag, cells primed in the presence of IL-12 showed a more diverse effector phenotype in comparison with that of cells primed in the presence of IL-2. This effector phenotype was derived from a significant increase in the simultaneous expression of IFN-γ and TNF-α in response to Ag (Fig. 6B). These results demonstrate the successful production of human tumor-specific T cells with an enhanced ability to mobilize effector cytokines when primed in the presence of IL-12. In addition, these results highlight the ability of a prototypic inflammatory cytokine as an important component in mediating T cell-based antitumor immunity.
FIGURE 6.
Priming of human PBMCs with IL-12 generates tumor-specific cells phenotypically similar to their murine counterparts. A, Human PBMCs transduced with the chimeric gp100 TCR were analyzed on day 8. The phenotype of CD3+ CD8+ T cells containing the mouse TCR β-chain (mTCRb) was analyzed for their expression of CD25 and CD62L. B, Human PBMCs transduced with the gp100 chimeric TCR were pulsed with gp100 peptide-loaded T2 cells for 4 h. CD3+ CD8+ mTCRb+ cells were analyzed for IFN-γ, TNF-α, and CD107a. Pie charts for each priming condition indicate the number of cytokines being secreted simultaneously. These experiments were conducted three times with similar results.
Discussion
Our current studies have demonstrated the superior activity of tumor-specific CD8+ T cells primed in the presence of IL-12 for adoptive transfer immunotherapy. Use of IL-12 during in vitro priming significantly altered the phenotype, function, and memory formation of CD8+ T cells. Increased expression of both the high-affinity IL-2R and its downstream transducer, STAT5, clearly potentiated the IL-2 signal. This efficient use of IL-2 translated into enhanced in vivo T cell expansion and tumor infiltration. More importantly, administration of IL-12–primed T cells led to decreased tumor burden in an s.c. tumor model and increased survival in an intracranial tumor model compared with administration of CD8+ T cells primed in IL-2. From these studies, we conclude that IL-12 priming imparts an instructional program to CD8+ T cells that results in the development of potent effector cells with the ability to perceive proliferative signals in vivo, traffic, persist, and induce antitumor immunity.
Recent studies have demonstrated the importance of cytokines in programming the differentiation of naive T cells into functional effector cells (2, 3, 55, 56). Our use of IL-12 in these studies highlights the need for an instructive third signal in the expansion and use of tumor-specific effector T cells for adoptive immunotherapy (17, 20). Recent studies in the viral and tumor literature have also suggested an important link between “polyfunctional” T cell responses and control of disease progression (48, 49, 57, 58). These polyfunctional T cells demonstrate the distinct ability to mobilize a diverse array of cytokines simultaneously in response to cognate Ag restimulation. Our results indicate that the IL-12 signal may potently diversify the functional repertoire. However, even more importantly, IL-12 signaling also potentiates the ability of T cells to become more sensitive to cytokines such as IL-2, which are important for expansion and survival in vivo. This combined phenotype may potentially be of significant use to the field of adoptive immunotherapy. Currently, high systemic doses of IL-2 are needed to support and maintain the in vivo proliferation of appropriately primed T cells (59). Often, these maximally tolerated doses of IL-2 come at the risk of inducing vascular leak syndrome and other systemic side effects (60). As a result of IL-12 priming, adoptive cell transfer therapies could potentially avoid the side effects associated with high-dose IL-2 and reduce the absolute number of T cells required for potent antitumor activity. As others have shown, production of IL-12 by a very low number of tumor-specific T cells increases their expansion even in the absence of IL-2 and vaccination (61). These characteristics could potentially enable adoptive immunotherapy approaches to overcome the technical and monetary hurdles of producing large numbers of functional tumor-specific T cells for clinical use.
Although IL-2 remains a necessary component for adoptive immunotherapy protocols, the mechanisms by which STAT5 signaling promotes proliferation and possibly antitumor immunity have not been defined. Recent studies have shown enhanced memory formation in the presence of IL-12. Our own studies point to the rapid expansion of IL-12–primed Ag-specific cells immediately after revaccination. However, such work has not distinguished whether IL-2 signaling influences in vivo T cell persistence, memory formation, or effector function in IL-12–primed T cells. Recent studies have implicated the strength of IL-2 signaling and the expression of CD25 as key determinants in formation of short-lived effector cells (62, 63). Nevertheless, we must take into account that CD8+ T cell effector programming by an inflammatory cytokine, such as IL-12, may uncouple IL-2 signaling from directing formation of memory T cells. Instead, enhanced IL-2 signaling may program highly proliferative IL-12– primed T cells with the ability to traffic into the bone marrow and remain as long-lived memory cells.
Notably, our results have also indicated an increased expression of CD62L. Compounded with increased sensitivity to IL-2, CD62L expression has the capability to enhance lymphoid organ trafficking and subsequent accessibility to APCs. This marker is expressed immediately after IL-12 priming but is slowly down-regulated after adoptive transfer (data not shown) and in vivo expansion. This initial upregulation of CD62L confers a central memory-like phenotype to IL-12–primed CD8+ T cells. Characteristically, long-lived memory T cells express a low level of differentiation that can be monitored by high levels of CD62L, CD127, and the transcription factors Eomes and Tcf7. However, IL-12 priming imparts a more differentiated CD8+ T cell phenotype, with high levels of the transcription factor Blimp-1 and the marker KLRG1. Furthermore, these T cells express high levels of CD25 and quickly mobilize IFN-γ upon peptide restimulation. This phenotype is most commonly associated with effector memory T cells, which more efficiently mobilize effector function than their quiescent central memory counterparts. Thus, IL-12 programs a distinct phenotype to CD8+ T cells that does not noticeably fit into traditional T cell memory classification. Instead, our results suggest that the use of CD8+ T cells for adoptive transfer with the ability to home efficiently to lymphoid organs and tumors sites, while remaining sensitive to homeostatic and proliferative cytokines, may be the critical determinant of therapeutic efficacy.
Supplementary Material
Acknowledgments
We thank Drs. Antoni Ribas, Steven Bengsinger, and David Brooks, for critical reading of the manuscript.
This work was supported in part by National Institutes of Health/National Cancer Institute Grants K01 CA111402 and RO1-CA123396 (to R.M.P.) and R01 CA 112358 (to L.M.L.) and by the Philip R. and Kenneth A. Jonsson Foundations (to L.M.L.). R.M.P. is the recipient of the Howard Temin National Cancer Institute Career Development award and STOP Cancer Career Development award. Flow cytometry was performed at the University of California Los Angeles Jonsson Comprehensive Cancer Center Core Facility, which is supported by National Institutes of Health Award CA16042.
Abbreviations used in this article
- DC
dendritic cell
- hgp10025–33
human gp10025–33
- MFI
median fluorescence intensity
- TIL
tumor-infiltrating lymphocyte
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The array data presented in this article have been submitted to the Gene Expression Omnibus database under accession number GSE22443.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macgregor JN, Li Q, Chang AE, Braun TM, Hughes DP, McDonagh KT. Ex vivo culture with interleukin (IL)-12 improves CD8 (+) T-cell adoptive immunotherapy for murine leukemia independent of IL-18 or IFN-gamma but requires perforin. Cancer Res. 2006;66:4913–4921. doi: 10.1158/0008-5472.CAN-05-3507. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, Miller JF, Liau LM. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J. Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 9.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002;27:571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 10.Schön MP, Schön M. Immune modulation and apoptosis induction: two sides of the antitumoral activity of imiquimod. Apoptosis. 2004;9:291–298. doi: 10.1023/b:appt.0000025805.55340.c3. [DOI] [PubMed] [Google Scholar]
- 11.Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, Reiter MJ, Vasilakos JP, Tomai MA. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell. Immunol. 1999;191:10–19. doi: 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- 12.Zhu KJ, Cen JP, Lou JX, Wang Q, Zhang X, Xu Y, Chen XZ, Cheng H. Imiquimod inhibits the differentiation but enhances the maturation of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2009;9:412–417. doi: 10.1016/j.intimp.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Hernández J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J. Exp. Med. 2002;196:323–333. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- 15.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 17.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 19.Abken H, Hombach A, Heuser C, Kronfeld K, Seliger B. Tuning tumor-specific T-cell activation: a matter of costimulation? Trends Immunol. 2002;23:240–245. doi: 10.1016/s1471-4906(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 20.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J. Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 21.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol. Today. 2000;21:455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 22.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fallarino F, Uyttenhove C, Boon T, Gajewski TF. Endogenous IL-12 is necessary for rejection of P815 tumor variants in vivo. J. Immunol. 1996;156:1095–1100. [PubMed] [Google Scholar]
- 24.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, Gri G, Wysocka M, Kim JE, Liu L, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 25.Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 26.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 27.Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H, Yang MY, Lin MS, Shelly S, Witte ON, et al. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol. Immunother. 2008;57:1279–1289. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, et al. Enhanced anti-tumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 31.Sampson JH, Carter, Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 32.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin. Cancer Res. 2010;16:4892–4898. doi: 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prins RM, Liau LM. Immunology and immunotherapy in neurosurgical disease. Neurosurgery. 2003;53:144–152. doi: 10.1227/01.neu.0000068865.34216.3a. discussion 152–143. [DOI] [PubMed] [Google Scholar]
- 34.Prins RM, Liau LM. Cellular immunity and immunotherapy of brain tumors. Front. Biosci. 2004;9:3124–3136. doi: 10.2741/1465. [DOI] [PubMed] [Google Scholar]
- 35.Yang MY, Zetler PM, Prins RM, Khan-Farooqi H, Liau LM. Immunotherapy for patients with malignant glioma: from theoretical principles to clinical applications. Expert Rev. Neurother. 2006;6:1481–1494. doi: 10.1586/14737175.6.10.1481. [DOI] [PubMed] [Google Scholar]
- 36.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Herndon, II JE, Lally-Goss D, McGehee-Norman S, Paolino A, Reardon DA, Friedman AH, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol. Cancer Ther. 2009;8:2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liau LM, Black KL, Martin NA, Sykes SN, Bronstein JM, Jouben- Steele L, Mischel PS, Belldegrun A, Cloughesy TF. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case report. Neurosurg. Focus. 2000;9:e8. doi: 10.3171/foc.2000.9.6.9. [DOI] [PubMed] [Google Scholar]
- 38.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 39.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin. Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanges R, Cordero F, Calogero RA. one Channel GUI: a graphical interface to Bio conductor tools, designed for life scientists who are not familiar with R language. Bioinformatics. 2007;23:3406–3408. doi: 10.1093/bioinformatics/btm469. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, Cooper LJ, Gelovani J, Hwu P. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc. Natl. Acad. Sci. USA. 2008;105:14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shu CJ, Guo S, Kim YJ, Shelly SM, Nijagal A, Ray P, Gambhir SS, Radu CG, Witte ON. Visualization of a primary anti-tumor immune response by positron emission tomography. Proc. Natl. Acad. Sci. USA. 2005;102:17412–17417. doi: 10.1073/pnas.0508698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prins RM, Vo DD, Khan-Farooqi H, Yang MY, Soto H, Economou JS, Liau LM, Ribas A. NK and CD4 cells collaborate to protect against melanoma tumor formation in the brain. J. Immunol. 2006;177:8448–8455. doi: 10.4049/jimmunol.177.12.8448. [DOI] [PubMed] [Google Scholar]
- 45.Craft N, Bruhn KW, Nguyen BD, Prins R, Liau LM, Collisson EA, De A, Kolodney MS, Gambhir SS, Miller JF. Bioluminescent imaging of melanoma in live mice. J. Invest. Dermatol. 2005;125:159–165. doi: 10.1111/j.0022-202X.2005.23759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Willinger T, Freeman T, Herbert M, Hasegawa H, McMichael AJ, Callan MF. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol. 2006;176:1439–1446. doi: 10.4049/jimmunol.176.3.1439. [DOI] [PubMed] [Google Scholar]
- 48.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl. Acad. Sci. USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur. J. Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J. Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. Il-12 enhances CD8 T cell homeostatic expansion. J. Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 52.Shu CJ, Radu CG, Shelly SM, Vo DD, Prins R, Ribas A, Phelps ME, Witte ON. Quantitative PET reporter gene imaging of CD8+ T cells specific for a melanoma-expressed self-antigen. Int. Immunol. 2009;21:155–165. doi: 10.1093/intimm/dxn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z, Zheng Z, Jones S, Restifo NP, Rosenberg SA, Morgan RA. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15:1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Díaz-Montero CM, El Naggar S, Al Khami A, El Naggar R, Montero AJ, Cole DJ, Salem ML. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8+CD62Lhi cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol. Immunother. 2008;57:563–572. doi: 10.1007/s00262-007-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 58.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.June CH. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 61.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.