Abstract
β-Adrenergic receptor (β-AR) stimulation increases extracellular ubiquitin (UB) levels, and extracellular UB inhibits β-AR-stimulated apoptosis in adult cardiac myocytes. This study investigates the role of exogenous UB in chronic β-AR-stimulated myocardial remodeling. l-Isoproterenol (ISO; 400 μg·kg−1·h−1) was infused in mice in the presence or absence of UB (1 μg·g−1·h−1). Left ventricular (LV) structural and functional remodeling was studied 7 days after infusion. UB infusion enhanced serum UB levels. In most parts, UB alone had no effect on morphometric or functional parameters. Heart weight-to-body weight ratios were increased to a similar extent in the ISO and UB + ISO groups. Echocardiographic analyses showed increased percent fractional shortening, ejection fraction, and LV circumferential stress and fiber-shortening velocity in the ISO group. These parameters were significantly lower in UB + ISO vs. ISO. Isovolumic contraction and relaxation times and ejection time were significantly lower in ISO vs. UB + ISO. The increase in the number of TUNEL-positive myocytes and fibrosis was significantly higher in ISO vs. UB + ISO. Activation of Akt was higher, whereas activation of GSK-3β and JNKs was lower in UB + ISO vs ISO. Expression of MMP-2, MMP-9, and TIMP-2 was higher in UB + ISO vs ISO. In isolated cardiac fibroblasts, UB enhanced expression of MMP-2 and TIMP-2 in the presence of ISO. Neutralizing UB antibodies negated the effects of UB on MMP-2 expression, whereas recombinant UB enhanced MMP-2 expression. UB activated Akt, and inhibition of Akt inhibited UB + ISO-mediated increases in MMP-2 expression. Thus, exogenous UB plays an important role in β-AR-stimulated myocardial remodeling with effects on LV function, fibrosis, and myocyte apoptosis.
Keywords: ubiquitin, heart, fibrosis, apoptosis, fibroblasts, myocytes
ubiquitin (UB), a highly conserved protein of ∼8.5 kDa, is found in all eukaryotic cells. The most important intracellular function of UB is to regulate protein turnover by the ubiquitin-proteasome pathway (15). The ubiquitin-proteasome pathway may regulate receptor internalization, hypertrophic response, apoptosis, and tolerance to ischemia and reperfusion in cardiac myocytes (56). UB is a normal constituent of plasma. Elevated levels of UB are described in the serum or plasma of patients with parasitic and allergic diseases (4), alcoholic liver disease (48), type 2 diabetes (1), β2-microglobulin amyloidosis (34), and chronic hemodialysis patients (2). Patients with traumatic brain injury have been shown to have increased UB levels in the cerebrospinal fluid (28). Extracellular UB has been proposed to have pleiotropic functions, including regulation of immune response and anti-inflammatory and neuroprotective activities (27, 29, 36), as well as growth regulation and apoptosis control in hematopoetic cells (9). The biological functions of extracellular UB in the heart remain largely unexplored. Specifically, the role of exogenous UB in myocardial remodeling has not yet been investigated.
Sympathetic nerve activity increases in the heart during cardiac failure. Prolonged stimulation of the β-adrenergic neurohormonal axis contributes to the progression of heart failure and mortality in animal models and human patients (13, 43). Stimulation of β-adrenergic receptor (β-AR) increases expression and activity of matrix metalloproteinase (MMP-2 and MMP-9) in cardiac myocytes in vitro and in vivo (23, 31). It induces apoptosis in cardiac myocytes in vitro and in vivo (19, 42, 43, 55). β-AR-stimulated apoptosis in adult rat ventricular myocytes (ARVMs) is demonstrated to occur via the GSK-3β/JNK-dependent mitochondrial death pathway (30, 39). Recently, we provided evidence that stimulation of β-AR increases extracellular levels of UB in ARVMs, and extracellular UB plays a protective role in β-AR-stimulated apoptosis via the inactivation of GSK-3β and JNK pathways (44).
Here, we investigated the in vivo role of exogenous UB in cardiac myocyte apoptosis and myocardial remodeling following chronic β-AR stimulation in mice. We report that exogenous UB plays an important role in β-AR-stimulated myocardial remodeling with effects on left ventricular function, fibrosis, and myocyte apoptosis. It may inhibit myocyte apoptosis via the activation of Akt and inactivation of GSK-3β and JNKs while also inhibiting fibrosis by modulating expression and activity of MMPs and tissue inhibitors of MMPs (TIMPs).
MATERIALS AND METHODS
Experimental animals.
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996). The animal protocol was approved by the East Tennessee State University Committee on Animal Care. Animals were anesthetized using a mixture of isoflurane (2.5%) and oxygen (0.5 l/min), and the heart was excised following a bilateral cut in the diaphragm. Mice were euthanized by exsanguination. The studies were performed using male Institute of Cancer Research (ICR) mice (25–30 g; purchased from Harlan Laboratories).
Mice treatment.
Mice were randomly assigned to four groups (sham, ISO, UB + ISO, and UB). The mice in UB + ISO and UB groups received intraperitoneal injection of UB (1 μg/g, U6253; Sigma) 1 h prior to pump implantation. The mice were infused with isoproterenol [ISO; 400 μg·kg−1·h−1 (ISO group)], UB + ISO (1 μg·g−1·h−1 + 400 μg·kg−1·h−1; UB + ISO group) and UB (1 μg·g−1·h−1; UB group) at the rate of 1.0 μl/h for 7 days using miniosmotic pumps (Alzet). l-Isoproterenol and UB were dissolved in acidified isotonic saline (0.001 N HCl). Sham animals were infused with acidified isotonic saline solution. The dose of ubiquitin and ISO were selected based on previously published reports (14, 23, 42).
Echocardiography.
Transthoracic two-dimensional M-mode echocardiogram and pulsed wave Doppler spectral tracings were obtained using a Toshiba Aplio 80 Imaging System (Tochigi, Japan) equipped with a 12-MHz linear transducer (12, 23). Echocardiographic procedures were performed prior to and 7 days after implantation of pumps. The animals were anesthetized during the procedure using a mixture of ISO (1.5%) and oxygen (0.5 l/min), and their body temperatures were maintained at ∼37°C using a heating pad. M-mode tracings were used to measure left ventricular (LV) end-systolic diameter (LVESD) and end-diastolic diameter (LVEDD). Percent fractional shortening (%FS) and ejection fraction percent (EF%) were calculated as described (12, 23). Doppler tracings of mitral and aortic flow were used to measure isovolumic relaxation time (IVRT), isovolumic contraction time (IVCT), and ejection time (ET). LV circumferential stress and fiber-shortening velocity (Vcf) were calculated as LVEDD − LVESD/LVEDD × LVET, where LVEDD and LVESD are LV diastolic and systolic diameters, respectively, and LVET is LV ejection time (38). All echocardiographic assessments were performed by the same investigator blinded to the treatments. A second person also performed measurements on a separate occasion using the same recordings, with no significant differences in interobserver variability.
Morphometric analyses.
Animals were euthanized, and the hearts were arrested in diastole using KCl (30 mM), followed by perfusion fixation with 10% buffered formalin. Cross-sections (4 μm thick) were stained with Masson's trichrome for the measurement of fibrosis using Bioquant image analysis software (Nashville, TN).
Measurement of serum UB levels.
Serum UB levels, 7 days after ISO infusion, were measured using ELISA kit (Novatein Biosciences).
Myocyte cross-sectional area.
Heart sections (4 μm thick) were deparaffinized and stained with tetramethyl rhodamine isothiocyanate-labeled wheat germ agglutinin. The sections were visualized using fluorescent microscopy (×20; Nikon), and images were recorded using Retiga 1300 color-cooled camera. Suitable area of the sections was defined as the one with nearly circular capillary profiles.
TUNEL staining.
To measure apoptosis, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining followed by Hoechst 33258 staining was carried out on 4-μm-thick paraffin-embedded sections as per the manufacturer's instructions (cell death detection assay kit; Roche). To identify apoptosis associated with cardiac myocytes, the sections were immunostained using α-sarcomeric actin antibodies (1:50, 5C5 clone; Sigma, St. Louis, MO). Hoechst 33258 (10 μM; Sigma) staining was used to count the total number of nuclei. TUNEL-positive nuclei that were clearly seen within cardiac myocytes were counted. The number of apoptotic cardiac myocyte nuclei was counted, and index of apoptosis was calculated as the percentage of apoptotic myocyte nuclei/total number of nuclei.
Fibroblast isolation and treatment.
Adult rat cardiac fibroblasts were isolated as described (53). The cells were grown to confluence and serum starved for 48 h before use. First- and second-passage cells were used for all of the experiments. The cells were pretreated with UB (10 μg/ml; Sigma) for 30 min, followed by treatment with ISO for the indicated time points. To neutralize effects of UB, cells were pretreated with anti-UB antibodies (5 μg/ ml, U-5379; Sigma) for 30 min. To verify effects of UB, cells were pretreated with recombinant UB (rUB; 10 or 100 μg/ml; Boston Biochem) for 30 min, followed by treatment with ISO for 48 h. To inhibit Akt, cells were pretreated with MK-2206 (1 μM; Chemie Tek) for 30 min and then with UB (10 μg/ml) for 30 min, followed by treatment with ISO.
Western analysis.
LV tissue lysates were prepared in RIPA buffer [10 mM Tris·HCl (pH 7.2), 158 mM NaCl, 1 mM EGTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and 0.2 mM phenylmethylsulfonyl fluoride] using a tissue homogenizer. Cell lysates were prepared using lysis buffer [10 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 0.5% Nonidet P-40, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride]. Equal amounts of total proteins (70 μg from tissue lysates or 20 μg from cell lysates) were resolved on 10% SDS-polyacrylamide gels. The proteins were transferred onto polyvinylidene difluoride membrane. The blots were then probed with primary antibodies directed against p-Akt (1:1,000; Cell Signaling Technology), p-GSK-3β (1:1,000; Cell Signaling Technology), p-JNK (1:1,000; Millipore), p-ERK1/2 (1:2,000; Cell Signaling Technology), MMP-2 (1:5,000; Millipore), MMP-9 (1:5,000; Millipore), or TIMP-2 (1:1,000; Santa Cruz Biotechnology) and appropriate secondary antibodies. Membranes were then stripped and probed with Akt, GSK-3β, JNKs, ERK1/2, actin (Chemicon), or GAPDH (Santa Cruz Biotechnology) antibodies to normalize protein loading. Band intensities were quantified using the Kodak photo documentation system (Eastman Kodak).
In-gel zymography.
Gelatin in-gel zymography in the concentrated conditioned media of fibroblasts (2 μg) or LV (50 μg) lysates was performed as described (53). Clear and digested regions representing MMP-2 activity were quantified using a Kodak documentation system.
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed using Student's t-test or a one-way analysis of variance followed by the Student-Newman-Keuls test. P values of <0.05 were considered to be significant.
RESULTS
Morphometric studies.
None of the animals died during the course of the experiment. No significant change in body weight (BW) in any group was observed during the experiment. ISO infusion increased heart weight (HW) as well as the HW/BW ratios in the ISO and UB + ISO groups compared with sham (P < 0.05; Table 1), with no significant difference between the two ISO groups. UB alone had no effect on HW or HW/BW ratio.
Table 1.
Morphometric measurements
| Parameters | Sham | ISO | UB + ISO | UB | P Value |
|---|---|---|---|---|---|
| BW, g | 30.27 ± 1.00 | 31.95 ± 0.86 | 31.60 ± 0.64 | 31.00 ± 0.76 | NS |
| HW, mg | 129.7 ± 6.74 | 167.4 ± 8.98* | 178.6 ± 7.46* | 144.5 ± 10.8 | <0.01* |
| HW/BW, mg/g | 4.29 ± 0.21 | 5.22 ± 0.17* | 5.65 ± 0.21* | 4.65 ± 0.29 | <0.01* |
Values are means ± SE; n = 6.
ISO, isoproterenol; UB, ubiquitin; HW, heart weight; BW, body weight; NS, not significant.
Comparison vs. sham.
Serum UB levels.
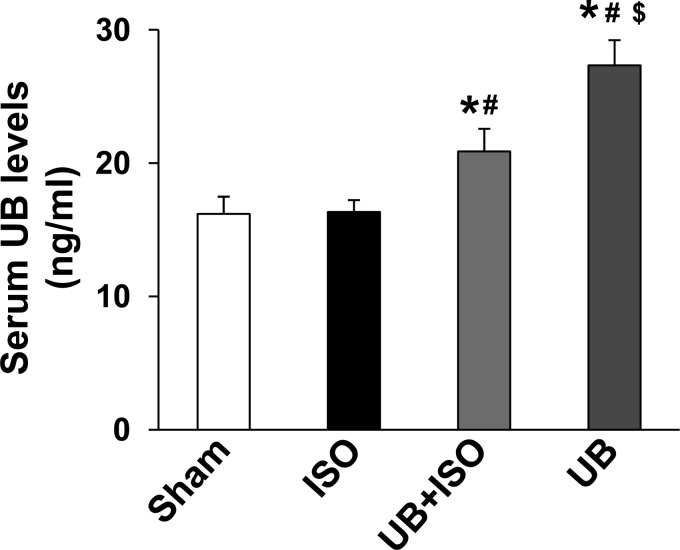
Measurement of serum UB levels using ELISA demonstrated the presence of 16.2 ± 1.3 ng/ml UB in sham animals. ISO had no effect on serum UB levels. Serum UB levels were increased in UB-infused groups compared with sham or ISO groups (P < 0.05; Fig. 1). However, the increase in serum UB levels was higher in UB alone compared with the UB + ISO group.
Fig. 1.
Serum ubiquitin (UB) levels. UB infusion increases serum UB levels. *P < 0.05 vs. sham; #P < 0.05 vs. isoproterenol (ISO); $P < 0.05 vs. UB + ISO; n = 5–6.
Echocardiographic measurements.
M-mode echocardiography showed no significant difference in LVESD, LVEDD, %FS, or EF% between the sham and UB groups. ISO infusion decreased LVESD in ISO but not in the UB + ISO group. LVEDD remained unchanged between the two groups. %FS, EF%, and Vcf were increased in the ISO not in the UB + ISO group compared with sham. In fact, %FS, %EF, and mean Vcf were significantly lower in the UB + ISO compared with the ISO group (Table 2).
Table 2.
M-mode echocardiographic measurements
|
P Value |
||||||
|---|---|---|---|---|---|---|
| Parameters | Sham (n = 6) | ISO (n = 6) | UB + ISO (n = 6) | UB (n = 5) | Vs. Sham | Vs. ISO group |
| LVESD, mm | 2.73 ± 0.04 | 2.36 ± 0.06* | 2.78 ± 0.06# | 2.74 ± 0.16 | <0.05 | <0.05 |
| LVEDD, mm | 3.90 ± 0.08 | 3.89 ± 0.02 | 4.11 ± 0.06 | 3.97 ± 0.16 | NS | NS |
| %FS | 30.01 ± 0.99 | 39.26 ± 1.55* | 32.50 ± 1.03# | 31.31 ± 1.88 | <0.01 | <0.05 |
| EF% | 57.73 ± 1.39 | 70.07 ± 2.02* | 61.14 ± 1.45# | 59.51 ± 2.84 | <0.01 | <0.05 |
| Mean Vcf, circ/s | 5.99 ± 0.23 | 10.10 ± 0.62* | 6.97 ± 0.28# | 6.09 ± 0.39 | <0.001 | <0.001 |
Values are means ± SE.
LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; %FS, percent fractional shortening; EF%, percent ejection fraction; Vcf, left ventricular circumferential stress and fiber-shortening velocity.
Comparison vs. sham;
comparison vs. ISO.
Doppler tracings revealed no difference in any parameters between sham and UB groups (Table 3). ISO infusion decreased IVRT, IVCT, and ET in both groups compared with sham. However, the decrease in these parameters was significantly lower in ISO compared with the UB + ISO group (P < 0.05; Table 3). ISO infusion increased heart rates in both groups, with no significant difference between the ISO and UB + ISO groups.
Table 3.
Doppler echocardiographic measurements
|
P Value |
||||||
|---|---|---|---|---|---|---|
| Parameters | Sham (n = 6) | ISO (n = 6) | UB + ISO (n = 6) | UB (n = 5) | Vs. sham | Vs. ISO group |
| IVRT, ms | 12.41 ± 0.01 | 5.07 ± 0.01* | 7.26 ± 0.01*# | 12.98 ± 0.01 | <0.05 | <0.01 |
| IVCT, ms | 11.98 ± 0.01 | 5.19 ± 0.01* | 7.67 ± 0.01*# | 13.11 ± 0.01 | <0.01 | <0.001 |
| ET, ms | 50.22 ± 0.01 | 39.07 ± 0.01* | 46.70 ± 0.01*# | 51.47 ± 0.01 | <0.05 | <0.001 |
| HR, beats/min | 428.37 ± 19.36 | 572.87 ± 23.7* | 506.98 ± 17.98* | 388.64 ± 18.31 | <0.05 | |
Values are means ± SE.
IVRT, isovolumetric relaxation time; IVCT, isovolumetric contraction time; ET, ejection time; HR, heart rate.
Comparison vs. sham; #comparison vs. ISO group.
Fibrosis, apoptosis, and hypertrophy.
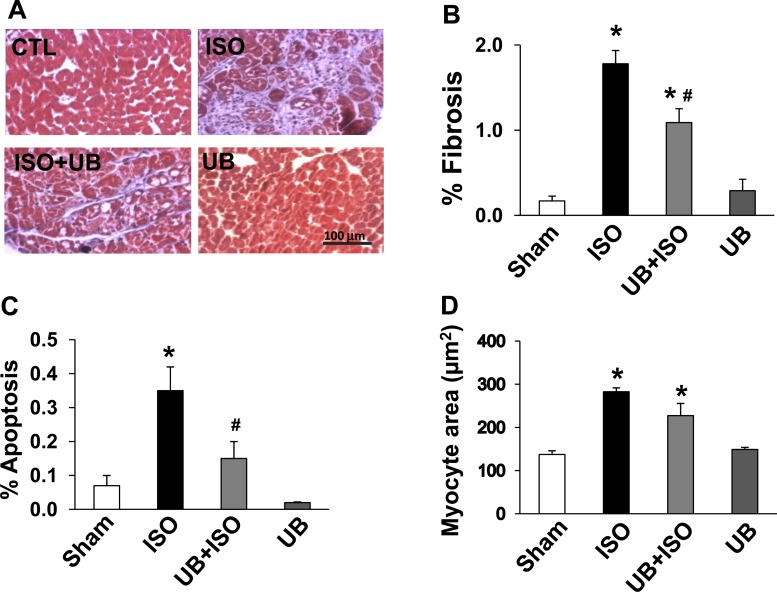
ISO infusion increased fibrosis in both groups (Fig. 2A). Quantitative analysis of trichrome-stained sections showed that the increase in fibrosis was significantly lower in the UB + ISO group compared with ISO (%fibrosis, WT sham: 0.17 ± 0.05; ISO: 1.78 ± 0.15; UB + ISO: 1.09 ± 0.16; UB: 0.29 ± 0.13; P < 0.01 vs. sham, P < 0.05 vs. ISO, n = 5–6; Fig. 2B).
Fig. 2.
Myocardial remodeling 7 days after ISO infusion. A and B: analysis of fibrosis. A: Masson's trichrome-stained sections 7 days after ISO infusion. B: quantitative analysis of fibrosis; n = 5–6. C: quantitative analysis of terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling-stained myocytes 7 days after ISO infusion; n = 3–5. D: quantitative analysis of myocyte cross-sectional area; n = 3–4. *P < 0.01 vs. sham; #P < 0.05 vs. ISO. CTL, control.
ISO infusion increased the number of TUNEL-positive myocytes in both groups. However, the percentage of apoptotic myocytes was significantly lower in the UB + ISO group compared with ISO (%apoptotic myocyte nuclei/total number of nuclei; sham: 0.07 ± 0.03; ISO: 0.35 ± 0.07; UB + ISO: 0.15 ± 0.05; UB: 0.02 ± 0.01; P < 0.01 vs. sham, P < 0.05 vs ISO, n = 3–5; Fig. 2C).
ISO infusion increased myocyte cross-sectional area to a similar extent in both groups compared with sham (μm2; sham: 137.5 ± 8.5; ISO: 297.4 ± 9.2; UB + ISO: 227.3 ± 28.5; UB: 149 ± 4.8; P < 0.01 vs. sham, n = 3–4; Fig. 2D).
Activation of Akt and GSK-3β.
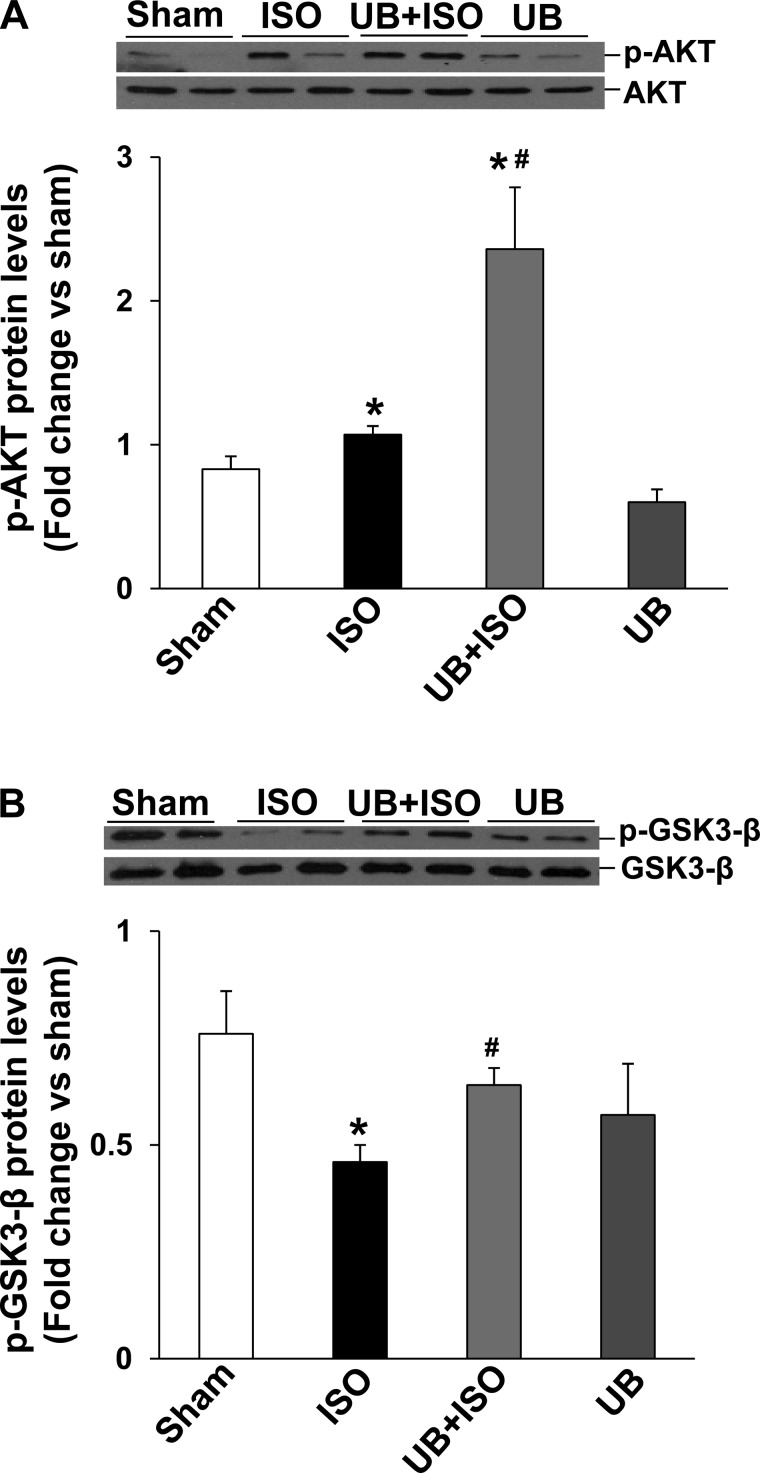
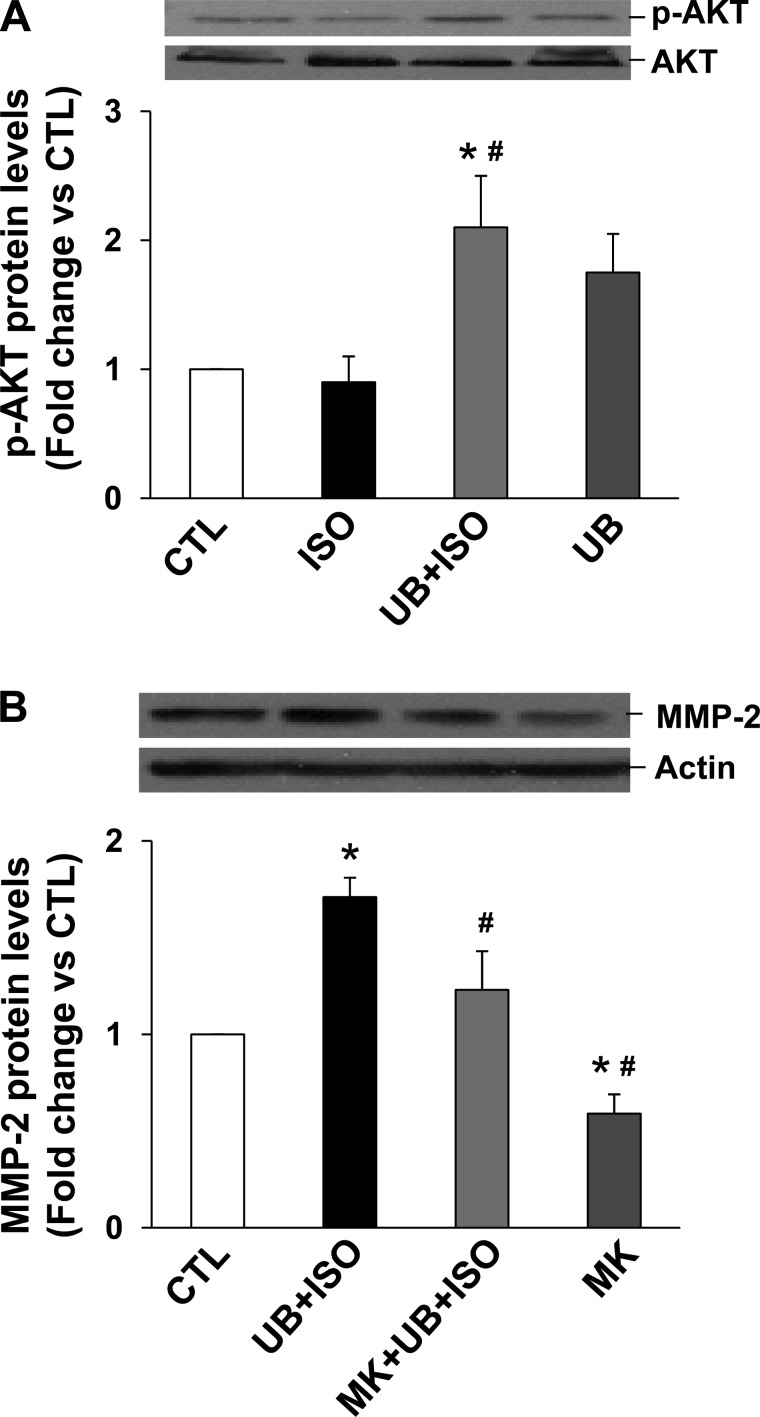
Previously, we provided evidence that inhibition of phosphatidylinositol(PI) 3-kinase inhibits the protective effects of UB in β-AR-stimulated apoptosis in ARVMs (44). PI 3-kinase activates Akt, and activation of Akt plays an antiapoptotic role (22). Western blot analysis of LV lysates using phosphospecific anti-Akt antibodies showed a significant increase in Akt phosphorylation (activity) in both ISO and UB + ISO groups compared with sham. However, the increase in Akt activity was significantly higher in UB + ISO group compared with ISO (P < 0.05; Fig. 3A). UB alone had no effect on Akt activity.
Fig. 3.
Activation of Akt and GSK-3β in the heart. Total left ventricular (LV) lysates (70 μg) were analyzed by Western blot using phosphospecific (p)-Akt (Ser473), total Akt, phosphospecific GSK-3β (Ser9) and total GSK-3β antibodies. A and B, bottom, exhibit mean data normalized to total Akt or GSK-3β. A: phosphorylation (activation) of Akt. *P < 0.05 vs. sham; #P < 0.05 vs. ISO; n = 6. B: phosphorylation (inactivation) of GSK-3β. *P < 0.05 vs. sham; #P < 0.01 vs. ISO; n = 4–7.
Activation of GSK-3β plays a proapoptotic role in β-AR-stimulated apoptosis (30). Phosphorylation of an NH2-terminal serine residue (Ser9) inactivates GSK-3β. Akt is one of the upstream kinases involved in phosphorylation (Ser9) and inactivation of GSK-3β (16). Western blot analysis of LV lysates using phosphospecific anti-GSK-3β antibodies showed decreased phosphorylation (activation) of GSK-3β in the ISO but not in the UB + ISO group. GSK-3β phosphorylation was significantly higher in the UB + ISO compared with the ISO group (WT sham: 0.76 ± 0.1; ISO: 0.46 ± 0.04; UB + ISO: 0.64 ± 0.04; UB: 0.57 ± 0.1; P < 0.05 vs. sham, P < 0.05 vs. ISO, n = 4–7; Fig. 3B). UB alone had no effect on GSK-3β phosphorylation.
Activation of JNKs and ERK1/2.
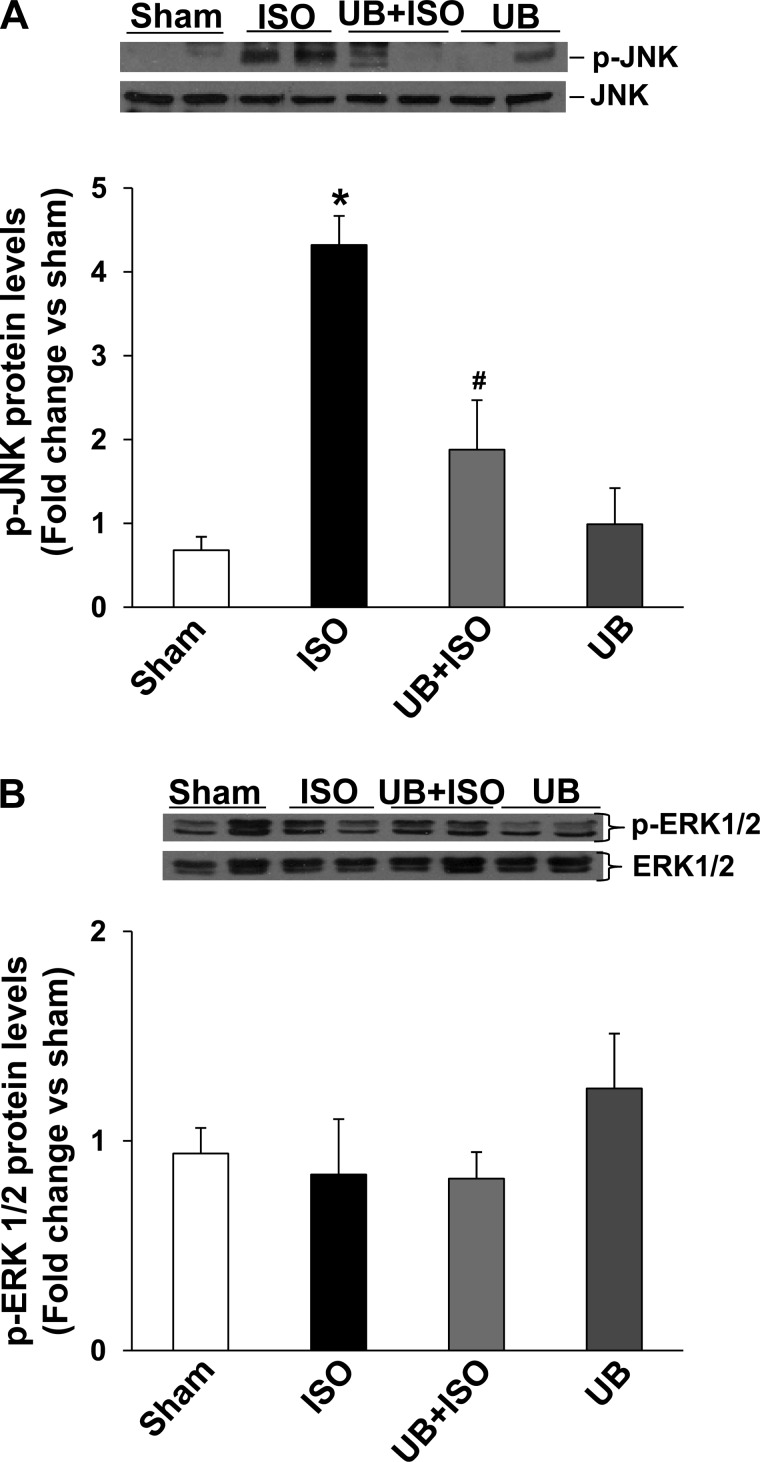
β-AR-stimulated activation of the JNK pathway is demonstrated to play a proapoptotic role in β-AR-stimulated apoptosis (39). Previously, we provided evidence that active GSK-3β may act upstream in the activation of JNKs (44). Western blot analysis of LV lysates using phosphospecific anti-JNK antibodies showed increased phosphorylation (activation) of JNKs in the ISO but not in the UB + ISO group. Activation of JNKs was significantly lower in UB + ISO group compared with ISO (WT sham: 0.68 ± 0.16; ISO: 4.32 ± 0.35; UB + ISO: 1.88 ± 0.59; UB: 0.99 ± 0.43; P < 0.05 vs. sham, P < 0.05 vs. ISO, n = 4–7; Fig. 4A). UB alone had no effect on JNK phosphorylation. ISO in the presence or absence of UB had no effect on the activation of ERK1/2 (Fig. 4B).
Fig. 4.
Activation of JNKs and ERK1/2 in the heart. Total LV lysates (70 μg) were analyzed by Western blot using p-JNKs, total JNKs, p-ERK1/2, and total ERK1/2. A and B, bottom, exhibit mean data normalized to total JNKs or ERK1/2. A: phosphorylation (activation) of JNKs. *P < 0.05 vs. sham; #P < 0.05 vs. ISO; n = 6. B: phosphorylation (activation) of ERK1/2; n = 5.
Expression and activation of MMP-2 and MMP-9.
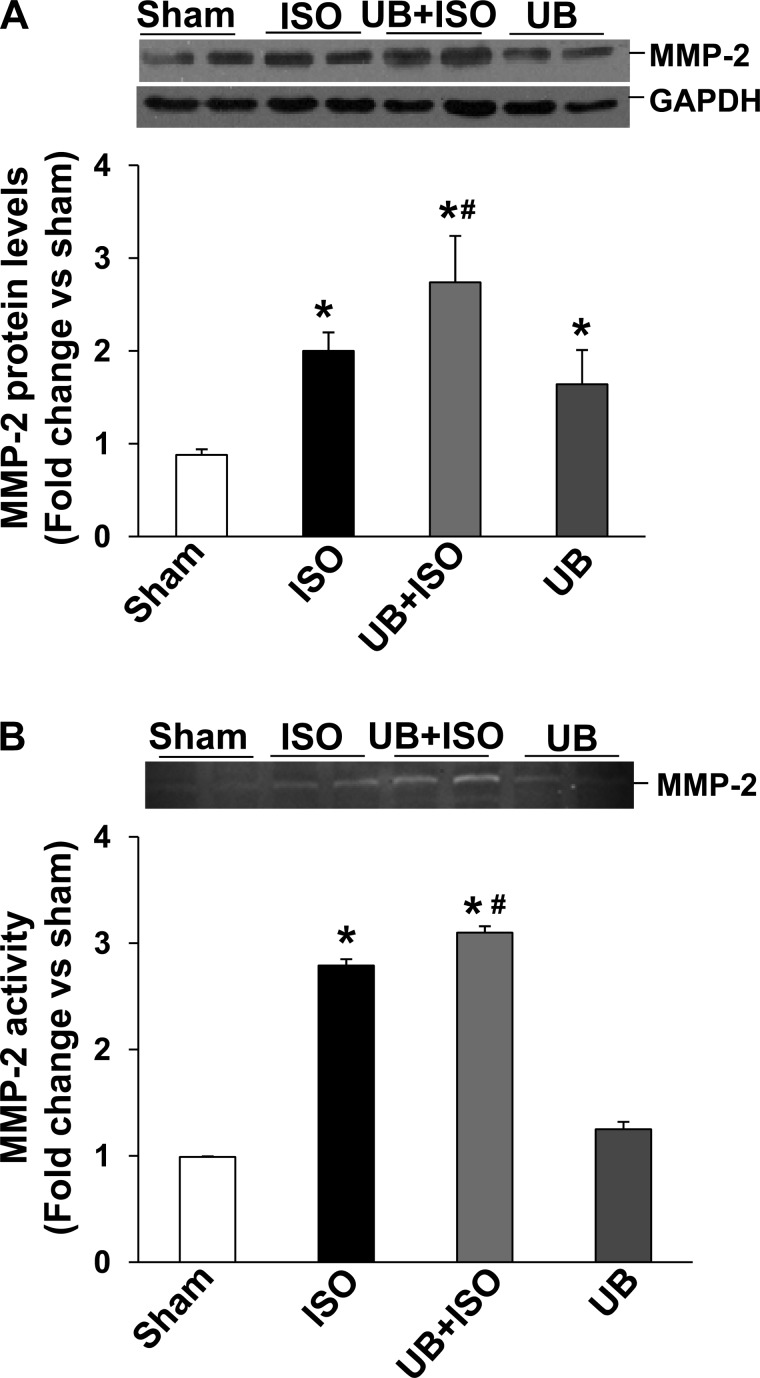
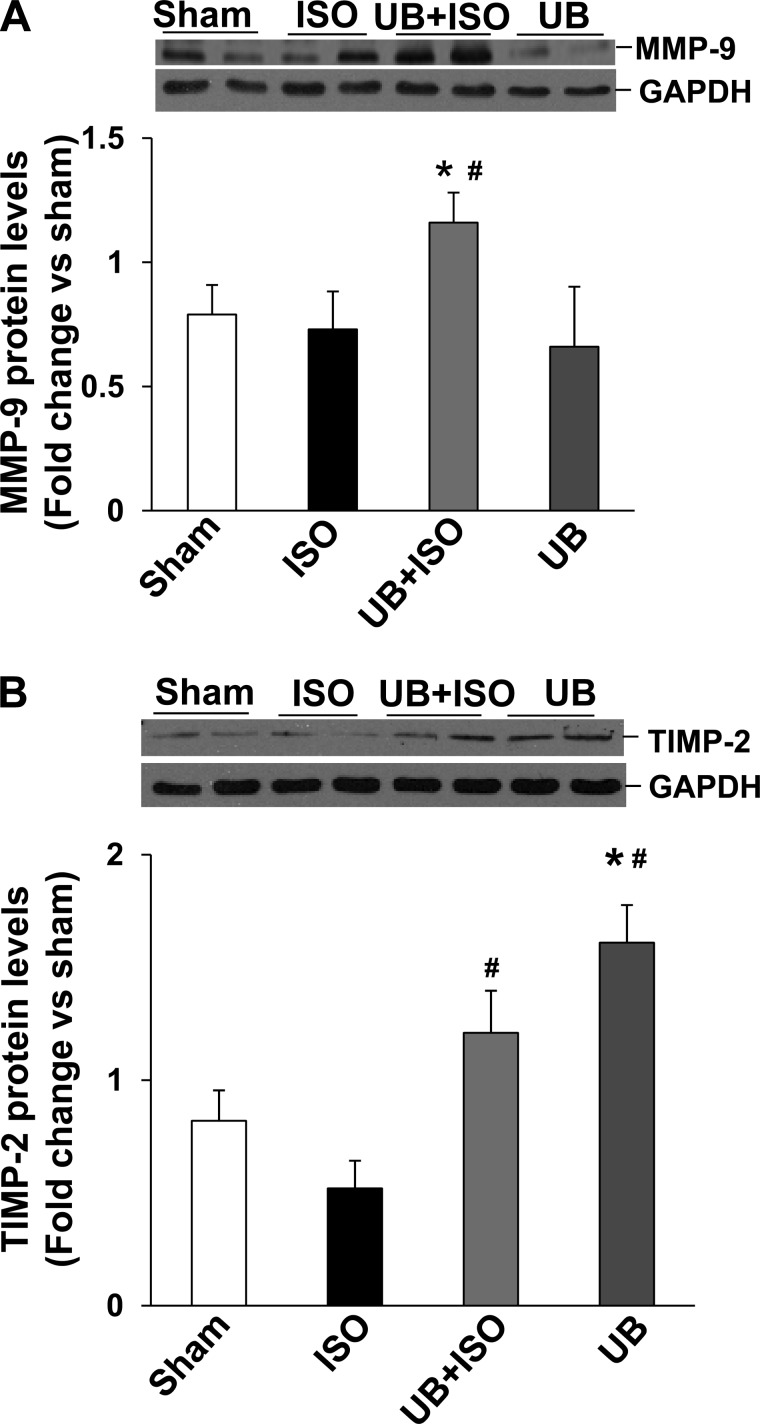
MMP-2 and MMP-9 play a significant role in myocardial fibrosis and remodeling (46). Western blot analysis of LV lysates demonstrated increased expression (protein levels) of MMP-2 in both ISO groups (Fig. 5A). However, the increase in MMP-2 expression was significantly higher in the UB + ISO group compared with ISO. In-gel zymography showed increased MMP-2 activity in both ISO groups. However, the increase in MMP-2 activity was significantly higher in the UB + ISO group compared with ISO (Fig. 5B). Increased MMP-9 expression was observed only in the UB + ISO group (WT sham: 0.79 ± 0.1; ISO: 0.73 ± 0.1; UB + ISO: 1.16 ± 0.1; UB: 0.66 ± 0.2; P < 0.05 vs. sham, P < 0.05 vs. ISO, n = 6; Fig. 6A). In-gel zymography failed to show active MMP-9 bands in the LV lysates from these groups (data not shown).
Fig. 5.
Expression and activation of matrix metalloproteinase-2 (MMP-2) in the heart. Total LV lysates (70 μg) were analyzed by Western blot (using anti-MMP-2 antibodies) or gelatin in-gel zymography. A: expression of MMP-2. GAPDH immunostaining indicates protein loading. *P < 0.05 vs. sham; #P < 0.05 vs. ISO; n = 6. B: MMP-2 activity. *P < 0.01 vs. sham; #P < 0.05 vs. ISO; n = 4.
Fig. 6.
Expression of MMP-9 and tissue inhibitor of MMP-2 (TIMP-2) in the heart. Total LV lysates (70 μg) were analyzed by Western blot using anti-MMP-9 or anti-TIMP-2 antibodies. GAPDH immunostaining indicates protein loading. A: expression of MMP-9. *P < 0.05 vs. sham; #P < 0.05 vs. ISO; n = 6. B: expression of TIMP-2. *P < 0.01 vs. sham; #P < 0.05 vs. ISO; n = 4.
Expression of TIMP-2 and TIMP-4.
TIMPs play an important role in regulation of MMP activity (32, 51). Western blot analysis of LV lysates demonstrated that ISO alone has no effect on TIMP-2 protein levels. However, UB alone or in the presence of ISO increased TIMP-2 protein levels significantly (Fig. 6B). ISO in the presence or absence of UB had no effect on protein levels of TIMP-4. UB alone increased TIMP-4 protein levels compared with ISO or UB + ISO groups (data not shown).
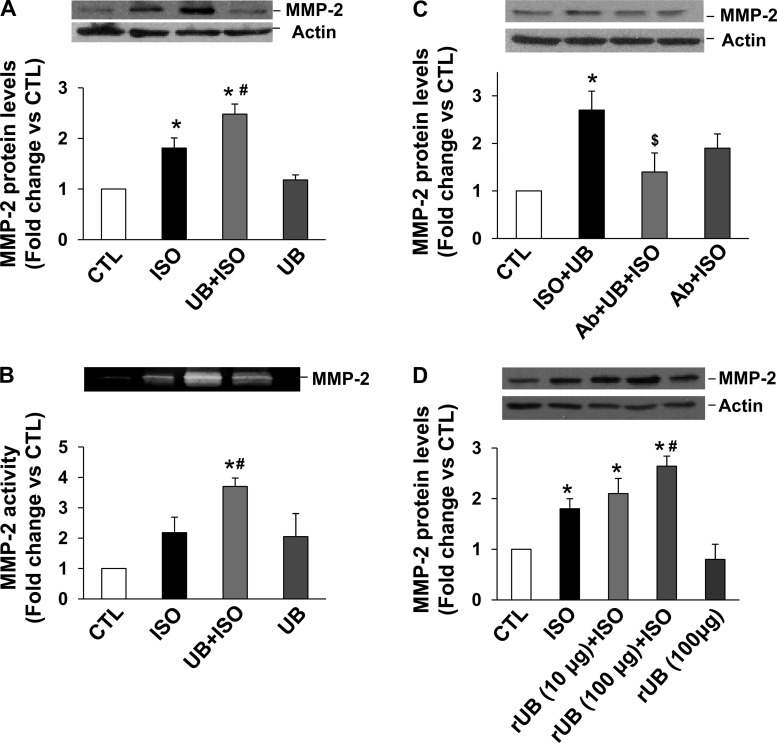
UB modulates expression of MMPs and TIMP-2 in cardiac fibroblasts.
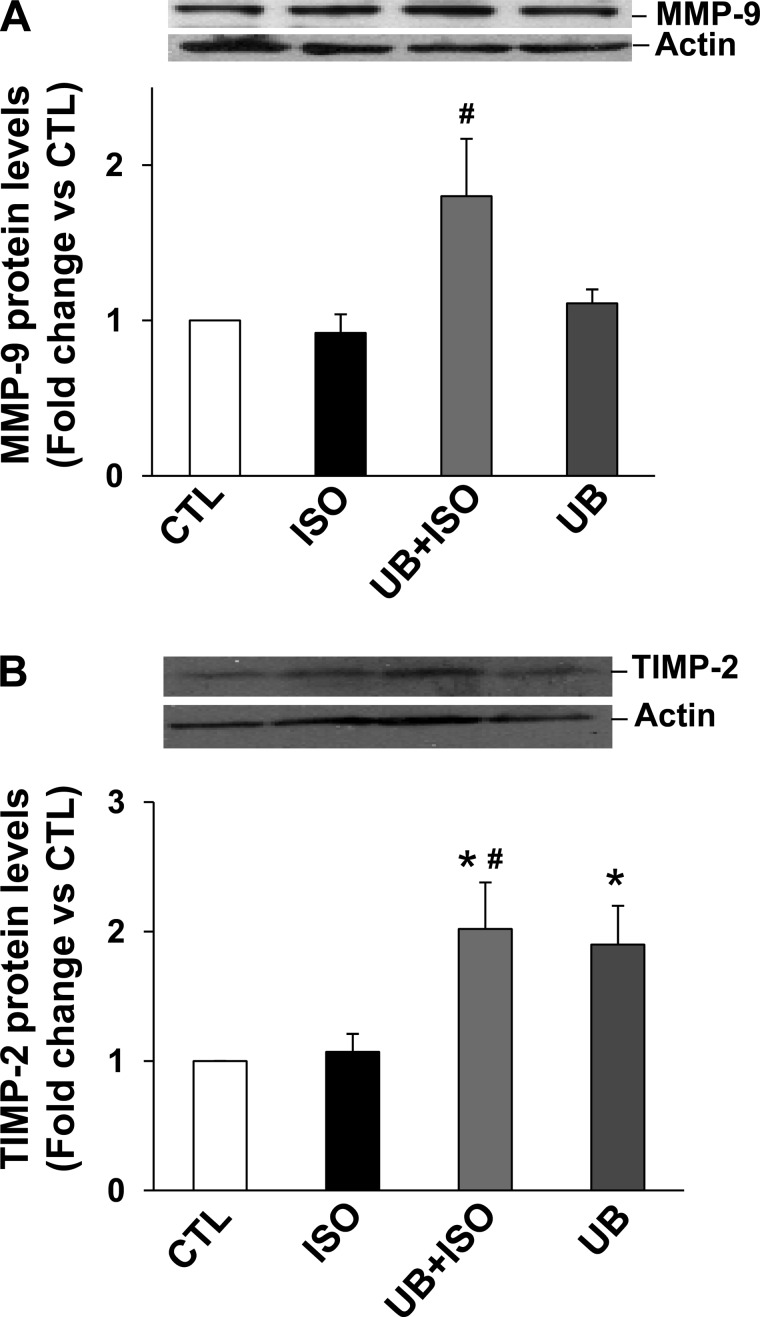
ISO treatment increased MMP-2 expression in adult cardiac fibroblasts, as analyzed by Western blots. However, the increase in MMP-2 expression was significantly higher in UB + ISO group compared with ISO (Fig. 7A). In-gel zymography showed a trend toward increased MMP-2 activity in response to ISO. However, MMP-2 activity was significantly higher in the UB + ISO group compared with control and ISO-treated samples (Fig. 7B). These effects of UB on MMP-2 expression were negated by pretreatment with neutralizing anti-UB antibodies (Fig. 7C). Treatment with rUB also enhanced ISO-mediated increases in MMP-2 expression (Fig. 7D). However, the effects were observed at a higher concentration (100 μg/ml). MMP-9 expression tended to be higher in the UB + ISO group compared with sham (P = 0.06, n = 6; Fig. 8A). ISO treatment had no effect on TIMP-2 protein levels. However, TIMP-2 protein levels were significantly higher in UB + ISO samples compared with control or ISO-treated samples (Fig. 8B).
Fig. 7.
Expression and activity of MMP-2 in cardiac fibroblasts. A and B: cardiac fibroblasts were pretreated with UB (10 μg/ml) for 30 min, followed by treatment with ISO (10 μM) for 48 h. A: cell lysates (20 μg) were analyzed by Western blot using anti-MMP-2 antibodies. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 11. B: concentrated conditioned media (2 μg) were analyzed by gelatin in-gel zymography. *P < 0.01 vs. CTL; #P < 0.05 vs. ISO; n = 3. C: cells were pretreated with neutralizing anti-UB antibody (Ab) for 30 min, followed by treatment with UB for 30 min. The cells were then treated with ISO for 48 h. Cell lysates (20 μg) were analyzed by Western blot using anti-MMP-2 antibodies. *P < 0.05 vs. CTL; $P < 0.05 vs. UB + ISO; n = 3. D: cells were pretreated with recombinant UB (rUB; 10 or 100 μg/ml) for 30 min, followed by treatment with ISO for 48 h. Cell lysates (20 μg) were analyzed by Western blot using anti-MMP-2 antibodies. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 3. Actin immunostaining indicates protein loading.
Fig. 8.
Expression of MMP-9 and TIMP-2. Cardiac fibroblasts were pretreated with UB (10 μg/ml) for 30 min, followed by treatment with ISO (10 μM) for 48 h. Cell lysates (20 μg) were analyzed by Western blot using anti-MMP-9 or anti-TIMP-2 antibodies. Actin immunostaining indicates protein loading. A: expression of MMP-9. #P = 0.06 vs. ISO; n = 6. B: expression of TIMP-2. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 3–4.
UB activates Akt, and inhibition of Akt inhibits MMP-2 expression in cardiac fibroblast.
To investigate whether UB alone or in combination with ISO activates Akt, cardiac fibroblasts were pretreated with UB followed by treatment with ISO for 15 min. Analysis of cell lysates using phosphospecific antibodies showed that UB or ISO alone had no effect on Akt activation. However, UB in the presence of ISO increased Akt activity significantly compared with control or ISO-treated samples (Fig. 9A). MMP-2 activation is shown to be dependent on activation of Akt in the heart (45). Inhibition of Akt using MK-2206 (18, 37) inhibited UB + ISO-mediated increases significantly in MMP-2 expression (fold change vs. CTL; UB + ISO: 1.71 ± 0.1; MK + UB + ISO: 1.23 ± 0.2; MK: 0.59 ± 0.3; P < 0.05 vs. sham, P < 0.05 vs. UB + ISO, n = 5; Fig. 9B).
Fig. 9.
Activation of Akt and its role in MMP-2 expression. A: cardiac fibroblasts were pretreated with UB (10 μg/ml) for 30 min, followed by treatment with ISO (10 μM) for 15 min to measure Akt activation. B: to measure MMP-2 expression, cardiac fibroblasts were pretreated with MK-2206 (MK; 1 μM) for 30 min and then with UB (10 μg/ml) for 30 min, followed by treatment with ISO (10 μM) for 48 h. Cell lysates (20 μg) were analyzed by Western blot using phosphospecific anti-Akt (Ser473) or anti-MMP-2 antibodies. A and B, bottom, exhibit mean data normalized to total Akt or actin. A: phosphorylation (activation) of Akt. *P < 0.05 vs. CTL; #P < 0.05 vs. ISO; n = 5. B: expression of MMP-2. *P < 0.05 vs. CTL; #P < 0.05 vs. UB + ISO; n = 5.
DISCUSSION
Previously, we provided evidence that β-AR stimulation increases levels of extracellular UB and that treatment with UB plays a protective role in β-AR-stimulated apoptosis in ARVMs (44). This is the first study investigating the role of exogenous UB in the heart specifically in response to chronic β-AR stimulation. Here, we confirm our previous finding of an antiapoptotic function for UB in vivo and show that UB plays an important role in β-AR-stimulated myocardial remodeling with effects on left ventricular function, fibrosis, and myocyte apoptosis. UB infusion enhanced serum UB levels. Exogenous UB depressed β-AR-stimulated increases in systolic and diastolic functional parameters of the heart. Increase in cardiac myocyte apoptosis and myocardial fibrosis was significantly lower in the presence of exogenous UB. Exogenous UB enhanced activation of the antiapoptotic kinase Akt, whereas it decreased the activation of the proapoptotic kinases GSK-3β and JNK. It increased protein levels of MMP-2, MMP-9, and TIMP-2 in the presence of ISO. In isolated cardiac fibroblasts, UB enhanced expression of MMP-2 and TIMP-2. It activated Akt, and inhibition of Akt decreased MMP-2 expression.
Sympathetic nerve activity increases in the heart during cardiac failure. Prolonged stimulation of the β-adrenergic neurohormonal axis contributes to the progression of heart failure and mortality in animal models and human patients (3, 13). Catecholamines, released during heightened adrenergic drive, accumulate in the interstitial space of the heart (6, 8). This accumulation of catecholamines may contribute to left ventricular dysfunction (10). UB is a normal constituent of plasma or serum (11, 29, 47). Levels of UB increase in plasma or serum of patients under a variety of pathological conditions (26). However, the role of plasma UB in the heart has not yet been investigated. Previously, we have shown that β-AR stimulation increases extracellular levels of UB in ARVMs (44). Here, we observed basal presence of UB in the serum of ICR mice. Infusion of UB enhanced serum UB levels in the presence or absence of β-AR stimulation. It was interesting to note that serum UB levels were lower in the presence of β-AR stimulation, and β-AR stimulation alone had no effect on serum UB levels. These observations suggest the possibility that β-AR stimulation may enhance UB absorption in vivo. Isoproterenol is shown to increase cardiac output without affecting renal blood flow in dog, human, or lamb (5, 40). However, the possibility of increased renal blood flow and renal clearance of UB in the presence of isoproterenol cannot be ruled out. A significant finding of this study is that exogenous UB has the potential to play an important role in the remodeling process of the heart by affecting β-AR-stimulated increases in myocyte apoptosis and myocardial fibrosis.
Ventricular hypertrophy is considered to be an important compensatory mechanism that allows the heart to maintain its output. Chronic β-AR stimulation is shown to induce hypertrophy and increase heart rate and LV systolic function (20, 49, 50). Indicators of hypertrophy such as HW/BW ratio and myocyte cross-sectional area were increased to a similar extent in both ISO groups. An interesting finding of this study is that exogenous UB in the presence of ISO restored systolic function to normal levels, as indicated by decreased %FS, %EF, and Vcf. It partially restored ISO-mediated decrease in IVRT, IVCT, and ET. Changes in heart rate are suggested to affect echocardiographic parameters in mice (52). As observed previously (23), ISO infusion increased heart rate in ICR mice. UB alone or in the presence of ISO had no effect on basal or ISO-mediated increases in heart rates. Therefore, the observed changes in echocardiographic parameters between ISO and UB + ISO groups may not be due to the changes in heart rate. Extracellular UB is shown to promote intracellular Ca++ flux and reduce cAMP levels through a G protein-coupled receptor in THP1 cells (41). Therefore, UB may affect systolic and diastolic parameters of the heart by modulating levels of intracellular Ca++ and/or cAMP. However, further investigations are needed to understand the mechanism by which exogenous UB modulates heart function in the presence of an β-AR agonist.
Cardiac myocyte apoptosis plays a crucial role in the pathogenesis of heart failure (17, 21, 33). β-AR-stimulated activation of JNKs and GSK-3β plays a proapoptotic role via the involvement of mitochondrial death pathway (30, 39). Previously, UB has been shown to activate PI 3-kinase and inhibit β-AR-stimulated activation of JNKs and GSK-3β and mitochondrial death pathway of apoptosis (44). PI 3-kinase is an upstream activator of Akt (35), whereas Akt acts upstream in the inactivation of GSK-3β (16). Active GSK-3β may act upstream in the activation of JNKs (44). Here, we show that exogenous UB enhances β-AR-stimulated activation of Akt, whereas it inhibits activation of GSK-3β and JNKs. These in vivo data confirm our previous in vitro findings and suggest that activation of the PI 3-kinase/Akt pathway may be a mechanism involved in antiapoptotic effects of exogenous UB.
Chronic sympathetic stimulation is shown to induce growth of interstitial tissue in the heart, leading to fibrosis (7). MMPs and TIMPs play an important role in the remodeling of extracellular matrix (46). Previously, we have shown that chronic β-AR stimulation increases myocardial fibrosis (23). Consistent with these observations, we observed increased fibrosis following chronic β-AR stimulation in ICR mice. The new finding of this study is that exogenous UB inhibits chronic β-AR-stimulated increases in myocardial fibrosis. It enhanced chronic β-AR-stimulated increases in the expression and activity of MMP-2. It also increased expression of MMP-9 and TIMP-2 in the heart. Use of isolated adult cardiac fibroblasts confirmed our in vivo findings with respect to the expression of MMPs and TIMP-2 in response to UB. Of note, a higher concentration of rUB (compared with bovine UB) was required to observe increased expression of MMP-2 in the presence of isoproterenol in fibroblasts (Fig. 7D). The reasons may include differential modification of UB from different sources. UB is shown to be modified by acetylation of lysines, oxidation of methionine, and nitration of tyrosine (25, 54). These modifications may influence interaction of extracellular UB with its receptor and/or other proteins. The concentration of TIMP-2 is suggested to determine the role of TIMP-2 in activation of MMP-2. At low concentrations, TIMP-2 may activate MMP-2 on the cell surface with the MT1/MMP/TIMP-2 complex serving as a receptor for proMMP-2, whereas at higher concentrations TIMP-2 may neutralize MT1/MMP and prevent activation of MMP-2 (24). The data presented here suggest that exogenous UB decreases chronic β-AR-stimulated myocardial fibrosis by modulating expression of MMPs and TIMP-2. Previously, we have reported that chronic β-AR stimulation increases MMP-9 expression in the heart (23). Here, we did not observe increased expression of MMP-9 in response to chronic β-AR stimulation. The observed discrepant findings may relate to the use of different mouse strains. A previous study used 129xblack Swiss mice, whereas the current study uses ICR mice (23).
Increased MMP-2 expression is suggested to be dependent on Akt activation in the rat heart during ischemia-reperfusion (45). In the current study, UB enhanced Akt activation in the heart and in isolated cardiac fibroblasts in the presence of β-AR stimulation. Inhibition of Akt decreased UB + ISO-mediated increases in MMP-2 expression. These data suggest a potential relationship between Akt activation in response to UB treatment and MMP-2 expression in the heart.
Perspective.
The data presented here are novel and of significant interest since exogenous UB modulates β-AR-stimulated myocardial function and inhibits myocardial fibrosis and cardiac myocyte apoptosis. The structural changes related to cardiac myocyte apoptosis and extracellular matrix play a significant role in modulation of myocardial function and in the progression to heart failure. Therefore, elucidation of processes that can shift the balance from myocyte apoptosis to survival may have clinical implications. In addition, analysis of components of extracellular matrix, including collagen type I and IV, laminin, fibronectin, etc., may provide insight into the modulation of heart function in the presence of exogenous UB. It should be emphasized that our data on heart function and signaling are obtained 7 days after ISO infusion. The experimental time point should be extended beyond 7 days to investigate long-term effects of exogenous UB.
GRANTS
This work is supported by the National Heart, Lung, and Blood Institute (grant nos. HL-091405 and HL-092459) and a Merit Review Award (no. BX000640) from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.D., C.R.F., S.Y., and S.D. performed the experiments; C.R.D., C.R.F., S.Y., S.D., and K.S. analyzed the data; C.R.D., W.L.J., M.S., and K.S. interpreted the results of the experiments; C.R.D. and C.R.F. drafted the manuscript; C.R.D., C.R.F., S.Y., S.D., W.L.J., M.S., and K.S. approved the final version of the manuscript; C.R.F. prepared the figures; C.R.F., M.S., and K.S. edited and revised the manuscript; M.S. and K.S. did the conception and design of the research.
REFERENCES
- 1. Akarsu E, Pirim I, Capoglu I, Deniz O, Akcay G, Unuvar N. Relationship between electroneurographic changes and serum ubiquitin levels in patients with type 2 diabetes. Diabetes Care 24: 100–103, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Akarsu E, Pirim I, Selcuk NY, Tombul HZ, Cetinkaya R. Relation between serum ubiquitin levels and KT/V in chronic hemodialysis patients. Nephron 88: 280–282, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Amin P, Singh M, Singh K. β-Adrenergic Receptor-Stimulated Cardiac Myocyte Apoptosis: Role of β1 Integrins. J Signal Transduct 2011: 179057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asseman C, Pancre V, Delanoye A, Capron A, Auriault C. A radioimmunoassay for the quantification of human ubiquitin in biological fluids: application to parasitic and allergic diseases. J Immunol Methods 173: 93–101, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Bartelds B, Gratama JW, Meuzelaar KJ, Dalinghaus M, Koers JH, Heikens WF, Zijlstra WG, Kuipers JR. Comparative effects of isoproterenol and dopamine on myocardial oxygen consumption, blood flow distribution and total body oxygen consumption in conscious lambs with and without an aortopulmonary left to right shunt. J Am Coll Cardiol 31: 473–481, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Behonick GS, Novak MJ, Nealley EW, Baskin SI. Toxicology update: the cardiotoxicity of the oxidative stress metabolites of catecholamines (aminochromes). J Appl Toxicol 21, Suppl 1: S15–S22, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bos R, Mougenot N, Findji L, Mediani O, Vanhoutte PM, Lechat P. Inhibition of catecholamine-induced cardiac fibrosis by an aldosterone antagonist. J Cardiovasc Pharmacol 45: 8–13, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Carlsson L, Abrahamsson T, Almgren O. Local release of myocardial norepinephrine during acute ischemia: an experimental study in the isolated perfused rat heart. J Cardiovasc Pharmacol 7: 791–798, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, Shibayama H, Ikeda H, Hibi M, Machii T, Hirano T, Kanakura Y. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 95: 2577–2585, 2000 [PubMed] [Google Scholar]
- 10. Downing SE, Chen V. Myocardial injury following endogenous catecholamine release in rabbits. J Mol Cell Cardiol 17: 377–387, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Earle SA, El Haddad A, Patel MB, Ruiz P, Pham SM, Majetschak M. Prolongation of skin graft survival by exogenous ubiquitin. Transplantation 82: 1544–1546, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Foster CR, Singh M, Subramanian V, Singh K. Ataxia telangiectasia mutated kinase plays a protective role in beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Mol Cell Biochem 353: 13–22, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fowler MB, Laser JA, Hopkins GL, Minobe W, Bristow MR. Assessment of the beta-adrenergic receptor pathway in the intact failing human heart: progressive receptor down-regulation and subsensitivity to agonist response. Circulation 74: 1290–1302, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Covarrubias L, Manning EW, 3rd, Sorell LT, Pham SM, Majetschak M. Ubiquitin enhances the Th2 cytokine response and attenuates ischemia-reperfusion injury in the lung. Crit Care Med 36: 979–982, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res 90: 1055–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Haunstetter A, Izumo S. Future perspectives and potential implications of cardiac myocyte apoptosis. Cardiovasc Res 45: 795–801, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9: 1956–1967, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 100: 305–311, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circ Res 78: 517–524, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Kajstura J, Bolli R, Sonnenblick EH, Anversa P, Leri A. Cause of death: suicide. J Mol Cell Cardiol 40: 425–437, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11: 701–713, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Krishnamurthy P, Subramanian V, Singh M, Singh K. Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension 49: 865–872, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46: 214–224, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Macdonald JM, Haas AL, London RE. Novel mechanism of surface catalysis of protein adduct formation. NMR studies of the acetylation of ubiquitin. J Biol Chem 275: 31908–31913, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Majetschak M. Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J Leukoc Biol 89: 205–219, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Majetschak M, Cohn SM, Nelson JA, Burton EH, Obertacke U, Proctor KG. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery 135: 536–543, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Majetschak M, King DR, Krehmeier U, Busby LT, Thome C, Vajkoczy S, Proctor KG. Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: clinical and experimental findings. Crit Care Med 33: 1589–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Majetschak M, Krehmeier U, Bardenheuer M, Denz C, Quintel M, Voggenreiter G, Obertacke U. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 101: 1882–1890, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Menon B, Johnson JN, Ross RS, Singh M, Singh K. Glycogen synthase kinase-3beta plays a pro-apoptotic role in beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes: Role of beta1 integrins. J Mol Cell Cardiol 42: 653–661, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menon B, Singh M, Singh K. Matrix metalloproteinases mediate β-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes. Am J Physiol Cell Physiol 289: C168–C176, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Mori S, Gibson G, McTiernan CF. Differential expression of MMPs and TIMPs in moderate and severe heart failure in a transgenic model. J Card Fail 12: 314–325, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92: 139–150, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Okada M, Miyazaki S, Hirasawa Y. Increase in plasma concentration of ubiquitin in dialysis patients: possible involvement in beta 2-microglobulin amyloidosis. Clin Chim Acta 220: 135–144, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res 82: 250–260, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Pancre V, Pierce RJ, Fournier F, Mehtali M, Delanoye A, Capron A, Auriault C. Effect of ubiquitin on platelet functions: possible identity with platelet activity suppressive lymphokine (PASL). Eur J Immunol 21: 2735–2741, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Pant A, Lee II, Lu Z, Rueda BR, Schink J, Kim JJ. Inhibition of AKT with the Orally Active Allosteric AKT Inhibitor, MK-2206, Sensitizes Endometrial Cancer Cells to Progestin. PLoS One 7: e41593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quinones MA, Gaasch WH, Cole JS, Alexander JK. Echocardiographic determination of left ventricular stress-velocity relations. Circulation 51: 689–700, 1975 [DOI] [PubMed] [Google Scholar]
- 39. Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res 92: 136–138, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Rosenblum R, Berkowitz WD, Lawson D. Effect of acute intravenous administration of isoproterenol on cardiorenal hemodynamics in man. Circulation 38: 158–168, 1968 [DOI] [PubMed] [Google Scholar]
- 41. Saini V, Marchese A, Tang WJ, Majetschak M. Structural determinants of ubiquitin-CXC chemokine receptor 4 interaction. J Biol Chem 286: 44145–44152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonnenblick EH. beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol Heart Circ Physiol 275: H961–H968, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 189: 257–265, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Singh M, Roginskaya M, Dalal S, Menon B, Kaverina E, Boluyt MO, Singh K. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res 86: 20–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spanikova A, Ivanova M, Matejikova J, Ravingerova T, Barancik M. Influence of ischemia/reperfusion and modulation of PI3K/Akt kinase pathway on matrix metalloproteinase-2 in rat hearts. Gen Physiol Biophys 29: 31–40, 2010 [PubMed] [Google Scholar]
- 46. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Takada K, Nasu H, Hibi N, Tsukada Y, Shibasaki T, Fujise K, Fujimuro M, Sawada H, Yokosawa H, Ohkawa K. Serum concentrations of free ubiquitin and multiubiquitin chains. Clin Chem 43: 1188–1195, 1997 [PubMed] [Google Scholar]
- 48. Takagi M, Yamauchi M, Toda G, Takada K, Hirakawa T, Ohkawa K. Serum ubiquitin levels in patients with alcoholic liver disease. Alcohol Clin Exp Res 23: 76S–80S, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Takemoto Y, Yoshiyama M, Takeuchi K, Omura T, Komatsu R, Izumi Y, Kim S, Yoshikawa J. Increased JNK, AP-1 and NF-kappa B DNA binding activities in isoproterenol-induced cardiac remodeling. J Mol Cell Cardiol 31: 2017–2030, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Tan TP, Gao XM, Krawczyszyn M, Feng X, Kiriazis H, Dart AM, Du XJ. Assessment of cardiac function by echocardiography in conscious and anesthetized mice: importance of the autonomic nervous system and disease state. J Cardiovasc Pharmacol 42: 182–190, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res 69: 604–613, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Wu J, Bu L, Gong H, Jiang G, Li L, Ma H, Zhou N, Lin L, Chen Z, Ye Y, Niu Y, Sun A, Ge J, Zou Y. Effects of heart rate and anesthetic timing on high-resolution echocardiographic assessment under isoflurane anesthesia in mice. J Ultrasound Med 29: 1771–1778, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1beta. J Biol Chem 279: 39513–39519, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Yi D, Perkins PD. Identification of ubiquitin nitration and oxidation using a liquid chromatography/mass selective detector system. J Biomol Tech 16: 364–370, 2005 [PMC free article] [PubMed] [Google Scholar]
- 55. Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation 102: 344–350, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Zolk O, Schenke C, Sarikas A. The ubiquitin-proteasome system: focus on the heart. Cardiovasc Res 70: 410–421, 2006 [DOI] [PubMed] [Google Scholar]