Abstract
Respiratory syncytial virus (RSV) is one of the most common causes of bronchiolitis and pneumonia among infants and young children worldwide. In previous investigations, we have shown that RSV infection induces rapid generation of reactive oxygen species (ROS), which modulate viral-induced cellular signaling, and downregulation of antioxidant enzyme (AOE) expression, resulting in oxidative stress in vitro and in vivo, which plays a pathogenetic role in RSV-induced lung disease. In this study, we determined whether pharmacological intervention with synthetic catalytic scavengers could reduce RSV-induced proinflammatory gene expression and oxidative cell damage in an in vitro model of infection. Treatment of airway epithelial cells (AECs) with the salen-manganese complexes EUK-8 or EUK-189, which possess superoxide dismutase, catalase, and glutathione peroxidase activity, strongly reduced RSV-induced ROS formation by increasing cellular AOE enzymatic activity and levels of the lipid peroxidation products F2-8-isoprostane and malondialdehyde, which are markers of oxidative stress. Treatment of AECs with AOE mimetics also significantly inhibited RSV-induced cytokine and chemokine secretion and activation of the transcription factors nuclear factor-κB and interferon regulatory factor-3, which orchestrate proinflammatory gene expression. Both EUKs were able to reduce viral replication, when used at high doses. These results suggest that increasing antioxidant cellular capacities can significantly impact RSV-associated oxidative cell damage and cellular signaling and could represent a novel therapeutic approach in modulating virus-induced lung disease.
Keywords: respiratory syncytial virus, airway epithelial cells, antioxidant enzyme mimetics, oxidative stress
respiratory syncytial virus (RSV) is the one of the most important causes of viral upper and lower respiratory tract infections (LRTI) in infants and young children. RSV is so ubiquitous in nature that it will infect 100% of children before the age of two. The number of children hospitalized each year in the United States with viral LRTI has recently been estimated at >200,000, with 500 deaths per year in children under age 5 years (24). Although the mechanisms of RSV-induced airway disease and associated long-term consequences remain incompletely defined, the lung inflammatory response is thought to play a fundamental role. Oxidative stress has been shown to play an important role in the pathogenesis of both acute and chronic lung inflammatory diseases (reviewed in Refs. 27, 29, and 33). Reactive oxygen species (ROS) are highly unstable molecules produced by the pulmonary epithelial and endothelial cells involved in many forms of tissue damage, including the damage caused to cellular components such as lipids, proteins, and DNA (reviewed in Refs. 1 and 11). We have previously shown that RSV infection of AECs induces ROS production, which is involved in transcription factor activation and chemokine gene expression (6, 26). We have also shown that rapid generation of ROS is associated with oxidative stress and lung damage in infected cells in both animals and children (8, 16, 17). Antioxidant treatment significantly ameliorates RSV-induced oxidative stress, clinical disease, and pulmonary inflammation in a mouse model of infection, suggesting a causal relationship between increased ROS production and lung disease (8). RSV infection leads to a significant decrease in the expression and activity of antioxidant enzymes (AOEs) in AECs, in lungs of RSV-infected mice, as well as in children with severe RSV-induced LRTI (16, 17), suggesting that oxidative damage associated with RSV infection results from an imbalance between ROS production and antioxidant cellular defenses.
The use of recombinant superoxide dismutase (SOD) and SOD mimetics has been explored as therapeutics in a variety of disease models either in vitro or in vivo. A number of SOD mimetics based around organo-manganese complexes have been developed. They include metalloporphyrin-based compounds, such as AEOL10113 and -10150, cyclic polyamine-based molecules, such as M40403 and -40419, and the salen compounds, such as EUK-8, -134 and -189, the latter ones possessing also significant catalase and peroxidase activity (reviewed in Ref. 3). Although EUKs have been used in a variety of disease models, there is no reported literature about their use in models of viral infections. Recently, we have shown that treatment of A549 cells with EUK-134 significantly inhibits RSV-induced interleukin (IL)-8 and regulated on activation normal T cell expressed and secreted (RANTES) secretion (17). In the present study, we found that EUK-8 and EUK-189 treatment of AECs significantly restored intracellular catalase and glutathione peroxidase (GPx) enzyme activities, which are significantly diminished by RSV infection, leading to reduced viral-induced ROS production and generation of lipid peroxidation markers, such as isoprostane and malondialdehyde (MDA). In addition, EUK administration significantly reduced secretion of a variety of proinflammatory molecules in response to RSV infection. At high concentration, both EUK-8 and -189 reduced viral replication, indicating that EUKs could represent a novel therapeutic approach to restore the prooxidant/antioxidant balance in favor of the latter, in the context of RSV infection, leading to reduced cellular oxidative stress, proinflammatory mediator secretion, and reduced viral replication.
MATERIALS AND METHODS
Materials.
Eukarion compounds (salen-manganese complexes) EUK-8 and EUK-189 were kindly provided by Susan Doctrow (Boston University, School of Medicine). 2′,7′-Dichlorodihydro-fluorescein diacetate (DCF-DA) was from Invitrogen (Molecular Probes, Eugene, OR); 3-amino-9-ehtyl carbazole was from Sigma (St. Louis, MO).
RSV preparation.
The RSV long strain was grown in Hep-2 cells and purified by centrifugation on discontinuous sucrose gradients as described elsewhere (39). The virus titer of the purified RSV pools was 8–9 log10 plaque-forming units (PFU)/ml using a methylcellulose plaque assay. No contaminating cytokines were found in these sucrose-purified viral preparations (31). Lipopolysaccharide (LPS), assayed using the limulus hemocyanin agglutination assay, was not detected. Virus pools were separated into aliquots, quick-frozen on dry ice/alcohol, and stored at −70°C until used.
Cell culture and infection of epithelial cells with RSV.
A549 cells, a human alveolar type II-like epithelial cell line (American Type Culture Collection, Manassas, VA), and small alveolar epithelial (SAE) cells (Clonetics, San Diego, CA), normal human AECs derived from terminal bronchioli, were grown according to the manufacturer's instructions. A549 and SAE were maintained in F12K and small airway epithelial cell (SAEC) growth medium, respectively, containing 10% (vol/vol) FBS, 10 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin for F12K medium and 7.5 mg/ml bovine pituitary extract, 0.5 mg/ml hydrocortisone, 0.5 μg/ml human epidermal growth factor, 0.5 mg/ml epinephrine, 10 mg/ml transferrin, 5 mg/ml insulin, 0.1 μg/ml retinoic acid, 0.5 μg/ml triiodothyronine, 50 mg/ml gentamicin, and 50 mg/ml BSA for SAEC medium. When SAE were used for RSV infection, they were changed to basal medium, not supplemented with growth factors, 6 h before and throughout the length of the experiment. At around 80–90% confluency, cell monolayers were infected with RSV at multiplicity of infection (MOI) of three (unless otherwise stated), as previously described (13). An equivalent amount of a 30% sucrose solution was added to uninfected A549 and SAE cells, as a control.
For the catalytic scavenger experiment, cells were treated with EUK-8 or EUK-189 either 1 h before and throughout the infection or at different times postinfection. Because EUKs were diluted in ethanol, equal amounts of ethanol were added to untreated cells, as control. Total number of cells and cell viability, following antioxidant treatment, were measured by trypan blue exclusion. There was no significant change in cell viability with both compounds at all doses tested.
Luciferase assay.
Logarithmically growing A549 cells were transfected in triplicate in 24-well plate dishes with Cignal Antioxidant Response Reporter from Qiagen, an optimized luciferase reporter construct that monitors both increases and decreases in the transcriptional activity of NF-E2-related factor 1 and NF-E2-related factor 2 (Nrf2), using Fugene 6 (Roche Diagnostic, Indianapolis, IN). Reporter gene plasmid (0.5 μg/well) and β-galactosidase (0.05 μg/well) expression plasmid were premixed with FuGene 6 and added to the cells in 1 ml of regular medium. The next morning, cells were infected with RSV in the presence or absence of EUKs and harvested at 24 h postinfection to independently measure luciferase and β-galactosidase reporter activity, as previously described (7). Luciferase activity was normalized to the internal control β-galactosidase activity. Results are expressed in arbitrary units. All experiments were performed at least two to three times.
Determination of lactate dehydrogenase activity.
Lactate dehydrogenase (LDH) activity in the medium, an index of cellular damage, was measured by a colorimetric LDH release assay using a commercially available kit (catalog no. 10008882; Cayman Chemical). The assay was carried out according to the kit's instructions.
Antiviral assay in the presence of EUKs.
A549 cells were seeded into 48-well plates at 5 × 104 cells/well. Cells were treated with EUKs in triplicate wells for 1 h and infected with RSV at a MOI of 0.01 PFU/cell. Following adsorption of virus for 1 h at 37°C and 5% CO2, viral inoculum was aspirated, the cells were washed three times with minimal essential medium (MEM), and then MEM with 2% FBS was added. Control or EUK-treated and infected cells were then incubated at 37°C and 5% CO2 for 24 h. RSV titers were determined by using polyclonal antibodies and a horseradish peroxidase (HRP) staining method, as previously described (23). Briefly, medium was aspirated, and the cells were fixed for 20 min using methanol and 2% H2O2. Cells were then incubated for 30 min with anti-RSV polyclonal antibody (Biogenesis, Kingston, NH) followed by HRP-conjugated anti-guinea pig secondary antibody (Zymed, San Francisco, CA). Plaques were visualized by the addition of 3-amino-9-ethyl-carbazole substrate and enumerated by light microscopy.
Quantitative real-time PCR.
RNA samples were quantified by using a Nanodrop Spectrophotometer (Nanodrop Technologies), and quality was analyzed on RNA Nano or Pico chip using the Agilent 2100 Bioanalyzer (Agilent Technologies). Synthesis of cDNA was performed with 1 µg of total RNA in a 20-µl reaction by using the reagents in the Taqman Reverse Transcription Reagents Kit from ABI (Applied Biosystems no. N8080234). The reaction conditions were as follows: 25°C 10 min, 48°C 30 min, 95°C 5 min. Quantitative real-time PCR amplifications (performed in triplicate) were done with 1 µl of cDNA in a total volume of 25 µl by use of the Faststart Universal SYBR Green Master Mix (Roche Applied Science no. 04913850001). The final concentration of the primers was 300 nM. 18S RNA was used as housekeeping gene for normalization. PCR assays were run in the ABI Prism 7500 Sequence Detection System with the following conditions: 50°C 2 min, 95°C 10 min and then 95°C 15 s, 60°C 1 min for 40 cycles. RSV N-specific RT primer contained a tag sequence from the bacterial chloroamphenicol resistance gene to generate the cDNA, because of self-priming exhibited by RSV RNA. Duplicate cycle threshold (CT) values were analyzed in Microsoft Excel by the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amount of target (2−ΔΔCT) was obtained by normalizing to endogenous reference (18S) sample. RSV N dT+Tag (RT primer): CTGCGATGAGTGGCAGGCTTTTTTTTTTTTAACTYAAAGCTC Cmr Tag. For PCR assay, RSV Tag (R primer): CTGCGATGAGTGGCAGGC. RSV N forward primer: ACTACAGTGTATTAGACTTRACAGCAGAAG.
Measurement of intracellular ROS.
A549 cells were grown in 96-well tissue culture plates and infected with RSV. At different times postinfection, cells were washed with Hanks' balanced salt solution (HBSS) and loaded with 10 μM 2,7 DCF-DA in HBSS medium containing 25 mM HEPES, pH 7.4, for 30 min at 37°C. The cells were then washed two times, and fluorescence intensity was determined at 485 nm excitation and 590 nm emission, using an automated fluorescence reader (Molecular Devices, Sunnyvale, CA).
Measurement of lipid peroxidation products.
Measurement of F2-8-isoprostane was performed using a competitive enzyme immunoassay from Cayman Chemical (Ann Arbor, MI) according to the manufacturer's instructions. Measurement of the lipid peroxidation marker MDA was carried out using a lipid peroxidation kit from Calbiochem/EMD Chemicals.
Western blotting.
Nuclear extracts of uninfected and infected cells were prepared using hypotonic/nonionic detergent lysis, according to the protocol of Schreiber et al. (35). To prevent contamination with cytoplasmic proteins, isolated nuclei were purified by centrifugation through 1.7 M sucrose buffer for 30 min, at 12,000 rpm, before nuclear protein extraction, as previously described (5). Total cell lysates were prepared from uninfected and infected A549 cells by adding ice-cold lysis buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.25% sodium deoxycholate, 1 mM Na3VO4, 1 mM NaF, 1% Triton X-100, and 1 μg/ml of aprotinin, leupeptin, and pepstatin). After incubation on ice for 10 min, the lysates were collected, and detergent-insoluble materials were removed by centrifugation at 4°C at 14,000 g. Proteins (10–20 μg/sample) were then boiled in 2× Laemmli buffer and resolved on SDS-PAGE. Proteins were transferred onto Hybond-polyvinylidene difluoride membrane (Amersham, Piscataway, NJ), and nonspecific binding sites were blocked by immersing the membrane in Tris-buffered saline-Tween (TBST) containing 5% skim milk powder or 5% BSA for 30 min. After a short wash in TBST, membranes were incubated with the primary antibody for 1 h or overnight at 4°C, depending on the antibody used, followed by HRP-conjugated secondary antibody (Sigma) diluted 1:10,000 in TBST for 30 min at room temperature. After being washed, proteins were detected using an enhanced chemiluminescence system (Amersham Life Science) and visualized through autoradiography. Antibodies used for Western blot assay are goat anti-RSV (Ab D SeroTec) and rabbit anti-p65, anti-Ser536 p65, and anti-IRF-3 (Cell Signaling Technology, Danvers, MA).
Biochemical assays.
Catalase, GPx, and SOD activities were determined using specific kits (catalog nos. 707002, 703102, and 706002, respectively, for catalase, GPx, and SOD; Cayman Chemical), according to the manufacturer's instructions, as previously described (17).
Bio-Plex.
Cell-free supernatant from EUK-8 and EUK-189-treated and virus- and mock-infected A549 and SAE cells were collected at 24 h postinfection to measure the production of cytokines and chemokines. Samples were tested for multiple cytokines using the Bio-Plex Cytokine Human Multi-Plex panel (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. IL-8 and RANTES were also quantified by enzyme-linked immunosorbent assay following the manufacturer's protocol (DuoSet; R&D Systems, Minneapolis, MN).
Statistics.
A two-tailed Student's t-test using 95% confidence levels was used when comparison of two groups was performed, whereas one-way ANOVA was used for multiple group comparison. Significance is designated by the following: *P < 0.05 and **P < 0.01.
RESULTS
Antioxidant mimetic treatment increases total SOD, catalase, and GPx enzyme activities in RSV-infected A549 cells.
In recent investigations, we have shown that RSV infection of AECs induces a significant decrease in SOD 1, SOD 3, catalase, and glutathione S-transferase (GST) expression with a concomitant increase of SOD 2. Total SOD activity was increased, but catalase, GPx, and GST activities, needed to detoxify H2O2 produced by SOD, were decreased following RSV infection (17). In this study, we tested whether treatment with the antioxidant mimetics EUK-8 and -189, which possess significant catalase and peroxidase activity in addition to SOD, could restore AOE capacity in RSV-infected AECs and thereby exert a protective effect against RSV-induced oxidative stress.
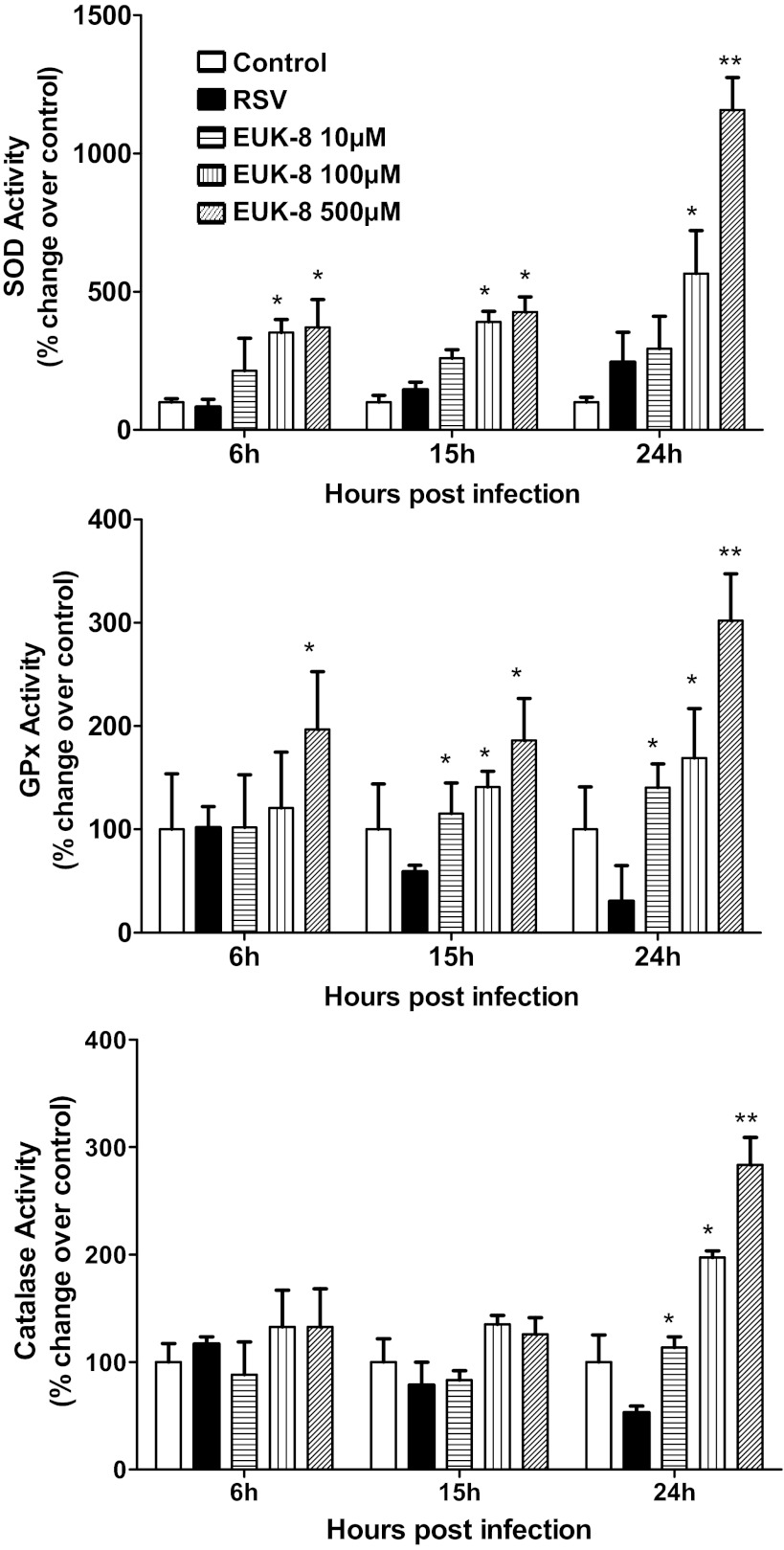
A549 cells were treated 1 h before infection and throughout the length of infection with increasing concentration of EUK and infected with RSV. Cells were harvested at a different time postinfection to measure AOE activity in the presence or absence of EUK treatment. RSV infection induced a progressive increase in SOD activity with a concomitant decrease in catalase and peroxidase activity (Fig. 1). EUK-8 treatment further increased SOD activity but, more importantly, reversed the loss of catalase and peroxidase activity observed in response to RSV infection, with the highest dose of EUK-8 increasing the latter two AOE activities above values of uninfected cells (Fig. 1). Similar results were obtained in cells treated with EUK-189 (data not shown).
Fig. 1.
Effect of EUK treatment on total superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) activity in respiratory syncytial virus (RSV)-infected cells. Total lysates were prepared from uninfected and RSV-infected treated or untreated with different doses of EUK-8 at 6, 15, and 24 h postinfection to measure total SOD, catalase, and GPx enzyme activities. Data are expressed as %change over control. Results are representative of two independent experiments. *P < 0.05 and **P < 0.01 compared with RSV-infected cells.
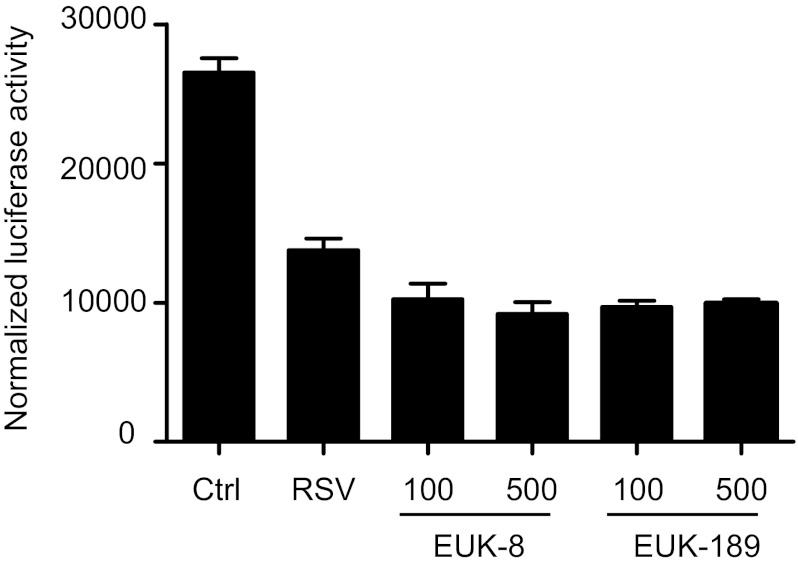
Transcription of many oxidative stress-inducible genes, including AOEs, is regulated in part through cis-acting antioxidant responsive element (ARE) sequences. Nrf2 is an important redox-responsive protein that binds to ARE promoter elements to induce gene transcription (19). We have recently shown that RSV infection decreases nuclear levels of Nrf2 in AECs (17), as well as in the lungs of infected mice (16), and reduces ARE-dependent gene transcription (12). To determine whether changes in AOE activity observed with EUK treatment affected Nrf2-dependent gene transcription and endogenous AOE expression, we performed reporter gene assays. A549 cells were transfected with an artificial ARE-driven promoter, linked to the luciferase reporter gene, and infected with RSV in the presence or absence of EUKs. As previously shown, RSV infection induced a substantial decrease in Nrf2-driven gene transcription, demonstrated by the decrease in luciferase activity, which was not rescued by EUK treatment (Fig. 2). In addition, we did not observe a significant increase in endogenous AOE protein levels in cells infected with RSV and treated with EUK (data not shown), suggesting that the increased AOE activities in EUK-treated cells are not due to an increase in the endogenous enzymes.
Fig. 2.
Effect of EUK treatment on NF-E2-related factor 2-dependent gene transcription. A549 cells were transiently transfected with an airway epithelial cell-luciferase reporter plasmid and β-galactosidase control plasmid. The next day, cells were infected with RSV in the presence or absence of either EUK-8 or -189, at 100 or 500 μM concentration, and harvested at 24 h postinfection to measure luciferase activity. Uninfected plates served as controls. For each plate, luciferase was normalized to the β-galactosidase activity. Data are expressed as means ± SE of normalized luciferase activity. Data are representative of two independent experiments performed in triplicates.
Effects of antioxidant mimetics on RSV-induced ROS formation and cellular oxidative stress.
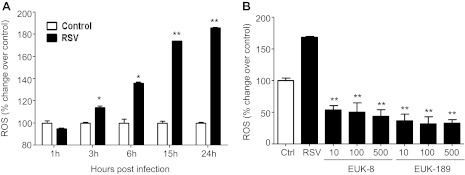
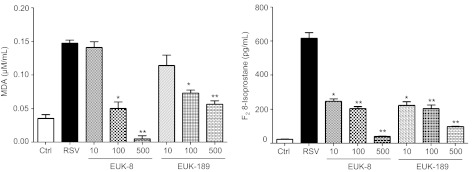
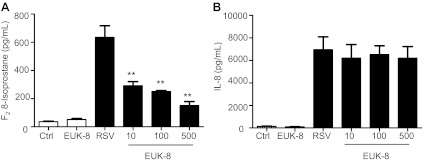
To determine whether EUK treatment could reduce RSV-induced ROS production and cellular oxidative stress, A549 cells were treated with different concentrations of the two antioxidant mimetics either 1 h before and throughout the length of infection, or at a different time postinfection Cells were harvested at 24 h postinfection to measure ROS generation and concentration of the lipid peroxidation markers MDA and F2-8-isoprostane. As previously reported (6), RSV infection of AECs induced a time-dependent increase in ROS generation, starting between 1 and 3 h postinfection (Fig. 3A), which was significantly reduced by pretreatment with both EUK-8 and EUK-189, in a dose-dependent manner (Fig. 3B). In agreement with the observed reduction in ROS production, EUK pretreatment of AECs significantly decreased the elevated cellular levels of the lipid peroxidation markers MDA and 8-isoprostane generated in response to RSV infection (Fig. 4), indicating that antioxidant mimetic pretreatment can effectively counteract viral-induced cellular oxidative stress. EUK treatment was still effective, leading to a significant reduction in 8-isoprostane generation, even after RSV infection was established (see Fig. 6A).
Fig. 3.
Effect of EUK treatment on RSV-induced reactive oxygen species (ROS) formation and cellular oxidative stress. A: A549 cells were infected with RSV and, at various time points after infection, cells were loaded with 2′,7′-dichlorodihydro-fluorescein diacetate (DCF-DA), and fluorescence was measured in control and infected cells. *P < 0.05 and **P < 0.01 compared with control cells. B: A549 cells were treated with different μM concentrations of EUK-8 and EUK-189, infected with RSV for 24 h, and harvested to measure DCF-DA fluorescence. Ctrl, control, uninfected cells. Mean fluorescence intensity is reported as % increase over control. Results are representative of two independent experiments. *P < 0.05 and **P < 0.01 compared with untreated RSV-infected cells.
Fig. 4.
Effect of EUK treatment on RSV-induced lipid peroxidation. A549 cells were treated with different μM concentrations of EUK-8 and EUK-189 and infected with RSV. Cell supernatants were harvested at 24 h postinfection to measure F2-isoprostanes and malondialdehyde (MDA). Results are expressed as means ± SE. Results are representative of two independent experiments run in triplicate. *P < 0.05 and **P < 0.01 compared with untreated RSV-infected cells.
Fig. 6.
Effect of EUK postinfection treatment on RSV-induced lipid peroxidation and IL-8 secretion. A549 cells were treated with different μM concentrations of EUK-8 at 3 h postinfection and harvested at 24 h postinfection to measure 8-isoprostane (A) and IL-8 (B). Results are expressed as means ± SE. Results are representative of two independent experiments run in triplicate. **P < 0.01 compared with untreated RSV-infected cells.
Effects of antioxidant mimetics on RSV-induced cellular signaling.
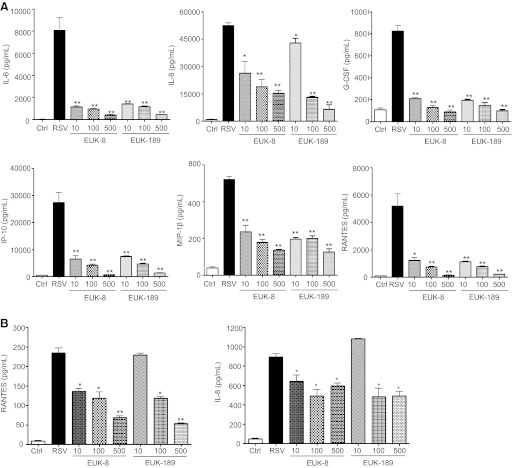
Because ROS generation plays a key role in RSV-induced cellular signaling, leading to transcription factor activation and expression of proinflammatory mediators (6, 20, 26), we investigated the effect of EUK treatment on viral-induced cytokine and chemokine secretion. A549 cells were treated either 1 h before and throughout the length of infection or at a different time postinfection with increasing concentrations of EUK-8 and -189 and infected with RSV. Cell supernatants were collected to measure levels of various cytokines and chemokines by Bio-Plex assay. As shown in Fig. 5A, EUK pretreatment caused a dose-dependent decrease in several cytokines, such as IL-6 and granulocyte colony-stimulating factor, and chemokines, such as IL-8, RANTES, macrophage inflammatory protein-1β (MIP-1β), and interferon-induced protein-10. Similar results were obtained in SAE cells, normal human AECs derived from cadaveric donor, which we have previously shown to behave very similarly to A549 cells in terms of chemokine/cytokine gene expression and transcription factor and signaling pathway activation after RSV infection (2, 6, 13, 16, 30, 32, 41) (Fig. 5B). On the other hand, administration of EUKs, when infection was already established, was not able to reduce proinflammatory mediator production, as shown for IL-8 in Fig. 6B.
Fig. 5.
Effect of EUK treatment on RSV-induced cytokine and chemokine production. A549 cells (A) or small alveolar epithelial (SAE) cells (B) were infected with RSV in the absence or presence of different μM concentrations of EUK-8 and EUK-189. Cell supernatants from uninfected and RSV-infected, treated or untreated, were assayed at 24 h postinfection for cytokine and chemokine secretion by Bio-Plex. IL, interleukin; G-CSF, granulocyte colony-stimulating factor; IP-10, interferon-induced protein-10; MIP-1β, macrophage inflammatory protein-1β; RANTES, regulated on activation normal T cell expressed and secreted. Results are expressed as means ± SE. Results are representative of two independent experiments run in triplicate. *P < 0.05 and **P < 0.01 compared with untreated RSV-infected cells.
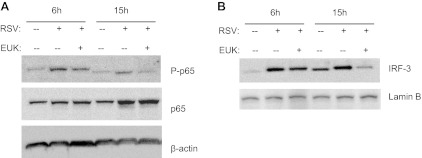
Cytokine and chemokine gene expression in AECs infected by RSV is orchestrated by activation of two key transcription factors, nuclear factor (NF)-κB and interferon regulatory factor (IRF)-3. A number of RSV-inducible inflammatory and immunoregulatory genes require NF-κB for their transcription and/or are dependent on an intact NF-κB signaling pathway (4, 38), and IRF-3 is necessary for viral induction of RANTES transcription and gene expression (25). We have previously shown that treatment of AECs with the antioxidant butylated hydroxyanisole blocks RSV-induced IRF-3 nuclear translocation and DNA binding to the RANTES interferon-stimulated responsive element (6), an event required for RSV-induced RANTES gene transcription. We have also shown that the antioxidants N-acetylcysteine or dimethyl sulfoxide significantly reduce RSV-dependent serine phosphorylation of the NF-κB subunit p65, resulting in the inhibition of RSV-induced expression of several NF-κB-dependent genes, without affecting its nuclear translocation (20). To determine whether EUK treatment was able to modulate viral-induced NF-κB and IRF-3 activation, A549 cells were pretreated with 100 μM EUK-8, infected with RSV, and harvested at 6 and 15 h postinfection to prepare either total cell lysates or nuclear extracts. IRF-3 nuclear levels or cellular levels of p65 serine phosphorylation were assessed by Western blot. As shown in Fig. 7, EUK-8 pretreatment significantly reduced activation of both transcription factors, in particular at the 15-h time point of infection. Taken together, these results indicate that increasing antioxidant cellular defenses can effectively modulate the strong proinflammatory cellular response induced by RSV infection.
Fig. 7.
Effect of EUK treatment on RSV-induced interferon regulatory factor (IRF)-3 and p65 activation. Total cell lysates (A) or nuclear extracts (B) were prepared from A549 cells, control and infected with RSV for 6 and 15 h, in the absence or presence of 100 μM EUK-8 and assayed for p65 phosphorylation and IRF-3 nuclear levels, respectively, by Western blot. Membranes were stripped and reprobed for either β-actin or lamin b as a control for equal loading of the samples. Blot is representative of two independent experiments with similar results.
Effects of antioxidant mimetics on viral replication.
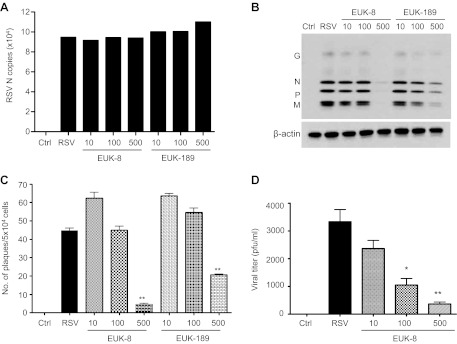
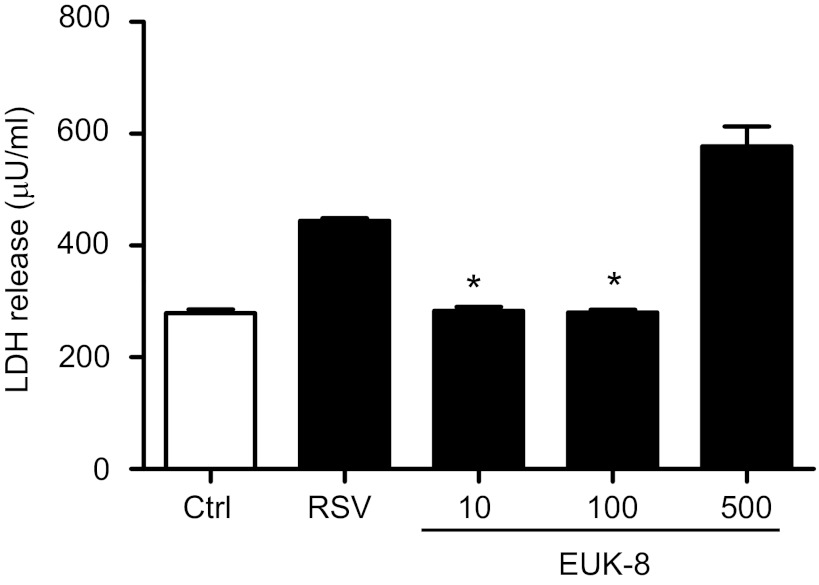
To determine whether antioxidant mimetic treatment of A549 cells affected viral replication, we used several approaches, including quantification of viral gene transcription, direct cell-based plaque immunostaining (23) and viral antigen detection, and determination of released infectious particles in the cell supernatants. As shown in Fig. 8A, there was no significant difference in the number of RSV N gene copies between untreated and EUK-pretreated cells at any given concentration. To further characterize the antiviral activity of EUKs, we assessed the expression of RSV proteins by Western blot. A549 cells were pretreated with different concentrations of EUKs and infected with RSV. At 24 h postinfection, cell extracts were prepared, and RSV proteins were detected by Western blot using a polyclonal antibody, as described previously (23). In RSV-infected cells, viral proteins, including G, N, P, and M, were expressed at comparable levels in untreated and EUK-treated cells using concentrations of 10 and 100 μM, whereas significant lower (EUK-189) or almost no expression (EUK-8) of RSV proteins was detected in infected cells treated with 500 μM of both compounds (Fig. 8B). A similar result was observed when the number of plaques was detected by direct immunostaining of infected cells (Fig. 8C). In addition, both 100 and 500 μM concentrations of EUK-8 significantly reduced virus infectious particle release from AECs, as shown by the decreased viral titers in cell supernatants (Fig. 8D). The reduction in viral replication was not due to cellular toxicity, as shown by LDH release assay in Fig. 9. Taken together, these results suggest that EUKs impair viral replication at a step subsequent to virus cellular binding and entry, and after initiation of viral gene transcription.
Fig. 8.
Effect of EUK treatment on viral replication. A549 cells were treated with different μM concentrations of EUK-8 and EUK-189, followed by infection with RSV. Cells were harvested at 24 h postinfection to prepare either total RNA to measure RSV N gene copies by real-time PCR (A) or total cell lysates to detect RSV proteins by Western blot. Membrane was stripped and reprobed with β-actin as a control for equal loading of the samples (B). Figures are representative of two independent experiments with similar results. A549 cells were treated with different μM concentrations of EUK-8 and EUK-189 followed by infection with RSV at a multiplicity of infection of 0.01. Viral replication was determined 24 h postinfection by either direct immunostaining (C) or by titration of viral infectious particles released in the cell supernatants by plaque assay (D). Data are representative of two independent experiments with similar results. *P < 0.05 and **P < 0.01 compared with untreated RSV-infected cells.
Fig. 9.
Effect of EUK treatment on RSV-induced lactate dehydrogenase (LDH) release. A549 cells were infected with RSV in the absence or presence of different μM concentrations of EUK-8. Cell supernatants from uninfected and RSV infected, treated or untreated, were assayed at 24 h postinfection for LDH activity. Results are expressed as means ± SE. Results are representative of two independent experiments run in triplicate. *P < 0.05 compared with untreated, RSV-infected cells.
DISCUSSION
Free radicals and ROS have been shown to function as cellular signaling molecules influencing a variety of molecular and biochemical processes, including expression of proinflammatory mediators, such as cytokines and chemokines (reviewed in Ref. 1). However, excessive ROS formation can lead to a condition of oxidative stress, which has been implicated in the pathogenesis of several acute and chronic airway diseases, such as asthma and chronic obstructive pulmonary disease (reviewed in Refs. 9 and 10). Inducible ROS generation has been shown following stimulation with a variety of molecules and infection with certain viruses like human immunodeficiency virus, hepatitis B, influenza, and rhinovirus (reviewed in Ref. 36). In the past few years, we have shown that RSV infection of AECs induces ROS production, in part through an NAD(P)H oxidase-dependent mechanism, inducing oxidative stress in vitro (17) and in vivo (8), which correlates with severity of disease (16), and that antioxidant treatment blocks transcription factor activation and chemokine gene expression in vitro (6, 18, 26) and ameliorates RSV-induced clinical illness in vivo (8), indicating a central role of ROS in RSV-induced cellular signaling and lung disease. RSV infection leads to a significant decrease in the expression and activity of AOEs in AECs, lungs of RSV-infected mice, as well as in children with severe RSV-induced LRTI (16, 17), likely because of decreased activation of Nrf2 (16, 17), which regulates basal and inducible expression of AOE genes (19), suggesting that oxidative damage associated with RSV infection results from an imbalance between ROS production and antioxidant cellular defenses.
Based on this strong supportive evidence that RSV-induced intracellular ROS formation regulates the expression of proinflammatory mediators and that oxidative stress likely represents an important pathogenetic mechanism of RSV-induced lung disease, antioxidant intervention would represent a rational approach for treatment of RSV LRTI. Two complementary approaches could be used to affect the outcome of RSV-associated LRTI. The first would be to increase airway antioxidant defenses by modulation of AOE expression/activity, and the second would be by enhancing nonenzymatic defenses through pharmacological intervention with molecules able to scavenge/detoxify ROS. Approaches that combine scavenging ROS by administration of antioxidant compounds or compounds able to increase lung antioxidant defenses, such as AOE mimetics or Nrf2 inducers, together with inhibitors of viral replication would likely be the most effective in modulating severe lung disease associated with RSV infection.
In the past few years, several classes of synthetic antioxidant mimetics have been generated and tested as a potential therapeutic approach to oxidant-related lung damage. The salen class of AOE mimetics includes compounds that have mainly SOD activity as well as compounds that, in addition, exhibit catalase and peroxidase activity. These molecules have been shown to be effective in preventing lung injury in animal models of oxidative stress, as well as to protect against damage of other organs, such as heart, kidney, and liver (reviewed in Ref. 22). EUK-8 administration has been shown to ameliorate LPS-induced lung injury in a porcine model of endotoxemia (14, 15) and to mitigate lung radiation injury (34). In this study, we found that pretreatment of AECs with EUK-8 and -189 effectively restored catalase and GPx enzyme activities, which were significantly decreased in response to RSV infection, leading to reduced viral-induced ROS production and generation of the lipid peroxidation markers isoprostanes and MDA, as well as reduced activation of the ROS-dependent signaling pathway involved in NF-κB and IRF-3 activation and proinflammatory gene expression. Administration of EUKs when infection was fully established was effective primarily in reducing cellular oxidative stress, but not in blocking ROS-dependent cellular signaling. This is the first report of the effect of EUKs on oxidative stress in a model of viral infection. We had previously reported that treatment of epithelial cells with EUK-134, which is similar to EUK-8 and -189, but not EUK-163, which lacks catalase or peroxidase activity, significantly inhibited RSV-induced IL-8 and RANTES secretion (17). This suggests that enhancement of cellular SOD activity alone in response to RSV infection cannot modulate ROS-mediated signaling and subsequent viral-induced gene expression, whereas increasing the levels of catalase and/or peroxidase activity is beneficial in reducing proinflammatory gene expression.
In addition, both EUKs were able to significantly reduce viral replication, which also represents a novel finding for this type of antioxidant compounds. We and others did not observe a similar effect when AECs were treated with other classes of antioxidants, such as N-acetylcysteine or dimethyl sulfoxide (20, 28). These initial studies suggest that EUK antiviral activity might affect different steps of the viral replication cycle, from viral protein synthesis to viral assembly and release. Cytoskeletal proteins, such as actin, play an important role in various stages of RSV replication (21), and their function is known to be affected by ROS production (37); therefore, it is possible that changes in specific ROS species, following EUK administration, lead to alteration in cytoskeletal structures, affecting ultimately viral replication. A previous report on administration of SOD 1 and 2 in a rodent model of RSV infection showed a significant reduction in lung viral titers, supporting the concept that increased SOD expression/activity can indeed be associated with antiviral activity (40). These results, together with our previous finding that antioxidant treatment attenuates symptoms and pathology in RSV infection (8), warrant further investigation of AOE mimetics as a novel therapeutic approach to modulate viral-induced pulmonary disease. Antioxidant supplementation would be successful only if available at the site of infection/inflammation; therefore, route of administration, bioavailability, and tissue distribution are all important parameters that will need to be taken into consideration when planning future therapeutic intervention.
GRANTS
This work was supported by National Institutes of Health Grants AI-062885 and N01 HV-00245; by Department of Defense Grant W81XWH1010146; and by Flight Attendant Medical Research Institute Clinical Innovator Awards to A. C (no. 072147) and R. P. G (no. 42253).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.M.H., N.K., N.M., T.L., and A.C. performed experiments; Y.M.H. and A.C. analyzed data; Y.M.H. and A.C. prepared figures; R.P.G. and A.C. conception and design of research; R.P.G. and A.C. interpreted results of experiments; R.P.G. and A.C. edited and revised manuscript; R.P.G. and A.C. approved final version of manuscript; A.C. drafted manuscript.
ACKNOWLEDGMENTS
We thank Susan Doctrow for the generous gift of the EUKs and Cynthia Tribble for assistance in manuscript editing and submission.
REFERENCES
- 1. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Rad Biol Med 28: 463–499, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bao X, Liu T, Spetch L, Kolli D, Garofalo RP, Casola A. Airway epithelial cell response to human metapneumovirus infection. Virology 368: 91–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 13: 877–918, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bitko V, Velazquez A, Yank L, Yang YC, Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kB and is inhibited by sodium salicylate and aspirin. Virology 232: 369–378, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Brasier AR, Spratt H, Wu Z, Boldogh I, Zhang Y, Garofal RP, Casola A, Pashmi J, Haag A, Luxon B, Kurosky A. Nuclear heat shock response and novel nuclear domain 10 reogranization in respiratory syncytial virus-infected A549 cells identified by high resolution 2D gel electrophoresis. J Virol 78: 11461–11476, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofal RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem 276: 19715–19722, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Casola A, Garofalo RP, Jamaluddin M, Vlahopoulos S, Brasier AR. Requirement of a novel upstream response element in RSV induction of interleukin-8 gene expression: stimulus-specific differences with cytokine activation. J Immunol 164: 5944–5951, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 174: 1361–1369, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122: 456–468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol 429: 251–262, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Gabbita SP, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 376: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Garofalo R, Kolli D, Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxid Redox Signal September 7 [Epub ahead of print] 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garofalo RP, Sabry M, Jamaluddin M, Yu RK, Casola A, Ogra PL, Brasier AR. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: Nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. J Virol 70: 8773–8781, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J Pharmacol Exp Ther 275: 798–806, 1995 [PubMed] [Google Scholar]
- 15. Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. Role of oxidant stress in the adult respiratory distress syndrome: evaluation of a novel antioxidant strategy in a porcine model of endotoxin-induced acute lung injury. Shock 6, Suppl 1: S23–S26, 1996 [PubMed] [Google Scholar]
- 16. Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 183: 1550–1560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosakote YM, Liu T, Castro SM, Garofalo RP, Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol 41: 348–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Indukuri H, Castro SM, Liao SM, Feeney LA, Dorsch M, Coyle AJ, Garofalo RP, Brasier AR, Casola A. Ikkepsilon regulates viral-induced interferon regulatory factor-3 activation via a redox-sensitive pathway. Virology 353: 155–165, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36: 1199–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol 83: 10605–10615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kallewaard NL, Bowen AL, Crowe JE., Jr Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 331: 73–81, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lai SH, Stein DA, Guerrero-Plata A, Liao SL, Ivanciuc T, Hong C, Iversen PL, Casola A, Garofalo RP. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol Ther 16: 1120–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J 21: 629–632, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lin R, Heylbroeck C, Genin P, Pitha PM, Hiscott J. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol Cell Biol 19: 959–966, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 279: 2461–2469, 2004 [DOI] [PubMed] [Google Scholar]
- 27. MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 429: 195–207, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Mastronarde JG, Monick MM, Hunninghake GW. Oxidant tone regulates IL-8 production in epithelium infected with respiratory syncytial virus. Am J Respir Cell Mol Biol 13: 237–244, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res 40: 393–404, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, Ogra PL, Garofalo RP. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol 72: 4756–4764, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel JA, Kunimoto M, Sim TC, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra PL, Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol 13: 602–609, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Pazdrak K, Olszewska-Pazdrak B, Liu B, Takizawa R, Brasier AR, Garofalo RP, Casola A. MAP-kinase activation is involved in post-transcriptional regulation of RSV-induced RANTES gene expression. Am J Physiol Lung Cell Mol Physiol 283: L364–L372, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem 11: 359–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells (Abstract). Nucleic Acids Res 17: 6419, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwarz KB. Oxidative stress during viral infection: a review. Free Rad Biol Med 21: 641–649, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Taulet N, Delorme-Walker VD, Dermardirossian C. Reactive oxygen species regulate protrusion efficiency by controlling actin dynamics. PLoS ONE 7: e41342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tian B, Zhang Y, Luxon B, Garofalo RP, Casola A, Sinha M, Brasier AR. Identification of NF-kB dependent gene networks in respiratory syncytial virus-infected cells. J Virol 76: 6800–6814, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama 32: 265–272, 1978 [PubMed] [Google Scholar]
- 40. Wyde PR, Moore DK, Pimentel DM, Gilbert BE, Nimrod R, Panet A. Recombinant superoxide dismutase (SOD) administered by aerosol inhibits respiratory syncytial virus infection in cotton rats. Antiviral Res 31: 173–184, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Zhang Y, Luxon B, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of RSV-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J Virol 75: 9044–9058, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]