Abstract
Mortality and morbidity of acute lung injury and acute respiratory distress syndrome remain high because of the lack of pharmacological therapies to prevent injury or promote repair. Mesenchymal stem cells (MSCs) prevent lung injury in various experimental models, despite a low proportion of donor-derived cell engraftment, suggesting that MSCs exert their beneficial effects via paracrine mechanisms. We hypothesized that soluble factors secreted by MSCs promote the resolution of lung injury in part by modulating alveolar macrophage (AM) function. We tested the therapeutic effect of MSC-derived conditioned medium (CdM) compared with whole MSCs, lung fibroblasts, and fibroblast-CdM. Intratracheal MSCs and MSC-CdM significantly attenuated lipopolysaccharide (LPS)-induced lung neutrophil influx, lung edema, and lung injury as assessed by an established lung injury score. MSC-CdM increased arginase-1 activity and Ym1 expression in LPS-exposed AMs. In vivo, AMs from LPS-MSC and LPS-MSC CdM lungs had enhanced expression of Ym1 and decreased expression of inducible nitric oxide synthase compared with untreated LPS mice. This suggests that MSC-CdM promotes alternative macrophage activation to an M2 “healer” phenotype. Comparative multiplex analysis of MSC- and fibroblast-CdM demonstrated that MSC-CdM contained several factors that may confer therapeutic benefit, including insulin-like growth factor I (IGF-I). Recombinant IGF-I partially reproduced the lung protective effect of MSC-CdM. In summary, MSCs act through a paracrine activity. MSC-CdM promotes the resolution of LPS-induced lung injury by attenuating lung inflammation and promoting a wound healing/anti-inflammatory M2 macrophage phenotype in part via IGF-I.
Keywords: cell therapy, lung injury, repair
despite improvements in management, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) remain major causes of morbidity and mortality in critically ill patients of all ages (63). The incidence of ALI/ARDS in the United States is 138:100,000 persons per year and is anticipated to double in the next 25 years (63). Treatment of ALI/ARDS remains primarily supportive (41).
Bone-marrow derived mesenchymal stem cells (MSCs) differentiate into cartilage, fat, and bone, but also into muscle, liver, kidney, heart, and brain cells (58, 59, 69). These properties have been harnessed for organ regeneration (69). In the lung, bone marrow-derived cells, including MSCs, engraft and adopt a lung epithelial cell phenotype (3, 21, 33, 34, 54, 62, 71). MSCs can also suppress local immune responses and have the capacity to avoid immune rejection in allotransplantation (29, 35, 38, 47, 59). These immunomodulatory properties might be of therapeutic benefit in ALI/ARDS and other lung diseases characterized by inflammation. Both intratracheal and systemic MSC administrations improve lipopolysaccharide (LPS)-induced ALI in mice (21, 44). These findings are congruent with the therapeutic benefits of MSCs described in other lung injury models (3, 5, 25, 37, 48, 53, 54, 62, 71). In all these studies, the engraftment rates of MSCs were low (32, 33, 62, 71). Together with the rapid therapeutic benefits seen within 48 to 72 h of MSC delivery in the ALI models (20, 44), these findings suggest that beyond cell replacement, MSCs may be releasing factors responsible for the beneficial effects of cell therapy. In addition, since the use of MSCs as whole-cell therapy may hold some risks to the patient (2, 38), we investigated the effects of MSC-derived conditioned medium (CdM) in LPS-induced ALI in mice.
MATERIALS AND METHODS
All procedures were approved by the Animal Welfare Committee of the University of Alberta.
MSCs and lung fibroblast isolation, culture, and characterization.
Bone marrow was harvested from adult (8–10 wk) C57BL/6 mice (Charles River), and MSCs were cultured and characterized as previously described (25). The femur and tibia were excised and the marrow was flushed with Dulbecco's modified Eagle medium (DMEM; Invitrogen, Burlington, ON, Canada) containing 10% fetal bovine serum and 1% penicillin-streptomycin-fungizone (PSF). The extracted marrow was dissociated and plated in a tissue culture flask. After 24 h, the medium was aspirated, and adherent cells were rinsed three times with PBS and replenished with fresh media. Cells were grown to ∼80% confluency, trypsinized, and reseeded at a density of 105 cells/cm2. Differentiation of MSCs was performed over 21 days on passage 2–3 cells (25). MSCs were evaluated for expression of a panel of surface markers according to established criteria (13, 19, 44, 46, 68). Antibodies against the following markers were obtained from Becton Dickinson (BD, Mississauga, ON, Canada): stem cell antigen-1 [Sca-1, fluorescein isothiocyanate (FITC)], CD31 [phycoerythrin (PE)], CD11b (FITC), CD45 (FITC), CD44 (FITC), CD73 (PE), CD14 (FITC), CD34 (FITC), c-kit (FITC), Flk-1 (PE), CD106 [vascular cell adhesion molecule-1 (VCAM1)], CD29 (PE). CD105 (PE) was obtained from BioLegend (San Diego, CA). MSCs between passages 7–11 were detached from culture surfaces, counted, and divided into aliquots of ∼0.5–1 × 106 cells/sample in 12 × 75 mm polystyrene round-bottom tubes (BD Falcon). Cells were washed twice with flow buffer (0.05% sodium azide, 0.1% bovine serum albumin in PBS), incubated with the respective antibodies at 4°C with gentle shaking for 30 min, washed twice, resuspended in flow buffer, and analyzed by flow cytometry (FACSCalibur, BD). Cellquest (BD) and FlowJo (version 5.7.2) software were used for analyses.
Lung fibroblasts were isolated from adult (8–10 wk) C57BL/6 mice (67). Fibroblast (Fib) identity was confirmed by immunofluorescence staining for the intermediate filament protein vimentin.
CdM preparation.
Passage 2–8 MSCs and fibroblasts were grown to >80% confluency. Medium (DMEM) was aspirated and cells were rinsed three times with PBS. Cells were cultured with serum-free DMEM (+ PSF) for 24 h. CdM was collected and filtered through a 0.2-μm filter to remove cellular debris. Adherent cells were trypsinized, stained with trypan blue, and counted. The medium from 5 × 106 cells yielded 15 ml of primary CdM that was further desalted and concentrated ∼25-fold, yielding 600 μl CdM, using ultrafiltration units with a 3-kDa molecular weight cutoff (Amicon Ultra-PL 3, Millipore, Billerica, MA). Similar to work by others (24), serum-free DMEM + PSF (desalted and concentrated 25-fold) was the vehicle control.
For IGF-I studies, IGF-I was quantified in DMEM, Fib-CdM, and MSC-CdM by using a commercially available ELISA kit (R&D Systems) according to manufacturer's instructions. Neutralizing antibody to IGF-I (R&D Systems, 100 ng/ml) was added to the serum-free medium to obtain IGF-I-neutralized CdM (Neut-CdM).
Murine LPS-induced ALI.
Eight- to 10-wk-old male C57BL/6 mice were anaesthetized with 5% isoflurane and injected intratracheally (i.t.) with 4 mg/kg LPS (Escherichia coli 055:B5, Sigma-Aldrich, Oakville, ON, Canada). Four hours post-LPS, mice were reanesthetized and received a 30-μl i.t. instillation of MSCs, Fib, MSC-CdM, CdM, or DMEM. We ensured equivalence between cell-based and CdM-based treatment by administering the same number of cells (250,000 cells/30 μl DMEM) that produced 30 μl concentrated CdM. For IGF-I studies, recombinant mouse IGF-I (rIGF-I, R&D Systems, 100 μg/kg) was administered i.t. in a total volume of 30 μl saline solution.
Mice (n ≥ 5 per group per endpoint) were euthanized via an intraperitoneal injection of pentobarbital at 48 h post-LPS for either bronchoalveolar lavage fluid (BALF) or lung histological analysis.
BALF analysis and AM isolation.
Lungs were lavaged with 2.5 ml ice-cold phosphate-buffered saline (PBS) injected at 0.5-ml increments via a 20-gauge catheter inserted in the trachea. BALF was centrifuged for 10 min at 400 g and BALF cells were enumerated by use of the Scepter automated cell counter (Millipore). Differential cell counts were performed on cytospin preparations (Thermo Shandon, Pittsburgh, PA) stained with Hema 3 Manual Staining System (Fisher Scientific, Nepean, ON, Canada) by counting 300 cells per cell smear and multiplying by total cell number per milliliter.
For alveolar macrophage (AM) isolation, an established protocol was followed (73). Briefly, BALF was centrifuged at 300 g for 10 min and the cellular pellet was washed with PBS, resuspended in red blood cell lysis buffer (8.3 g NH4Cl, 1 g KHCO3, 1.8 ml of 5% EDTA in 1 liter of distilled water) for 5 min at room temperature, and centrifuged again at 300 g for 10 min. The pellet was resuspended in RPMI-1640 medium and plated at a density of 600,000 cells/ml in a 24-well tissue culture plate. After 2 h, medium was removed and adherent cells were washed three times with PBS. AMs were stained with Hema 3 Manual Staining System for morphological assessment. AMs were evaluated for the expression of characteristic surface markers (22) with use of antibodies against CD11b (FITC) and CD11c [allophycocyanin (APC)] (BD Biosciences) by flow cytometry according to the same protocol described for MSC characterization.
Assessment of lung permeability.
Lung edema due to LPS-induced increase in lung permeability was measured by using the wet-to-dry weight ratio of lung lobes as previously described (21). Briefly, lungs were weighed upon excision (wet weights), homogenized in 1 ml of water, and placed in a drying oven at 55°C for 24 h; dry weights were recorded; and wet-to-dry ratio was calculated.
Lung histological analysis.
Lungs were inflated and fixed with 4% formaldehyde solution through a tracheal catheter at a constant pressure of 20 cmH2O (25). Lungs were processed and paraffin embedded, and 4-μm-thick serial sections were stained with hematoxylin and eosin. Images were captured (Openlab, Improvision, version 5.0.2.) with Leica CTRMIC microscope, and 40 high-powered fields per lung were examined by a blinded investigator to quantify the histopathology score. Parameters assessed were alveolar septal congestion, alveolar hemorrhage, intra-alveolar fibrin, and intra-alveolar infiltrates. A score from 0 to 3 was given for each criterion and a total score was established, all according to a previously published protocol (43, 44). Briefly, lung injury score = [(alveolar hemorrhage points/no. of fields) + 2 × (alveolar infiltrate points/no. of fields) + 3 × (fibrin points/no. of fields) + (alveolar septal congestion/no. of fields)]/total number of alveoli counted.
AM LPS exposure and AM phenotype characterization.
Freshly isolated AMs were cultured with RPMI-1640 media containing 10% FBS in a 12-well plate. After overnight adherence, RPMI-1640 medium was replaced with either DMEM alone, DMEM+LPS (1 μg/ml), or MSC-CdM+LPS (1 μg/ml). After 48 h in culture, medium was aspirated and cells were rinsed 3× with PBS. The monolayer of cells was scraped, cells were spun down, and pellets were frozen. Arginase activity in AM was measured by determining the amount of urea generated by the enzyme (11, 65). Briefly, the frozen AM pellets were thawed and homogenized in 100 μl per sample of lysis buffer [0.1% Triton X-100 with protease inhibitors: phenyl-methyl-sulfonyl fluoride (PMSF) (1 mM), leupeptin (0.5 μg/ml), aprotinin (5 μg/ml), EDTA (2 mM)]. The samples were sonicated twice for 3–5 s and stored at −80°C. Samples were thawed and 50 μl of the homogenate was added to 50 μl Tris·HCl (25 mM, pH 7.5) containing MnCl2 (5 mM) and incubated at 56°C for 10 min to activate the enzyme. Then 50 μl of this heat-activated supernatant mixture was incubated with 50 μl l-arginine (0.5 mol/l) at 37°C for 60 min. The reaction was stopped by adding 400 μl of an acid mixture (1 H2SO4:3 H3PO4:7 H2O), and 25 μl of α-isonitrosopropiophenone (9% dissolved in 100% ethanol) was added to the above mixture and incubated at 100°C for 45 min for color development. The mixture was cooled at room temperature in the dark for 10 min. A standard curve for urea (0–30 μg) was prepared. Urea concentration in the homogenate was measured by using a colorimeter at 550/540 nm with 200 μl of the aliquot. Arginase activity was expressed as units per milligram protein per hour.
For further macrophage phenotype characterization by immunodetection (Western blot) and multicolor flow cytometry, AMs were isolated from experimental animals and allowed to adhere to plastic as described above. The following antibodies were used: CD11b (Pacific Blue), CD11c (PE, both from Biolegend), rabbit anti-mouse inducible nitric oxide synthase (iNOS; Abcam) with donkey anti-rabbit secondary conjugated with FITC (Biomeda, Foster City, CA) or streptavidin (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-mouse Ym1 (R&D Systems) and donkey anti-goat secondary [Cy5 (Biomeda) or streptavidin (Santa Cruz)].
Immunodetection was performed as previously described (70). For flow cytometry, the staining was performed as described for MSC characterization, with the addition of a 30-min incubation of the cells at 4°C in Cytofix/Cytoperm Buffer (BD), followed by resuspension in permeabilization buffer (BD) before incubation with primary antibodies. Analysis was performed using FACSCanto with FACS Diva software (BD).
Antibody array of MSC-CdM.
MSC-CdM and Fib-CdM were prepared, concentrated, and desalted as described above. Samples were analyzed for a panel of 96 factors by use of an antibody array (RayBio Mouse Antibody Array G Series 1000, RayBiotech, Norcross, GA). Relative intensity of each factor in MSC-CdM and Fib-CdM is reported compared with a positive and negative control, as well as fold change of specific factors in MSC-CdM vs. Fib-CdM.
Statistical analysis.
Data are expressed as means ± SE. Group comparisons were analyzed by one-way ANOVA with a Fisher's least significant difference post hoc test. An unpaired t-test was used when appropriate. Data was assessed with statistical software Statview (version 5.0.1, SAS Institute, Cary, NC). Values were considered significant with P < 0.05.
RESULTS
MSC display functional characteristics and surface marker phenotype of murine MSCs.
Bone marrow-derived MSCs differentiated into adipogenic, osteogenic, and chondrogenic mesenchymal lineages (Fig. 1A). Cell surface antigen phenotype was assessed by flow cytometry. MSCs expressed high levels of Sca-1 (92.93% of cells) and CD29 (89.99%) and moderate levels of CD105 (33.92%), CD106 (14.36%), CD11b (12.10%), and CD45 (11.62%) and were considered negative for CD14 (0.30%), c-kit (0.53%), CD34 (1.13%), CD73 (1.49%), Flk-1 (3.01%), and CD31 (4.08% of cells) (Fig. 1, B and C).
Fig. 1.
Characterization of mesenchymal stem cells (MSCs) isolated from C57BL/6 mice. A: MSCs differentiated along adipogenic, osteogenic, and chondrogenic lineages. Top: differentiated MSCs. Bottom: control MSCs. Left to right, respectively: oil red O staining (adipocytes), alizarin red (osteocytes), safranin O (chondrocytes). Size bar: 60 μm. B: representative flow-cytometry histograms. C: quantification of MSC surface marker expression. Values are expressed as means ± SE.
MSC-CdM decreased lung inflammation and lung vascular permeability in LPS-induced lung injury.
LPS significantly increased the total cell and neutrophil count in the BALF compared with uninjured controls (Fig. 2A). This inflammatory influx was attenuated by MSCs and MSC-CdM treatment, but not by treatment with DMEM, the vehicle control, Fib, or Fib-CdM (Fig. 2A). MSCs and MSC-CdM, but not DMEM, Fib, or Fib-CdM, prevented the LPS-induced increase in lung vascular permeability, as assessed by lung wet-to-dry ratio (Fig. 2B).
Fig. 2.
MSC-conditioned medium (CdM) decreased bronchoalveolar lavage fluid (BALF) total cell and neutrophil number in LPS-induced lung inflammation. A: LPS-DMEM mice (n = 10) had significantly more inflammatory cells than uninjured controls (n = 5) and control-CdM (n = 6) mice. Treatment with MSCs (n = 5) or MSC-CdM (n = 10), but not fibroblast (Fib; n = 5) or Fib-CdM (n = 5), significantly attenuated lung cells influx. BALF of mice treated with DMEM had 2 times more polymorphonuclear leukocytes (PMNs) than those treated with MSC-CdM. There were no differences in mononuclear cells (MN) number. *P < 0.05 control, control-CdM vs. LPS-MSC CdM; #P < 0.01 control, control-CdM vs. all other LPS groups (LPS-DMEM, LPS-Fib, LPS-Fib CdM, LPS-MSC); §P < 0.01 LPS-MSC vs. LPS-DMEM, LPS-Fib, LPS-Fib CdM. B: LPS-DMEM (n = 5) lungs had increased wet-to-dry weight ratios compared with control (n = 5) and control-CdM (n = 5) lungs. LPS-MSCs (n = 4) and LPS-MSC-CdM (n = 5), but not LPS-Fib (n = 4) or LPS-Fib-CdM (n = 5), had significantly decreased wet-to-dry weight ratios compared with LPS-DMEM. *P < 0.05 LPS-DMEM vs. LPS-MSC; †P < 0.05 LPS-Fib and LPS-Fib CdM vs. LPS-MSC CdM; #P < 0.01 control, control-CdM vs. LPS-DMEM, LPS-Fib, LPS-Fib CdM, LPS-MSC CdM; §P < 0.01 LPS-MSC vs. LPS-Fib and LPS-Fib CdM.
MSC-CdM failed to prevent LPS-induced body weight loss.
All mice given LPS were lethargic and had reduced activity and decreased body weight over 48 h compared with control mice (Fig. 3A).
Fig. 3.
MSC-CdM reduced LPS-induced lung injury but failed to prevent body weight loss. A: no effect of treatment on LPS-induced body weight loss. Control (n = 13), control-CdM (n = 6), LPS-DMEM (n = 36), LPS-Fib (n = 9), LPS-Fib-CdM (n = 10), LPS-MSC (n = 8), LPS-MSC CdM (n = 35). **P < 0.01 control groups vs. each LPS group. B: LPS-MSC (n = 5) and LPS-MSC-CdM (n = 8) lungs had improved lung injury score compared with LPS-DMEM (n = 8), LPS-Fib (n = 5), and LPS-Fib CdM (n = 8). **P < 0.01 control groups vs. each LPS group; §P < 0.01 LPS-MSC and LPS-MSC CdM vs. LPS-DMEM, LPS-Fib, and LPS-Fib CdM. C: representative images of lungs from experimental animals. Size bar: 130 μm.
MSC-CdM improved LPS-induced lung injury.
Histological assessment of lung injury by using a semiquantitative histopathology score revealed that mice treated with LPS had increased septal thickening, alveolar hemorrhage, alveolar infiltrates, and fibrin strands compared with controls, whereas treatment with MSCs or MSC-CdM significantly attenuated these features (Fig. 3, B and C).
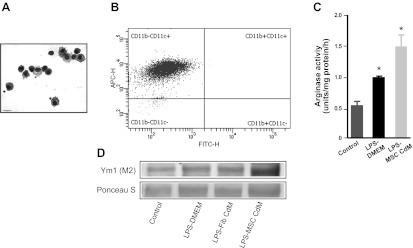
MSC-CdM determined alternative activation of AMs following LPS exposure in vitro and in vivo.
Isolated AMs were macroscopically (Fig. 4A) and phenotypically (CD11c+CD11b−) (Fig. 4B) consistent with AMs. In vitro, AMs were exposed to LPS to test the role of MSC-CdM on the induction and maintenance of the M2 phenotype in AMs. LPS increased arginase activity in AMs compared with non-LPS-exposed cells, and MSC-CdM further elevated these levels compared with LPS-exposed cells cultured with DMEM (Fig. 4C). MSC-CdM also increased Ym1 protein expression compared with LPS-DMEM and LPS-Fib-CdM (Fig. 4D).
Fig. 4.
MSC-CdM promotes the M2 alveolar macrophage (AM) phenotype. A: representative photomicrograph of Hema3-stained AMs. Size bar: 15 μm. B: representative scatterplots of 4-color-stained AMs from experimental lungs. AMs were 98.2% CD11c+ and CD11b−. FITC-H, fluorescein isothiocyanate; APC-H, allophycocyanin. C: AMs exposed to LPS for 24 h had greater arginase activity compared with control AMs. MSC-CdM enhanced arginase activity compared with DMEM (n = 4/group). *P < 0.05 control vs. LPS-DMEM and LPS-MSC-CdM; LPS-DMEM vs. LPS-MSC-CdM. D: immunoblots of AM show enhanced induced Ym1 expression in LPS-MSC-CdM compared with LPS-DMEM and LPS-Fib-CdM (n = 4 samples/lane).
To further investigate the effects of MSC-CdM in vivo, AMs were isolated from LPS-exposed animals that had received cell or CdM treatment. MSC-CdM induced an iNOS-Ym1+ phenotype in AMs from both control (11.6 vs. 0.8%) and LPS-treated animals (69 vs. 6.8%). This effect was enhanced in LPS-MSC recipients (79.9%). Fib and Fib-CdM markedly induced an iNOS+Ym1− phenotype (52 and 41.9%, respectively) compared with control AMs (1.3%), similar to the proportion found in LPS-DMEM AMs (41.8%) (Fig. 5).
Fig. 5.
MSC-CdM promotes the M2 AM phenotype in vivo. A: representative scatterplots of 4-color-stained AMs from experimental lungs. a.u., Arbitrary units. B: AMs from LPS-DMEM, LPS-Fib, LPS-Fib CdM lungs displayed an iNOS+Ym1− (gate P3; M1) phenotype. AMs from LPS-MSC and LPS-MSC CdM showed an iNOS-Ym1+ (gate P2; M2) phenotype (n = 5/group). Q1-1, Q1-2, Q1-3, Q1-4: quadrants resulted from quadrant gating. For M1 macrophages: no significant differences in control vs. control-CdM and LPS-MSC, control-CdM vs. LPS-MSC CdM, or LPS-DMEM vs. LPS-Fib CdM. P < 0.05 LPS-MSC vs. control-CdM and LPS-MSC CdM. P < 0.01 for all other group pairings. For M2 macrophages: no significant differences in control vs. LPS-Fib; LPS-DMEM vs. LPS-Fib CdM. P < 0.01 for all other group pairings.
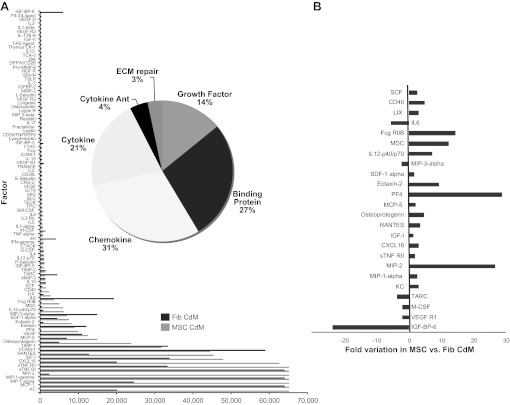
MSC-CdM contains soluble factors that may convey therapeutic benefit.
The levels of 96 different factors were compared between MSC-CdM and Fib-CdM (Fig. 6). Cluster analysis of both CdMs revealed the following distribution: chemokines (31%), binding proteins (27%), cytokines (21%), growth factors (14%), cytokine antagonists (4%), and molecules involved in extracellular matrix repair (3%) (Fig. 6A). Significant fold variations of certain factors were found (Fig. 6B). MSC-CdM contained higher concentrations of stem cell factor (SCF), IL-12p40/p70, stromal-derived factor-1α (SDF-1α), IGF-I, and osteoprotegerin and lower concentrations of IL-6, macrophage inflammatory protein-3α (MIP-3α), and thymus, and activation-regulated chemokine (TARC).
Fig. 6.
A: MSC secretome analysis. Antibody array-based comparison between MSC-CdM and Fib-CdM. B: significant differences in MSC- vs. Fib-CdM levels of factors highlighted. Ant, antagonists.
IGF-I decreased lung LPS-induced lung injury and determined alternative AMs activation.
IGF-I levels were confirmed to be at significantly higher levels in MSC-CdM compared with Fib-CdM by ELISA (Fig. 7A). Administration of rIGF-I significantly attenuated the LPS-induced BALF inflammatory cell influx (Fig. 7B), increase in lung vascular permeability (Fig. 7C), and lung injury (Fig. 7, D–H).
Fig. 7.
IGF-I decreases LPS-induced lung injury. A: ELISA measurement showing that IGF-I is present at higher levels in MSC-CdM compared with Fib-CdM. *P < 0.05 control; #P < 0.01. B–D: treatment with recombinant mouse IGF-I (rIGF-I; n = 5) significantly attenuated BALF cells influx (B), lung permeability (C), and lung injury score (D) compared with LPS-DMEM (n = 5). *P < 0.05 control vs. LPS groups; #P < 0.05 LPS-rIGF-I vs. LPS-DMEM. E: representative images of lungs from experimental animals. Size bar: 130 μm.
To further investigate the effects of IGF-I in vivo, AMs were isolated from LPS-exposed animals that had received rIGF-I or MSC-CdM with IGF-I neutralizing antibody. rIGF-I induced a iNOS−Ym1+ phenotype in AMs in LPS-treated animals, whereas neutralization of IGF-I in MSC-CdM attenuated the induction of a M2 phenotype (Fig. 8).
Fig. 8.
IGF-I promotes the M2 AM phenotype. A: representative scatterplots of 4-color-stained AMs from experimental lungs. B: LPS-rIGF-I treatment enhanced Ym1 expression and prevented inducible nitric oxide synthase (iNOS) induction compared with LPS-DMEM. LPS-Neut-CdM (LPS-MSC CdM +nAb IGF-I) attenuated Ym1 expression in AMs and was less effective in preventing iNOS induction (n = 5/group).
DISCUSSION
We provide evidence that the therapeutic benefits of MSCs are attributable to a paracrine mechanism. In vivo, administration of MSC-CdM alone attenuated lung neutrophil influx and improved lung histology. This effect was comparable with administration of an equivalent number of MSC and absent in mice treated with control cells (lung fibroblasts) or control cell CdM. MSC-CdM induced an M2 phenotype in AMs exposed to LPS in vitro and in vivo in LPS-exposed animals. We also suggest that MSC-CdM contains soluble factors capable of attenuating lung injury. Identification of these soluble factors secreted by MSCs may yield new therapeutic options for ALI/ARDS.
Recent studies have shown that MSCs modulate immune cell function (28, 39, 46) and have cell-protective effects through the release of cytokines and growth factors (17, 18, 24, 37). MSC-CdM decreased hypoxia-induced cell death and improved tube formation of human aortic endothelial cells (24). CdM derived from MSCs engineered to overexpress the prosurvival gene Akt protected cardiomyocytes from hypoxia-induced cell death and limited myocardial infarct size in vivo (17, 18). MSC-CdM improved healing in an excisional wound splinting model in mice (9), oxygen-induced AT2 and pulmonary microvascular endothelial cell injury in vitro (71), neonatal oxygen-induced lung injury in vivo (3), and reversed hepatocyte death and increased survival in Gal-N-induced fulminant hepatic failure (55). Moreover, human MSC-CdM attenuated endotoxin-induced lung injury in an ex vivo perfused human lung model (37) and mouse MSC-CdM prevented the development of murine asthma (25).
In the present study, the therapeutic benefit seen with CdM mirrored the protective effects described with whole cell therapy. Similar to previous findings with whole cell therapy after LPS injury (21, 44), we observed that a single intratracheal injection of MSC-CdM or MSCs 4 h after LPS administration decreased lung neutrophil influx and lung permeability and improved the lung histopathology score compared with control media, lung fibroblasts, or their CdM. These findings open new therapeutic options by identifying potential healing molecules contained in MSC-derived CdM and understanding their mechanism of action.
Our data suggest that MSC-CdM promote a M2 “healer” macrophage phenotype. In the development of ALI/ARDS, neutrophils and macrophages are activated to eliminate pathogens but also contribute to tissue injury through the release of antimicrobial compounds (72). Macrophages are phenotypically heterogeneous because they respond to stimuli in their microenvironment and they also differ genetically (20). Classically activated macrophages (M1) kill invading microorganisms and tumors and promote type I immunity by secreting high levels of proinflammatory cytokines and low levels of anti-inflammatory cytokines (40). In contrast, alternatively activated macrophages (M2) secrete lower levels of proinflammatory cytokines and higher levels of anti-inflammatory cytokines. M2 macrophages promote type II immunity and are thought to dampen the immune response and to promote wound healing, angiogenesis, and debris scavenging (12). M1 and M2 macrophages can be characterized by receptor expression, effector function, cytokine and chemokine production (26, 40), or a set of marker genes: M1 macrophages generate nitric oxide by upregulation of iNOS, whereas M2 macrophages express Ym1 [also known as T-lymphocyte-derived eosinophil chemotactic factor (ECF-L) and chitinase 3-like 3 (CHI3L3)], FIZZ1 [found in inflammatory zone-1, also known as resistin-like molecule alpha (RELM-α)], and arginase-1 (Arg-1) (20, 50). The upregulation of arginase-1 expression and activity is crucial in the metabolic switch from M1 to M2 phenotype in mice (60). In our LPS-induced inflammation model, we screened for the effect of MSC-CdM on AM phenotype in vitro. AMs isolated from healthy mice and subsequently exposed to LPS followed by MSC-CdM had higher Arg-1 activity and expressed higher levels of Ym1, findings characteristic of M2 macrophages. To more thoroughly test for the occurrence of alternative macrophage activation in vivo, we isolated AMs from experimental animals and found that LPS, as well as Fib and FibCdM, increased the numbers of macrophages expressing iNOS in the absence of Ym1 (a pattern corresponding with the M1 phenotype), whereas MSCs and MSC-CdM upregulated Ym1+ macrophages lacking iNOS expression (M2). These data support the immunomodulatory capacity of MSC-CdM shifting the immune environment from pro- to anti-inflammatory via the induction of a “healer” M2 AM phenotype from a “killer” M1 macrophage. Our results are in line with several recent reports indicating that MSCs exert anti-inflammatory properties via macrophage reprogramming (31, 39, 49, 52). However, the murine MSC populations differ amongst these reports, mainly because of the current lack of consensus in establishing the universal murine MSC phenotype (7, 56). Comparison is also limited by differences in experimental protocols and variations in CdM preparation methods.
The paracrine mechanism of action of MSCs opens new therapeutic perspectives. Indeed, several paracrine mediators that can mediate restorative effects of MSCs have been identified, including interleukin-10, IL-1 receptor antagonist (IL-1ra), and keratinocyte growth factor (KGF). IL-1ra was identified as an MSC-derived paracrine factor that reduced the severity of bleomycin-induced lung injury (53). Allogeneic human MSCs or their CdM attenuated endotoxin-induced lung injury in an ex vivo perfused human lung model partly through KGF, a well-known growth factor to reduce lung injury (37). In our study, we found that MSC-CdM contained higher levels of KGF and HGF than Fib-CdM. IL-10 was shown to be responsible for the therapeutic benefits of exogenous MSCs in murine sepsis (49). Our group has recently reported that bone marrow MSC (BMSC)-derived adiponectin contributes to the antiasthmatic effect of BMSC CdM in a murine ovalbumin-induced asthma model (25). Human cord blood-derived MSCs, but not fibroblasts, produce high levels of angiotensin converting enzyme 2, an exopeptidase recently shown to be lung protective (61). Recent findings suggest the possibility that cell-to-cell communication mediated by transfer of exosomes/microvesicles or whole organelles contributes to the therapeutic benefit of MSCs (1, 10). Indeed, MSC-derived microvesicles protect from kidney injury (57) and MSC mitochondria transfer mediates the protective effects of MSC in ALI (27). Further identification of cellular communication mediators, including soluble factors, may lead to the development of critically missing pharmacological therapies for ALI/ARDS and other inflammatory diseases (6).
In an attempt to broaden the search for candidate soluble factors, we performed a multiplex analysis screening for 96 factors present in MSC-CdM and showed that their secretory profile differed from that of lung fibroblasts. In a similar manner, Schinköthe et al. (66) screened human MSCs to attain the first large-scale description of factors they secrete and categorized these into functional groups: antiapoptotic, immunosuppressive, proproliferative, and angiogenic modulating. Among factors that may account for the alternative macrophage activation, we found that MSC-CdM contained several known M2 activators, whereas Fib-CdM contained higher amounts of the potent classical (M1) activator IL-6, whereas MSC-CdM had higher levels of factors that have recently been shown to contribute to the promotion of an M2 environment. We have previously reported that adiponectin, which may exert similar M2-activating effects (50) was found in our MSCs CdM from both WT C57/BL6 and Balb/C mouse strains but was undetectable in Fib-CdM by ELISA (25).
The factors with the highest differential expression in MSC-CdM compared with Fib-CdM included platelet factor 4 (PF4) and macrophage inflammatory protein 2 (MIP-2). PF4-induced macrophages are distinct from M1 macrophages and M2 macrophages and have been proposed to have specific proatherogenic capacities (16), suggesting that PF4 does not induce a healer macrophage phenotype. With regard to the role of MIP-2 as a phenotype marker, the existing literature is unclear. MIP-2 has been presented as both an “M1 marker” (30) and a factor produced by M2 macrophages (14). Conversely, IGF-I has recently been shown to aid in creating an M2-favorable environment (8). Thus we explored the contribution of IGF-I to the therapeutic benefit by administering rIGF-I alone as in vivo treatment. Administration of rIGF-I partially reproduced the beneficial effects of MSC-CdM.
MSC-CdM also contained markedly higher levels of potential protective factors compared with Fib-CdM, suggesting that additional mechanisms could explain the therapeutic benefit of MSC-CdM in this model. SCF is increased almost threefold in MSC-CdM compared with Fib-CdM. SCF improves survival, proliferation, and differentiation of hematopoietic stem and progenitor cells and antiapoptotic effects reported in kidney tubular epithelial cells (7, 15). Another molecule, FcγRIIB, is increased 14-fold in MSC-CdM compared with Fib-CdM. FcγRIIB mediates the anti-inflammatory benefits of intravenous gamma globulin in a murine model of immune thrombocytopenia (64). We also found that IL-12p40, a natural antagonist of IL-12, which is a cytokine responsible for the production of IFN-γ, was increased sevenfold in MSC-CdM compared with Fib-CdM. IL-12p40 has protective effects in bacterial pneumonitis in mice (23) and selectively inhibits airway hyperresponsiveness and peribronchial fibrosis in murine asthma (52). A twofold increase in soluble TNF-α receptor II (sTNFRII), an inhibitor of TNF-α and a possible regulator of inflammatory activity, was observed in MSC-CdM compared with Fib-CdM. sTNFRII may contribute to dampening of the activity of TNF-α in LPS injury (45).
In conclusion, our study provides direct in vivo evidence that MSCs exert their therapeutic benefit through a paracrine activity. Identification of pneumoprotective soluble factors in MSC-CdM holds promise for the discovery of new pharmacological therapies for lung diseases. Although MSCs may have a unique ability to monitor the microenvironment of injured tissues and respond appropriately, therapies with the proteins or cytokines produced by activated MSCs may be more practical than cell therapies (2, 38). In vitro priming of MSCs to optimize the production of protective factors may combine the advantages of both cell and small molecule therapy.
GRANTS
B. Thébaud is a Canada Research Chair and supported by the Canada Foundation for Innovation (CFI), the Alberta Innovates-Health Solutions (AIHS), the Canadian Institutes for Health Research (CIHR), the Canadian Stem Cell Network, and the Stollery Children's Hospital Foundation. L. I. Ionescu and R. N. Byrne were supported by CIHR/IHDCYH-sponsored Maternal Fetal Neonatal Health Training Programs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.I., R.N.B., F.E., and B.T. conception and design of research; L.I., R.N.B., T.v.H., A.V., R.S.A., G.J.R.-P., G.W., A.H., and F.E. performed experiments; L.I., R.N.B., T.v.H., A.V., R.S.A., G.J.R.-P., G.W., A.H., F.E., and B.T. analyzed data; L.I., R.N.B., T.v.H., A.V., R.S.A., G.J.R.-P., G.W., A.H., F.E., and B.T. interpreted results of experiments; L.I., R.N.B., F.E., and B.T. prepared figures; L.I., R.N.B., and B.T. drafted manuscript; L.I., R.N.B., T.v.H., A.V., R.S.A., F.E., and B.T. edited and revised manuscript; L.I., R.N.B., T.v.H., A.V., R.S.A., G.J.R.-P., G.W., A.H., F.E., and B.T. approved final version of manuscript.
REFERENCES
- 1. Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, Dubois-Randé JL, Rodriguez AM. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 29: 812–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6: e1000029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180: 1122–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bengatta S, Arnould C, Letavernier E, Monge M, de Preneuf HM, Werb Z, Ronco P, Lelongt B. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol 20: 787–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonfield TL, Koloze M, Lennon DP, Zuchowski B, Yang SE, Caplan AI. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol 299: L760–L770, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 21: 119–143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25: 2739–2749, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr, Wynn TA, Gause WC. An essential role for T(H)2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18: 260–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3: e1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho YM, Kim JH, Kim M, Park SJ, Koh SH, Ahn HS, Kang GH, Lee JB, Park KS, Lee HK. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One 7: e32778, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 174: 231–235, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Dal-Pizzol F. Alternative activated macrophage: a new key for systemic inflammatory response syndrome and sepsis treatment? Crit Care Med 32: 1971–1972, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE2 catabolism in myeloid cells. J Leukoc Biol 88: 839–848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galli MC, Giardina PJ, Migliaccio AR, Migliaccio G. The biology of stem cell factor, a new hematopoietic growth factor involved in stem cell regulation. Int J Clin Lab Res 23: 70–77, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Gleissner CA. Macrophage phenotype modulation by CXCL4 in atherosclerosis. Front Physiol 3: 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 11: 367–368, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol 482: 281–294, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 296: L936–L946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutierrez JG, Valdez SR, Di Genaro S, Gomez NN. Interleukin-12p40 contributes to protection against lung injury after oral Yersinia enterocolitica infection. Inflamm Res 57: 504–511, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 25: 2363–2370, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Ionescu LI, Alphonse RS, Arizmendi N, Morgan B, Abel M, Eaton F, Duszyk M, Vliagoftis H, Aprahamian TR, Walsh K, Thebaud B. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol 46: 207–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF, 4th, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, Kunkel SL. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 14: 3244–3254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18: 759–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 8: 569–581, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181: 4389–4396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joshi AD, Raymond T, Coelho AL, Kunkel SL, Hogaboam CM. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J Leukoc Biol 83: 314–324, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37: 1445–1453, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 33: 328–334, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128: 5181–5188, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369–377, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 262: 509–525, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus host disease: a phase II study. Lancet 371: 1579–1586, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol 43: 1018–1023, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5: e9252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Matthay MA, Thompson BT, Read EJ, McKenna DH, Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138: 965–972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol 158: 153–161, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 4: e269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 151: 1548–1561, 1993 [PubMed] [Google Scholar]
- 46. Morrison AC, Correll PH. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol 168: 853–860, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499–3506, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Gorham JD, Bundoc VG, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-β to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci USA 107: 5652–5657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol 6: 275–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA 105: 14638–14643, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Onari Y, Yokoyama A, Haruta Y, Nakashima T, Iwamoto H, Hattori N, Kohno N. IL-12p40 is essential for the down-regulation of airway hyperresponsiveness in a mouse model of bronchial asthma with prolonged antigen exposure. Clin Exp Allergy 39: 290–298, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE 2: e941, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103: 1662–1668, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Plotnikov EY, Khryapenkova TG, Galkina SI, Sukhikh GT, Zorov DB. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp Cell Res 316: 2447–2455, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17: 939–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity 23: 361–374, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Rey-Parra GJ, Vadivel A, Coltan L, Hall A, Eaton F, Schuster M, Loibner H, Penninger JM, Kassiri Z, Oudit GY, Thébaud B. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J Mol Med 90: 637–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 33: 145–152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291: 484–486, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res 85: 194–203, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev 17: 199–206, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Sugiura H, Liu X, Duan F, Kawasaki S, Togo S, Kamio K, Wang XQ, Mao L, Ahn Y, Ertl RF, Bargar TW, Berro A, Casale TB, Rennard SI. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. Am J Respir Cell Mol Biol 37: 424–430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc 40: 2649–2654, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Vadivel A, van Haaften T, Alphonse RS, Rey-Parra GJ, Ionescu L, Haromy A, Eaton F, Michelakis E, Thébaud B. Critical role of the axonal guidance cue EphrinB2 in lung growth, angiogenesis, and repair. Am J Respir Crit Care Med 185: 564–574, 2012 [DOI] [PubMed] [Google Scholar]
- 71. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thebaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 180: 1131–1142, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Zhang C, Wang SH, Lasbury ME, Tschang D, Liao CP, Durant PJ, Lee CH. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect Immun 74: 1857–1864, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]