Abstract
Conduct disorder (CD) and depression co-occur at far greater levels than chance, despite largely separate diagnostic criteria. One potential shared mechanism of this comorbidity is emotion dysregulation, which characterizes both internalizing and externalizing disorders. Previous research demonstrates that respiratory sinus arrhythmia (RSA)—a peripheral biomarker of emotion regulation—is attenuated among children with CD, and among children with depression. However, few studies have examined biomarkers of emotion regulation as a function of heterotypic comorbidity. We evaluated longitudinal patterns of RSA and RSA reactivity to emotion evocation across three annual assessments among 207 children diagnosed at ages 8–12 years with CD (n=30), depression (n=28), comorbid CD and depression (n=80), or no psychiatric condition (n=69). Using continuous symptom counts as predictors, Depression × CD interactions were observed for both Time 1 resting RSA and Time 1 RSA reactivity. Conduct disorder, depression, and their interaction were all associated with low resting RSA at Time 1. In addition, concurrently elevated CD and depression scores predicted the greatest RSA reactivity to emotion evocation. Psychopathology scores were unrelated to developmental changes in RSA and RSA reactivity over time.
Keywords: conduct disorder, depression, heterotypic comorbidity, respiratory sinus arrhythmia, heart rate variability
Despite divergent presentations at the symptom level, conduct disorder (CD) and depression are highly comorbid conditions in childhood and adolescence (Angold, Costello, & Erkanli, 1999). Conduct disorder is defined by persistent and repeated violations of social rules and the rights of others, whereas depression is characterized by sadness and anhedonia, often accompanied by anxiety (American Psychiatric Association, 2000). Nevertheless, youth with CD experience much higher rates of depression—and youth with depression experience much higher rates of CD—than found in the general population. Epidemiologic samples, for example, yield CD co-occurrence rates ranging from 15% to 24% among depressed youth (Zoccolillo, 1992).
There are two somewhat divergent perspectives on risk associated with comorbidity of internalizing and externalizing disorders. According to some reports, such heterotypic comorbidity is associated with greater functional impairment than either CD or depression alone (see e.g., Goodman, Schwab-Stone, Lahey, Shaffer, & Jensen, 2000; Kopp & Beauchaine, 2007). Comorbid CD and depression may portend lower academic achievement (Marmorstein & Iacono, 2004), poorer social competence and more involvement with deviant peers (Ingoldsby, Kohl, McMahon, & Lengua, 2006), and greater risk for substance abuse (Brenner & Beauchaine, 2011) than either disorder alone. Furthermore, some longitudinal studies indicate especially poor clinical trajectories (Beyers & Loeber, 2003), high risk for recurrent depression (Rohde, Clarke, Lewinsohn, Seeley, & Kaufman, 2001), more suicide attempts (Fombonne et al., 2001), and greater levels of adult criminal behavior (Copeland, Miller-Johnson, Keeler, Angold, & Costello, 2007). Given the poor outcomes associated with heterotypic comorbidity, it may be especially important to identify vulnerability early, which might improve prevention outcomes (e.g., Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2008).
In contrast, a smaller but growing literature suggests that comorbid internalizing disorders may offer partial protection from especially poor outcomes among children and adolescents with CD and related externalizing disorders. For example, symptoms of anxiety predict better response to treatment among children with ADHD and CD (Jensen et al., 2001). Furthermore, some studies indicate that youth with CD and comorbid anxiety are less aggressive physically, regarded less negatively by their peers, and experience fewer police contacts than youth with CD alone (Walker et al., 1991). Neuroanatomically, symptoms of anxiety and depression are associated with less severe structural compromises in brain regions subserving motivation and behavior regulation—including the caudate, hippocampus, and anterior cingulated cortex—among externalizing males (Sauder, Beauchaine, Gatzke-Kopp, Shannon, & Alyward, in press). These findings are consistent with the hypothesis that internalizing symptoms serve to dampen excessive approach behaviors, including conduct problems and aggression, among those predisposed to externalizing conduct (e.g., Beauchaine, 2001).
One potentially fruitful strategy for increasing our understanding of heterotypic comorbidity, and disentangling alternative viewpoints such as those articulated above, is by evaluating biomarkers of emotion regulation/dysregulation (e.g., Beauchaine & Gatzke-Kopp, in press). As we have described in detail elsewhere, emotion dysregulation characterizes both internalizing and externalizing disorders, at both behavioral and physiological levels of analysis (see e.g., Beauchaine, 2001; Beauchaine, Gatzke-Kopp, & Mead, 2007; Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009; Vasilev, Crowell, Beauchaine, Mead, & Gatzke-Kopp, 2009). For example, children with clinical levels of conduct problems and children with clinical levels of depression show consistently lower respiratory sinus arrhythmia (RSA) at baseline, and consistently greater RSA reactivity to emotion evocation than controls (e.g., Beauchaine, Katkin, Strassberg, & Snarr, 2001; Boyce et al., 2001; Mezzacappa et al., 1997; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007)1. To date, however, most of this research has evaluated main effects of CD or depression on autonomic functioning, ignoring possible CD × Depression interactions. In addition, few studies conducted to date have evaluated autonomic markers of emotion dysregulation longitudinally. In this study, we evaluate longitudinal patterns of RSA and RSA reactivity in a cohort of middle school children who were diagnosed at ages 8–12 years with CD, depression, comorbid CD and depression, or no psychiatric condition.
Studying the development of RSA and RSA reactivity is important given an expanding literature linking patterns of RSA responding to (1) central nervous system substrates of emotion regulation/dysregulation (e.g., Porges, 1995, 2007; Beauchaine et al., 2007; Thayer & Lane, 2000), (2) socialization processes within families (Kupper et al., 2004, 2005), and (3) self-reports of developing ER skills across middle childhood and adolescence (Vasilev et al., 2009). In turn, strong emotion regulation skills offer protection from emerging psychopathology (see Beauchaine & Gatzke-Kopp, in press; Shannon et al., 2007). Nevertheless, very few longitudinal studies have examined autonomic nervous system-linked cardiac activity/reactivity within this age range—particularly among children with clinical levels of conduct problems and depression.
Ordinarily, RSA indexes parasympathetic nervous system (PNS)-linked cardiac activity (see Berntson et al., 1997). RSA quantifies naturally occurring oscillations in heart rate across the respiratory cycle. Phasic decreases in RSA occur during the experience of strong emotions, including empathy, sadness, panic, anger, and fight/flight/freeze responding (see e.g., Beauchaine, 2001; George et al., 1989; Marsh, Beauchaine, & Williams, 2008). Among other functions, such decreases in RSA facilitate increased cardiac output to cope with real or perceived stressors (see Beauchaine et al., 2007; Porges, 1995).
Studies of normative samples indicate consistent positive associations between baseline RSA and competent social behavior and self-regulation (e.g., Eisenberg et al., 1995; Fox & Field, 1989). In contrast, although studies of typically developing children have linked modest decreases in RSA during a host of challenges to positive outcomes including protection from psychopathology (e.g., Blandon et al., 2008; Calkins & Keane, 2004; El-Sheikh et al., 2009; Gottman & Katz, 2002), excessive RSA reactivity to such challenges, as noted above, is observed consistently in psychiatric samples of internalizing and externalizing children, adolescents, and adults (Beauchaine et al., 2001; Beauchaine & Gatzke-Kopp, in press; Boyce et al., 2001; Crowell et al., 2005; Mezzacappa et al., 1997; Shannon et al., 2007). This seeming inconsistency is likely because modest decreases in RSA reflect competent attention allocation, whereas excessive RSA reactivity reflects emotional lability and fight/flight/freeze responding (see Beauchaine, 2009; Porges, 1995; Suess, Porges, & Plude, 1994). As already noted, however, little attention has been paid to the association between RSA and comorbid CD and depression.
In this article, we analyze data from a longitudinal study of developing conduct problems, depression, and heterotypic comorbidity in children who were 8–12 years old at the first of three annual assessments. Consistent with the literature reviewed above, we anticipated that both CD and depression would be associated with compromised parasympathetic-linked cardiac function compared with controls. In addition, we expected children with comorbid CD and depression to exhibit particularly low baseline RSA and especially high RSA reactivity to emotion-induction. In other words, we predicted a CD × Depression interaction in predicting low RSA and high RSA reactivity across development, consistent with the notion that heterotypic comorbidity is synergistic—not protective.
Method
Participants
All procedures were approved by the University of Washington Institutional Review Board. Consent and assent were obtained from the parent and child respectively, at each assessment. Data were collected as part of a longitudinal study examining the development of conduct problems, depression, and heterotypic comorbidity in middle childhood. Families were assessed at three time points (T1, T2, T3), each separated by one year. They received a $175 incentive at T1, which included an extensive diagnostic battery, described below. At each subsequent assessment (T2, T3), families were paid $100.
Participants between the ages of 8 and 12 years (M = 9.9, SD = 1.52) were recruited from urban Seattle neighborhoods through flyers posted at clinics and community centers, and through advertisements in local newspapers, radio spots, city busses, and school newsletters. Interested parents responded to advertisements by telephone and completed a 20–30 min structured interview to evaluate their child’s fit for the study. The telephone interview included portions of the Child Symptom Inventory (CSI; Gadow & Sprafkin, 1997) and the Child Behavior Checklist (CBCL; Achenbach, 1991), two frequently used measures of child psychopathology with adequate to excellent reliability. The CSI provides a dimensional score and diagnostic cutoffs for DSM-IV (2000) disorders. Each diagnostic criterion is rated on a 4-point scale (0 = never, 1 = sometimes, 2 = often, 3 = very often), with ratings of 2 or higher considered positive for each criterion. For purposes of the phone screen, administered CSI scales included CD, attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), major depressive disorder, and dysthymia (DYS). In the most recent validation sample, internal consistencies for these scales ranged from .79 to 91 (Sprafkin, Gadow, Salisbury, Schneider, & Loney, 2002).
Parents also completed the aggression, attention problems, and anxious/depressed subscales of the CBCL. Internal consistencies for these scales range from .73 to .84 (Achenbach, 1991). Based on results from the telephone interview, children were placed into either a control group (n=69) or one of the three clinical groups (CD, n=30; DEP, n=28; comorbid, n=80). Control participants were excluded if they met criteria for any disorder on the CSI and/or scored T ≥ 70 on any CBCL scale. Children placed into the three clinical groups were required to meet CSI criteria for their respective disorder(s). In addition, children in the CD group were required to score at or above the 95th percentile on the CBCL aggression subscale, children in the DEP groups were required to score at or above the 85th percentile on the CBCL anxious/depressed subscale, and children in the comorbid group had to meet criteria on both the aggression and anxious/depressed subscales.
At the T1 lab visit, symptom severity was assessed via structured clinical interview using the self-report version of the Diagnostic Interview Schedule for Children (DISC; Shaffer, 2000), which was administered by a graduate research assistant. The DISC is a widely used diagnostic interview for children ages 8 to 19. It is based on DSM-IV diagnostic criteria and assesses both impairment and severity. For purposes of this study, participants were pooled into a single group to evaluate the correspondence between continuous symptom counts and measures of physiological responding using regression-based techniques (see below). Of the 207 participants at T1, 178 (86%) returned at T2, and 159 (76%) returned at T3.
Psychophysiological Assessment
Task
RSA was collected during a 5-min resting baseline and 3-min video clip from the movie The Champ. This film clip portrays a child witnessing and reacting to his father’s death. This video is effective in inducing both sadness (Gross & Levenson, 1995) and individual differences in RSA reactivity (Crowell et al., 2005; Marsh et al., 2008).
Respiratory sinus arrhythmia
Parasympathetic-linked cardiac activity was assessed at each annual assessment using identical hardware, procedures, and settings. Children were seated alone in a comfortable, sound-attenuated room equipped with audio and video monitoring equipment. Spot electrodes were placed on each child using the modified Lead II configuration described by Qu, Zhang, Webster, and Tompkins (1986). The electrocardiographic (ECG) signal was acquired using a HIC 2000 impedance cardiograph (Chapel Hill, NC). The ECG waveforms was digitized at 1 kHz for later scoring.
RSA was indexed by extracting the high frequency component (> 0.15 Hz) of the R-R time series using spectral analysis. High-frequency spectral densities were calculated in 30-s epochs using the Medistar Nevrokard software system and normalized through natural log transformations, as is common practice. RSA is a well-validated measure of PNS-linked cardiac activity (see Berntson et al., 1997)2. Increases in RSA reflect greater PNS activation. Spectral densities were calculated in 30 s epochs and averaged to obtain a single value. Although 30-s epochs are shorter than sometimes recommended for calculating RSA, averaging epochs together provides adequately stable and reliable estimates (Berntson et al., 1997). The final 2 min of the resting baseline were used for analysis to ensure children were habituated to their surroundings. RSA reactivity was measured as the difference between participants' resting baseline scores (averaged across four 30-s epochs) and their reactivity scores (averaged across six 30-s epochs).
Data Analyses
Analyses were conducted by constructing multilevel models in Hierarchical Linear Modeling, version 6.04 (HLM; Raudenbush, Bryk, & Congdon, 2007). Multilevel modeling provides estimates of both within- and between-participants effects on longitudinal growth trajectories in outcomes, and uses full maximum likelihood to accommodate missing data. Accordingly, all participants who provided data at T1 were included in the HLM models. Two-level models were constructed for both baseline RSA and RSA reactivity. At Level 1, RSA and RSA reactivity were modeled as random effects (in separate models) for each participant. Participant’s age in months was included at Level 1 as a time-varying covariate. At Level 2, symptoms of depression, CD, and the CD × Depression interaction were modeled as predictors of Level 1 slopes and intercepts in baseline RSA and RSA reactivity scores across years (T1, T2, T3). Continuous psychopathology scores were used instead of group-based analyses both to increase statistical power (see MacCallum, Zhang, Preacher, & Rucker, 2002), and to accommodate the multilevel modeling framework. Importantly, skew values were acceptable for all variables (see Table 1). Analyses tested the hypothesis that heterotypic comorbidity (i.e., the CD × Depression interaction) would account for RSA and RSA reactivity, over-and-above the effects of depression and CD symptoms alone. Since the DISC was administered only at T1, psychopathology symptom counts were used as Level 2 predictors instead of as time-varying covariates at Level 1. The baseline RSA model is presented below:
Level 1: baseline RSAij = π0j + π1j (ageij) + rij
Level 2: π0j = β00 + β01 (depression) + β02 (CD) + β03 (CD × Depression) + u0 π1j = β10 + β11 (depression) + β12 (CD) + β13 (CD × Depression) + u1j
Table 1.
Descriptive Statistics and Bivariate Correlations among Analyzed Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | M | SD | skew | kurtosis | range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. age in months at time 1 | - | 123.95 | 18.45 | 0.09 | −1.28 | 87 – 156 | |||||||
| 2. depression | .02 | - | 7.12 | 5.06 | 0.34 | −0.89 | 0 – 19 | ||||||
| 3. conduct problems (square root transformed) | .28** | .32** | - | 0.93 | 0.99 | 0.73 | −0.35 | 0 – 4 | |||||
| 4. T1 baseline RSA | −.07 | .03 | −.07 | - | 7.06 | 1.10 | 0.23 | −0.17 | 4.52 – 10.12 | ||||
| 5. T2 baseline RSA | −.12 | −.17* | −.01 | .41** | - | 7.11 | 1.14 | −0.08 | −0.13 | 3.90 – 9.84 | |||
| 6. T3 baseline RSA | .07 | −.17* | .04 | .35** | .54** | - | 6.86 | 1.20 | −0.24 | −0.07 | 3.30 – 9.33 | ||
| 7. T1 RSA reactivity | −.10 | −.19* | −.13 | −.08 | −.01 | −.05 | - | 0.03 | 0.94 | 0.66 | 0.71 | −1.84 – 3.06 | |
| 8. T2 RSA reactivity | .00 | .03 | −.07 | .09 | .01 | .03 | .10 | - | −0.14 | 0.85 | −0.81 | 2.77 | −3.66 – 1.90 |
| 9. T3 RSA reactivity | .07 | −.01 | .11 | .00 | −.01 | .09 | −.14 | .01 | −0.31 | 0.73 | −0.19 | 1.85 | −2.68 – 2.08 |
Notes. RSA = respiratory sinus arrhythmia; CD = conduct disorder.
p <.05.
p <.01
Results
Descriptive Statistics
Means, standard deviations, and correlations for all study variables are shown in Table 1. Although age was somewhat kurtotic given our recruitment strategy, all other variables met assumptions for multiple linear regression.
Baseline RSA
Consistent with our hypothesis, depression scores were associated with intercepts in resting RSA, b = −0.04, t(197) = −2.51, p = .013, as were CD scores, b = −0.09, t(197) = −2.46, p = .015. In both cases, higher psychopathology scores at T1 predicted lower baseline RSA, consistent with previous research. In addition, the CD × Depression interaction predicted intercepts in resting RSA (T1), over-and-above all main effects in the model, b = 0.01, t(197) = 3.16, p = .002. Thus, depression, CD, and comorbid depression and CD all predicted lower resting RSA scores than observed among those who scored low on measures of psychopathology. However, neither CD scores, depression scores, nor the CD × Depression interaction term predicted slopes in resting RSA from T1 to T3, all bs ≤ 0.004, all ts(197) ≤ 1.46, all ps ≥ .15. In addition, although age was associated with higher resting RSA at T1, b = 7.30, t(197)=55.71, p < .001, it did not predict slopes in resting RSA from T1 to T3, b < 0.001, t(197) = −0.12, p < .907.
Change in RSA during The Champ
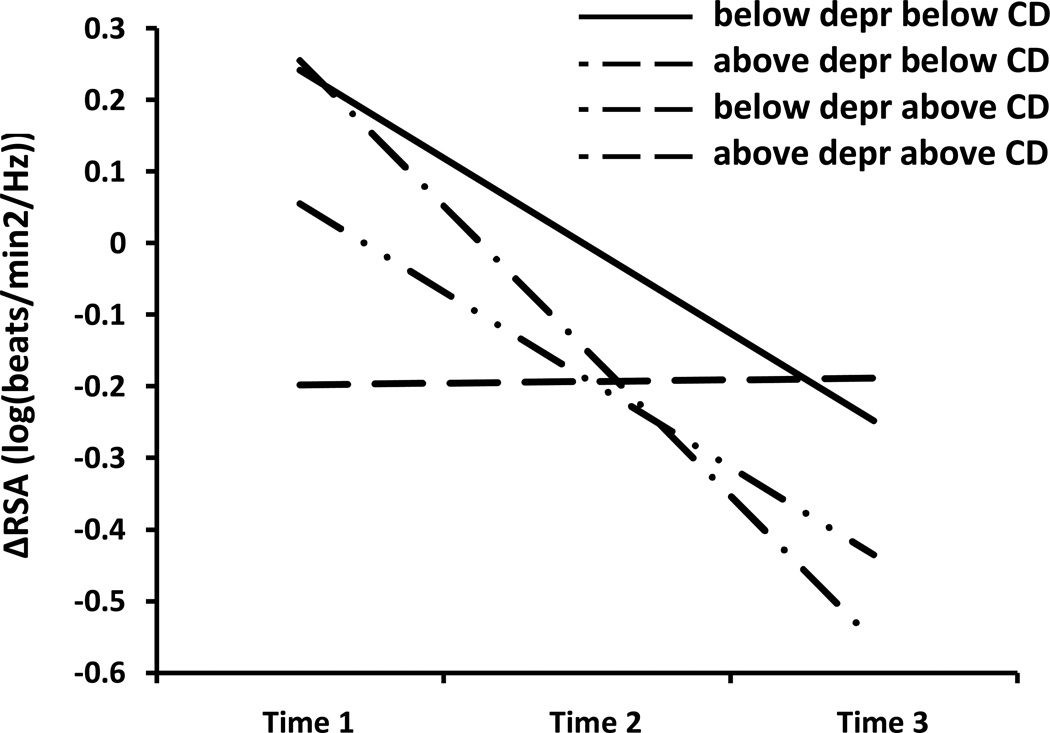
Similar to baseline RSA, both DISC depression scores, b = −0.02, t(196)= −2.16, p = .032, and CD scores, b = −0.07, t(196)= −2.26, p = .025, were associated with T1 intercepts in RSA reactivity. Thus, depression and CD were associated independently with increases in RSA during emotion elicitation at T1. In addition, the CD × Depression interaction term predicted RSA reactivity, over-and-above all main effects in the model b = 0.006, t(196)=2.78, p = .007., Concurrently high CD and depression scores predicted RSA withdrawal to emotion evocation, in contrast to the other groups. Neither CD scores, depression scores, nor the CD × Depression interaction term predicted slopes in RSA reactivity from T1 to T3, all bs ≤ 0.002, all ts(196) ≤ 0.77, all ps ≥ .44. In addition, although age did not predict RSA reactivity at T1, b = 0.04, t(196)=0.48, p = .63, it did predict more RSA withdrawal to emotion evocation over time, b < −0.021, t = −2.29, p < .023. This pattern is depicted in Figure 1, for which we divided the sample into four groups solely for graphing purposes (see Aiken & West, 1991). These groups included (1) those below the sample median on both CD and depression, (2) those above the sample median on CD and below on depression, (3) those below the sample median on depression and above on CD, and (4) those above the sample median on both CD and depression. The figure illustrates both (a) group differences in RSA reactivity at baseline, and (b) increasing RSA withdrawal to emotion evocation across years (T1-T3).
Figure 1.
Longitudinal patterns of respiratory sinus arrhythmia (RSA) reactivity to emotion induction among children scoring (1) below the sample median on both depression and CD, (2) above the sample median on depression and below on CD, (3) below the sample median on depression and above on CD, and (4) above the sample median on both depression and CD. Median splits were performed for graphing purposes only; all analyses were run using continuous depression scores, CD scores, and a Depression × CD product vector, consistent with established guidelines for testing interactions (Aiken & West, 1991).
Discussion
As reviewed above, many (though not all) studies suggest that comorbid CD and depression portends poorer long-term functional outcomes than either CD or depression alone. However, most studies evaluating autonomic and central nervous system correlates of internalizing and externalizing behavior have focused on main effects of CD or depression—ignoring CD × Depression interactions (see Sauder et al., in press). In this study, we evaluated longitudinal patterns of RSA and RSA reactivity in a cohort of children and adolescents with CD, depression, comorbid CD and depression, or no psychiatric condition. At T1, depression, conduct problems, and their interaction were all associated with lower resting RSA. Furthermore, comorbid CD and depression predicted the greatest RSA withdrawal to emotion evocation at T1.
Our findings linking both baseline RSA and RSA reactivity to CD and depression are consistent with previous research (Beauchaine et al., 2001; Boyce et al., 2001; Crowell et al., 2005; Mezzacappa et al., 1997; Shannon et al., 2007). Compromises in parasympathetic-linked cardiac function have been associated consistently with emotional lability, which characterizes both internalizing and externalizing disorders (Beauchaine, 2001, 2009; Beauchaine et al., 2009). Heterotypic comorbidity also predicted low baseline RSA. To our knowledge, this is a new contribution to the literature, and is more consistent with models of additive vulnerability among those who score high on measures of both internalizing and externalizing psychopathology.
As expected, heterotypic comorbidity also predicted greater RSA withdrawal to emotion-evocation at T1, indicating a synergistic effect of concurrent CD and depression on autonomic functioning. Excessive RSA reactivity to emotion evocation is a hallmark of emotional lability (Beauchaine, 2001), and is observed consistently among those with clinical levels of both internalizing and externalizing symptoms (e.g., Beauchaine, 2009; Beauchaine et al., 2007; Crowell et al., 2005). Developmental increases in RSA withdrawal were also observed from T1 to T3, yet these were unrelated to depression scores, conduct disorder scores, or their interaction. Of note, developmental increases in baseline RSA were not observed in our sample, a finding that may appear to be inconsistent with previous research. A careful reading of the literature, however, suggests that differences in our findings may be due to the specific developmental epoch examined. In general, baseline RSA shows mean level increases up to about age 7, with much less change among children and adolescents ages 8 and older (e.g., Alkon et al., 2006; El-Sheikh, 2005; Salomon, 2005).
Limitations and Future Directions
Among other limitations, DISC self-reports of psychopathology were assessed only at T1, so we could not determine whether or how patterns of psychopathology covaried with autonomic functioning over time. Similarly, the effects of psychopathology on longitudinal autonomic functioning remain purely speculative. Future research should include diagnostic measures of CD and depression at all time points, which will provide for assessment of cross-lag correlations between psychophysiological responding and symptom expression. In addition, we did not collect self-reports of affect at any point during the emotion-eliciting task. Such information might contribute to our understanding of participant’s subjective experience of emotion, and to our interpretation of patterns of RSA reactivity.
We were also unable to assess sex effects. Although doing so would have been of interest and potential importance (Beauchaine, Hong, & Marsh, 2008), it would have required us to evaluate three-way interactions to test our hypotheses. Higher-order interactions are woefully underpowered in samples of this size, and often produce spurious results (see Beauchaine & Mead, 2006; Whisman & McClelland, 2005).
Finally, although autonomic and other biological vulnerabilities are critical to consider when studying the development of psychopathology, they represent only one level of analysis in the long chain of causal factors spanning genes to environments (Beauchaine & Gatzke-Kopp, in press). In this study, we did not assess potentially important environmental influences—such as family and peer socialization processes—that shape and maintain patterns of internalizing, externalizing, and heterotypic symptom presentation.
Despite these limitations, our findings demonstrate that examining internalizing and externalizing symptoms in isolation is likely to provide only partial understanding of physiological correlates of psychopathology. In contrast, by evaluating the Internalizing × Externalizing symptom interactions, we are in a better position to understand key aspects of etiology among hetertypically comorbid youth.
Acknowledgments
Work on this article was supported by grant MH63699 from the National Institute of Mental Health to Theodore P. Beauchaine.
Footnotes
One reviewer noted that a fair number of papers (e.g., Dietrich et al., 2007) indicate positive rather than negative associations between RSA reactivity and conduct problems in community samples. As we have noted elsewhere (Beauchaine, 2009), such differential findings across normative vs. clinical samples are remarkably consistent. This consistency suggests different mechanism for RSA–behavior relations at the extreme end of externalizing distributions than in the normal range. In fact, normative variation in externalizing scores may capture temperamental exuberance rather than psychopathology (e.g., Degnan et al., 2011). Thus, RSA-psychopathology relations are likely to be obscured in normative samples because so few individuals score at or above the 95th percentile—the cutoff for inclusion in this study and in all of our work.
There is ongoing controversy in the psychophysiology literature over the need to control for respiration when estimating RSA. Although the specifics of this controversy are beyond the scope of this article, the general argument is that RSA only reflects efferent traffic through the vagus nerve when respiration rate and/or tidal volume are controlled, either statistically or using paced breathing (see Grossman, Karemaker, & Wieling, 1991; Ritz, 2009). It is important to note that this concerns the construct validity of RSA as an accurate index of cardiac vagal tone. In contrast, in the present study we are concerned with the predictive validity of RSA as a marker of psychopathology, regardless of its neural origin. Accordingly, we do not control for individual differences in respiration. Nevertheless no group differences in respiration were found.
Contributor Information
Karen C. Pang, University of Washington
Theodore P. Beauchaine, Washington State University
References
- Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991a. [Google Scholar]
- Aiken LS, West SG. Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Alkon A, Lippert S, Vujan N, Rodriquez ME, Boyce WT, Eskenazi B. The ontogeny of autonomic measures in 6-and 12-month- old infants. Developmental Psychobiology. 2006;48:197–208. doi: 10.1002/dev.20129. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., Text Revision. Washington, DC: Author; 2000. [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Some difficulties in interpreting psychophysiological research with children. Monographs of the Society for Research in Child Development. 2009;74:80–88. doi: 10.1111/j.1540-5834.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp LM. Instantiating the multiple levels of analysis perspective into a program of study on externalizing behavior. Development and Psychopathology. doi: 10.1017/S0954579412000508. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal Theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Mead HK. Some recommendations for testing mediating and moderating effects in treatment-outcome research. International Journal of Psychology Research. 2006;1:97–117. [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2008;20:745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Beyers JM, Loeber R. Untangling developmental relations between depressed mood and delinquency in male adolescents. Journal of Abnormal Child Psychology. 2003;31:247–266. doi: 10.1023/a:1023225428957. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O'Brien M. Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children's physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology. 2011:1588–1596. doi: 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Miller-Johnson S, Keeler G, Angold A, Costello EJ. Childhood psychiatric disorders and young adult crime: A prospective, population-based study. American Journal of Psychiatry. 2007;164:1668–1675. doi: 10.1176/appi.ajp.2007.06122026. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Degnan K, Hane AA, Henderson HA, Moas OL, Reeb-Sutherland BC, Fox NA. Longitudinal stability of temperamental exuberance and social–emotional outcomes in early childhood. Developmental Psychology. 2011;47:765–780. doi: 10.1037/a0021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FE, Greaves-Lord K, van Roon AM, Ormel J, Rosmalen JGM. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P. The role of emotionality and regulation in children's social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal tone exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005;114:735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children's externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74:1–69. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E, Wostear G, Cooper V, Harrington R, Rutter M. The Maudsley long-term follow-up of child and adolescent depression: 2. Suicidality, criminality and social dysfunction in adulthood. British Journal of Psychiatry. 2001;179:218–223. doi: 10.1192/bjp.179.3.218. [DOI] [PubMed] [Google Scholar]
- Fox NA, Field TM. Individual differences in preschool entry behavior. Journal of Applied Developmental Psychology. 1989;10:527–540. [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent Symptom Inventory 4 Screening manual. Stony Brook, NY: Checkmate Plus; 1997. [Google Scholar]
- George DT, Nutt DJ, Walker WV, Porges SW. Lactate and hyperventilation substantially attenuate vagal tone in normal volunteers: A possible mechanism of panic provocation? Archives of General Psychiatry. 1989;46:153–156. doi: 10.1001/archpsyc.1989.01810020055009. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Schwab-Stone M, Lahey BB, Shaffer D, Jensen PS. Major depression and dysthymia in children and adolescents: Discriminant validity and differential consequences in a community sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:761–770. doi: 10.1097/00004583-200006000-00015. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF. Children's emotional reactions to stressful parent-child interactions: The link between emotion regulation and vagal tone. Marriage and Family Review. 2002;34:265–283. [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Ingoldsby EM, Kohl GO, McMahon RJ, Lengua L. Conduct problems, depressive symptomatology and their co-occurring presentation in childhood as predictors of adjustment in early adolescence. Journal of Abnormal Child Psychology. 2006;34:603–621. doi: 10.1007/s10802-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, Vitiello B. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Kopp LM, Beauchaine TP. Patterns of psychopathology in the families of children with conduct problems, depression, and both psychiatric conditions. Journal of Abnormal Child Psychology. 2007;35:301–312. doi: 10.1007/s10802-006-9091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper N, Willemsen G, Posthuma D, De Boer D, Boomsma DI, De Geus EJC. A genetic analysis of ambulatory cardiorespiratory coupling. Psychophysiology. 2005;42:202–212. doi: 10.1111/j.1469-8986.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Kupper NHM, Willemsen G, vandenBerg M, deBoer D, Posthuma D, Boomsma DI, deGeus EJC. Heritability of ambulatory heart rate variability. Circulation. 2004;110:2792–2796. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR, Iacono WG. Major depression and conduct disorder in youth: associations with parental psychopathology and parent-child conflict. Journal of Child Psychology and Psychiatry. 2004;45:377–386. doi: 10.1111/j.1469-7610.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- Marsh P, Beauchaine TP, Williams B. Dissociation of sad facial expressions and autonomic nervous system responding in boys with disruptive behavior disorders. Psychophysiology. 2008;45:100–110. doi: 10.1111/j.1469-8986.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage: A Polyvagal Theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu MH, Zhang YJ, Webster JG, Tompkins WJ. Motion artifact from spot and band electrodes during impedance cardiography. IEEE Transactions in Biomedical Engineering. 1986;33:1029–1036. doi: 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM 6.04: Hierarchical linear and non-linear modeling. Lincolnwood, IL: Scientific Software International; 2007. [Google Scholar]
- Ritz T. Studying non-invasive indices of vagal control: The need for respiratory control and the problem of target specificity. Biological Psychology. 2009;80:158–168. doi: 10.1016/j.biopsycho.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rohde P, Clarke GN, Lewinsohn PM, Seeley JR, Kaufman NK. Impact of comorbidity on a cognitive-behavioral group treatment for adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:795–802. doi: 10.1097/00004583-200107000-00014. [DOI] [PubMed] [Google Scholar]
- Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology. 2005;24:68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Sauder C, Gatzke-Kopp LM, Shannon KE, Aylward E. Neuroanatomical correlates of heterotypic comorbidity in externalizing adolescent males. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2012.658612. (in press) [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. Further evidence of reliability and validity of the Child Symptom Inventory-4: Parent checklist in clinically referred boys. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;31:513–524. doi: 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Vasilev CA, Crowell SE, Beauchaine TP, Mead HK, Gatzke-Kopp LM. Correspondence between physiological and self-report measures of emotion dysregulation: A longitudinal investigation of youth with and without psychopathology. Journal of Child Psychology and Psychiatry. 2009;50:1357–1364. doi: 10.1111/j.1469-7610.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- Walker JL, Lahey BB, Russo MF, Frick PJ, Christ MAG, McBurnett K, Green SM. Anxiety, inhibition, and conduct disorder in children: I. Relations to social impairment. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:187–191. doi: 10.1097/00004583-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. Journal of Family Psychology. 2005;19:111–120. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M. Co-occurrence of conduct disorder and its adult outcomes with depressive and anxiety disorders: A review. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:547–556. doi: 10.1097/00004583-199205000-00024. [DOI] [PubMed] [Google Scholar]