Abstract
Background
Red blood cell distribution width (RDW) is a measure of heterogeneity in erythrocytes size used in the differential diagnosis of anemia. High levels are associated with elevated cardiovascular biomarkers and increased mortality. The hypothesis of this study is that high RDW levels on admission are associated with higher recourse to coronary artery bypass graft (CABG) in unstable angina (UA) or non ST-elevation myocardial infarction (NSTEMI) patients.
Methods
An observational, cross-sectional study of all adult patients undergoing coronary angiography admitted to an urban tertiary care center in 2007 with UA or NSTEMI was conducted. Data was gathered by review of inpatient charts. RDW was considered “high” if it exceeded the 95th percentile (16.3%).
Results
Among the 503 subjects included in the analysis, high RDW was independently associated with higher recourse to CABG versus a non-surgical approach (OR = 2.39 (1.04–5.50); p = 0.041) but not with conservative management (OR = 0.97 (0.51–1.84); p = 0.922) or percutaneous coronary intervention (PCI) (OR = 0.67 (0.36–1.25); p = 0.208).
Conclusions
This study of patients with UA or NSTEMI demonstrated an independent association of elevated RDW with higher recourse to CABG. RDW should be considered in the stratification of patients presenting with UA or NSTEMI.
Keywords: Coronary, Red, Blood, Cell, Width, Bypass
Background
Red blood cell distribution width (RDW) is an objective measure of the heterogeneity in red blood cell (RBC) size (coefficient of variability of red blood cell volume) obtained from the RBC size distribution. Higher values reflect greater heterogeneity in cell sizes. It is automatically calculated by most hemocytometers by dividing the standard deviation of RBC volume by the mean corpuscular volume and multiplying by 100 to express the result as a percentage[1]. A cutoff value of 14% corresponds to the 95th percentile of RDW for the reference population in the Third National Health and Nutrition Examination Surveys (NHANES III) study, however the normal range can vary based on the type of hemocytometer used[2, 3]. Routinely reported as part of the complete blood count, it is mainly used as an auxiliary index in the differential diagnosis of anemia.
Higher levels of RDW are associated with increased mortality among patients with heart failure, myocardial infarction, coronary artery disease (CAD) or undergoing angiography[4–11]. A high RDW is also associated with elevated cardiovascular biomarkers and cardiac enzymes[12]. The goal of this project was to assess the independent relation between RDW, an inexpensive, readily available measure and the therapeutic decision adopted in unstable angina (UA) or non ST-elevation myocardial infarction (NSTEMI) patients. The rationale for selecting this population lies in the difference of treatment algorithms between ST-elevation myocardial infarction (STEMI) that is driven by a time-sensitive emergent revascularization of a likely culprit lesion and UA/NSTEMI which given the nature of the process at hand can legitimately lead to any of the three therapeutic strategies (conservative medical management, percutaneous coronary intervention (PCI), or coronary artery bypass graft surgery (CABG)). The hypothesis of this study was that high RDW levels are associated with higher recourse to CABG in this patient population.
Methods
STUDY POPULATION
This observational, cross-sectional study was conducted at the Beth Israel Medical Center, a tertiary care center in New York City. It included all patients aged 18 years or older, admitted to the telemetry floors between January 1 and December 31, 2007 with a diagnosis of UA or NSTEMI who underwent a coronary angiography during that admission. The choice of admission to the telemetry floors as an inclusion criterion allowed us to avoid including patients who developed cardiac complaints or complications during their inpatient stay as our target population were patients presenting with UA or NSTEMI who by hospital policy are sent to monitored beds. All patients who needed critical unit level of care, presented with STEMI, admitted to non telemetry floors, or did not require (or did not undergo) coronary angiography were excluded.
DEFINITIONS
Patients in this study presented to our medical center with chief complaints of chest pain, shortness of breath or decreased exercise tolerance for which they were admitted to our telemetry floors and underwent coronary angiography regardless of the presence or absence of electrocardiogram (EKG) changes. They were divided into 2 groups: UA if they did not have detectable cardiac biomarkers (troponin I <0.03 ng/mL) and NSTEMI if they had a troponin I level ≥0.03 ng/mL without EKG changes qualifying for STEMI. End-points were treatment strategies: Medical therapy, PCI, or CABG. Revascularization strategy was decided at the discretion of the treating physician. RDW level on admission was considered “high” if it were greater than the 95th percentile per the normal interval of the institution’s laboratory (16.3%), and “normal” if ≤16.3%. This grouping was based on the calibration and the reported normal range of our institution’s laboratory which uses the COULTER® LH 750 Hematology Analyzer (Beckman Coulter, Inc., Brea, CA).
DATA COLLECTION
Information on our subjects’ demographics (age, gender, and race), angiographic findings, clinical history (heart failure, CAD, hypertension (HTN), diabetes, dyslipidemia, smoking, family history of CAD (FHx), and previous CABG and stenting), laboratory measurements on admission (white blood cell count (WBC), hemoglobin, RDW, platelets, C-reactive protein (CRP), B-type natriuretic peptide (BNP), and creatinine), iron level, ferritin, and total iron binding capacity (TIBC), maximal troponin I level (Tn Max), diagnosis (UA or NSTEMI), and outcomes (medical therapy, PCI, or CABG) was collected by conducting a retrospective chart review of all patients admitted to our tertiary care center from January 1, 2007 till December 31, 2007 using internal electronic databases.
ETHICS
The study was approved by the Beth Israel Medical Center Institutional Review Board. The requirement for obtaining an informed consent was waived. There were no conflicts of interest.
STATISTICAL ANALYSIS
Data were analyzed using Stata version 11.2 (StataCorp, College Station, TX). Categorical data were analyzed using Chi-Square or Fisher’s Exact tests. Continuous variables whose distribution followed the normality assumptions were analyzed using the Student’s t-test and the Analysis of Variance (ANOVA). Variables whose distribution did not approximate normality were analyzed using the non-parametric Wilcoxon Rank-Sum and Kruskal-Wallis tests. Multivariable logistic regression analyses were used to determine the independent associations of medical therapy alone, PCI, and CABG respectively. The multivariate models adjusted for all the variables that were of statistical significance in the univariate and bivariate analyses for each of the endpoints of interest. Assessment for effect modifiers was performed and none were detected. Regression diagnostics and assessment of the fit of the models were conducted via the Hosmer and Lemeshow goodness of fit test which showed that they fit well. P-values<0.05 were considered statistically significant.
Results
Among the 1,631 patients who underwent cardiac catheterization in 2007, 536 fulfilled the selection criteria. Thirty-three had incomplete or missing data and were excluded from the analysis (Figure). The baseline characteristics and the diagnoses of the remaining 503 subjects are listed in Table 1. On average the study population was 65 years of age, and had a high prevalence of comorbidities. Fifty-six percent (n=280) were male, 28% (n=139) had a prior stent placed and 14% (n=72) had prior CABG. Baseline laboratory showed normal WBC (8 ± 3 1,000/µL), hemoglobin (13 ± 2 g/dL), RDW (14.3 (2.2) %) and platelet (244 ± 87 1,000/µL) levels. The study population showed racial diversity (with relative (35%) Hispanic preponderance).
Figure.
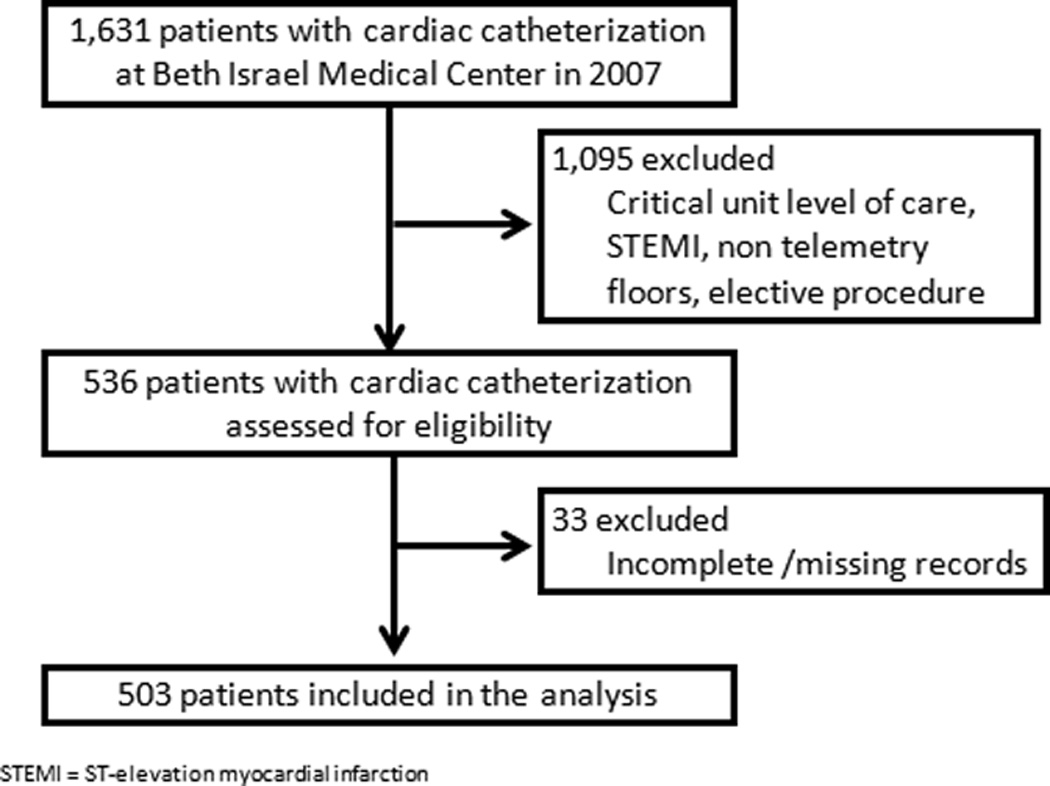
Study Sample Selection Flow Diagram
Table 1.
Characteristics of the Study Population and by Therapeutic Strategy*
| Characteristics | Overall (n = 503) |
Medical therapy (n = 270) |
PCI (n = 180) |
CABG (n = 53) |
p |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 65 ± 13 | 63 ± 14 | 68 ± 12 | 70 ± 12 | <0.001 |
| Male | 280 (56%) | 147 (54%) | 101 (56%) | 32 (60%) | 0.721 |
| Race | 0.258 | ||||
| Caucasian | 106 (21%) | 52 (19%) | 40 (22%) | 14 (26%) | |
| Black | 63 (13%) | 33 (12%) | 24 (13%) | 6 (11%) | |
| Hispanic | 178 (35%) | 105 (39%) | 55 (31%) | 18 (34%) | |
| Asian | 40 (8%) | 27 (10%) | 11 (6%) | 2 (4%) | |
| Other | 116 (23%) | 53 (20%) | 50 (28%) | 13 (25%) | |
| Laboratory Measurements | |||||
| WBC (1,000/µL) | 8 ± 3 | 8 ± 3 | 8 ± 2 | 9 ± 4 | 0.043 |
| Hemoglobin (g/dL) | 13 ± 2 | 13 ± 2 | 13 ± 2 | 13 ± 2 | 0.763 |
| High RDW | 101 (20%) | 58 (21%) | 27 (15%) | 16 (30%) | 0.037 |
| Platelets (1,000/µL) | 244 ± 87 | 253 ± 95 | 235 ± 66 | 235 ± 103 | 0.068 |
| Tn Max (ng/mL) | 0.01 (0.00–0.12) | 0.00 (0.00–0.06) | 0.01 (0.00–0.42) | 0.04 (0.00–0.45) | 0.022 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.1 (0.9–1.3) | 1.1 (1.0–1.8) | 0.025 |
| Anemia | 224 (45%) | 126 (47%) | 75 (42%) | 23 (43%) | 0.570 |
| Angiographic Findings | <0.001 | ||||
| Normal or non obstructive disease | 167 (33%) | 162 (60%) | 5 (3%) | 0 (0%) | |
| 1 vessel-disease | 109 (22%) | 41 (15%) | 62 (34%) | 6 (11%) | |
| 2 vessel-disease | 89 (18%) | 26 (10%) | 52 (29%) | 11 (21%) | |
| ≥ 3 vessel-disease | 138 (27%) | 41 (15%) | 61 (34%) | 36 (68%) | |
| Medical History | |||||
| Heart Failure | 173 (34%) | 97 (36%) | 52 (29%) | 24 (45%) | 0.064 |
| CAD | 287 (57%) | 131 (49%) | 128 (71%) | 28 (53%) | <0.001 |
| HTN | 392 (78%) | 203 (75%) | 144 (80%) | 45 (85%) | 0.209 |
| Diabetes | 218 (43%) | 106 (39%) | 86 (48%) | 26 (49%) | 0.137 |
| Dyslipidemia | 228 (45%) | 114 (42%) | 94 (52%) | 20 (38%) | 0.057 |
| Smoking | 155 (31%) | 80 (30%) | 53 (29%) | 22 (42%) | 0.204 |
| FHx | 106 (21%) | 44 (16%) | 47 (26%) | 15 (28%) | 0.017 |
| Prior Interventions | |||||
| Previous CABG | 72 (14%) | 27 (10%) | 43 (24%) | 2 (4%) | <0.001 |
| Previous PCI | 139 (28%) | 72 (27%) | 57 (32%) | 10 (19%) | 0.163 |
| Diagnosis | 0.006 | ||||
| UA | 406 (81%) | 232 (86%) | 134 (74%) | 40 (75%) | |
| NSTEMI | 97 (19%) | 38 (14%) | 46 (26%) | 13 (25%) |
n = 503. Continuous variables are reported as mean ± standard deviation if normally distributed, median (range) if not. Categorical variables are reported as frequency (percentage). WBC = White Blood Cell count; High RDW = Red Blood Cell Distribution Width > 16.3%; Tn Max = Maximal Troponin I; CAD = Coronary Artery Disease; HTN = Hypertension; FHx = Family History of Coronary Artery Disease; CABG = Coronary Artery Bypass Graft; PCI = Percutaneous Coronary Intervention; UA = Unstable Angina; NSTEMI = Non ST Elevation Myocardial Infarction. P values<0.05 are in boldface.
After the diagnostic coronary angiography, 270 patients (53%) were treated with medical therapy alone whereas the remaining 233 underwent either PCI (180 (36%)) or CABG (53 (11%)). Compared to the subjects undergoing medical therapy and PCI respectively, patients requiring CABG were on average older (70 years versus 63 and 68, p<0.001), less likely to have prior CABG (4% versus 10% and 24%, p<0.001), had a higher prevalence of elevated RDW (30% versus 21% and 15%, p = 0.037). Patients with CABG had a more extensive atherosclerotic burden (in terms of number of diseased vessels by catheterization findings). A higher prevalence of heart failure was noted in the CABG group compared to the other two (45% versus 36% and 29% respectively) with borderline statistical significance (p = 0.064). High RDW (>16.3%) was associated with anemia (77% versus 36%, p<0.001) and heart failure (50% versus 31%, p<0.001) (Table 2). The prevalence of a higher atherosclerotic burden was noted in the high RDW group compared to the normal one (32% and 20% versus 27% and 17% respectively) but it reached only borderline statistical significance (p = 0.066). Race did not show any statistically significant difference across the RDW or the outcome divides (p = 0.772 and p = 0.258 respectively).
Table 2.
Characteristics of the Study Population by RDW Group*
| Characteristics | Normal RDW (n = 402) |
High RDW (n = 101) |
p |
|---|---|---|---|
| Demographics | |||
| Age (y) | 65 ± 13 | 67 ± 14 | 0.091 |
| Male | 233 (58%) | 47 (47%) | 0.039 |
| Race | 0.772 | ||
| Caucasian | 89 (22%) | 17 (17%) | |
| Black | 48 (12%) | 15 (15%) | |
| Hispanic | 142 (35%) | 36 (35%) | |
| Asian | 31 (8%) | 9 (9%) | |
| Other | 92 (23%) | 24 (24%) | |
| Laboratory Measurements | |||
| WBC (1,000/µL) | 8 ± 3 | 8 ± 4 | 0.548 |
| Hemoglobin (g/dL) | 13 ± 2 | 11 ± 2 | <0.001 |
| Platelets (1,000/µL) | 240 ± 71 | 263 ± 131 | 0.017 |
| Tn Max (ng/mL) | 0.01 (0.00–0.10) | 0.02 (0.00–0.28) | 0.053 |
| Creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.2 (0.8–1.8) | 0.002 |
| Anemia | 146 (36%) | 78 (77%) | <0.001 |
| Angiographic Findings | 0.066 | ||
| Normal or non obstructive disease | 130 (32%) | 37 (36%) | |
| 1 vessel-disease | 97 (24%) | 12 (12%) | |
| 2 vessel-disease | 69 (17%) | 20 (20%) | |
| ≥ 3 vessel-disease | 106 (27%) | 32 (32%) | |
| Medical History | |||
| Heart Failure | 123 (31%) | 50 (50%) | <0.001 |
| CAD | 237 (59%) | 50 (50%) | 0.086 |
| HTN | 314 (78%) | 78 (77%) | 0.849 |
| Diabetes | 172 (43%) | 46 (46%) | 0.617 |
| Dyslipidemia | 192 (48%) | 36(36%) | 0.029 |
| Smoking | 129 (32%) | 26 (26%) | 0.217 |
| FHx | 94 (23%) | 12 (12%) | 0.011 |
| Prior Interventions | |||
| Previous CABG | 58 (14%) | 14 (14%) | 0.884 |
| Previous PCI | 113 (28%) | 26 (26%) | 0.634 |
| Diagnosis | 0.320 | ||
| UA | 328 (82%) | 78 (77%) | |
| NSTEMI | 74 (18%) | 23 (23%) | |
| Treatment | 0.037 | ||
| Medical Therapy only | 212 (53%) | 58 (57%) | |
| PCI | 153 (38%) | 27 (27%) | |
| CABG | 37 (9%) | 16 (16%) |
n = 503. Continuous variables are reported as mean ± standard deviation if normally distributed, median (range) if not. Categorical variables are reported as frequency (percentage). WBC = White Blood Cell count; Tn Max = Maximal Troponin I; CAD = Coronary Artery Disease; HTN = Hypertension; FHx = Family History of Coronary Artery Disease; CABG = Coronary Artery Bypass Graft; PCI = Percutaneous Coronary Intervention; UA = Unstable Angina; NSTEMI = Non ST Elevation Myocardial Infarction. P values<0.05 are in boldface.
In multivariable logistic regression analyses, and after adjusting for age, gender, race, hemoglobin, atherosclerotic burden, heart failure, smoking, family history of coronary artery disease, and previous CABG, a high RDW was not found to be statistically significantly associated with neither conservative management (non-invasive, i.e. medical treatment only) versus recourse to any form of revascularization (PCI or CABG) (OR = 0.97 (0.51–1.84); p = 0.922) nor with discrimination between PCI and non-PCI approaches (OR = 0.67 (0.36–1.25); p = 0.208). It was however found to be independently associated with recourse to CABG versus non-surgical strategy (OR = 2.39 (1.04–5.50); p = 0.041). This finding was similar after excluding all patients who had undergone previous CABG (Table 3). This association had a sensitivity of 30%, a specificity of 81%, and a negative predictive value of 91%. Few patients had CRP, BNP, iron, ferritin, TIBC, and ejection fraction measurements performed for these variables to be properly tested in our regression analyses.
Table 3.
Multivariable Logistic Regression Models For High RDW*
| Outcome = Medical Management | p | |
|---|---|---|
| Unadjusted OR (95%CI) | 1.21 (0.78–1.88) | 0.399 |
| Adjusted OR (95%CI)† | 0.97 (0.52–1.81) | 0.916 |
| Adjusted OR (95%CI)‡ | 0.97 (0.51–1.84) | 0.922 |
| Outcome = PCI* | p | |
| Unadjusted OR (95%CI) | 0.59 (0.37–0.96) | 0.035 |
| Adjusted OR (95%CI)† | 0.69 (0.38–1.25) | 0.222 |
| Adjusted OR (95%CI)‡ | 0.67 (0.36–1.25) | 0.208 |
| Adjusted OR (95%CI)⍰ | 0.60 (0.34–1.06) | 0.078 |
| Outcome = CABG* | p | |
| Unadjusted OR (95%CI) | 1.86 (0.99–3.49) | 0.055 |
| Adjusted OR (95%CI)† | 2.33 (1.02–5.32) | 0.044 |
| Adjusted OR (95%CI)‡ | 2.39 (1.04–5.50) | 0.041 |
| Adjusted OR (95%CI)° | 2.43 (1.02–5.82) | 0.045 |
High RDW = Red Blood Cell Distribution Width >16.3%; PCI = Percutaneous Coronary Intervention. CABG = Coronary Artery Bypass Graft. P values<0.05 are in boldface.
Adjusted for age, gender, race, atherosclerotic burden, anemia, heart failure, smoking, family history of coronary artery disease, and previous CABG.
Adjusted for age, gender, race, hemoglobin, atherosclerotic burden, heart failure, smoking, family history of coronary artery disease, and previous CABG.
Adjusted for age, gender, atherosclerotic burden, coronary artery disease, dyslipidemia, family history of coronary artery disease, previous CABG, and diagnosis.
n= 431 after excluding patients with previous CABG. Adjusted for age, gender, race, hemoglobin, atherosclerotic burden, heart failure, smoking, and family history of coronary artery disease.
Discussion
RDW reflects variability in the size of circulating red cells (anisocytosis) and is routinely reported by automated laboratory equipment for complete blood counts. Although its use had been limited to the narrowing of the differential diagnosis of anemia mounting evidence suggests additional roles for this measurement. The present study demonstrated an independent association between high RDW on admission and recourse to CABG in patients presenting with UA or NSTEMI. We found this association to be independent of multiple potential confounding factors, such as anemia, heart failure, age, race and smoking. RDW did not demonstrate any statistically significant association with medical therapy alone or PCI. No deaths were reported in this study. The likely reason for this finding is the lower acuity of the selected patient population which excluded STEMI and intensive care patients.
This study extends previous work on the role and impact of RDW. In an analysis of 15,852 adult participants in NHANES III Perlstein et al determined that higher RDW (with a median of 14.35 in the highest quintile) was associated with increased risk of all-cause mortality independently of CVD risk[4]. Patel et al found similar results in a meta-analysis on older populations (aged 73.6 to 79.1 years). They noted a graded increased risk of death associated with higher RDW values. The RDW–mortality association occurred in all major demographic, disease, and nutritional risk factor subgroups examined[5]. Tonelli et al had similar conclusions regarding all-cause mortality but also with increased risk of coronary death/nonfatal myocardial infarction, new symptomatic heart failure, and stroke[6]. The relation of high RDW and greater CHD risk was also the main theme of the study conducted by Zalawadiya et al[7]. An RDW level greater than the seventy-fifth percentile in both anemic and nonanemic participants was a significant predictor of greater CHD risk. As for cardiovascular disease, a high RDW has been reported as associated with worse prognosis in patients with heart failure, acute myocardial infarction, and those referred for percutaneous coronary interventions[8–11]. The study by Lippi et al analyzed the RDW values on admission of patients presenting to the local emergency department for chest pain suggestive of acute coronary syndrome (ACS). They found that RDW levels were highly correlated with troponin T levels, and an RDW greater than 14% had a clinical sensitivity and specificity for diagnosing ACS of 79% and 50%, respectively. When combined, troponin T and RDW levels had a diagnostic sensitivity of 99%[12]. They concluded that RDW might be considered alongside other conventional cardiac markers for the risk stratification of ACS patients admitted to emergency departments.
Despite cumulative reports associating RDW and prognosis, the underlying biological mechanism for this relation remains unknown. Chronic subclinical inflammation is the most frequently hypothesized pathway as it is a well-established entity preceding de novo cardiovascular events and could adversely influence erythropoiesis by a variety of mechanisms, including direct myelosuppression of erythroid precursors, reducing renal erythropoietin production and the bioavailability of iron, increasing erythropoietin resistance in erythroid precursor cell lines, promoting cell apoptosis, and red blood cell membrane deformability, factors that might increase anisocytosis[13]. It is less likely to be the case though as RDW was not found to be correlated with inflammatory markers such as C-reactive protein (CRP)[14]. However in patients with conditions characterized by increased levels of oxidative stress, such as Down syndrome, poor pulmonary function, and dialysis, RDW values are elevated[15–17].
Decreased serum antioxidant levels, including carotenoids, selenium, and vitamin E, were also associated with increased RDW[18]. An elevated RDW can also be a sign of ‘‘reticuloendothelial block’’ or impaired iron metabolism that may directly contribute to disease progression[19]. It is conceivable that there are subclinical disease processes that cause a subtle dysregulation of erythrocyte homeostasis that is expressed in RDW.
Several limitations should be considered in the interpretation of the results. This study is a retrospective nonrandomized analysis and has inherent limitations of a retrospective design. Data on medication, previous admissions, as well as factors required for disease burden scoring (whether as separate variables or as a score) was not present in the medical records collected and information on ejection fraction, iron profile, CRP and BNP was not consistent enough to allow for a proper analysis of these factors. This was a single center study of UA and NSTEMI patients only, a potential hindrance to external validity. Since our study population was fairly diverse from clinical and racial perspectives this study should have reasonable generalizability within the target population. Baseline characteristics between the RDW and outcome groups were reasonably similar, but certain differences, especially high-RDW patients having for example a higher prevalence of anemia and heart failure, had to be adjusted for. Despite adjustment for multiple variables and assessment of potential confounding factors and effect modifiers, residual unrecognized confounding variables could influence the observed differences in outcomes between the groups. As with all analyses of observational data, this study cannot distinguish causality from association. However, the hypothesis that elevated RDW measurements were associated with higher recourse to CABG (based on review of the available literature in this regard) had been formulated before performing analyses on our database. This approach reduced the risk of spurious conclusions. Nevertheless RDW was assessed on a single occasion (admission) that might be influenced by biologic variability or measurement error, although such errors are likely to attenuate the observed association between RDW and outcome. The cutoff value for high RDW differed from that of larger studies such as NHANES III but it was consistent with our laboratory’s range. The actual angiograms were not reviewed to verify the reported findings especially among those with no (or nonobstructive) CAD. There was no standardized evaluation of atherosclerotic burden such as the SYNTAX score[20] as the required data was not available in the medical records at hand. The revascularization strategy (especially CABG) was at the discretion of the treating physician and depended on the patients’ consent. The treating physician was also aware of the actual RDW value with what that entails as potential bias to the results. However in view of the retrospective nature of this investigation it is less likely to be the case. We also could not account for patients who were considered high risk for surgery as we did not have reliable means to assess non-atherosclerotic disease burden. These factors were a likely source of bias and may be the reason behind this relatively low frequency of CABG (11%) for a tertiary care center. However findings such as the marked prevalence of higher atherosclerotic burden and of heart failure in subjects undergoing CABG are consistent with the current recommendations[21] and therefore indicate a trend towards adherence to the established guidelines rather than personal or group practice behavior. Finally our study does not provide a mechanism for the association between RDW and the observed outcome, but to date no biological pathway for RDW’s role has been clearly established.
In conclusion this study has demonstrated an independent association of elevated RDW with recourse to CABG. Our findings are concordant with, and extend, previous reports about the potential roles of RDW in patients with cardiovascular disease. Despite its low sensitivity but considering its high negative predictive value, its availability to clinicians as part of the complete blood count, and lack of additional costs, RDW should be considered as a potential additional tool for stratification of patients presenting to emergency departments with UA or NSTEMI. A potential benefit from such early classification may be the successful avoidance of thienopyridine loading in patients who are more likely to be CABG candidates as this may incur a 5- to 7-day delay in surgery. In view of the retrospective, observational, and chart abstraction design of this investigation further prospective studies are required to confirm the association between RDW and clinical outcomes and explain its underlying mechanism.
ACKNOWLEDGMENT
We would like to thank Steven R. Bergmann, M.D., Ph D, for his contribution to this article.
This publication was supported in part by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), through CTSA grant numbers UL1RR025750, KL2RR025749 and TL1RR025748. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. 1991;9(Suppl 1):71–74. doi: 10.1016/0736-4679(91)90592-4. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC, Gunter EW, Johnson CL. Methods to assess iron status in various NHANES surveys. Nutr Rev. 1995;53:246–254. doi: 10.1111/j.1753-4887.1995.tb05481.x. [DOI] [PubMed] [Google Scholar]
- 3.Van hove L, Schisano T, Brace L. Anemia diagnosis, classification, and monitoring using Cell-Dyn Technology reviewed for the new millennium. Lab Hematol. 2000;6:93–108. [Google Scholar]
- 4.Perlstein TS, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009 Mar 23;169(6):588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel KV, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010 Mar;65(3):258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, et al. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008 Jan 15;117(2):163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 7.Zalawadiya SK, et al. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010 Oct 1;106(7):988–993. doi: 10.1016/j.amjcard.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Allen LA, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010 Mar;16(3):230–238. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabbah S, et al. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010 Feb 1;105(3):312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Poludasu S, et al. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb Haemost. 2009 Sep;102(3):581–587. doi: 10.1160/TH09-02-0127. [DOI] [PubMed] [Google Scholar]
- 11.Cavusoglu E, et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int J Cardiol. 2010 May 28;141(2):141–146. doi: 10.1016/j.ijcard.2008.11.187. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, et al. Clinical usefulness of measuring red blood cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47(3):353–357. doi: 10.1515/cclm.2009.066. [DOI] [PubMed] [Google Scholar]
- 13.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl JMed. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 14.Fukuta, et al. Elevated plasma levels of B-Type natriuretic peptide but not C-reactive protein are associated with higher red blood cell distribution width in patients with coronary artery disease. Int Heart J. 2009 May;Vol. 50(No. 3):301–312. doi: 10.1536/ihj.50.301. [DOI] [PubMed] [Google Scholar]
- 15.Garcez ME, Peres W, Salvador M. Oxidative stress and hematologic and biochemical parameters in individuals with Down syndrome. Mayo Clin Proc. 2005;80:1607–1611. doi: 10.4065/80.12.1607. [DOI] [PubMed] [Google Scholar]
- 16.Grant BJ, et al. Relation between lung function and RBC distribution width in a population-based study. Chest. 2003;124:494–500. doi: 10.1378/chest.124.2.494. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi S, et al. Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int. 2003;63:1881–1887. doi: 10.1046/j.1523-1755.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 18.Patel KV, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783e8. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 20.Serruys PW, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009 Mar 5;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 21.Hillis LD, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: Executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 Dec 6;124(23):2610–2642. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]


