Abstract
The intracellular mitogen-activated protein kinase (MAPK) pathway in the brain is necessary for the formation of a variety of memories including conditioned taste aversion (CTA) learning. However, the functional role of MAPK activation in the amygdala during lithium chloride (LiCl)-induced CTA learning has not been established. In the present study, we investigated if local microinjection of SL327, a MAPK kinase inhibitor, into the rat amygdala could alleviate LiCl-induced CTA learning. Our results revealed that acute administration of a high dose of LiCl (0.15 M, 12 ml/kg, i.p.) rapidly increased the level of phosphorylated MAPK (pMAPK)-positive cells in the central nucleus of the amygdala (CeA) and nucleus of the solitary tract (NTS) of rats as measured by immunohistochemistry. Local microinjection of SL327 (1 µg/0.5 µl/hemisphere) into the CeA 10 min before LiCl administration decreased both the strength of LiCl-induced CTA paired with 0.125% saccharin and the level of LiCl-induced pMAPK-positive cells in the CeA, but not in the NTS. Our data suggest that the intracellular signaling cascade of the MAPK pathway in the CeA plays a critical role in the processing of visceral information induced by LiCl for CTA learning.
Keywords: Mitogen-activated protein kinase, Conditioned taste aversion, Lithium chloride, SL327, Central nucleus of the amygdala, Nucleus of the solitary tract
1. Introduction
Conditioned taste aversion (CTA) is a form of associative learning in which an animal avoids a taste after pairing of a novel taste (conditioned stimulus, CS) with a toxin (unconditioned stimulus, US; Garcia et al., 1974). Toxicity of the US, e.g. lithium chloride (LiCl), evokes illness like nausea, diarrhea or physical discomfort in animals. The amygdala has been implicated in many forms of emotional and aversive conditioning (LeDoux, 1993; Gallagher and Chiba, 1996), and the amygdala is involved in the central processing of the toxic US. It has been reported that lesions (Lasiter and Glanzman, 1985; Yamamoto et al., 1995; Schafe and Bernstein, 1996) or blockade of synaptic transmission of the amygdala (Roldan and Bures, 1994) impaired CTA induced by LiCl in rats. LiCl toxicity also increases expression of some activator protein 1 (AP-1) transcription factors in the central nucleus of the amygdala (CeA) (Yamamoto et al., 1992; Swank, 1999; Spencer and Houpt, 2001; Kwon et al., 2008; Kwon and Houpt, 2010a and b). LiCl also induces c-Fos in the brainstem nucleus of the solitary tract (NTS), which projects directly and indirectly to the CeA (Yamamoto et al., 1992; Houpt et al., 1994; Swank and Bernstein, 1994; Swank, 1999). The gene expression and protein synthesis in the amygdala following LiCl is necessary for the formation of CTA memory because administration of anisomycin, a protein synthesis inhibitor, into the rat amygdala impaired CTA acquisition (Lamprecht and Dudai, 1996).
LiCl-induced gene expression in the amygdala may result from the molecular signaling cascades that are induced by LiCl-induced chemoreceptive stimulation. The visceral pathway of LiCl toxicity is indirectly relayed to the amygdala from the NTS and area postrema (Schafe and Bernstein, 1996 and 1998; Sakai and Yamamoto, 1999) via the release of neurotransmitters such as glutamate (Yasoshima et al., 2000; Miranda et al., 2002) and glucagon-like peptide-1 (Seeley et al., 2000; Kinzig et al., 2002). It has been reported that acute administration of a high dose of LiCl activated the intracellular signaling pathway involving mitogen-activated protein kinases (MAPKs) in the CeA (Swank, 2000a), NTS (Swank, 2000b) and insular cortex (Swank, 2000a). Conversely, novel taste experience did not activate MAPKs in the CeA (Swank, 2000a) and NTS (Swank et al., 1996), but taste stimulation did activate MAPKs in the insular cortex (Berman et al., 1998; Belelovsky et al., 2005; Yefet et al., 2006). This suggests that MAPK activity in the CeA and NTS is more associated with the visceral stimulation induced by LiCl than the gustatory stimulation induced by a novel taste.
MAPKs are a family of protein kinases that phosphorylate threonine and tyrosine residues of target proteins or themselves. MAPKs are also known as extracellular signal-regulated kinases (ERKs), which amplify and transfer the information of extracellular signals from the membrane to the cytoplasm and nucleus. MAPKs are activated by phosphorylation by their upstream kinases, MAPK/ERK kinases (MEKs), as the result of a variety of extracellular stimuli such as growth factors, stress, osmotic and heat shock, hormones, and cytokines (Clayton and Mahadevan, 2003). MAPKs also have a broad number of downstream targets such as transcription factors, cytoskeletal proteins, regulatory enzymes and kinases. In addition, the MAPK signaling pathway in the brain plays an important role in formation of a variety of memories (e.g. fear conditioning (Atkins et al., 1998; Schafe et al., 1999 and 2000; Villarreal and Barea-Rodriguez, 2006), spatial water maze learning (Selcher et al., 1999; Blum et al., 1999), aversive olfactory learning (Zhang et al., 2003) and recognition memory (Kelly et al., 2003)) including synaptic and neuronal plasticity (Sweatt, 2001; Adams and Sweatt, 2002; Thomas and Huganir, 2004; Peng et al., 2010).
Previous studies have provided evidence that the MAPK pathway is critical for LiCl-induced CTA learning. Systemic injection of a MEK inhibitor attenuated LiCl-induced CTA learning in mice (Swank and Sweatt, 2001) and rat pups (Languille et al., 2009). Local microinjection of a MEK inhibitor into the rat insular cortex (Berman et al., 1998) and mouse fourth ventricle (Swank, 2000b) before conditioning attenuated LiCl-induced CTA learning. However, the functional role of MAPK activation in the amygdala during LiCl-induced CTA learning has not been established.
In the present study, we investigated if local microinjection of SL327, a MEK inhibitor, into the rat amygdala could alleviate LiCl-induced CTA learning. Our results revealed that LiCl rapidly increased the level of phosphorylated MAPK (pMAPK) in the CeA and NTS of rats. Local microinjection of SL327 into the CeA before LiCl administration decreased both the strength of LiCl-induced CTA and the level of LiCl-induced pMAPK in the CeA, but not in the NTS. Our data suggest that the MAPK signaling pathway in the CeA plays a critical role in LiCl-induced CTA learning.
2. Materials and Methods
2.1 Animals
Adult male Sprague-Dawley rats (300–450 g, Charles River Laboratories, Wilmington, MA) were individually housed under a 12-h light –12-h dark cycle (lights on 07:00) at 25 °C with free access to Purina rodent chow and distilled water. All experiments and procedures were conducted in the first half of the lights-on period. Anesthesia (isoflurane and sodium pentobarbital) was used to minimize pain and discomfort. All experiments were approved by the Florida State University institutional animal care and use committee.
2.2 Intra-amygdala cannulation
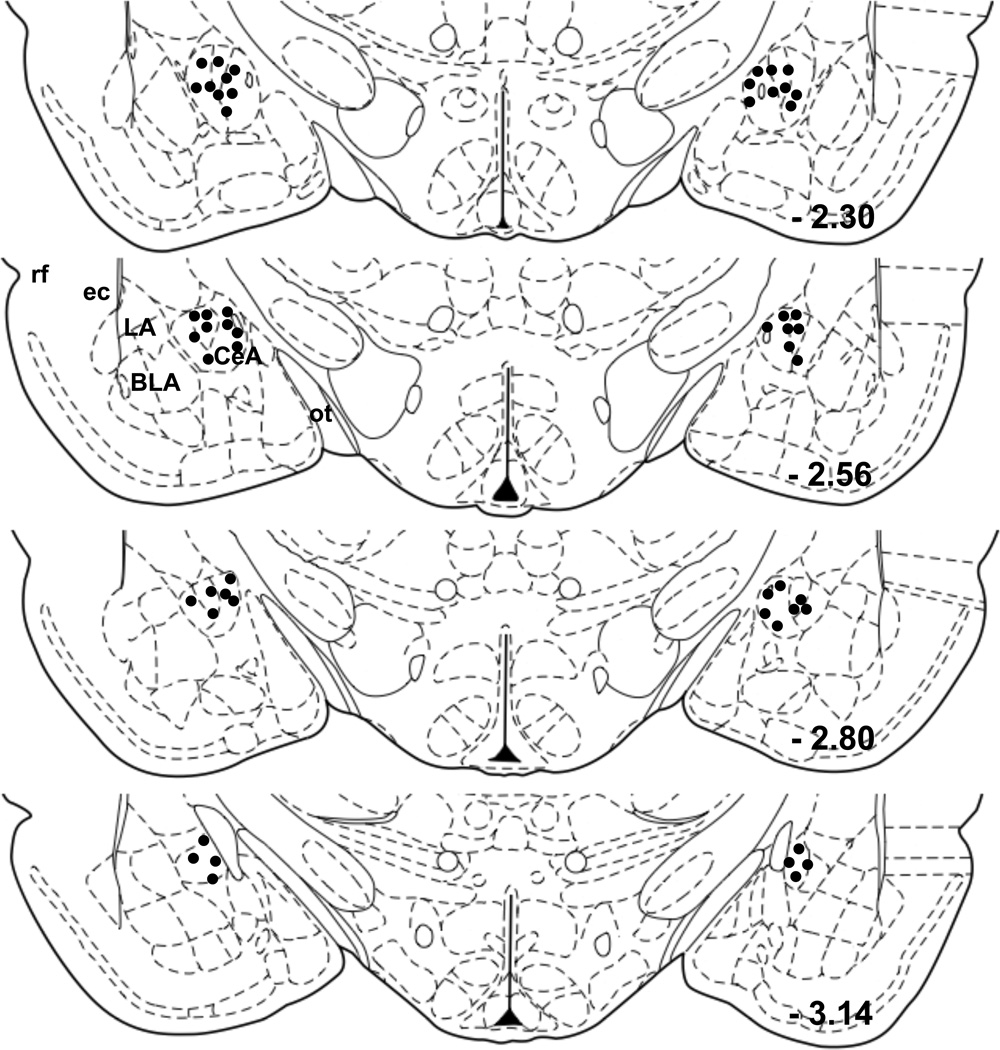
Under isoflurane anesthesia, rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and bilateral 22-gauge guide cannulae (Plastics One, Inc., Roanoke, VA) were implanted. Cannulae were directed at the CeA, with the tip of the guide cannula positioned 2.5 mm anteroposterior, 4.3 mm mediolateral from bregma and 8.5 mm dorsoventral from the skull surface (stereotaxic coordinates based on the rat brain atlas (Paxinos and Watson, 1997)). Dummy cannulae extending 1.0 mm beyond the guide cannula were inserted to prevent clogging. Screws were anchored to the skull and the assembly was fixed in place using dental acrylic. As an analgesic and anti-inflammatory drug, all rats received ketoprofen subcutaneously (2.5 mg/0.5 ml). After surgery, rats were given at least 10 days for recovery. To confirm cannula orientations, brain sections were examined under a microscope at the end of all experiments (See Figures 4 and 6). Rats showing the cannula placement outside CeA were excluded in the present study. Animal numbers shown in experiment 2 and 3 are those who had the cannula placement inside CeA (see Figure 4).
Figure 4.
Diagram showing the location of bilateral SL327 microinjection sites within the CeA. Black dots indicate the location of the tip of guide cannulae for rats whose data were used in analyses in experiment 2 and 3 (coronal rat brain sections, spanning −2.30 mm through −3.14 mm relative to bregma; modified from Paxinos and Watson, 1997). rf, rhinal fissure; ec, external capsule; ot, optic tract.
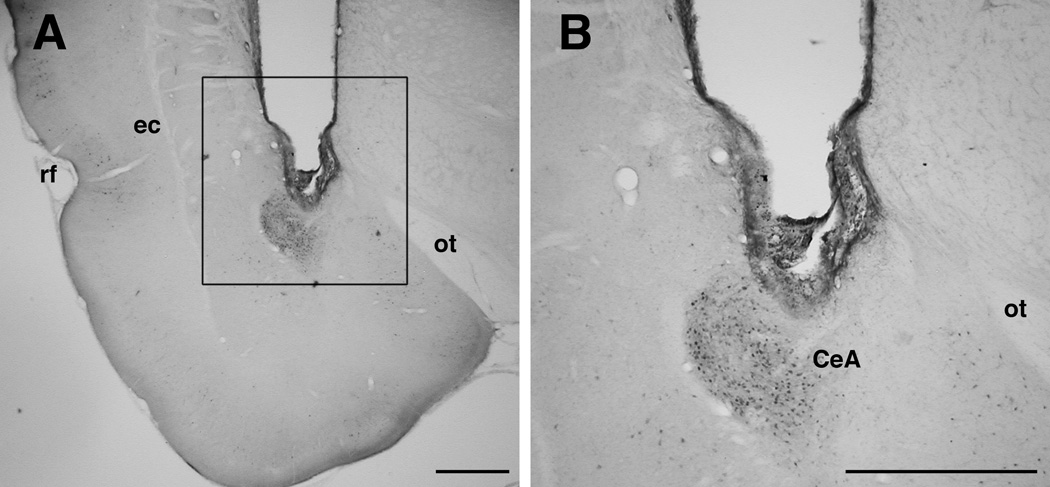
Figure 6.
Photomicrograph of cannula placement and pMAPK immunohistochemistry in the CeA after vehicle injection into the CeA and systemic LiCl injection. B is magnification of inset in A. rf, rhinal fissure; ec, external capsule; ot, optic tract. Scale bars, 1 mm.
2.3 Immunohistochemistry
Rats were anesthetized with sodium pentobarbital and perfused first with 100 ml of isotonic saline containing 0.5% sodium nitrite and 1000 U heparin, and then with 400 ml phosphate-buffered 4% paraformaldehyde. Brains were dissected out and post-fixed for 3 h, then cryoprotected in 30% sucrose for 2–3 days. Brain sections were cut at 40 µm at − 20°C by microtome, and washed twice in 0.1 M phosphate-buffered saline (PBS) for 10 min. After PBS washes, sections were washed in 0.2% Triton-1% bovine serum albumin (BSA)-PBS for 30 min, and washed in PBS-BSA for 10 min twice. Sections were incubated with primary antibodies in PBS-BSA at room temperature for 20 h. The primary antisera used were anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) rabbit mAb (#4370, 1:500, Cell Signaling Technology) and anti-c-Fos (Ab-5, 1:20,000, Oncogene Research). Sections were washed in PBS-BSA for 10 min twice, and incubated for 1 h with the biotinylated goat anti-rabbit antibody (Vector Laboratories) at a dilution of 1:200 in PBS-BSA. After washes in PBS-BSA for 10 min twice, antibody complexes were amplified using the Elite Vectastain ABC kit (Vector Laboratories), and visualized by a 5-min reaction in 0.05% 3,3-diaminobenzidine tetrahydrochloride. Sections were immediately washed twice in 0.1 M phosphate buffer and mounted on gelatin-coated slides. Sections on the slides were stained with Methyl Green Nuclear Counterstain (Vector Laboratories) and coverslipped with Permount.
2.4 Quantification and statistical analysis
For the chromogenic immunohistochemistry, cells expressing darkly-positive, nuclear staining were quantified with custom software (MindsEye, T. Houpt). Regions were digitally-captured at 40× magnification on a Macintosh computer using an Olympus Provis AX-70 microscope with a Dage-MTI DC-330 CCD camera and Scion LG-3 framegrabber. Counting was restricted to the BLA, CeA, LA and NTS as delineated by a hand-drawn outline based on the rat brain atlas (Paxinos and Watson, 1997). Bilateral cell counts were averaged for 6 sections of the amygdala and NTS for each rat. The individual mean counts for each region were then averaged across rats within experimental groups.
Significant effects across treatment groups were detected by one-way or two-way ANOVA and Newman-Keuls post-hoc tests (Kaleidagraph, Synergy Software). All data are presented as the mean ± standard error of the mean.
2.5 Experiment 1. The level of pMAPK after LiCl
In order to investigate whether LiCl increases the level of pMAPK in the amygdala and NTS, rats were sacrificed 10 min, 30 min, 1 h and 3 h after LiCl injections (0.15 M, 12 ml/kg, i.p., n=6 per time point) or NaCl injections (0.15 M, 12 ml/kg, i.p., n=4 per time point). Their brains were processed for immunohistochemistry to measure the level of pMAPK as described above.
2.6 Experiment 2. Effect of SL327 on CTA learning
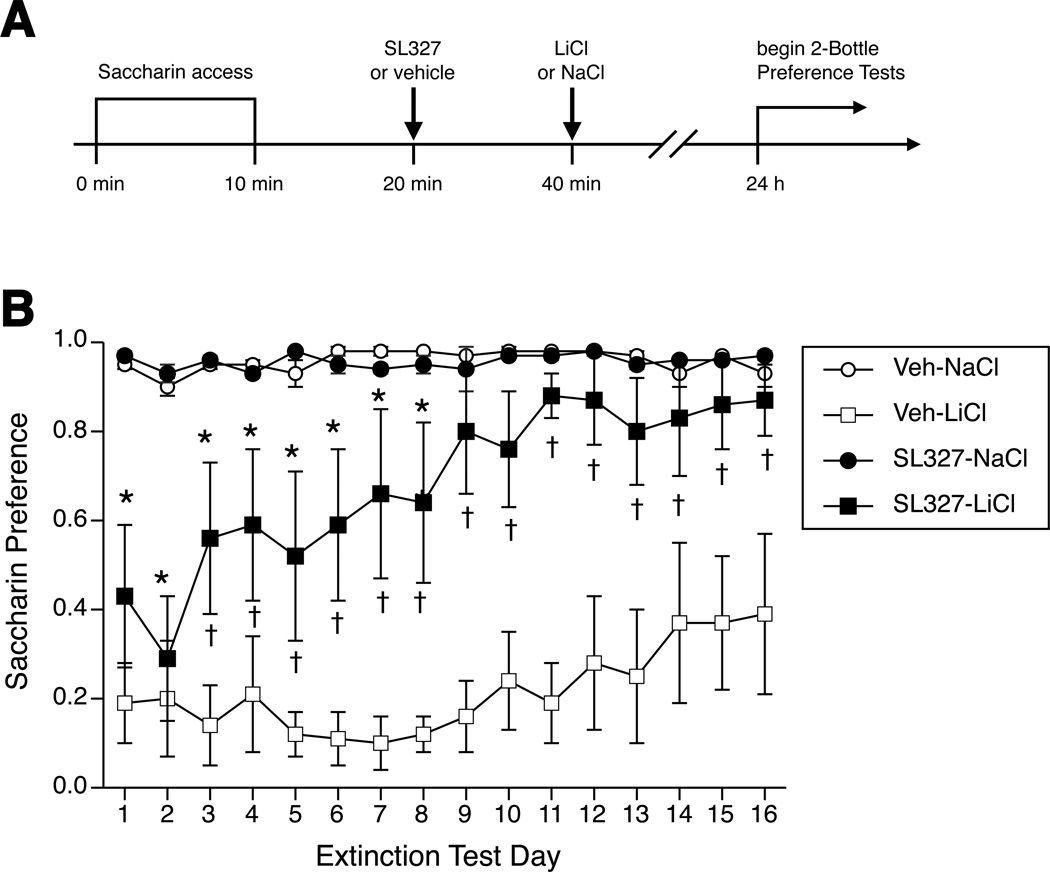
A schematic of timeline for Experiment 2 is shown in Figure 5A. Eight days prior to the CTA conditioning day, the rats were placed on a water deprivation schedule under which they received daily water access in one drinking session. The initial session was 3 h in length and the session times were diminished each day so that the day before conditioning the rats received their water in a 10-min session. On the conditioning day, rats were divided into 4 different groups (Vehicle (Veh)-NaCl, Veh-LiCl, SL327-NaCl, and SL327-LiCl) and given a 10-min access to a bottle containing 0.125 % saccharin solution. Ten minutes after the end of saccharin intake, the SL327-LiCl (n=6) and the SL327-NaCl groups (n=5) were injected bilaterally with a MEK inhibitor (SL327, Sigma, 1 µg/0.5 µl/hemisphere in 50% DMSO in saline). This dose was chosen according to previous reports showing that microinjection of U0126, a structural analog of SL327, into the BLA or hippocampus had a significant effect on the memory of fear conditioning and MAPK activation in rats (Schafe et al., 2000) and mice (Fischer et al., 2007). The Veh-NaCl (n=4) and Veh-LiCl (n=6) groups were microinjected bilaterally with vehicle (50% DMSO in saline) via 28 gauge injectors. The bilateral microinjections of SL327 or vehicle were performed for 1 min by a mechanical pump. The injectors were left in place 1 min before being withdrawn to avoid backflow along the injection tract. Ten minutes after microinjections of SL327 or vehicle into the CeA, the Veh-LiCl and SL327-LiCl groups were injected with LiCl (0.15 M, 12 ml/kg, i.p.) and the Veh-NaCl and SL327-NaCl groups were injected with NaCl (0.15 M, 12 ml/kg, i.p.). Three hours after conditioning, rats received ad libitum access to water overnight. The next day, CTA acquisition and extinction was measured with 24-h, 2-bottle preference tests. Rats were given 24-h free access to both saccharin and water bottles. Bottles were placed side by side, and the placement of the bottles was alternated each day to observe possible position bias. Consumption of each solution was measured by weighing the bottles daily for 16 days. The preference score was calculated for each rat for each day by dividing the saccharin consumed by the total fluid consumed (saccharin/(water + saccharin)). A score of 1.0 indicates that all fluid intake was saccharin. A low preference score indicates intake largely of water, and thus an aversion to saccharin.
Figure 5.
A. Timeline for Experiment 2. Water-restricted rats were given 10-min access to 0.125% saccharin, then injected bilaterally with either SL327 (1 µg/0.5 µl/hemisphere in 50% DMSO in saline) or vehicle into the CeA, followed by a systemic injection of LiCl or NaCl (0.15 M, 12 ml/kg, i.p.) The day after conditioning 2-bottle preference tests were begun to assess CTA. B. Saccharin preference scores measured by 24-h, 2-bottle preference tests during CTA extinction. Rats injected with vehicle or SL327 before NaCl (Veh-NaCl and SL327-NaCl group) showed a high preference for saccharin. Rats injected with vehicle before LiCl (Veh-LiCl group) showed a significantly lower preference for saccharin across all 16 test days compared to the Vehicle-NaCl group. Rats injected with SL327 before LiCl initially showed a significantly lower saccharin preference compared to the Veh-NaCl group, but showed a significantly higher saccharin preference compared to the Veh-LiCl group from test day 9 onwards such that their CTA rapidly extinguished. * p <0.05 SL327-LiCl vs. Veh-NaCl, † p < 0.05 SL327-LiCl vs. Veh-LiCl.
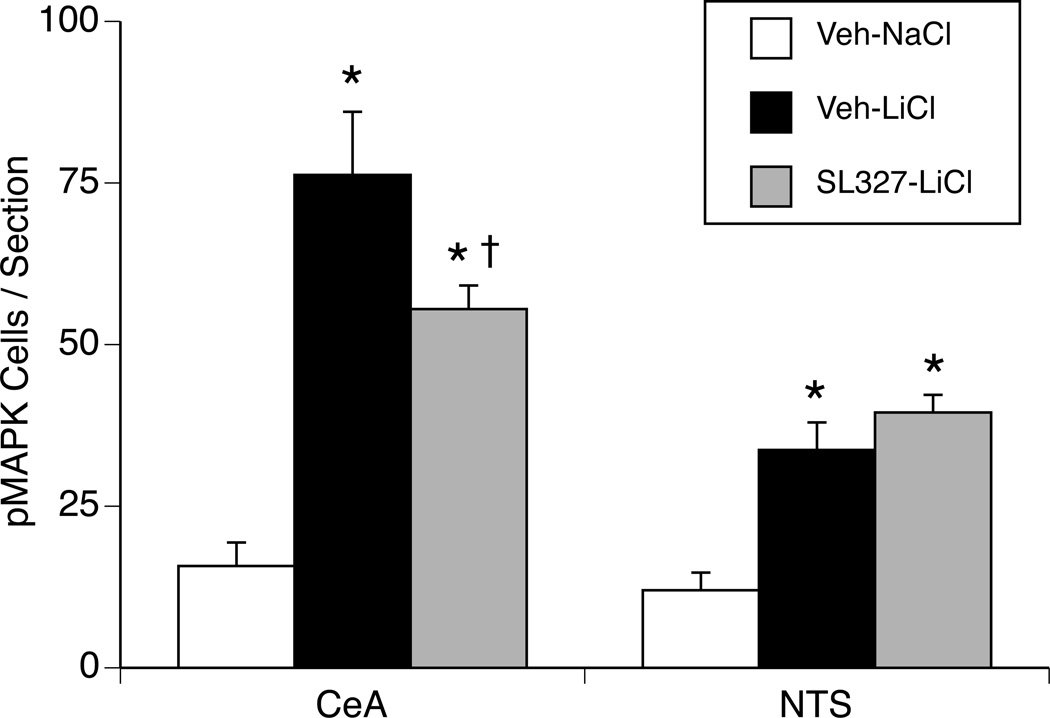
2.7 Experiment 3. Effect of SL327 on LiCl-induced pMAPK
In order to confirm that SL327 decreases the level of LiCl-induced pMAPK in the amygdala, rats were microinjected with SL327 into the CeA before a LiCl injection. On the experiment day, rats were divided into three different groups (Veh-NaCl, Veh-LiCl and SL327-LiCl, n=4 per group). The SL327-LiCl group was microinjected bilaterally with SL327 (1 µg/0.5 µl/hemisphere in 50% DMSO in saline). The Veh-NaCl and Veh-LiCl groups were microinjected bilaterally with vehicle (50% DMSO in saline). Intra-amygdala microinjection was performed as described in experiment 2. Ten minutes after microinjections of SL327 or vehicle into the CeA, the Veh-LiCl and SL327-LiCl groups were injected with LiCl (0.15 M, 12 ml/kg, i.p.) and the Veh-NaCl group was injected with NaCl (0.15 M, 12 ml/kg, i.p.). All rats in the three groups were sacrificed 45 min after LiCl or NaCl injections. Their brains were processed for immunohistochemistry to measure the levels of pMAPK as described above. This time point (45 min after LiCl or NaCl) was originally selected to measure the levels of both pMAPK and c-Fos expression. However, we found a high basal level of c-Fos induction around cannulation areas, which might be caused by the tissue damage (Herrera and Robertson, 1996) rather than LiCl stimulation. (There was no comparable induction of pMAPK immunoreactivity by cannulation per se, and pMAPK staining was clearly localized in the CeA; see Figure 7) Thus, the results of c-Fos immunohistochemistry were excluded in the present study.
Figure 7.
Quantification of pMAPK-positive cells in the CeA and NTS. Rats were injected with SL327 or vehicle into the CeA 10 min before LiCl or NaCl. Both LiCl-injected groups (Veh-LiCl and SL327-LiCl) showed a significant increase in the number of pMAPK-positive cells in the CeA and NTS compared to the NaCl-injected group (Veh-NaCl). Injection of SL327 into the CeA significantly decreased the number of LiCl-induced pMAPK-positive cells in the CeA, but not in the NTS. * p <0.005 vs. Veh-NaCl, † p < 0.05 vs. Veh-LiCl.
3. Results
3.1 Experiment 1. LiCl increases pMAPK in the CeA and NTS
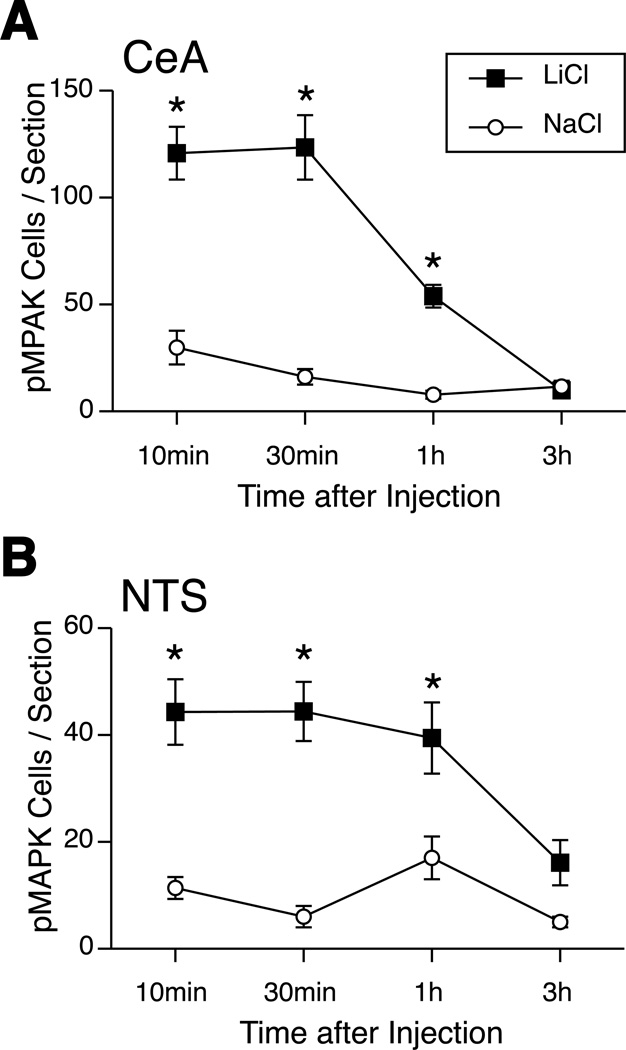
The number of pMAPK-positive cells in the amygdala was quantified by immunohistochemistry 10 min, 30 min, 1 h and 3 h after either LiCl or NaCl injections (Figure 1). In the CeA, two-way ANOVA showed that there was a significant interaction of treatment and time on the number of pMAPK-positive cells (F(3,47) = 19.85, p < 0.0001; Figure 3A). LiCl significantly increased the number of pMAPK-positive cells in the CeA at 10 min, 30 min, and 1 h compared to the NaCl-injected groups. The number of pMAPK-positive cells returned to the level of the NaCl-injected group at 3 h.
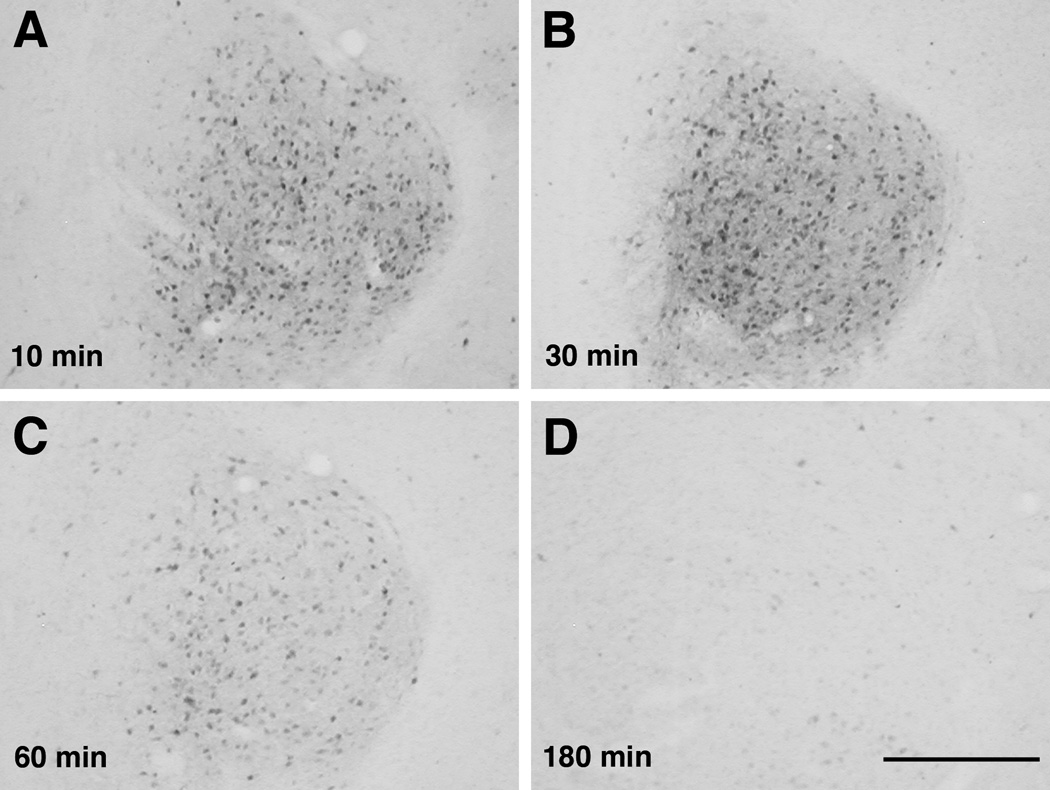
Figure 1.
Photomicrographs of pMAPK immunohistochemistry in the CeA after LiCl administration. Rats were sacrificed 10, 30, 60 or 180 min after LiCl (0.15 M, 12 ml/kg, i.p.). LiCl greatly increased the number of pMAPK-positive cells at 10–60 min; at 3 h after LiCl the number of pMAPK-positive cells returned to the level of NaCl-injected rats (not shown). Scale bar, 500 µm.
Figure 3.
Quantification of pMAPK-positive cells in the CeA and NTS at 10 min, 30 min, 1 h and 3 h following LiCl (0.15 M, 12 ml/kg, i.p.) or NaCl. LiCl greatly increased the number of pMAPK-positive cells in the CeA (A) and NTS (B) from 10 to 60 min compared to the NaCl-injected groups. * p <0.005 vs. NaCl.
LiCl did not increase the number of pMAPK-positive cells in the BLA and LA (data not shown). The number of pMAPK-positive cells was very low in the BLA (<15 cells/section) and LA (<6 cells/section) at all time points.
LiCl also significantly increased the number of pMAPK-positive cells in the NTS at 10 min, 30 min, and 1 h compared to the NaCl-injected group (Figure 2). The greatest density of pMAPK-positive cells was found medially in the subpostremal and intermediate NTS (abutting the fourth ventricle); very few cells were seen caudal to the obex or in the rostral (gustatory) NTS. Two-way ANOVA showed that there was a significant interaction of treatment and time on the number of pMAPK-positive cells (F(3,34) = 2.88, p < 0.05; Figure 3B). The number of pMAPK-positive cells returned to the level of the NaCl-injected group at 3 h.
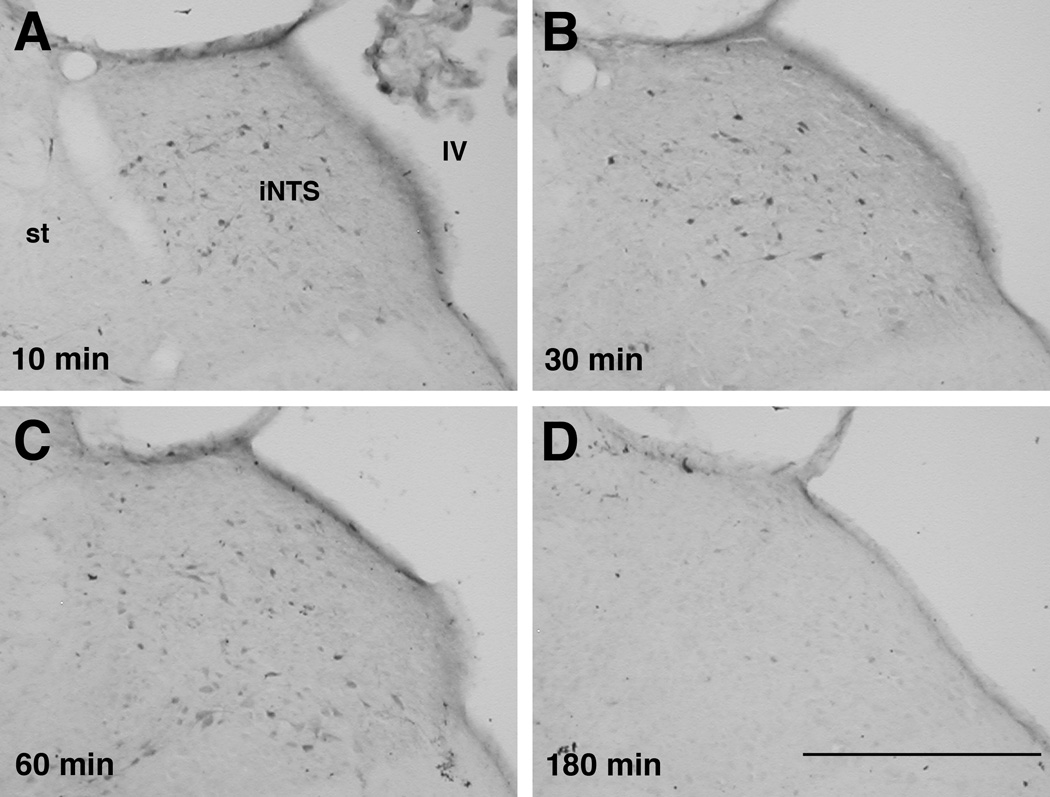
Figure 2.
Photomicrographs of pMAPK immunohistochemistry in the NTS after LiCl administration. As in the CeA, LiCl greatly increased the number of pMAPK-positive cells in the medial intermediate and subpostremal NTS at 10–60 min, which by 3 h returned to the level of NaCl-injected rats. st, solitary tract; iNTS, medial intermediate NTS; IV, fourth ventricle. Scale bar, 500 µm.
The area postrema and dorsal motor nucleus of the vagus were also present on the brainstem sections adjacent to the NTS. Despite the critical role of the area postrema in neuronal activation by systemic LiCl (e.g. Spencer et al. 2011), only diffuse staining was seen in the area postrema that did not distinctly label soma, and it did not appear to vary among the groups. In the dorsal motor nucleus, large soma were very lightly stained but also did not vary among the groups.
3.2 Experiment 2. Microinjection of SL327 into the CeA decreases LiCl-induced CTA learning
Because it is known that SL327 decreases stimuli-induced MAPK activation by inhibiting upstream kinases of MAPK, the effect of SL327 microinjection into the CeA on LiCl-induced CTA was examined. The location of cannula tips within the amygdala is diagrammed in Figure 4.
On the day of conditioning, average intake of saccharin was 12.7 ± 0.7 g. On the first day of 2-bottle testing, one-way ANOVA showed a significant effect of treatment (F(3,17) = 12.9, p < 0.0005; Figure 5B), such that both the LiCl-injected groups (Veh-LiCl and SL327-LiCl) were not different from each other but showed a significantly decreased preference for saccharin compared to the NaCl-injected groups (Veh-NaCl and SL327-NaCl).
Across the 16 days of 2-bottle preference testing, 2-way ANOVA found a significant interaction of group and test day (F(45, 55 = 1.5, p < 0.05; Figure 5B). Rats in the Veh-NaCl and SL327-NaCl groups showed a high preference for saccharin. Both LiCl-injected groups initially showed very low preference for saccharin, demonstrating acquisition of CTA against saccharin. The saccharin preference of the Veh-LiCl rats showed almost no extinction of their CTA, and remained significantly lower than the Veh-NaCl group across all 16 days. The preference of the SL327-LiCl rats, however, rapidly increased; by day 3 they showed a significantly higher preference than the Veh-LiCl rats and their saccharin preference was not significantly different from the control Veh-NaCl rats by day 9. Thus, the SL327-LiCl rats showed only a transient CTA and rapid extinction.
3.3 Experiment 3. Microinjection of SL327 into the CeA decreases LiCl-induced pMAPK
To determine if SL327 decreases the level of LiCl-induced pMAPK, rats were microinjected with SL327 into the CeA 10 min before LiCl. An example of cannula placement and pMAPK immunoreactivity in the CeA is shown in Figure 6. One-way ANOVA showed that there was a significant effect of treatment for the numbers of pMAPK-positive cells in the CeA (F(2,11) = 25.16, p < 0.0005; Figure 7). Both the LiCl-injected groups (Veh-LiCl and SL327-LiCl) showed a significant increase in the number of pMAPK-positive cells in the CeA compared to the NaCl-injected group (Veh-NaCl). However, microinjection of SL327 into the CeA significantly decreased the number of LiCl-induced pMAPK-positive cells in the CeA (Veh-LiCl vs. SL327-LiCl).
The numbers of pMAPK-positive cells in the NTS were significantly increased by LiCl (F(2,11) = 15.45, p < 0.005; Figure 7). There was no difference in the levels of pMAPK in the NTS between the LiCl-treated groups (Veh-LiCl vs. SL327-LiCl). Thus, MEK inhibition in the CeA did not alter pMAPK induction in the NTS.
4. Discussion
In the present study, we established that systemic administration of a high dose of LiCl greatly increased the level of pMAPK in the CeA and NTS. Local microinjection of SL327, a MEK inhibitor, into the CeA decreased both the strength of LiCl-induced CTA and the level of LiCl-induced pMAPK. This is the first demonstration showing that the MAPK signaling pathway in the CeA plays a critical role in LiCl-induced CTA learning.
We first investigated the time course of MAPK activation in the amygdala and NTS following LiCl administration. LiCl rapidly increased the numbers of pMAPK-positive cells in the CeA and NTS, but not in the BLA and LA, as measured by immunohistochemistry. This result is consistent with the previous reports showing that MAPK is activated in the CeA (Swank, 2000a) and NTS (Swank, 2000b) after LiCl injection. The pattern of LiCl-induced MAPK activation in this study closely corresponded to that of c-Fos expression in the CeA and NTS as a neuronal activation marker following LiCl administration (Houpt et al., 1994; Swank and Bernstein, 1994; Swank, 2000a and 2000b; Spencer and Houpt, 2001; Spencer et al., 2011). This supports the schema that LiCl-induced chemoreceptive stimulation reaches the amygdala via ascending transynaptic input from the NTS; intracellular signaling at both the forebrain and hindbrain sites involves the activation of the MAPK signaling pathway.
LiCl-induced pMAPK may be downstream of the protein kinase A (PKA) pathway that activates cAMP response element (CRE)-binding protein (CREB) and other transcription factors to induce gene expression (Sweatt, 2001; Adams and Sweatt, 2002; Waltereit and Weller, 2003). Previous studies have provided evidence that the intracellular signaling pathway involving cAMP, PKA, and CREB is activated in the amygdala after LiCl administration, perhaps upstream of c-Fos induction (Lamprecht et al., 1997; Swank, 2000a; Koh et al., 2002 and 2003). Thus, c-Fos induction in the CeA after LiCl stimulation may be regulated by the activation of MPAK. Unfortunately, in the present study we were unable to examine the level of LiCl-induced c-Fos in the CeA after reduction of MAPK activity by SL327 because a high basal level of c-Fos induction was detected around cannulation areas, which might be caused by the tissue damage (Herrera and Robertson, 1996) rather than LiCl stimulation.
To establish a functional role for MAPK of the CeA in CTA learning, we microinjected SL327, a MEK inhibitor, into the CeA just before LiCl administration. Reduction of LiCl-induced MAPK activity by SL327 in the CeA decreased the strength of LiCl-induced CTA during extinction. SL327 by itself did not induce CTA. This result is similar with previous reports showing that MAPK activity in the insular cortex (Berman et al., 1998) and fourth ventricle (Swank, 2000b) is critical for LiCl-induced CTA learning. In addition, other studies (Swank and Sweatt, 2001; Languille et al., 2009) also demonstrated that systemic injection of a MEK inhibitor attenuated LiCl-induced CTA learning. Blockade of the MAPK signaling pathway in the CeA, insular cortex or brain stem during CTA learning may block processing or consolidation of the visceral and gustatory information, which results in attenuated or impaired CTA acquisition.
Unlike in the insular cortex, however, gustatory stimulation by a novel taste does not increase the MAPK activity in the CeA (Swank, 2000a). This suggests that MAPK activity in the CeA is correlated with LiCl-induced visceral stimulation while MAPK activity in the insular cortex is correlated with gustatory stimulation induced by a novel taste. Thus, blockade of the MAPK signaling pathway in the CeA may block the processing of the visceral information, and its association with taste during CTA acquisition. It is also possible that acute blockade of MAPK signaling facilitated extinction, rather than attenuating acquisition. The effects of a MAPK inhibitor on extinction at a later time points (i.e. after acquisition occurred) was not explicitly tested, however.
Microinjection of SL327 into the CeA significantly decreased the level of LiCl-induced pMAPK-positive cells (~30%) in this study. The reduction of LiCl-induced MAPK activity in the CeA was enough to attenuate the strength of CTA during extinction although MAPK activity in the NTS was intact. However, the reduction of LiCl-induced MAPK activity did not completely block CTA acquisition, suggesting that this level of MAPK in the CeA was enough to produce CTA. Previous evidence suggests that the levels of molecules such as c-Fos, phospho-CREB, PKA and phospho-acetylated histone H3 in the amygdala following LiCl stimulation may regulate or reflect the strength of CTA (Lamprecht and Dudai, 1996; Lamprecht et al., 1997; Swank, 2000a; Koh et al., 2002; Koh et al., 2003; Koh and Bernstein, 2003; Kwon and Houpt, 2010b). Thus, the MAPK in the CeA may be one of the critical factors modulating the strength of LiCl-induced CTA.
Many intracellular factors in the CeA have been shown to be engaged by acute lithium. In recent years our laboratory has demonstrated that the AP-1 family members c-Fos, Fra2, c-Jun, and JunD are expressed in the CeA after systemic LiCl (Spencer and Houpt 2001; Kwon et al., 2008). Transcriptional activation of the CeA by systemic LiCl is dependent, in part, on transynaptic input from the area postrema (Spencer et al., 2011). CREB phosphorylation and expression of inducible cAMP early repressor (ICER) support a role for cAMP and CREB (Spencer and Houpt 2001). Endogenous PP1/PP2A phosphatase activity serves as a constraint on CREB phosphorylation and CTA learning (Oberbeck et al., 2010). Similarly, histone deactylase activity constrains both c-Fos induction in the CeA and CTA learning (Kwon and Houpt, 2010b). Taken together with the work of others, these results demonstrate that the CeA contributes gene expression induced by LiCl via CREB and AP-1 transcription factors to the process of CTA acquisition. A critical issue remains, however. The acquisition and consolidation of a CTA must require a unique pattern or combination of gene expression induced by the contingent pairing of novel taste and LiCl toxicity. This unique pattern has not yet been identified.
Highlights.
Conditioned taste aversion (CTA) involves activation of the amygdala by toxins.
Activated phosphorylated MAP kinase (pMAPK) may be a critical intracellular signal.
LiCl induced pMAPK in the central n. of the amygdala and n. of the solitary tract.
MEK inhibition by SL327 in the amygdala attenuated CTA and pMAPK induction.
Acknowledgements
This work was supported by the National Institute on Deafness and other Communication Disorders grant R01DC03198 (TAH) and a research grant from the B.W. Robinson Memorial Endowment for the Neurosciences (BSK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annual Review of Pharmacology and Toxicology. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. Review. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nature Neuroscience. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Belelovsky K, Elkobi A, Kaphzan H, Nairn AC, Rosenblum K. A molecular switch for translational control in taste memory consolidation. European Journal of Neuroscience. 2005;22:2560–2568. doi: 10.1111/j.1460-9568.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. Journal of Neuroscience. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. Journal of Neuroscience. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AL, Mahadevan LC. MAP kinase-mediated phosphoacetylation of histone H3 and inducible gene regulation. FEBS Letters. 2003;546:51–58. doi: 10.1016/s0014-5793(03)00451-4. Review. [DOI] [PubMed] [Google Scholar]
- Fischer A, Radulovic M, Schrick C, Sananbenesi F, Godovac-Zimmermann J, Radulovic J. Hippocampal Mek/Erk signaling mediates extinction of contextual freezing behavior. Neurobiology of Learning and Memory. 2007;87:149–158. doi: 10.1016/j.nlm.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA. The amygdala and emotion. Current Opinion in Neurobiology. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Progress in Neurobiology. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Philopena JM, Wessel TC, Joh TH, Smith GP. Increased c-Fos expression in the rat nucleus of the solitary tract after conditioned taste aversion formation. Neuroscience Letters. 1994;172:1–5. doi: 10.1016/0304-3940(94)90648-3. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. Journal of Neuroscience. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. Journal of Neuroscience. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: interference with extinction or reconsolidation of a memory? Neuroreport. 2003;14:405–407. doi: 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- Koh MT, Clarke SN, Spray KJ, Thiele TE, Bernstein IL. Conditioned taste aversion memory and c-Fos induction are disrupted in RIIbeta-protein kinase A mutant mice. Behavioural Brain Research. 2003;143:57–63. doi: 10.1016/s0166-4328(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Koh MT, Thiele TE, Bernstein IL. Inhibition of protein kinase A activity interferes with long-term, but not short-term, memory of conditioned taste aversions. Behavioral Neuroscience. 2002;116:1070–1074. [PubMed] [Google Scholar]
- Kwon B, Glotz M, Houpt TA. Expression of AP-1 family transcription factors in the amygdala during conditioned taste aversion learning: role for Fra-2. Brain Research. 2008;1207:128–141. doi: 10.1016/j.brainres.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B, Houpt TA. A combined method of laser capture microdissection and X-Gal histology to analyze gene expression in c-Fos-specific neurons. Journal of Neuroscience Methods. 2010a;186:155–164. doi: 10.1016/j.jneumeth.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B, Houpt TA. Phospho-acetylation of histone H3 in the amygdala after acute lithium chloride. Brain Research. 2010b;1333:36–47. doi: 10.1016/j.brainres.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Differential modulation of brain immediate early genes by intraperitoneal LiCl. Neuroreport. 1995;7:289–293. [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learning and Memory. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. Journal of Neuroscience. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languille S, Davis S, Richer P, Alcacer C, Laroche S, Hars B. Extracellular signal-regulated kinase activation is required for consolidation and reconsolidation of memory at an early stage of ontogenesis. European Journal of Neuroscience. 2009;30:1923–1930. doi: 10.1111/j.1460-9568.2009.06971.x. [DOI] [PubMed] [Google Scholar]
- Lasiter PS, Glanzman DL. Cortical substrates of taste aversion learning: involvement of dorsolateral amygdaloid nuclei and temporal neocortex in taste aversion learning. Behavioral Neuroscience. 1985;99:257–276. doi: 10.1037//0735-7044.99.2.257. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional memory systems in the brain. Behavioural Brain Research. 1993;58:69–79. doi: 10.1016/0166-4328(93)90091-4. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Ferreira G, Ramirez-Lugo L, Bermudez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proceedings of the National Academy of Sciences USA. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbeck DL, McCormack S, Houpt TA. Intra-amygdalar okadaic acid enhances conditioned taste aversion and CREB phosphorylation in rats. Brain Research. 2010;1348:84–94. doi: 10.1016/j.brainres.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Peng S, Zhang Y, Zhang J, Wang H, Ren B. ERK in learning and memory: a review of recent research. International Journal of Molecular Sciences. 2010;11:222–232. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan G, Bures J. Tetrodotoxin blockade of amygdala overlapping with poisoning impairs acquisition of conditioned taste aversion in rats. Behavioural Brain Research. 1994;65:213–219. doi: 10.1016/0166-4328(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiological Reviews. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. Review. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neuroscience Research. 1999;35:53–61. doi: 10.1016/s0168-0102(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. Journal of Neuroscience. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion: I. The amygdala. Brain Research. 1996;741:109–116. doi: 10.1016/s0006-8993(96)00906-7. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Forebrain contribution to the induction of a brainstem correlate of conditioned taste aversion. II. Insular (gustatory) cortex. Brain Research. 1998;800:40–47. doi: 10.1016/s0006-8993(98)00492-2. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learning and Memory. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. Journal of Neuroscience. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learning and Memory. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Houpt TA. Dynamics of c-Fos and ICER mRNA expression in rat forebrain following lithium chloride injection. Molecular Brain Research. 2001;93:113–126. doi: 10.1016/s0169-328x(01)00173-5. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Nardos R, Eckel LA, Houpt TA. Area postrema lesions attenuate LiCl-induced c-Fos expression correlated with conditioned taste aversion learning. Physiology and Behavior. 2011;105:151–160. doi: 10.1016/j.physbeh.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Research. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Swank MW, Ellis AE, Blaker WD. Selective disruption of CS and US inputs by pharmacological agents: the brainstem as a site of CS-US associativity in taste aversion learning. Society for Neuroscience Abstract. 1996;22:1129. [Google Scholar]
- Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. Journal of Neuroscience. 2001;21:3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW. Coordinate regulation of Fos and Jun proteins in mouse brain by LiCl. Neuroreport. 1999;10:2685–2689. doi: 10.1097/00001756-199911260-00041. [DOI] [PubMed] [Google Scholar]
- Swank MW. Phosphorylation of MAP kinase and CREB in mouse cortex and amygdala during taste aversion learning. Neuroreport. 2000a;11:1625–1630. doi: 10.1097/00001756-200006050-00006. [DOI] [PubMed] [Google Scholar]
- Swank MW. Pharmacological antagonism of tyrosine kinases and MAP kinase in brainstem blocks taste aversion learning in mice. Physiology & Behavior. 2000b;69:499–503. doi: 10.1016/s0031-9384(00)00225-0. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of Neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. Review. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nature Reviews Neuroscience. 2004;5:173–183. doi: 10.1038/nrn1346. Review. [DOI] [PubMed] [Google Scholar]
- Villarreal JS, Barea-Rodriguez EJ. ERK phosphorylation is required for retention of trace fear memory. Neurobiology of Learning and Memory. 2006;85:44–57. doi: 10.1016/j.nlm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Molecular Neurobiology. 2003;27:99–106. doi: 10.1385/MN:27:1:99. Review. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Bernstein IL. Conditioning method determines the pattern of c-fos expression following novel taste-illness pairings. Behavioural Brain Research. 2006;169:93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neuroscience Research. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S. c-Fos expression in the rat brain after intraperitoneal injection of lithium chloride. Neuroreport. 1992;3:1049–1052. doi: 10.1097/00001756-199212000-00004. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Morimoto T, Yamamoto T. Different disruptive effects on the acquisition and expression of conditioned taste aversion by blockades of amygdalar ionotropic and metabotropic glutamatergic receptor subtypes in rats. Brain Research. 2000;869:15–24. doi: 10.1016/s0006-8993(00)02397-0. [DOI] [PubMed] [Google Scholar]
- Yefet K, Merhav M, Kuulmann-Vander S, Elkobi A, Belelovsky K, Jacobson-Pick S, Meiri N, Rosenblum K. Different signal transduction cascades are activated simultaneously in the rat insular cortex and hippocampus following novel taste learning. European Journal of Neuroscience. 2006;24:1434–1442. doi: 10.1111/j.1460-9568.2006.05009.x. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Okutani F, Inoue S, Kaba H. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway leading to cyclic AMP response element-binding protein phosphorylation is required for the long-term facilitation process of aversive olfactory learning in young rats. Neuroscience. 2003;121:9–16. doi: 10.1016/s0306-4522(03)00392-0. [DOI] [PubMed] [Google Scholar]