Abstract
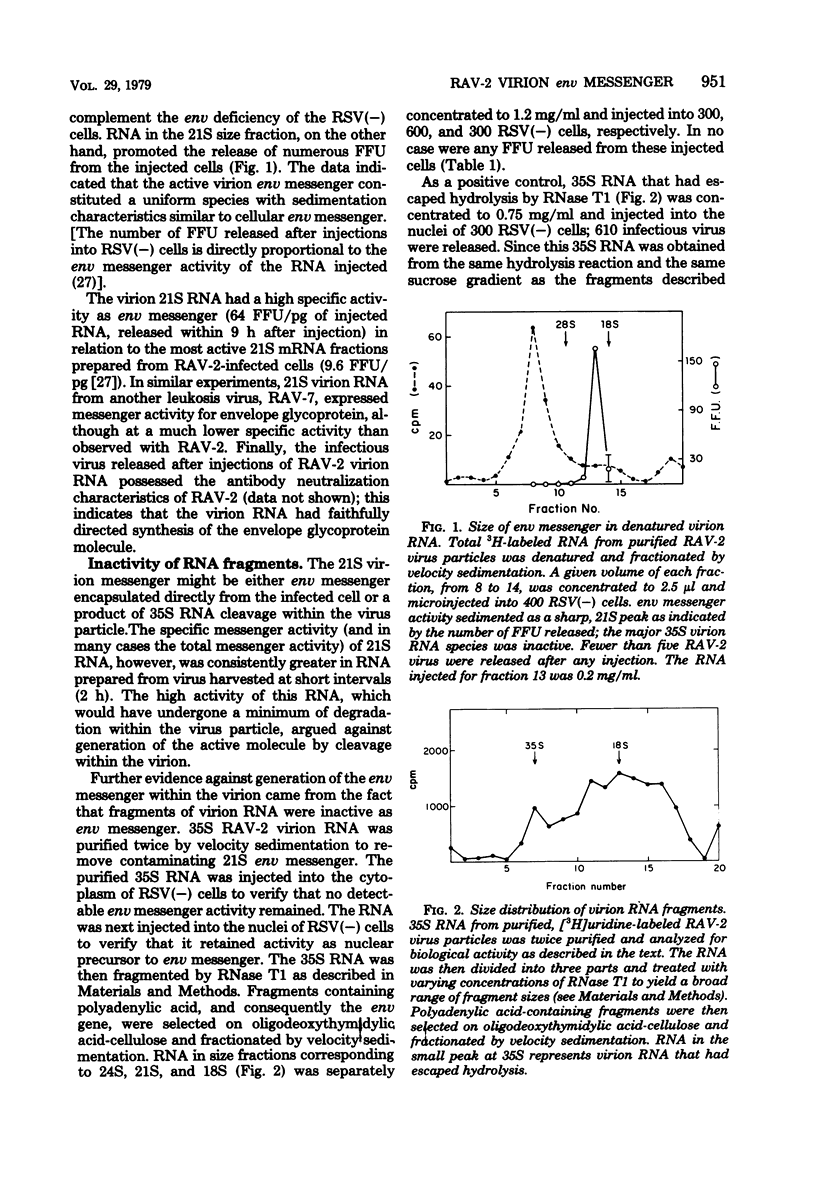
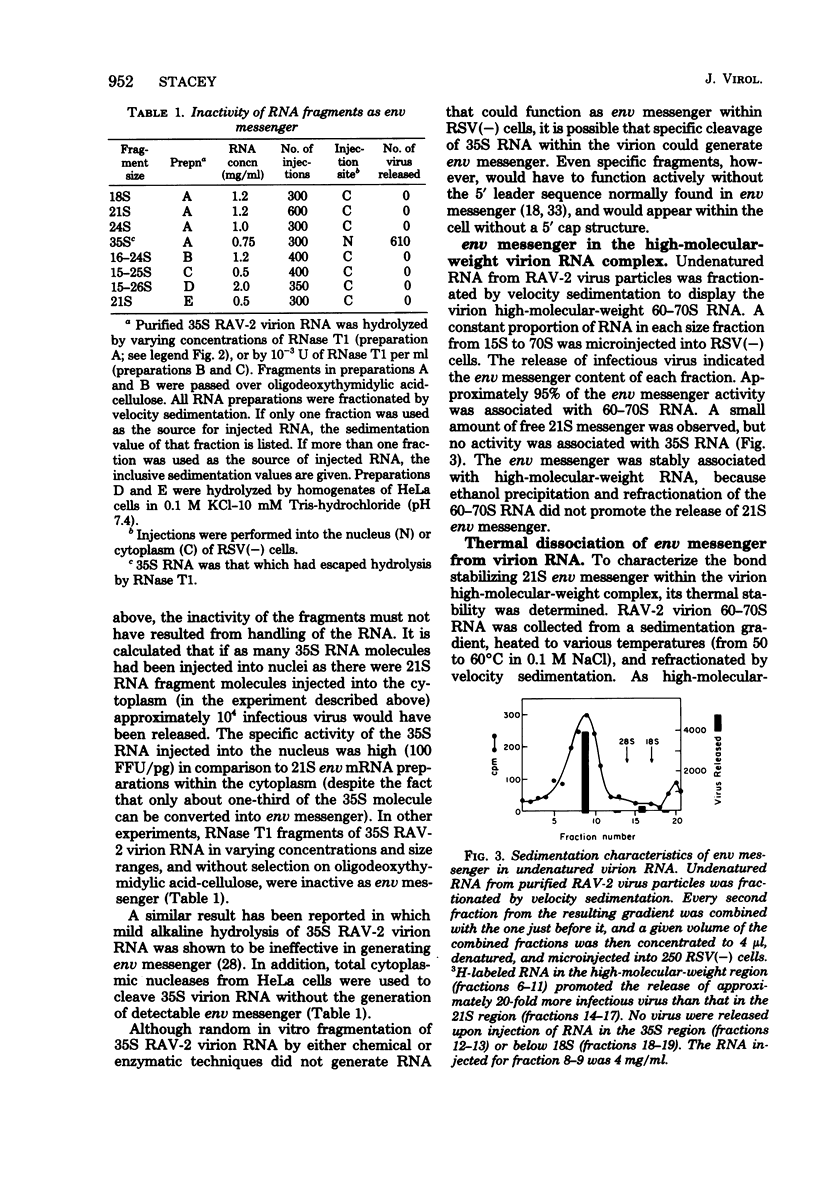
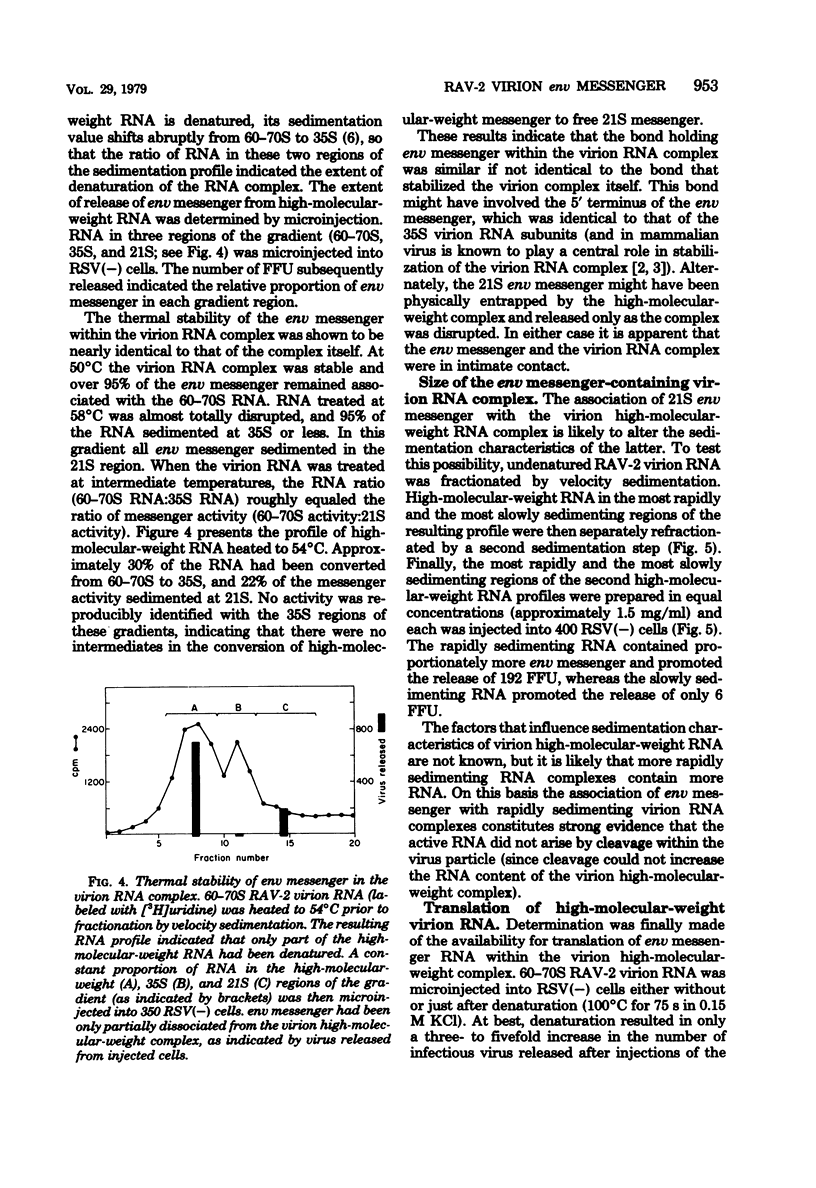
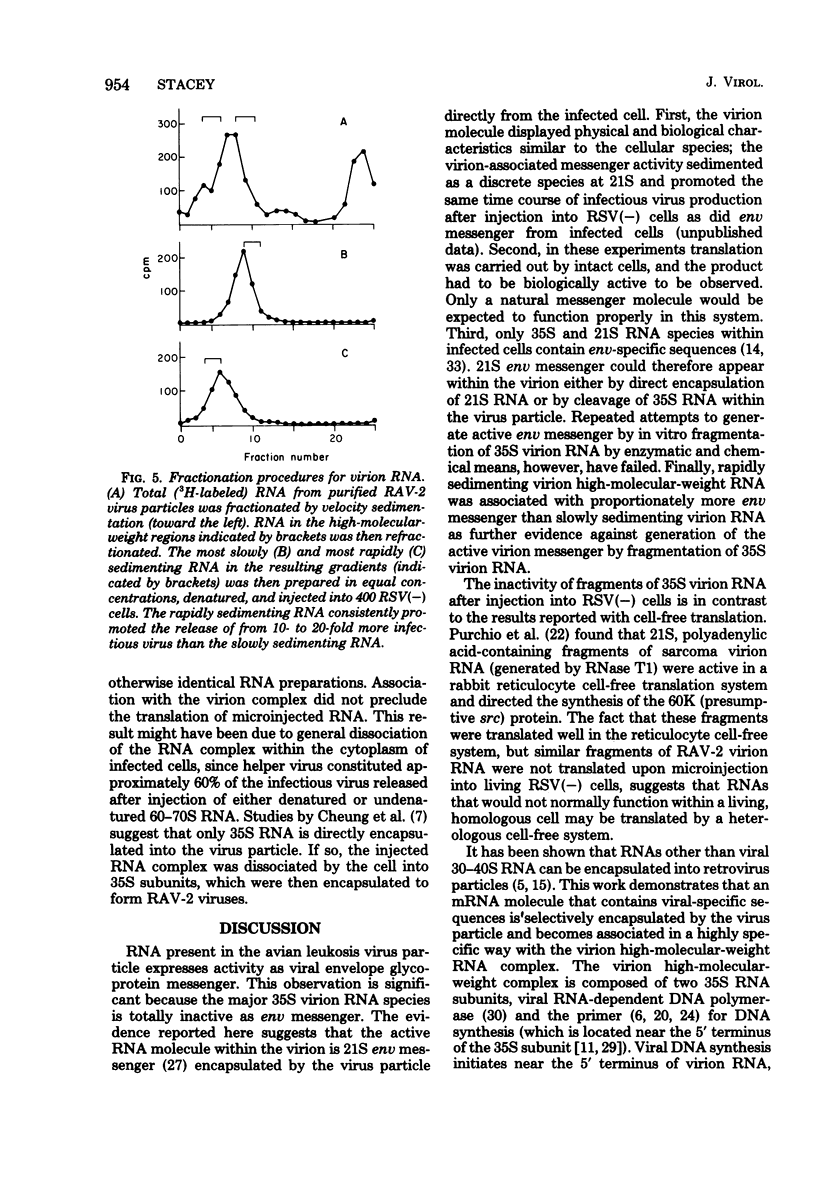
An intracellular assay for viral envelope glycoprotein (env) messenger was employed to analyze the RNA from virus particles of Rous-associated virus type 2. For this assay RNA was microinjected into cells infected by the env-deficient Bryan strain of Rous sarcoma virus [RSV(−) cells]. Only when the injected RNA could be translated by the recipient cells to produce viral envelope glycoprotein was the env deficiency of the RSV(−) cells complemented, enabling them to release focus-forming virus. RNA in a 21S size fraction from the Rous-associated virus particle promoted the release of numerous focus-forming virus from RSV(−) cells, whereas the major 35S virion RNA species was inactive. The env messenger activity sedimented as a sharp peak with high specific activity. RNase T1-generated fragments of virion 35S RNA were unable to promote the release of infectious virus from RSV(−) cells. Consequently, the active molecule was most likely to be env messenger which had been encapsulated by the virus particle from the cytoplasm of infected cells. Approximately 95% of the env messenger within the virion was associated with the virion high-molecular-weight RNA complex. The temperature required to dissociate env messenger from the high-molecular-weight complex was indistinguishable from the temperature required to disrupt the complex itself. Virion high-molecular-weight RNA that was associated with env messenger sedimented slightly more rapidly than the bulk virion RNA; this was the strongest evidence that the 21S messenger had been encapsulated directly from the infected cells. These data are considered along with a related observation [concerning the prolonged expression of env messenger after injection into RSV(−) cells] to raise the possibility that virus-encapsulated env messenger can become expressed within subsequently infected cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Chien Y. H., Chattopadhyay S., Vogt P. K., Gardner M. B., Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978 Mar;25(3):888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Evidence for circularization of the avian oncornavirus RNA genome during proviral DNA synthesis from studies of reverse transcription in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1329–1332. doi: 10.1073/pnas.73.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacumakos E. G. Methods for micromanipulation of human somatic cells in culture. Methods Cell Biol. 1973;7:287–311. doi: 10.1016/s0091-679x(08)61783-5. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J. The methionyl transfer RNAs of Rous sarcoma virus. Virology. 1975 Feb;63(2):583–588. doi: 10.1016/0042-6822(75)90330-x. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Ross J., Leder P. An association between globin messenger RNA and 60S RNA derived from Friend leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1154–1158. doi: 10.1073/pnas.71.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Naso WANG C. S., Tsai S., Arlinghaus R. B. Ribosomes from Rauscher leukemia virus-infected cells and their response to Rauscher viral RNA and polyuridylic acid. Biochim Biophys Acta. 1973 Oct 26;324(3):346–364. doi: 10.1016/0005-2787(73)90280-3. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G. Microinjection studies of duck globin messenger RNA translation in human and avian cells. Cell. 1976 Dec;9(4 Pt 2):725–732. doi: 10.1016/0092-8674(76)90136-7. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Hanafusa H. Nuclear conversion of microinjected avian leukosis virion RNA into an envelope-glycoprotein messenger. Nature. 1978 Jun 29;273(5665):779–782. doi: 10.1038/273779a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]



