Abstract
A series of α-alkoxy carbamates that cleave under mild conditions to release alcohols has been synthesized through a multicomponent process. The relationship between structural features in these compounds and the rate of alcohol release in the presence of basic hydrogen peroxide has been studied. The preparation of carbamates that cleave under other conditions has been demonstrated.
Introduction
Stimulus-responsive cleavage reactions have found broad use for applications in temporally-controlled drug release, chemical sensing, and material decomposition processes.1 Carbamates have frequently been used to release amines in these processes due to the diverse array of stimuli that can be employed for the decomposition step. We have initiated a program by which α-alkoxy carbamate cleavage can serve to release alcohols in response to externally-applied stimuli, as shown in Scheme 1.
Scheme 1.
Stimulus-promoted cleavage of α-alkoxy carbamates.
The capacity to direct drug release at a particular location would be a significant attribute for a drug-delivery agent. The benefit of employing α-alkoxy carbamates as delivery agents2 is that the carbamate structure contains a branch that can be used to append cell-targeting agents. Thus a single molecule can contain directing, triggering, and cargo groups (Fig. 1). This design has been implemented in elegant studies from the Shabat,3 Shin,4 and Sinha5 groups. These successful constructs highlight the utility of the design yet reveal that enhanced synthetic accessibility would be desirable for rapid property optimization. In this manuscript we demonstrate that several α-alkoxy carbamates can be prepared rapidly through a multicomponent reaction and illustrate the manner in which this protocol can be used to access compounds that release alcohols at varying rates. Compounds with different triggering mechanisms have been prepared to demonstrate the merits of the multicomponent reaction for the preparation of these structures.
Fig. 1.
Schematic of a cargo-releasing composite structure that contains localization and triggering groups.
Results and discussion
Our approach to α-alkoxy carbamate synthesis employed a sequence of nitrile hydrozirconation, acylation, and alcohol addition.6 This protocol has successfully been applied to rapid syntheses of cyclic amides,7 the natural products pederin and psymberin, and many analogs.8 Conducting the multicomponent reaction at a late stage in the synthetic sequence provides abundant opportunities for optimizing molecular properties through structural diversification.
The synthesis of a representative cleavable α-alkoxy carbamate is shown in Scheme 2. We chose to explore peroxide-triggered compounds in consideration of the potential for drug delivery to oxidant-rich medical conditions such as cancer9 and neurodegeneration.10 Our design was based on Chang’s peroxide-released fluorophores11 in which boronate oxidation forms a phenoxide that releases its cargo through p-quinone methide formation.12 Thus, known benzylic alcohol 1 was treated with a solution of phosgene in toluene to form chloroformate 2. Hydrozirconation of tBuCN (3) with Cp2Zr(H)Cl followed by the addition of freshly-prepared 2 yielded intermediate acylimine 4. The synthesis was completed through the addition of neopentyl alcohol to provide 5 in 64% yield.
Scheme 2.
Multicomponent approach to α-alkoxy carbamate synthesis.
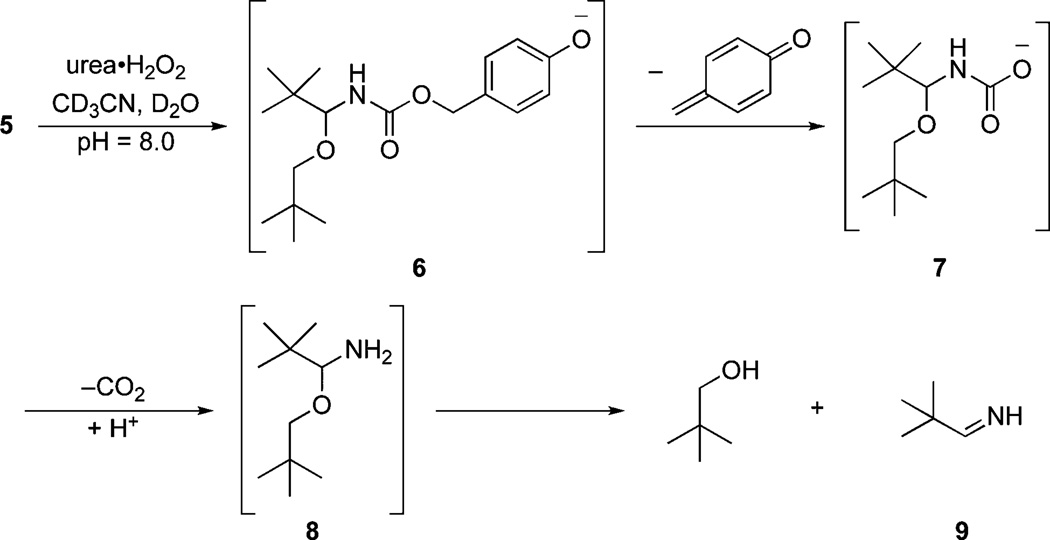
The breakdown of compound 5 was effected by treatment with urea·H2O2 at pH = 8.0 (phosphate buffer) in a mixture of CD3CN and D2O (Scheme 3). This allowed for direct monitoring of starting material consumption and product formation by 1H NMR. This experiment showed that the oxidation of the boronate to phenoxide 6 was rapid, as indicated by the subtle change in the chemical shifts of the diastereotopic hydrogens of the neopentyl ether group after 1 min (Fig. 2). Acyl aminal breakdown proceeded through quinone methide loss to form 7, decarboxylation to yield 8, and aminal breakdown to yield neopentyl alcohol and imine 9. The imine was not observed in this reaction due to its rapid reaction with water to yield the corresponding aldehyde. Aldehydes oxidize rapidly with basic hydrogen peroxide, making the loss of this component difficult to monitor in this experiment. Under different conditions, however, this decomposition process could be used to release alcohol and aldehyde cargo through a single triggering event. Alcohol release was slower than phenoxide formation, indicating that oxidation is not the rate-determining step when high peroxide concentrations are employed for the breakdown.
Scheme 3.
Peroxide-mediated alcohol release. [5]0 = 6 mM, [H2O2]0 = 90 mM, T = 300 K.
Fig. 2.
Time dependent release of neopentyl alcohol from 5. The AB pattern arises from 5 and the growing singlet arises from neopentyl alcohol. See Scheme 3 for experimental conditions.
Neopentyl alcohol was released at a similar rate to the decomposition of the carbamate (Fig. 2), as determined by integrating the growing methylene singlet from the alcohol and the AB system from the carbamate and comparing the intensities to THF, which was added as an internal standard. The yield of neopentyl alcohol was approximately 60% as determined by 1H NMR, but subsequent experiments that released less volatile alcohols showed that isolated yields are much higher (see below), suggesting that yield determination can be influenced by relaxation rate differences in comparison to the internal standard. Therefore determinations of the half lives for product formation provides a valid comparison for determining the relative reactivities of different analogs. We used the conversion at 35 min as the end point for t1/2 determinations. Using conversion levels at later times does not significantly change the values. The half life for the release of neopentyl alcohol from 5 was 12 min.
This facile approach to α-alkoxy carbamate synthesis allowed us to prepare a number of analogous structures (Fig. 3)13 in accord with our objective of determining the influence of structural perturbations in the triggering group, the nitrile group, and the alcohol on the rate of peroxide-mediated alcohol release. Steric hindrance around the leaving group was altered by preparing 10 from acetonitrile, while the electronics of the nitrile component were altered by synthesizing 11 and 12 from the corresponding electron-poor and electron-rich aromatic nitriles. The carbamate group was altered through the incorporation of a fluorine, a methoxy group, and a benzylic methyl branch (13–15). Carbamate 16 was prepared to study the impact of increasing the leaving group ability of the cargo. The selection of alcohols in these analogs was based on the presence of methylene groups that appear as AB quartets in the 1H NMR spectra of the carbamates then collapse to singlets upon release, thereby allowing decomposition to be monitored readily. Vinyl boronate 17 was prepared from the corresponding allylic chloroformate to determine the relative merits of utilizing the β-elimination of an enolate intermediate14 to promote acyl aminal breakdown. Benzyl carbamate 18 was synthesized as a control compound to verify that alcohol release can be attributed to boronate oxidation.
Fig. 3.
Structurally-varied carbamates.
The versatility of the synthetic protocol was further probed through the preparation of a number of additional structures that contain different triggering groups. Acetoxy carbamate 19 was prepared for cargo release in response to esterase-mediated cleavage. Azido carbamate 20 was prepared for cargo release in thioredoxin-rich environments. Nitro-substituted carbamate 21 was prepared for cargo release in the presence of nitrido reductase. The latter two compounds were designed for release in hypoxic environments that are associated with many forms of cancer.15
We exposed compounds 10, 11, and 12 to the oxidative fragmentation conditions to determine the influence of the group on the nitrile fragment on the rate of alcohol release. As shown in Fig. 4 very little difference was observed for these compounds, with 10 showing a t1/2 of 9 min, 11 showing a t1/2 of 12 min, and 12 showing a t1/2 of 9 min. The similarities of these numbers contrasts with the significant steric and electronic differences between the structures and demonstrates that the rate determining step in this process is not the release of the neopentyl alcohol from a tetrahedral intermediate similar to 8. The stability of control compound 18 toward oxidative fragmentation is also confirmed in Fig. 4. This demonstrates that the nitrile-derived subunit of these structures can be selected to facilitate the synthesis or to optimize a different property without consequence on the release rate.
Fig. 4.
Comparison of the rates of alcohol release from structurally-varied nitrile precursors. See Scheme 3 for experimental conditions. Experiments were run in triplicate.
The next phase of this project was to determine the impact of perturbations to the alkoxy group of the carbamate on the rate of alcohol release. The peroxide-mediated breakdowns of 13, 14, and 15 were compared to 5 (Fig. 5). While the incorporation of a fluorine atom to the benzene ring did not result in a significant change in the rate of alcohol release (t1/2 for 13 = 11 min), the addition of a methoxy group to the arene provided a substantial rate increase, with a t1/2 for 14 being 2 min. The addition of a methyl group at the benzylic site resulted in a noticeable rate increase, with a t1/2 of 6 min for 15. These results are consistent with benzyl cleavage being the rate determining step in this process.16 The addition of a branching alkyl group at the benzylic position and the incorporation of an electron-donating methoxy group to the arene facilitate the departure of the carboxyl group. These observations show that factors that stabilize a benzylic cation will promote breakdown. The negligible impact of incorporating a fluorine into the carbamate is therefore somewhat surprising since this substitution should inductively blunt the electron-donating capacity of the phenol that forms from boronate oxidation. We propose that this inductive stabilization can be balanced by the higher concentration of the more reactive phenoxide that results from the lower pKa of the fluorinated analog. The decomposition rates for 5 and 14 did not vary significantly when the substrate concentration was 3 mM and [H2O2] was 30 mM (see ESI†).
Fig. 5.
Impact of carbamate variation on alcohol release. See Scheme 3 for experimental conditions. Experiments were run in triplicate.
The influence of alcohol nucleofugacity on the cleavage rate was determined by comparing the rates of peroxide-mediated breakdowns of 5 and aryl ether 16. These processes were shown to proceed with essentially identical rates (t1/2 = 10 min for 16), as shown in Fig. 6, despite the greater stability of the phenoxide leaving group in comparison to an alkoxide leaving group. This outcome is consistent with the hypothesis that the facility of benzylic carbon–oxygen bond cleavage, and not tetrahedral intermediate breakdown, determines the rate of alcohol release. The decomposition of 16 appears to proceed in higher yield than the decomposition of 5, though this can be attributed to a difference in 1H relaxation rates in these NMR-derived plots.
Fig. 6.
Release as a function of alcohol structure. See Scheme 3 for experimental conditions. Experiments were run in triplicate.
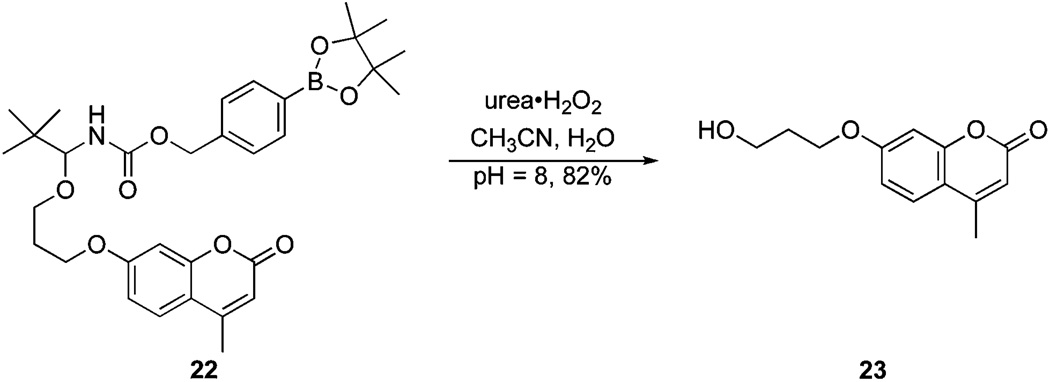
Allylic carbamate 17 differs from the previous compounds in that its peroxide-mediated oxidation forms an enolate rather than a phenoxide. β-Elimination leads to cargo release and the formation of acrolein. The breakdown of this compound, in comparison to rapidly reacting substrate 14 and parent structure 5, is shown in Fig. 7. This process is extremely fast, with a t1/2 of <2 min, showing that this structural variant will be useful for applications where extremely rapid cargo release is desirable. We prepared carbamate 22 in an effort to determine the yield of the released alcohol.13 The alkoxy group in 22 was selected to be sufficiently non-volatile and UV-active to facilitate isolation. Exposing 22 to the standard cleavage conditions (Scheme 4) for 1 h provided alcohol 23 in an 82% isolated yield, demonstrating that cargo release proceeds more effectively than the 1H NMR experiments would indicate.
Fig. 7.
Release from the allylic carbamate. See Scheme 3 for experimental conditions. Experiments were run in triplicate.
Scheme 4.
Determination of an isolated yield.
This method was applied to the preparation of a compound that is designed to deliver an anti-oxidant to mitochondria17 in an effort to prepare agents that mitigate radiation damage by suppressing the formation of reactive oxygen species.18 The design of the compound and its synthesis are shown in Scheme 5. The boronate carbamate group was employed to trigger compound release in the presence of the high peroxide concentrations in mitochondria.19 Pentamethyl chromanol (PMC, 24) was selected for release due to its ability to mitigate the effects of cytochrome c-mediated mitochondrial damage.20 The triphenylphosphonium ion was included to promote passage through mitochondrial membranes.21 Hydrozirconation of nitrile 25 followed by acylation with 2 and nucleophilic addition of 24 provided carbamate 26 in 58% yield. Removal of the silyl group followed by acylation with commercially available acid 27 provided 28 in good yield. The rapid synthesis of this compound shows that a more stable linkage to the phosphonium ion could be incorporated if the ester linkage is susceptible to esterase cleavage in cellular studies. Benzyl carbamate 29 was prepared as a control compound for comparison in breakdown studies.
Scheme 5.
Application to the synthesis of a potential mitigator of radiation damage.
The peroxide-mediated breakdown of 28 to release PMC was studied in aqueous media (phosphate buffer containing 5% EtOH) at pH 7.2 and 8.0 (the pH of the mitochondrial matrix).22 These studies were performed with micromolar concentrations of the carbamate and H2O2 at 37 °C to mimic biological conditions. These concentrations required that the reactions be monitored by HPLC using an electrochemical detector. Treating 28 (40 µM) with varying concentrations of H2O2 (10, 20, and 40 µM) resulted in the liberation of PMC in a dose-dependant manner (Fig. 8). The release rate was notably accelerated when the reaction was performed at higher pH. A pH = 8.0 reaction mixture containing 40 µM of 28 and H2O2 generated approximately 20 µM of PMC within 30 min, whereas the reaction at pH = 7.2 required 90 min to reach a similar concentration. The necessity of the boronate ester for carbamate breakdown and alcohol release was also demonstrated as PMC accumulation was not observed following exposure of 29 to H2O2 and the small amounts of PMC that were detected were not distinguishable from noise. The release of PMC from 28 proceeded much more slowly than the release of neopentyl alcohol from 5. This can be attributed to the rate reduction for boronate oxidation that results from the low concentrations. The rate of alcohol release is dependent on the rate of quinone methide formation, and the rate of quinone methide formation is dependent upon the concentration of the phenoxide intermediate that forms through a bimolecular reaction between the substrate and the hydroperoxide anion, thereby explaining the low release rates in these reactions.
Fig. 8.
PMC release from 28 in response to variations in pH and H2O2 concentration. Reactions were performed using 40 µM of 28 or 29 in 95% aqueous phosphate buffer/5% EtOH at 37 °C. Experiments were run in triplicate.
Conclusions
We have shown that the multicomponent sequence of nitrile hydrozirconation, acylation, and alcohol addition can be used for the rapid preparation of structurally diverse α-alkoxy carbamates. These carbamates have been designed to release the alcohol component in response to a precise chemical signal as an entry into the development of drug delivery vehicles. The utility of this approach has been demonstrated in a quick study of the relationship between structural features and the rate of peroxide-mediated alcohol release from boronate-substituted benzylic carbamates. This work showed that the release rate is controlled by the rate of quinone methide formation rather than boronate oxidation or tetrahedral intermediate collapse. Carbamates that cleave in the presence of nucleophiles and reducing agents have also been prepared to highlight the generality of the pathway. A complex that contains a triphenyl phosphonium ion for mitochondrial transport, a boronate-substituted benzylic carbamate as a peroxide-mediated trigger, and a chromanol derivative has been prepared for the delivery of anti-oxidants for studies on the directed mitigation of damage from reactive oxygen species. The ability of 28 and related structures to act as radiation mitigators is currently under investigation and the results will be reported elsewhere. We anticipate that the facile preparation of structurally diverse agents that can release cargo under defined conditions will prove to be useful for applications in targeted drug delivery and for controlled tailoring of materials.
Supplementary Material
Acknowledgements
This work was supported by generous funding from the National Science Foundation (CHE-0848299) and the National Institutes of Health (AI068021). We thank Ms Stephanie Garrell and Mr Akira Shimizu (recipient of a Brackenridge undergraduate research fellowship) for assistance in chloroformate synthesis. We thank Dr Detcho Stoyanovsky (Department of Occupational Health and Safety, University of Pittsburgh) for helpful discussions and technical assistance.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ob26571k
Notes and references
- 1.(a) Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite DW. Polymer Chem. 2011;2:773. [Google Scholar]; (b) Shabat D, Amir RJ, Gopin A, Pessah N, Shamis M. Chem.–Eur. J. 2004;10:2626. doi: 10.1002/chem.200305715. [DOI] [PubMed] [Google Scholar]
- 2.For examples of alcohol and thiol release through N-acyl aminal cleavage, see: Böhm G, Dowden J, Rice DC, Burgess I, Pilard J-F, Guilbert B, Haxton A, Hunter RC, Turner NJ, Flitsch SL. Tetrahedron Lett. 1998;39:3819. Meyer Y, Richard J-A, Delest B, Noack P, Renard P-Y, Romieu A. Org. Biomol. Chem. 2010;8:1777. doi: 10.1039/b926316k.
- 3.Gopin A, Pessah N, Shamis M, Rader C, Shabat D. Angew. Chem., Int. Ed. 2003;42:327. doi: 10.1002/anie.200390108. [DOI] [PubMed] [Google Scholar]
- 4.Lee M-R, Baek K-H, Jin HJ, Jung Y-G, Shin I. Angew. Chem., Int. Ed. 2004;43:1675. doi: 10.1002/anie.200353204. [DOI] [PubMed] [Google Scholar]
- 5.Abraham S, Guo F, Li L-S, Rader C, Liu C, Barbas CF, III, Lerner RA, Sinha SC. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5584. doi: 10.1073/pnas.0700223104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Wan S, Green ME, Park J-H, Floreancig PE. Org. Lett. 2007;9:5385. doi: 10.1021/ol702184n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeBenedetto MV, Green ME, Wan S, Park J-H, Floreancig PE. Org. Lett. 2009;11:835. doi: 10.1021/ol802764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Xiao Q, Floreancig PE. Org. Lett. 2008;10:1139. doi: 10.1021/ol8000409. [DOI] [PubMed] [Google Scholar]; (b) Lu C, Xiao Q, Floreancig PE. Org. Lett. 2010;12:5112. doi: 10.1021/ol102246d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Wu F, Green ME, Floreancig PE. Angew. Chem., Int. Ed. 2011;50:1131. doi: 10.1002/anie.201006438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wan S, Wu F, Rech JC, Green ME, Balachandran R, Horne WS, Day BW, Floreancig PE. J. Am. Chem. Soc. 2011;133:16668. doi: 10.1021/ja207331m. [DOI] [PubMed] [Google Scholar]
- 9.Fruehauf JP, Meysken FL., Jr Clin. Cancer Res. 2007;13:789. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 10.DiMauro S, Schon EA. Annu. Rev. Neurosci. 2008;31:91. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 11.(a) Lippert AR, Gschneidtner T, Chang CJ. Chem. Commun. 2010;46:7510. doi: 10.1039/c0cc01560a. [DOI] [PubMed] [Google Scholar]; (b) Srikun D, Miller EW, Domaille DW, Chang CJ. J. Am. Chem. Soc. 2008;130:4596. doi: 10.1021/ja711480f. [DOI] [PubMed] [Google Scholar]
- 12.For other examples of peroxide-mediated fragmentation reactions of aryl boronates, see: Lippert AR, Van de Bittner GC, Chang CJ. Acc. Chem. Res. 2011;44:793. doi: 10.1021/ar200126t. Major Jourden JL, Cohen SM. Angew. Chem., Int. Ed. 2010;49:6795. doi: 10.1002/anie.201003819. Broaders KE, Grandhe S, Fréchet JMJ. J. Am. Chem. Soc. 2011;133:756. doi: 10.1021/ja110468v. Sella E, Shabat D. Chem. Commun. 2008:5701. doi: 10.1039/b814855d. Nuñez SA, Yeung K, Fox NS, Phillips ST. J. Org. Chem. 2011;76:10099. doi: 10.1021/jo2018763. Karton-Lifshin N, Segal E, Omer L, Portnoy M, Satchi-Fainaro R, Shabat D. J. Am. Chem. Soc. 2011;133:10960. doi: 10.1021/ja203145v.
- 13.Please see the ESI† for details on the syntheses of these compounds.
- 14.Song F, Watanabe S, Floreancig PE, Koide K. J. Am. Chem. Soc. 2008;130:16640. doi: 10.1021/ja805678r. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WR, Hay MP. Nat. Rev. Cancer. 2011;11:393. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 16.For similar fragmentation rate effects, see: Hay MP, Sykes BM, Denny WA, O’Connor CJ. J. Chem. Soc., Perkin Trans. 1. 1999:2759. Schmid KM, Jensen L, Phillips ST. J. Org. Chem. 2012;77:4363. doi: 10.1021/jo300400q.
- 17.Hoye AT, Davoren JE, Wipf P, Fink MP, Kagan VE. Acc. Chem. Res. 2008;41:87. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- 18.(a) Zhao K, Zhao G-M, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. J. Biol. Chem. 2004;279:34682. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]; (b) Kanai A, Zabbarova I, Amoscato A, Epperly M, Xiao J, Wipf P. Org. Biomol. Chem. 2007;5:307. doi: 10.1039/b613334g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaban RS, Nemeto S, Finkel T. Cell. 2005;120:483. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 20.(a) Samhan-Arias AK, Tyurina YY, Kagan VE. J. Clin. Biochem. Nutr. 2011;48:91. doi: 10.3164/jcbn.11-009FR. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Staniek K, Rosenau T, Gregor W, Nohl H, Gille L. Biochem. Pharmacol. 2005;70:1361. doi: 10.1016/j.bcp.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 21.(a) Smith RAJ, Porteous CM, Coulter CV, Murphy MP. Eur. J. Biochem. 1999;263:709. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]; (b) Muratovska A, Lightowlers RN, Taylor RW, Wilce JA, Murphy MP. Adv. Drug Delivery Rev. 2001;49:189. doi: 10.1016/s0169-409x(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 22.(a) Chacon E, Reece JM, Nieminen A-L, Zahrebelski G, Herman B, Lemasters JJ. Biophys. J. 1994;66:942. doi: 10.1016/S0006-3495(94)80904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6803. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.