Abstract
Prenatal ethanol significantly heightens later alcohol consumption, but the mechanisms that underlie this phenomenon are poorly understood. Little is known about the basis of this effect of prenatal ethanol on the sensitivity to ethanol’s reinforcing effects. One possibility is that prenatal ethanol exposure makes subjects more sensitive to the appetitive effects of ethanol or less sensitive to ethanol’s aversive consequences. The present study assessed ethanol-induced second-order conditioned place preference (CPP) and aversion and ethanol-induced conditioned taste aversion (CTA) in infant rats prenatally exposed to ethanol (2.0 g/kg) or vehicle (water) or left untreated. The involvement of the κ opioid receptor system in ethanol-induced CTA was also explored. When place conditioning occurred during the ascending limb of the blood-ethanol curve (Experiment 1), the pups exposed to ethanol in utero exhibited greater CPP than untreated controls, with a shift to the right of the dose-response curve. Conditioning during a later phase of intoxication (30–45 min post-administration; Experiment 2) resulted in place aversion in control pups exposed to vehicle during late gestation but not in pups that were exposed to ethanol in utero. Ethanol induced a reliable and similar CTA (Experiment 3) in the pups treated with vehicle or ethanol during gestation, and CTA was insensitive to κ antagonism. These results suggest that brief exposure to a moderate ethanol dose during late gestation promotes ethanol-mediated reinforcement and alters the expression of conditioned aversion by ethanol. This shift in the motivational reactivity to ethanol may be an underlying basis of the effect of prenatal ethanol on later ethanol acceptance.
Keywords: prenatal ethanol exposure, ethanol, second-order conditioning, aversion, preference
Introduction
Drinking during pregnancy and the consequent fetal exposure to alcohol are associated with fetal alcohol syndrome (de Sanctis et al., 2011). Gestational exposure to ethanol, even at moderate levels that do not result in the full-blown expression of fetal alcohol syndrome, significantly affects the recognition, discrimination, and acceptance of ethanol later in life. Several longitudinal epidemiological studies indicated that adolescents whose mothers drank heavy amounts of alcohol during pregnancy were at risk for exacerbated alcohol consumption and alcohol-related problems (Alati et al., 2006; Baer et al., 2003). Another study that used adoptees with or without prenatal alcohol exposure revealed similar findings when alcoholism-related symptoms were assessed in adulthood (Yates et al., 1998). These epidemiological studies confirmed the results of experimentally controlled animal studies (for review, see Spear and Molina, 2005; Chotro et al., 2007; Abate et al., 2008).
The mechanisms that underlie the increase in ethanol preference after prenatal ethanol exposure remain elusive. The detection of ethanol’s taste and flavor in the womb has been suggested to allow for associative learning to occur, in which the chemosensory properties of ethanol experienced after maternal ethanol intoxication become associated with the pharmacologically reinforcing effects of the drug. This process may lead to heightened ethanol seeking and intake during adolescence or adulthood. Chotro and Arias (2003) suggested that the acquisition of this early learning depends on ethanol-induced activation of the opioid system. A recent study by Díaz-Cenzano and Chotro (2010) supported this possibility. As few as two episodes of alcohol exposure (2.0 g/kg) on gestational day (GD) 19–20 resulted in increased ethanol intake during infancy and adolescence, and the effect was blocked when the general opioid receptor antagonist naloxone was co-administered with ethanol during gestation.
Another possibility that complements rather than is an alternative to the acquisition of ethanol-mediated chemosensory learning is that prenatal ethanol exposure makes subjects more sensitive to the appetitive effects of ethanol or less sensitive to ethanol’s aversive consequences. These motivational effects of ethanol are known to modulate ethanol seeking and intake (Cunningham et al., 2000). Repeated exposure to ethanol in the womb could induce tolerance to the hypothermic (e.g., Abel et al., 1981) or malaise-inducing effects of ethanol, likely components of the aversive effects of ethanol. Prenatal ethanol could also enhance the psychomotor effects of ethanol (Becker et al., 1995), although a recent study revealed similar acute motor-activating effects of ethanol in preweanling rats derived from ethanol-exposed and -unexposed dams (Arias et al., 2008). Nizhnikov et al. (2006) studied 3- to 4-h-old rat pups and found that attachment to an empty surrogate nipple previously paired with ethanol was altered after prenatal exposure to 1.0 g/kg ethanol. This study, however, only focused on the appetitive effects of ethanol and did not assess the persistence of the prenatal effect later in life.
To our knowledge, the general hypothesis of altered motivational sensitivity to ethanol after gestational exposure to the drug has not been assessed using conventional measures of appetitive and aversive reinforcement, such as conditioned place preference (CPP), conditioned place aversion (CPA), or conditioned taste aversion (CTA). The present study analyzed ethanol-induced second-order CPP and CPA in Experiments 1 and 2, respectively, in infant rats derived from pregnant rats that were administered ethanol (0.0 g/kg for the vehicle control or 2.0 g/kg, intragastric) during GD17–20 or remained untreated (i.e., untreated controls). Ethanol-induced CTA after gestational ethanol exposure was assessed in Experiment 3.
Conditioned place preference, CPA, and CTA studies involve the pairing of a salient environmental cue (CPP/CPA) or novel taste (CTA) and the pharmacological effect of ethanol. Ethanol-induced reinforcement is then indexed by measuring seeking or avoidance behavior in response to these stimuli. Second-order CPP is a variant of the conventional CPP paradigm, in which subjects are given pairings of ethanol’s effects and an intraoral stimulus (typically water, infused in a pulsate fashion) that serves as a conditioned stimulus (CS1). The CS1 is then paired with a tactile cue (CS2). Preference for or aversion to the CS2 is then measured in a two-way, tactile preference test.
Experiment 1 paired the intraoral CS1 after intubation (i.e., 5–20 min post-administration) when ethanol is presumably appetitive (Molina et al., 2006, 2007). Our expectation was that prenatal ethanol would exacerbate second-order appetitive CPP when using relatively low ethanol doses (0.5–1.0 g/kg) and that 2.0 g/kg ethanol would induce CPP only after prenatal ethanol exposure. Experiment 2 paired the CS1 30–45 min after the administration of 2.0–3.0 g/kg ethanol. This combination of dose and timing parameters has been shown to induce aversive second-order CPA in adolescent and adult animals (Pautassi et al., 2011). A hypothesis was that pups with a history of ethanol exposure in the womb would exhibit few, if any, signs of CPA. In Experiment 3, we expected to observe the diminished expression of CTA in pups exposed to ethanol in utero than in counterparts exposed to vehicle.
The ethanol doses were selected based on previous second-order CPP and CTA studies of ethanol reinforcement in preweanling and adolescent outbred rats. Specifically, 0.5 g/kg ethanol has been shown to induce reliable second-order appetitive CPP, particularly when the CS1 is presented during the onset of intoxication. The 2.0 g/kg dose, in contrast, induces mild or no second-order CPP during the ascending limb of the blood-ethanol curve (Pautassi et al., 2012a, b; but see Molina et al., 2006). Higher doses (e.g., 3.25 g/kg; Pautassi et al., 2011) have been shown to induce second-order CPA in adolescent and adult rats, particularly when conditioning captures the late phase of intoxication. The 2.5 g/kg dose used in Experiment 3 typically results in reliable ethanol-mediated conditioned aversion in preweanling rats (Arias et al., 2011).
Experiment 3 also analyzed the involvement of the κ opioid receptor (KOR) system in the aversive effects of ethanol. The pups were tested for CTA after nor-binaltorphimine (nor-BNI)-induced blockade of KOR function or vehicle injections. The rationale for choosing this specific transmitter subsystem was that previous studies have shown that acute activation of the KOR system may help mediate the aversive effects of ethanol (e.g., Land et al., 2009; Pautassi et al., 2012b).
In addition to pups born to vehicle-treated dams, Experiments 1 and 2 included pups derived from untreated dams. The rationale for adding this control group was that the procedure for the gestational administration of vehicle can be a considered a mild stressor. Specifically, the protocol shares some features with prenatal stress, a preparation in which pregnant rats are subjected to daily stress events, usually during late pregnancy (Campbell et al., 2009). Pups reared by these dams exhibit enhanced responsiveness to stressors and differential sensitivity to ethanol (Campbell et al., 2009). Therefore, unclear was how preweanlings born to vehicle-treated dams would process ethanol in the second-order CPP paradigm. One possibility was that the stress of prenatal vehicle administration would inhibit later appetitive learning or exacerbate the aversive effects of ethanol. This was the rationale for adding another, untreated control condition in the second-order CPP experiments (i.e., Experiments 1 and 2). Previous unpublished studies conducted in our laboratory found that vehicle treatment during gestation did not affect the expression of ethanol-mediated CTA.
Materials and Methods
Subjects
Five-hundred eleven Wistar rat pups were used. These animals were derived from 69 litters born and reared in the vivarium of the Instituto de Investigaciones Médicas M. y M. Ferreyra (INIMEC-CONICET, Argentina). The vivarium has a 12 h/12 h light/dark cycle, with lights on at 8:00 AM, and controlled temperature (22–24°C) and humidity. Vaginal smears of adult female rats were analyzed. When proestrus was detected, the females were mated with adult males. The presence of sperm in vaginal smears the next morning indicated fecundity (i.e., GD0). Births were checked daily, and the day of parturition was considered postnatal day 0 (PD0). The pups were housed with their dams and had ad libitum access to water and lab chow (Cargill, Buenos Aires, Argentina). On PD1, the litters were culled to 10 animals (five males and five females). The litter representation and number of pups in each experiment were the following: Experiment 1 (189 animals; 25 litters, with eight litters pre-exposed to ethanol during late pregnancy [PE group], eight litters given vehicle during late gestation [PV group], and nine litters that remained untreated throughout gestation [UT group]), Experiment 2 (179 animals; 24 litters, with eight litters per prenatal condition), and Experiment 3 (143 animals; 20 litters, with nine PE litters and 11 PV litters). The experimental procedures were approved by the animal care committee at INIMEC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Drug preparation and administration
Intragastric ethanol administration was conducted using a 12 or 8 cm section of polyethylene tubing (Clay-Adams, Parsippany, NJ) connected to a syringe (1 or 12 ml for pups and dams, respectively). The tubing (polyethylene-50 or -10) was gently inserted into the oral cavity of the animal and manually guided into the stomach. Ethanol doses of 0.5, 1.0, 2.0, 2.5, and 3.0 g/kg were achieved by intragastrically administering 0.015 ml of 4.2%, 8.4%, 16.8%, 21.0%, and 25.2% v/v ethanol solutions, respectively (190-proof ethanol; Porta Hnos, Cordoba, Argentina; vehicle: tap water), per gram of body weight.
The nor-BNI dose of 2.5 mg/kg (Sigma-Aldrich, Buenos Aires, Argentina) was administered intraperitoneally in an injection volume of 0.01 ml/g and derived from a 1 mg/ml solution (vehicle: 0.9% saline). Injections were performed with 1 ml tuberculin syringes mounted with a 27-gauge disposable needle that was replaced after each injection. Intraperitoneal injections took less than 10 s, and the doses were selected from a recent study (Pautassi et al., 2012b).
Prenatal ethanol treatment (Experiments 1–3)
Ethanol administration during pregnancy closely followed the procedures described in Pueta et al. (2011). Briefly, pregnant females were administered 0.0 (vehicle) or 2.0 g/kg ethanol during GD17–20 (PV and PE groups, respectively). Untreated dams (UT group) did not experience drug administration during gestation and were only exposed to normal animal facility rearing conditions. Home cages and pine shaving bedding were changed twice per week for all litters (i.e., PV, PE, and UT groups) by professionally trained animal care personnel. The ethanol dose and timing of gestation were selected on the basis of previous studies that showed that these experimental parameters result in the fetal processing of ethanol’s chemosensory and unconditioned properties, influence the postnatal discrimination of the drug, and heighten ethanol intake (Spear and Molina, 2005).
Second-order conditioned place preference (Experiment 1) or aversion (Experiment 2)
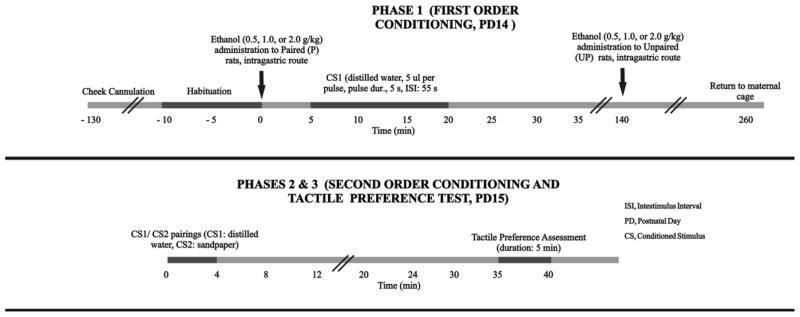
A three-step, second-order CPP procedure was used (see Figure 1). This variant of the CPP paradigm was originally devised by Molina et al. (2006, 2007) and later used in adolescents by Pautassi et al. (2008, 2010, 2011). It has been recently used to assess the role of KORs (Pautassi et al., 2012a) and effects of early maternal separation in ethanol-induced reinforcement (Pautassi et al., 2012b).
Figure 1.
Methods for analyzing the effects of prenatal exposure to ethanol on ethanol-mediated second-order conditioning, as conducted on Experiment 1. Phase 1, first-order conditioning, postnatal day (PD) 14: Pups were removed from the maternal cage, cannulated and then briefly (10 min) habituated to the experimental context. Paired pups were then given ethanol (0.5, 1.0 or 2.0 g/kg), and stimulated with a conditioned stimulus (CS1) consisting of intraoral pulses of water. CS1 delivery occurred 5–20 min after ethanol. Unpaired pups were given ethanol administration 120 minutes after termination of CS1. Phase 2, second-order conditioning, postnatal day 15: Animals were stimulated with water pulses while placed in a sandpaper-lined compartment (CS2). Phase 3, tactile preference test, postnatal day 15: Time spent on sandpaper was recorded during a 5 min preference test. Experiment 2 employed similar procedures. In Experiment 2, however, CS1 delivery occurred 30–45 min after administration of 2.0 or 3.0 g/kg ethanol. The figure and legend were adapted with permission from Pautassi et al., 2011.
Phase 1 (first-order conditioning, PD14)
The pups were removed from their dams at 8:30–9:00 AM and immediately implanted with a small piece of polyethylene-10 tubing in the cheek. The cannulation procedure took approximately 10 s per animal, was accompanied by few signs of stress, and has been extensively described (e.g., Abate et al., 2000; Ponce et al., 2008). The animals were then placed in same-sex pairs in holding cages (30 × 20 × 20 cm) kept warm with a heating pad. Two hours after cannulation, the pups were placed for 10 min in individual square Plexiglas chambers (10 × 10 × 12 cm) lined with cotton (Estrella, Buenos Aires, Argentina). This non-reinforced session sought to familiarize the pups with the chambers. All of the pups were weighed to the nearest 0.01 g (Ohaus, Buenos Aires, Argentina) at the end of the habituation session. Paired (P) animals were then given ethanol (Experiment 1: 0.5, 1.0, or 2.0 g/kg; Experiment 2: 2.0 or 3.0 g/kg).
Conditioning occurred in the square chambers 5–20 min post-administration (Experiment 1) or 30–45 min post-administration (Experiment 2). The preweanling animals were stimulated with fifteen 5 s pulses (5 μl per pulse; interstimulus interval, 55 s) of distilled water. The delivery of this CS1 was performed by connecting the intraoral cannula to thicker polyethylene-50 tubing that was connected to an infusion pump (KD Scientific, Holliston, MA). The cannulae were removed immediately after the termination of the infusion, and the pups were returned to their holding chambers. Unpaired controls (UP) experienced habituation, weighing, and intraoral infusion but were administered ethanol 120 min after CS1 exposure. Thus, the P and UP groups had comparable levels of exposure to the CS and US but differed in the contiguity (or lack thereof) between these stimuli. All of the pups were reunited with their dams 2 h after the administration of ethanol in unpaired controls.
In summary, Phase 1 included first-order conditioning of intraoral pulsed water as the CS and the postingestive consequences of ethanol delivered intragastrically.
Phase 2 (second-order conditioning, PD15)
The animals were removed from their dams, cannulated at approximately 8:30 AM and kept in pairs in warmed holding chambers for sixty minutes. They were then individually placed in the conditioning chambers used during Phase 1, which were now lined with coarse, 60 grit sandpaper (Norton, Rio Grande do Sul, Brazil). While in contact with the sandpaper (hereinafter referred to as CS2), the animals were given pulsed distilled water every 55 s (5 μl volume; pulse duration, 5 s). The session lasted 5 min, and the animals were returned to the holding chambers immediately after termination of the session. In summary, in Phase 2 the rats were given pairings of the CS1 (intraoral pulsed water) and CS2 (sandpaper flooring).
Phase 3 (tactile preference test, PD15)
A 5 min, two-way tactile preference test was conducted 30 min after the termination of Phase 2. The animals were tested in a rectangular Plexiglas chamber (28 × 13 × 15.5 cm). Half of the floor of this chamber was lined with the sandpaper CS2, and the remaining floor surface was lined with smooth cardboard. Both textures were replaced in each new test, which was conducted under red light provided by an overhead 40 W bulb. The time spent over each section of the apparatus was recorded in 1 min bins by an experimenter who was blind to the treatment of each animal. The middle section (15% of the entire surface) was considered a neutral area and not considered for the data collection or analysis. The dependent variable was the percent time spent on sandpaper: ([total time spent over sandpaper]/[total time spent over sandpaper + total time spent over smooth floor]) × 100.
Conditioned taste aversion procedure (Experiment 3)
The pups were given nor-BNI injections (0.0 or 2.5 mg/kg) on PD13. Conditioning sessions then occurred on PD14 and PD15. The pups were separated from their dams, and an intraoral cannula was implanted in the right cheek. The pups were then placed in pairs into a warmed holding chamber. Two hours later, the pup’s bladders were voided by brushing the anogenital area, and body weights were recorded. The animals were subsequently placed into individual Plexiglas chambers (10 × 10 × 12 cm), and intraoral saccharin was infused (0.05% w/v; CS; duration, 10 min). The total administration volume was equivalent to 3% of the subject’s pre-infusion body weight. Saccharin was delivered at a constant rate by means of a 10-syringe infusion pump (KD Scientific, Holliston, MA).
The cheek cannula was removed after the infusion, and the animal’s weight was recorded to estimate the saccharin consumption score. The dependent variable was the percentage of body weight gain: ([postinfusion weight − preinfusion weight]/preinfusion weight) × 100. Paired animals were then given intragastric ethanol administration (0.5 or 2.5 g/kg). Unpaired animals received the intubation 2 h after termination of the infusion. The animals remained in the warmed holding chamber for ethanol clearance and were returned to their dams 3 h after the unpaired ethanol intubation. Conditioning session 2 also served as a test session because the animals were infused with saccharin and assessed for intake prior to receive the corresponding ethanol dose.
In summary, beginning 24 h after an injection of 2.5 mg/kg nor-BNI, the pups were given two daily pairings of saccharin and intragastric ethanol. They were then tested for saccharin intake on PD16. Testing followed similar procedures as those of conditioning, but no ethanol administration was performed.
Experimental design
Experiment 1 assessed ethanol-mediated second-order appetitive conditioning after gestational ethanol exposure and was defined by a 3 (prenatal condition: PE, PV, or UT) × 2 (sex: male or female) × 2 (CS1 paired or unpaired with ethanol’s effects) × 3 (ethanol dose during conditioning: 0.5, 1.0, or 2.0 g/kg) factorial, with 4–7 animals in each group. A similar design was used in Experiment 2, with the difference that the ethanol doses were 2.0 and 3.0 g/kg, and the groups had 4–9 subjects each.
Experiment 3 analyzed ethanol-induced CTA and used a 2 (sex) × 2 (prenatal condition: PE or PV) × 2 (dose of nor-BNI on PD13: 0.0 or 2.5 mg/kg) × 2 (saccharin paired or unpaired with ethanol’s effects) × 2 (ethanol dose during conditioning: 0.5 or 2.5 g/kg) factorial. Each group had 4–6 animals.
Potential litter effects were controlled by always assigning no more than one animal per litter to a given cell of the design.
Statistical analysis
The preliminary analysis indicated that sex did not exert significant main effects across experiments and dependent variables and was not involved in any significant interaction. This lack of effect was not unexpected, however, because almost all studies that have assessed ethanol-mediated learning in preweanling animals (e.g., Arias et al., 2008; Pautassi et al., 2012b; Miranda-Morales et al., 2011) have observed similar responding in males and females. The data were, thus, collapsed across sex for representation in the figures and for the subsequent analyses.
The percent time in contact with the sandpaper CS (Experiments 1 and 2) during the tactile preference test was analyzed using a three-way analysis of variance (ANOVA). The between factors were prenatal treatment, conditioning (paired or unpaired), and ethanol dose.
The dependent variable for Experiment 3 was saccharin acceptance, expressed as percent body weight gain (%BWG). A two-way ANOVA was conducted on session 1 saccharin intake scores to confirm the lack of baseline differences in CS acceptance as a function of prenatal treatment and nor-BNI dosing. Saccharin acceptance during Sessions 2 and 3 was then analyzed using a four-way mixed ANOVA, with the between factors prenatal treatment, nor-BNI treatment, conditioning (paired or unpaired), and ethanol dose and the within-factor Sessions 2 and 3.
The loci of significant main effects or interactions were further examined using Fisher’s Least Mean Significant post hoc comparisons. Planned comparisons were also conducted if justified by previous hypotheses. The alpha level was set at < 0.05.
Results
Experiment 1
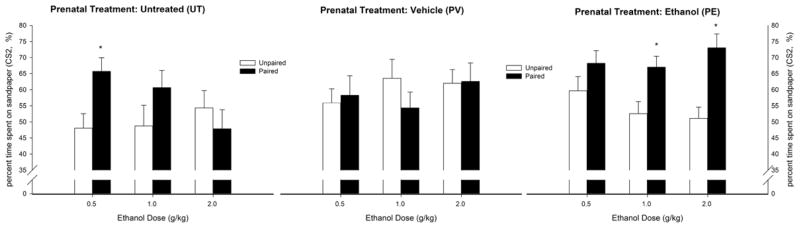
Fig. 2 appears to indicate that PE and UT pups, but not vehicle-treated pups, exhibited ethanol-induced CPP. Furthermore, pups given ethanol in utero exhibited the greatest magnitude of appetitive reinforcement among all of the groups. This effect was apparently driven by PE pups that exhibited CPP at the higher doses of 1.0 and 2.0 g/kg, whereas UT pups only exhibited CPP after the lower, 0.5 g/kg dose. The ANOVA supported these impressions. The analysis of the percent sandpaper preference revealed significant main effects of prenatal ethanol treatment and conditioning treatment (F2,171 = 3.91, p < 0.05, and F1,171 = 8.90, p < 0.005, respectively). The two-way interaction between prenatal ethanol treatment and conditioning treatment also achieved significance (F2,171 = 4.57, p < 0.05), whereas the prenatal treatment × conditioning treatment × ethanol dose interaction approached significance (p = 0.05). The post hoc tests indicated significantly greater CS2 preference in PE and UT paired pups but not PV animals compared with their respective, unpaired controls. Moreover, the level of sandpaper preference was greater in paired pups exposed to ethanol during pregnancy than in their paired UT or PV counterparts, a result that suggests the increased appetitive effects of ethanol after prenatal ethanol.
Figure 2.
Ethanol-induced second-order conditioning in infant rats derived from pregnant rats that were given 2.0 g/kg ethanol or vehicle (water) intragastrically (PE and PV groups, respectively) on gestational days 17 to 20 or were untreated (UT group), with the percent time spent on the rough CS2 texture (sandpaper) during the test as the dependent variable. During conditioning on PD14, the animals were given ethanol (0.5, 2.0, or 0.0 g/kg, i.g.) and stimulated 5–20 min post-administration with intraoral pulses of water (CS1; paired group) or experienced both stimuli separated by 120 min (unpaired groups). The next day, the animals experienced CS1–CS2 pairings and were then tested for CS2 preference. The figure depicts the percent time spent on sandpaper as a function of prenatal treatment, treatment during conditioning (i.e., paired or unpaired) and ethanol dose given at conditioning. To facilitate data visualization, the data were collapsed across sex. The latter factor did not affect tactile preference scores or significantly interact with the remaining factors. Asterisks (*) indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars indicate the SEM.
Guided by our a priori hypothesis and to better understand the dose-response profile, planned comparisons between paired and unpaired pups were conducted for the 0.5, 1.0, and 2.0 g/kg doses in each prenatal condition. UT paired pups exhibited greater CS2 preference than unpaired pups at 0.5 g/kg but not 1.0 or 2.0 g/kg ethanol. Conversely, paired pups exposed to ethanol in utero (PE animals) exhibited CPP with 2.0 and 1.0 g/kg ethanol but not with the lower, 0.5 g/kg dose. The planned comparisons revealed no difference between paired and unpaired subjects in the PV prenatal condition at any dose. Percent sandpaper preference as a function of prenatal treatment, conditioning treatment, and ethanol dose is depicted in Figure 2.
Experiment 2
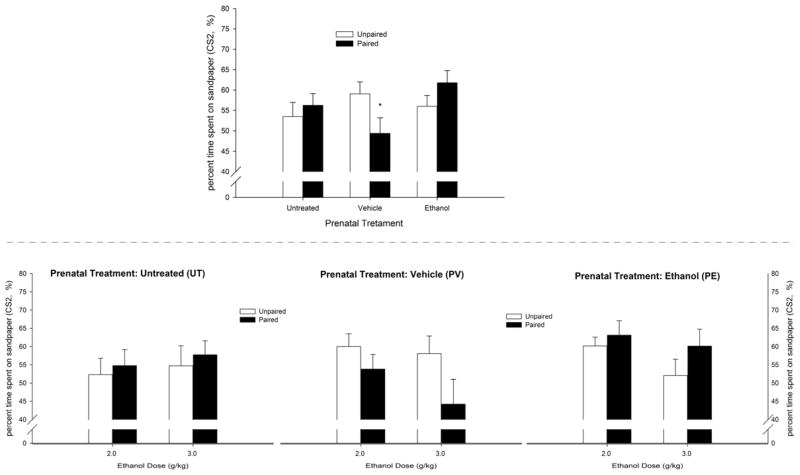
Experiment 2 used higher ethanol doses and tested conditioning during a period of the blood ethanol curve in which ethanol’s aversive effects at these doses appear to be dominant (Pautassi et al., 2011). Under these conditions, control pups exposed to vehicle during late gestation exhibited avoidance of the sandpaper CS2. This effect was inhibited in pups exposed to prenatal ethanol and absent in UT pups. Fig. 3 depicts these results.
Figure 3.
Ethanol-induced second-order conditioning in infant rats derived from pregnant rats that were given 2.0 g/kg ethanol or vehicle (water) intragastrically (PE and PV groups, respectively) on gestational days 17 to 20 or were untreated (UT group), with the percent time spent on the rough CS2 texture (sandpaper) during the test as the dependent variable. During conditioning on PD14, the animals were given ethanol (2.0 or 3.0 g/kg, i.g.) and stimulated 30–45 min post-administration with intraoral pulses of water (CS1; paired group) or experienced both stimuli separated by 120 min (unpaired groups). The next day, the animals experienced CS1–CS2 pairings and were then tested for CS2 preference. The upper panel depicts the percent time spent on sandpaper as a function of prenatal treatment and treatment during conditioning. The lower panel depicts these data disaggregated by ethanol dose given at conditioning. To facilitate data visualization, the data were collapsed across sex. The latter factor did not affect tactile preference scores or significantly interact with the remaining factors. Asterisks in the upper panel (*) indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars indicate the SEM.
These impressions were supported by the corresponding ANOVA, revealing a significant conditioning × prenatal ethanol interaction (F2,167 = 3.98, p < 0.05). Subsequent post hoc comparisons indicated significantly lower CS2 preference in paired UT pups than in unpaired UT pups. This result indicates that ethanol-induced CPA was not observed in PE or UT animals. Interestingly, paired pups exposed to ethanol during late gestation exhibited significantly greater CS2 preference than paired PV and unpaired UT animals.
Experiment 3
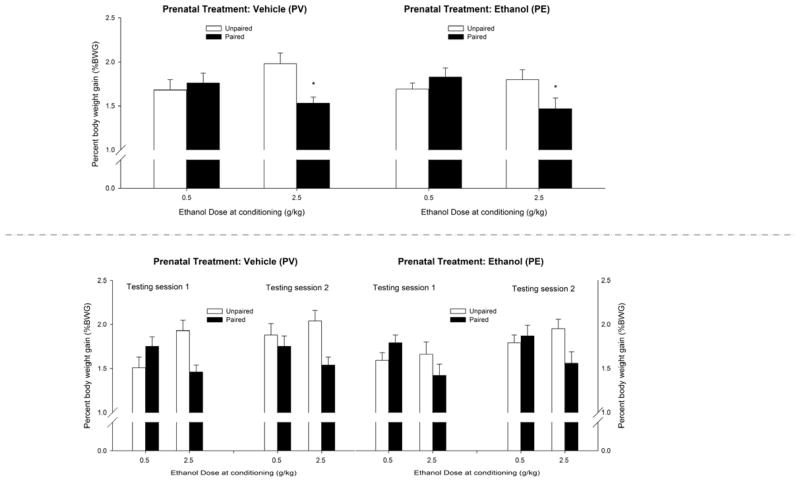
During the conditioning session on PD14, saccharin acceptance was fairly similar between PE and PV animals, regardless of nor-BNI treatment the previous day. The ANOVA revealed a lack of significant main effects or interactions. The mean ± SEM %BWG in animals that were exposed to ethanol during gestation was 1.54 ± 0.08% and 1.65 ± 0.09%, whereas the mean ± SEM %BWG in subjects reared by vehicle-treated dams was 1.65 ± 0.08% and 1.70 ± 0.07% in pups that received 0.0 and 2.5 mg/kg nor-BNI, respectively.
On the test days on PD15–16, the ANOVA revealed a significant conditioning treatment × ethanol dose interaction (F1,133 = 11.08, p < 0.005). The post hoc tests revealed that the pups given pairings of 2.5 g/kg ethanol but not the pups given 0.5 g/kg ethanol exhibited significantly less saccharin intake than their unpaired controls. This result indicates that ethanol-mediated CTA was fairly similar in PE and PV pups and in animals given 0.0 or 2.5 mg/kg nor-BNI on PD13. The highest ethanol dose appeared to endow the sweet taste with aversive incentive properties. This aversive learning, however, was similar across prenatal treatment and insensitive to KOR antagonism.
The ANOVA also revealed a significant day of assessment × conditioning treatment interaction (F1,133 = 7.88, p < 0.01). The post hoc tests revealed that overall drinking scores were lower in paired subjects than in unpaired subjects during the final test session but not during the session conducted on PD15 and that nor-BNI treatment did not exert a significant main effect and was not involved in significant interactions. Saccharin intake scores during the test sessions are depicted in Fig. 4 as a function of prenatal treatment and ethanol dose.
Figure 4.
Ethanol-induced conditioned taste aversion in infant rats derived from pregnant rats that were given 2.0 g/kg ethanol or vehicle (water) intragastrically (PE and PV groups, respectively) on gestational days 17 to 20, with CS (saccharin) intake depicted as percent body weight gain (%BWG) in a 10 min test. Twenty-four hours prior to conditioning, the pups were treated with the KOR antagonist nor-BNI (0.0 or 2.5 mg/kg, i.p.). During conditioning (PD14–15), the pups were exposed to a saccharin solution and intubated with ethanol (0.5 or 2.5 g/kg) immediately following the infusion. Conditioning session 2 on PD15 also served as a test session because the animals were infused with saccharin and assessed for intake prior to receiving the corresponding ethanol dose. A second test session was conducted on PD16. The upper panel depicts the average saccharin acceptance (%BWG) across the test sessions as a function of prenatal treatment and ethanol dose during conditioning. The lower panel depicts these data disaggregated by test session (1 or 2). To facilitate data visualization, the data were collapsed across sex and nor-BNI treatment. The latter factors did not affect avoidance scores or significantly interact with the remaining factors. Asterisks (*) in the upper panel indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars indicate the SEM.
Discussion
The main result of the present study was that moderate and relatively brief prenatal exposure to ethanol significantly enhanced responding to the appetitive motivational effects of ethanol during the second week of life in the rat. Prenatal ethanol exposure also modulated the aversive effects of ethanol measured by second-order CPA but not CTA.
Several previous studies (Molina et al., 2006, 2007; Pautassi et al., 2012a, b) found that preweanling rats displayed second-order conditioning by ethanol. Pups exposed to ethanol in utero, however, displayed significantly greater ethanol-induced second-order CPP than controls reared by untreated dams. Perhaps more importantly, the dose-response curve for ethanol-induced CPP apparently shifted to the right, suggesting development of tolerance after prenatal ethanol exposure. Consistent with recent results from our laboratory (Pautassi et al., 2012a, b), untreated pups displayed CPP with 0.5 g/kg but not 1.0 or 2.0 g/kg ethanol. Conversely, the higher but not lower ethanol dose resulted in CPP in pups exposed to ethanol in utero. This confirms the major results of Nizhnikov et al. (2006) with neonatal rats, the only other study of the effects of moderate doses of ethanol on the pharmacologically appetitive effects of ethanol postnatally. The study by Nizhnikov et al. assessed conditioned (i.e., ethanol-induced) attachment to an artificial nipple in cesarean-delivered 3- to 4-h-old rat pups and found that prenatal ethanol (1 g/kg) widened the range of ethanol doses that the newborns found reinforcing. The present study confirmed a positive modulatory role for gestational ethanol exposure on ethanol’s appetitive effects and indicated that this effect persists when measured by CPP during the second week of life in the rat. The finding of an apparent shift in postnatal sensitivity from lower to higher ethanol doses after in utero ethanol exposure is also relevant because higher doses are more likely to result in binge-like intoxication and detrimental effects on the central nervous system. For example, Murawski and Stanton (2011) observed dose-related impairment in a hippocampus-dependent task after ethanol intoxication in preweanling rats.
Important information can also be derived from the behavior of animals born to dams given vehicle during late gestation (i.e., PV group). Unlike untreated controls, PV animals failed to exhibit ethanol-induced second-order CPP. The procedure for administering vehicle to the dams shares some features (e.g., daily handling that involves brief restraint and exposure to aversive events, such as gastric intubations) with prenatal stress, a paradigm in which pregnant dams are exposed to unpredictable stress during the last phase of pregnancy, resulting in behavioral changes later in the pup’s (Lee et al., 2007; Harmon et al., 2009). In the present study, prenatal stress appeared to make the animals less sensitive to ethanol-induced reinforcement. This finding makes the increased appetitive responding in PE animals even more remarkable. Prenatal ethanol not only heightened motivational responding to ethanol compared with the untreated group but also reversed the apparent inhibitory effect that prenatal stress exerted in PV pups.
Maternal stress could also have lessened the ability of PV pups to learn an appetitive second-order schedule of appetitive reinforcement. Memory deficits have often been observed after prenatal stress (e.g., Gonzalez-Perez et al., 2011). This possibility, however, is unlikely in the present study. These stress-related detrimental effects often require prolonged exposure to stress (e.g., 1 h/day for 7 days; Hosseini-Sharifabad and Hadinedoushan, 2007). Furthermore, PV pups readily acquired first- and second-order learning in Experiments 2 and 3.
In Experiment 2, place conditioning with relatively high ethanol doses occurred during a late phase of intoxication (i.e., 30–45 min post-administration), and prenatally untreated rats exhibited neither significant conditioned aversion nor preference. Employing similar doses and timing of conditioning, Pautassi et al. (2011) reported ethanol-induced second-order conditioned aversion in adolescent and adult rats. Therefore, the present results are consistent with previous suggestions that preweanling animals exhibit lower sensitivity to ethanol-induced aversion than older, more mature animals (Truxell et al., 2007). Perhaps more importantly, animals from dams treated with vehicle during gestation exhibited avoidance of the ethanol-related texture. This behavior indicates that ethanol-induced conditioned aversion was absent in the animals exposed to ethanol in utero. Prenatal stress appeared to alter the balance between ethanol’s appetitive and aversive effects, promoting the acquisition of conditioned aversion induced by ethanol, and this effect was blocked by prenatal exposure to the drug. It should be noted, however, that the aversive conditioning in PV pups given ethanol-CS1 pairings 30–45 min post-intubation was relatively weak. This may be explained by poor contiguity between ethanol’s effects and CS1.
In Experiment 3, ethanol induced reliable CTA at the 2.5 g/kg dose but not at the lower, 0.5 g/kg dose. This effect was fairly similar in PE and PV animals. One could speculate why differences between these conditions emerged when ethanol-induced aversion was measured using second-order CPP and not when measured using CTA. Memories acquired through taste conditioning paradigms are known for their strength and biological preparation (Dellarosa Cummins and Cummins, 1998), and perhaps they are less susceptible to the effects of early life experiences than memories acquired through higher-order conditioning paradigms or pairings of ethanol with exteroceptive stimuli rather than flavors. Previous studies also showed that prenatal (Abate et al., 2001) or postnatal (Pautassi et al., 2005) exposure to ethanol did not disrupt the expression of postnatal conditioned aversion to chemosensory stimuli paired with ethanol.
Our hypothesis, in which we proposed that taste conditioning may be affected by pretreatment with a KOR agonist, was not confirmed. The rationale for using a KOR antagonist prior to taste aversion conditioning with an ethanol US was based on recent studies that suggested a role for KOR in ethanol-mediated aversion. κ opioid receptor antagonism, for example, inhibits ethanol-induced motor depression and reduces ethanol-induced hypothermia (Pillai and Ross, 1986; Pohorecky et al., 1989). Another study (Pautassi et al., 2012b) found that animals untreated in terms of prenatal treatment exhibited second-order CPP induced by ethanol (2.0 g/kg) only if treated with a KOR antagonist before conditioning. Future studies should further analyze the modulatory role of KORs in this class of ethanol’s motivational effects, which has been rarely studied.
The present results suggest that prenatal ethanol made subjects more sensitive to the positive appetitive rewarding effects of this drug. An alternative explanation, however, is that prenatal ethanol induced tolerance to the motivational effects of alcohol. It is possible that the higher ethanol doses (i.e., 1.0 and 2.0/kg) induced aversive effects that interfered with the appetitive conditioning in untreated animals. The pattern of the present findings, therefore, is consistent with prenatal ethanol decreasing ethanol’s aversive effects and facilitating expression of appetitive effects of ethanol at the 1.0 and 2.0 g/kg doses. Under this explanation the lack of second-order appetitive conditioning in PE pups given 0.5 g/kg ethanol may be due to the development of tolerance to the appetitive effects of ethanol.
It is also possible prenatal ethanol or its associated maternal stress simply enhanced the ability of animals to acquire, store, and express associative memories, such as those involved in second-order CPP. This explanation, however, appears to be unlikely in the present study. Prenatal ethanol exposure potentiated the appetitive effects of ethanol but not the aversive effects of the drug. When given pairings of a CS1 and high-dose ethanol (Experiment 2), the animals reared by dams given only water in utero displayed second-order aversion, whereas animals given prenatal exposure to ethanol did not. Furthermore, other studies that employed similar prenatal treatment as the treatments in the present work found that prenatal ethanol did not alter the detection or discrimination of or habituation to novel or familiar tastes (Abate et al., 2000, 2001). Specifically, the latter study found that prenatal ethanol exposure did not affect water consumption, the habituation to intraoral pulses of milk, or the ability to exhibit associative learning. Moreover, Pueta et al. (2011) found no conventional teratological effects in the offspring of dams given 1.0 or 2.0 g/kg ethanol on GD17–20 (i.e., prenatal ethanol did not alter the number of cells in the granular cell layer of the main olfactory bulb).
One possibility is that the behavioral differences observed in Experiments 1 and 2 may be attributable to an effect of prenatal ethanol on ethanol metabolism or the rate of habituation to the chamber in Phase 1 of second-order CPP. Preliminary, unpublished data from our laboratory, however, indicated that 13-day-old pups reared by dams given ethanol or vehicle or were untreated during late pregnancy exhibited similar levels of habituation to a novel environment. Arias et al. (2008) gave pregnant dams 2.0 or 0.0 g/kg ethanol on GD17–20 and then assessed blood ethanol levels in offspring on PD14 after 0.5–2.0 g/kg ethanol at several time points after intubation. No differences in the level of intoxication were observed as a function of prenatal treatment. A lack of effect of prenatal ethanol exposure on postnatal blood ethanol concentration was similarly found by Pueta et al. (2011).
Altogether, the present results add to a growing body of literature (for review, see Abate et al., 2008) that indicates that subjects exposed to moderate doses of ethanol in utero, doses unlikely to induce conventional teratology in terms of morphology or physiology, exhibit differential sensitivity to ethanol-induced reinforcement. This could be one the factors that underlie the facilitating effect of gestational ethanol on later ethanol intake.
Acknowledgments
Acknowledgments and Funding Sources
This work was a collaborative project between the Research Foundation of SUNY Binghamton and Instituto Ferreyra (Argentina), supported by NIAAA grants AA011960, AA01309, and AA017823 to NES and AA018164 and AA017823 to MN and grants PIP CONICET 2010–2012, PICT-PRH3, and SECYT-UNC to RMP. The authors would like to thank the animal care personnel at INIMEC-CONICET for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med. 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–998. [PubMed] [Google Scholar]
- Abel EL, Bush R, Dintcheff BA. Exposure of rats to alcohol in utero alters drug sensitivity in adulthood. Science. 1981;26:1531–1533. doi: 10.1126/science.7233243. [DOI] [PubMed] [Google Scholar]
- Alati R, Mamum AA, Williams GM, O’ Callaghan M, Najman JM, Bor W. In Utero Alcohol Exposure and Prediction of Alcohol Disorders in Early Adulthood. Arch Gen Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89:608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Pautassi RM, Molina JC, Spear NE. A comparison between taste avoidance and conditioned disgust reactions induced by ethanol and lithium chloride in preweanling rats. Dev Psychobiol. 2010;52:545–557. doi: 10.1002/dev.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Becker HC, Weathersby RT, Hale RL. Prenatal ethanol exposure alters sensitivity to the effects of apomorphine given alone and in combination with ethanol on locomotor activity in adult male mouse offspring. Neurotoxicol Teratol. 1995;17:57–64. doi: 10.1016/0892-0362(94)00055-i. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE. Contribution of early environmental stress to alcoholism vulnerability. Alcohol. 2009;43:547–554. doi: 10.1016/j.alcohol.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal Exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- de Sanctis L, Memo L, Pichini S, Tarani L, Vagnarelli F. Fetal alcohol syndrome: new perspectives for an ancient and underestimated problem. J Matern Fetal Neonatal Med. 2011;24(Suppl 1):34–37. doi: 10.3109/14767058.2011.607576. [DOI] [PubMed] [Google Scholar]
- Dellarosa Cummins D, Cummins R. Biological preparedness and evolutionary explanation. Cognition. 1997;73:B37–B53. doi: 10.1016/s0010-0277(99)00062-1. [DOI] [PubMed] [Google Scholar]
- Díaz-Cenzano E, Chotro MG. Prenatal binge ethanol exposure on gestation days 19–20, but not on days 17–18, increases postnatal ethanol acceptance in rats. Behav Neurosci. 2010;124:362–369. doi: 10.1037/a0019482. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Gutiérrez-Smith Y, Guzmán-Muñiz J, Moy-López NA. Intrauterine stress impairs spatial learning in the progeny of Wistar rats. Rev Invest Clin. 2011;63:279–286. [PubMed] [Google Scholar]
- Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. The effects of prenatal stress on motivation in the rat pup. Stress. 2009;12:250–258. doi: 10.1080/10253890802367265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Sharifabad M, Hadinedoushan H. Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anat Sci Int. 2007;82:211–217. doi: 10.1111/j.1447-073X.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Morales RS, Molina JC, Spear NE, Abate P. Naloxone attenuation of ethanol-reinforced operant responding in infant rats in a re-exposure paradigm. Psychopharmacology. 2012;219:235–246. doi: 10.1007/s00213-011-2402-5. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear NE. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcohol Clin Exp Res. 2011;35:1160–70. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Ethanol induces second-order aversive conditioning in adolescent and adult rats. Alcohol. 2011;45:45–55. doi: 10.1016/j.alcohol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Naloxone blocks ethanol-mediated appetitive conditioning and locomotor activation in adolescent rats. Behav Brain Res. 2010;216:262–269. doi: 10.1016/j.bbr.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE. Early role of the 3 opioid receptor in ethanol-induced reinforcement. Physiol Behav. 2012b;105:1231–1241. doi: 10.1016/j.physbeh.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Fabio MC, Spear NE. Early maternal separation affects ethanol-induced conditioning in a nor-BNI insensitive manner, but does not alter ethanol-induced locomotor activity. Pharmacol Biochem Behav. 2012a;100:630–638. doi: 10.1016/j.pbb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Ponce LF, Molina JC. Efectos de la exposición temprana al etanol sobre subsiguientes aprendizajes mediados por los atributos incondicionales de la droga [Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug] Rev Latinoam Psicol [Latin American Journal of Psychology] 2005;37:131–149. [Google Scholar]
- Pillai NP, Ross DH. Ethanol-induced hypothermia in rats: possible involvement of opiate kappa receptors. Alcohol. 1986;3:249–253. doi: 10.1016/0741-8329(86)90033-9. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Patel V, Roberts P. Effects of ethanol in an open field apparatus: modification by U50488H and WIN 44441–3. Physiol Behav. 1989;45:273–287. doi: 10.1016/0031-9384(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacol Biochem Behav. 2008;90:640–650. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueta M, Rovasio RA, Abate P, Spear NE, Molina JC. Prenatal and postnatal ethanol experiences modulate consumption of the drug in rat pups, without impairment in the granular cell layer of the main olfactory bulb. Physiol Behav. 2011;102:63–75. doi: 10.1016/j.physbeh.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]