Abstract
In this study, we tested the hypotheses that endothelial cells (ECs) derived from human umbilical cord blood (hCB-ECs) exhibit low permeability, which increases as hCB-ECs age and undergo senescence, and that the change in the permeability of hCB-ECs is due to changes in tight junction protein localization and the activity of exchange protein activated by cAMP (Epac)1. Albumin permeability across low-passage hCB-EC monolayers on Transwell membranes was 10 times lower than for human aortic ECs (HAECs) (P < 0.01) but similar to in vivo values in arteries. Expression of the tight junction protein occludin and tyrosine phosphorylation of occludin were less in hCB-ECs than in HAECs (P < 0.05). More hCB-ECs than HAECs underwent mitosis (P < 0.01). hCB-ECs that underwent >44 population doublings since isolation had a significantly higher permeability than hCB-ECs that underwent <31 population doublings (P < 0.05). This age-related increase in hCB-EC permeability was associated with an increase in tyrosine phosphorylation of occludin (P < 0.01); permeability and occludin phosphorylation were reduced by treatment with 2 μM resveratrol. Tyrosine phosphorylation of occludin and cell age influence the permeability of hCB-ECs, whereas levels of EC proliferation and expression of tight junction proteins did not explain the differences between hCB-EC and HAEC permeability. The elevated permeability in late passage hCB-ECs was reduced by 25–40% by elevation of membrane-associated cAMP and activation of the Epac1 pathway. Given the similarity to in vivo permeability to albumin and the high proliferation potential, hCB-ECs may be a suitable in vitro model to study transport-related pathologies and cell aging.
Keywords: vascular biology, cell aging, occludin, endothelial progenitor cell, permeability
the vascular endothelium plays a key role in the regulation of transport of macromolecules and water into tissues and blood vessels. An increase in the permeability of the endothelial layer to proteins influences the development and progression of atherosclerosis (38, 43). Localized sites of elevated endothelial permeability occur at lesion-prone regions of arteries before the onset of atherosclerosis (27). Such sites of increased permeability likely represent increased transport through endothelial cell junctions (13, 27, 43).
As cells age, morphological and physiological changes occur that may alter macromolecular transport and subsequent disease development. Aging at the cellular level is associated with replicative senescence or stress-induced senescence (22). Senescent cells become more frequent in aging individuals, exhibit an altered phenotype, contribute to a loss of tissue homeostasis, and play a role in the development of age-associated pathologies (10). Elevated levels of β-galactosidase, a histochemical marker for senescence, have been found in atherosclerotic lesions (36). Because endothelial cells (ECs) at sites that develop atherosclerosis often experience high cell turnover, ECs in these regions exhibit greater replicative senescence (9, 39), which may be linked to altered permeability. Additionally, environmental stresses in atheroprone regions of blood vessels may induce premature senescence (22).
The deacetylase sirtuin 1 (SIRT1) promotes proliferation and prevents senescence by targeting liver kinase B1 (LKB1), a serine/threonine kinase and tumor suppressor, in primary porcine aortic ECs (53). Senescence can be reversed by knocking down LKB1 with small interfering RNA or by downregulating LKB1 via activation of SIRT1 with resveratrol (53). Resveratrol indirectly activates SIRT1 via competitive inhibition of cAMP-degrading phosphodiesterases (PDEs) (40). Elevated levels of membrane-associated cAMP activate the cAMP-regulated guanosine nucleotide exchange factor exchange protein activated by cAMP (Epac)1, which, in turn, leads to GTP binding to the small G protein Rap1 (40). GTP-bound Rap1 activates SIRT1 but also causes changes to the cortical cytoskeleton and organization of vascular endothelial (VE-)cadherin in the endothelial junctions (19), leading to reduced endothelial permeability (24). Thus, changes that occur during cell aging that affect the pathway leading to SIRT1 activity may also elevate permeability through endothelial junctions.
ECs derived from late outgrowth endothelial progenitor cells (EPCs) in human umbilical cord or adult blood represent a promising source of the endothelium for applications in regenerative medicine (1, 7, 8). These cells express many of the molecular markers found on the large-vessel endothelium (7, 28, 30) and respond to flow in the same manner as the large vessel endothelium (1, 7). ECs that possess the high proliferative potential of late outgrowth EPCs can be isolated from the arterial endothelium (29). Circulating EPCs do not appear to contribute significantly to the endothelium overlying atherosclerotic lesions (26). Progenitor cells from young animals injected into older animals preferentially localize to sites of endothelial dysfunction and can reverse atherosclerosis in older animals (42). This result suggests that a decline in progenitor cell adhesion, incorporation into the endothelium, and function with age may influence the onset and progression of atherosclerosis. Furthermore, endogenous bone marrow-derived circulating EPCs adhere to sites of acute endothelial injury, such as vein grafts (51, 52).
In this study, we tested the hypotheses that 1) human ECs derived from EPCs isolated from umbilical cord blood (hCB-ECs) exhibit low permeability, which increases as the hCB-ECs age and undergo senescence, and 2) the change in the permeability of hCB-ECs is due to changes in tight junction protein localization and the activity of Epac1 (24). To address these hypotheses, we determined the permeability to the serum protein albumin of hCB-ECs and assessed the extent to which cell replication, occludin expression and phosphorylation, and cell age influence permeability differences of hCB-ECs and human aortic ECs (HAECs). We also examined the effect of agents that specifically activate Epac1 and SIRT1 upon the permeability in late passage hCB-ECs.
METHODS
Cell culture.
hCB-ECs and human adult peripheral blood-derived ECs (hPB-ECs) were isolated from blood as previously described by Ingram et al. (30). For the isolation of hCB-ECs, umbilical cord blood was obtained from the Carolina Cord Blood Bank (n = 5). Before receipt, all patient identifiers were removed. For the isolation of hPB-ECs, peripheral blood samples were obtained from young, healthy, nonsmoking volunteers taking no medicine (n = 1). The Institutional Review Board of Duke University approved the protocol for the collection and use of human blood used in this study.
After collection, blood was diluted 1:1 with HBSS (Invitrogen), placed into Histopaque 1077 (Sigma), and centrifuged at 740 g for 30 min. Buffy coat mononuclear cells were collected and washed three times with “complete EC growth medium,” which was composed of 8% (vol/vol) FBS added to endothelial basal medium (EGM)-2 (Cambrex) supplemented with EGM-2 SingleQuots (containing 2% FBS plus growth factors, Cambrex) and 1% antibiotic/antimycotic solution (Invitrogen). Mononuclear cells were plated on plastic six-well 35-mm diameter plates coated with collagen type I (rat tail, BD Biosciences) in complete EC growth medium. Medium was exchanged every 24 h for the first week in culture to remove nonadherent cells. Colonies of EPC-derived ECs appeared 7–10 days after the initial isolation. Colonies were trypsinized, and 200 cells were plated in a collagen-coated T25 flask and labeled as passage 1.
HAECs were obtained from Cambrex/Lonza. HAECs were obtained at passage 3 and, as noted by the supplier, had undergone 17 total population doublings at the time of purchase. HAECs were also cultivated in complete EC growth medium.
hCB-ECs, hPB-ECs, and HAECs were grown separately in T75 flasks using MCDB131 growth media supplemented with l-glutamine, penicillin-streptomycin, a EGM-2 Singlequot kit, and 10% FBS (10% complete media). Media were changed every other day until the time of experiment. hCB-ECs, hPB-ECs, and HAECs were passaged 1:10 into new T75 flasks upon reaching confluence. Cells were then subsequently split 1:10. The number of population doublings that occurred between each passage was adjusted based on a 75% attachment rate and calculated according to the following formula: ln(10)/ln(2) × (4/3) = 4.43, as previously described (46).
Flow cytometry.
To characterize the hCB-ECs used in this study, flow cytometry was performed for the surface markers CD31, CD34, CD45, and CD115 (10 μl antibody/100,000 cell sample, preconjugated with FITC and phycoerythrin, Biolegend). hCB-ECs were passaged using 0.025% trypsin-EDTA (Invitrogen) at 80% confluence. Approximately 100,000 hCB-ECs were resuspended in 1% BSA buffered with Dulbecco's PBS with calcium and magnesium (GIBCO). hCB-ECs were incubated at room temperature in the dark for 30 min with 2 μg of preconjugated antibody before a wash step with 1% BSA solution buffered in Dulbecco's PBS with calcium and magnesium (GIBCO). Cells were collected after centrifugation at 400 g for 7 min and fixed using 0.5% paraformaldehyde (J. T. Baker) before storage at −20°C until analysis. For each sample, 9,000 events were collected. Mouse IgG1 was used as a control (Biolegend).
Permeability experiments.
hCB-ECs or HAECs were seeded with 10% complete FBS media onto the luminal (top) chambers of 0.4-μm pore diameter, polyester, 12-well Transwell plates (Corning) at a density of 100,000 cells/cm2, which ensured the development of a confluent monolayer 1–2 days postplating. The abluminal (bottom) chamber contained serum-free media. Permeability was measured 2, 3, and 7 days postplating. Before the experiment, cells were incubated with serum-free media for 1 h. Unlabeled BSA (1 mg/ml) was added to the abluminal chamber, and FITC-BSA (1 mg/ml) was added to the luminal chamber at volumes that would ensure no differences in hydrostatic or osmotic pressure. Samples (10 μl) were taken from the abluminal chamber every 20 min for 2 h. The change in hydrostatic pressure due to a decrease in the volume of the abluminal chamber was 1.5 Pa, a level that would generate a very low fluid flow relative to physiological pressure drops between 1,333 and 6,667 Pa. There was no change in osmotic pressure during the experiment because the albumin concentration did not change with sampling. The albumin content of the sample was determined by a fluorimeter using a calibration curve. Permeability coefficients were determined using the following expression previously described by Albelda et al. (2):
| (1) |
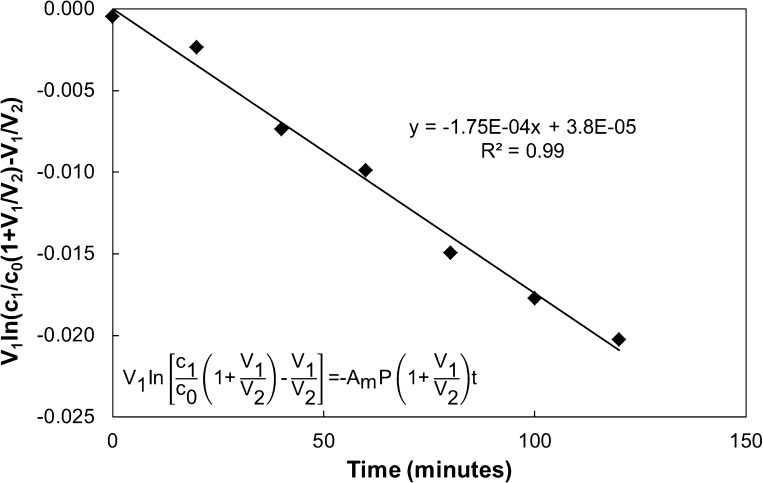
where V1 and V2 are the volumes in the luminal and abluminal chamber, respectively, C0 is the concentration of labeled albumin initially in the luminal chamber, C1 is the concentration of labeled albumin in the luminal chamber at time t, P is the diffusive permeability, and Am is the area of the membrane. Permeability was obtained from the slope of a linear regression of the natural logarithm of the bracketed term in Eq. 1 versus time for a given experiment. The R2 value of the trendline ranged from 0.94 to 0.99 and was used to assess goodness of fit. Figure 1 shows a representative plot. Permeability coefficients were calculated from the slope of the trendline. This permeability term represents contributions from both the cells and membrane. To calculate the permeability of the cells alone, the permeability of the cell layer and membrane were treated as resistances in series, as follows:
| (2) |
The permeability of the Transwell membrane alone was 3.1 ± 0.2 × 10−5 cm/s (n = 9), 13–100 times greater than the endothelial permeability.
Fig. 1.
Representative plot of data from the Transwell permeability experiments. A semi-log plot of the albumin concentration versus time using Eq. 1 was used to calculate the permeability. This permeability coefficient represents contributions from both the membrane and cells, which were treated as resistances in series (Eq. 2) to determine the permeability of the cell layer alone. In the equations, V1 and V2 are the volumes in the luminal and abluminal chamber, respectively, C0 is the concentration of labeled albumin initially in the luminal chamber, C1 is the concentration of labeled albumin in the luminal chamber at time t, P is the diffusive permeability, and Am is the area of the membrane.
Proliferation assays.
The Click-iT EdU Alexa fluor 488 imaging kit (Invitrogen) was used to measure the number of cells in the S phase. Cells were plated on Transwell plates as described above in the permeability experiments. The EdU label was incubated with the cells for 2 h. Detection of the label was performed as described in the manufacturer's protocol. Hoechst 33342 stain was used to detect all nuclei. The membrane of the Transwell insert was cut out and mounted on a glass slide for imaging. The stain was visualized with a Nikon Eclipse inverted microscope system. Cells in the M phase of mitosis were distinguished based on typical morphological features including condensed and segregating chromosomes (32). The percentage of cells in the S or M phase was reported as the total number of EdU-labeled cells or cells in the M phase, respectively, divided by the total number of cells that contained the Hoechst 33342 stain.
Western blot analysis.
Western blots were performed to determine the expression of the tight junction proteins occludin and zonula occludens (ZO)-2 in both cell types. Cells were grown in T25 flasks for 4 days postconfluence. Cells were harvested with a cell scraper and subjected to a solution of CellLytic-M (Sigma) and protease inhibitor cocktail (Sigma). Protein quantification was performed with a BCA protein assay kit (Pierce). Protein (10 μg) was loaded into each well for SDS-PAGE (10% gel, Bio-Rad). Afterward, protein bands were transferred to polyvinylidene difluoride membranes. Membranes were blocked with Tris-buffered saline with 0.1% Tween 20 (TBST) containing 5% milk at room temperature for 1 h. Primary antibodies for mouse ZO-2 (1:1,000 dilution, clone 3E8D9, Invitrogen), mouse occludin (1:2,000 dilution, clone OC-3F10, Invitrogen), and mouse β-actin (1:1,000 dilution, clone AC-15, Invitrogen) were diluted in TBST containing 1% milk and incubated with the membrane overnight at 4°C. The goat anti-mouse secondary antibody (1:2,500 dilution, Invitrogen) was incubated with the membrane for 45 min at room temperature. After being washed, immunoreactive bands were detected by luminography using Supersignal chemiluminescent substrate (Pierce). Subsequently, expression of phosphorylated occludin was detected by stripping the immunoblot and then reprobing with mouse anti-β-actin (1:1,000 dilution, Invitrogen) and mouse anti-phosphotyrosine (1:500 dilution, clone PY-7E1, PY20, Invitrogen) diluted in TBST containing 1% milk. The secondary antibody and chemiluminescent substrates were added in the normal manner. Integrated band density was determined by densitometry after scanning onto autoradiographic films (Kodak) and evaluated by ImageJ (National Institutes of Health). Protein expression was reported as the normalized intensity of the band of interest compared with the band of the housekeeping protein, β-actin.
Immunofluorescence.
hCB-ECs and HAECs were seeded at a density of 100,000 cells/cm2 on 35-mm glass dishes. At 6 days postplating, cells were fixed and permeabilized for 3 min with methanol. Samples were blocked with PBS-0.02% Tween 20 (Bio-Rad)-10% goat serum (GIBCO) for 1 h at room temperature. Samples were then incubated with antibodies to mouse occludin (1:250 dilution, clone OC-3F10, Invitrogen), mouse ZO-1 (1:250 dilution, clone ZO1-1A12, Invitrogen), or mouse ZO-2 (1:250 dilution, clone 3E8D9, Invitrogen) overnight at 4°C. This was followed by an appropriate secondary antibody incubation with goat anti-mouse Alexa fluor 488 (1:250 dilution, Invitrogen) or goat anti-mouse Alexa fluor 546 (1:250 dilution, Invitrogen) for 1 h at room temperature. All antibodies were diluted in PBS-10% goat serum. Stains were visualized with a Leica SP5 confocal microscope (Zeiss).
β-Galactosidase staining.
hCB-ECs were plated at 100,000 cells/cm2 on 24-well plates. Cells were then fixed at 4 days postplating. For resveratrol experiments, hCB-ECs were plated at 100,000 cells/cm2 and treated with 2 or 20 μM Resveratrol (25) 1 day postplating and were fixed 4 days postplating. The senescence β-galactosidase staining kit (Cell Signaling Technology) was used according to the manufacturer's protocol to stain for senescent cells. The stain was visualized with a Nikon Eclipse inverted microscope system. The percentage of senescent cells was calculated as the total number of cells that contained the blue β-galactosidase stain divided by the total number of cells in the field of view.
Telomerase activity.
The TRAPEZE RT telomerase detection kit (Millipore/Chemicon) was used to measure telomerase activity in cell samples. Lysis buffer (100 μl) was incubated with 500,000 cells for 30 min. The tube was then centrifuged at 4°C for 20 min. The supernatant was removed, and protein was quantified using the BCA assay kit (Pierce). Protein (200 ng) was used in each sample, and samples were performed in triplicate. A no template control of PCR-grade water (minus telomerase control of lysis buffer), positive telomerase control cell line, and heat-inactivated samples were used as controls. The assay was completed according to the manufacturer's protocol. According to the manufacturer, the sensitivity of the assay is 0.004 aM.
Statistical analysis.
n values represent the number of cell donors. For Figs. 2–4 and 6, ANOVA tests were performed to compare different data sets. Post hoc Tukey tests were performed for additional comparisons. For Fig. 7, two-factor repeated-measures ANOVA for cell age and treatment followed by a post hoc Tukey test was used. For Tables 2 and 3, repeated-measures ANOVA followed by a post hoc Tukey test was used. All data presented are means ± SE. P < 0.05 was interpreted to denote statistical significance.
Fig. 2.
Human cord blood-derived endothelial cells (hCB-ECs) that underwent <44 population doublings have lower permeability than human aortic endothelial cells (HAECs) that underwent <35 population doublings due to changes in phosphorylated occludin. A: albumin permeability of hCB-ECs was significantly lower than that of HAECs. B: Western blot and quantitative analyses of occludin and zonula occludens (ZO)-2 protein expression normalized to β-actin. C: Western blot and quantitative analyses of phosphorylated occludin. pTyr, phosphotyrosine. D: occludin localized to cell-cell junctions (arrows) in hCB-ECs but not HAECs. E: ZO-1 localization in hCB-ECs and HAECs. F: ZO-2 localization in hCB-ECs and HAECs. Data are means ± SE; n = 3–4. *P < 0.05; #P < 0.01.
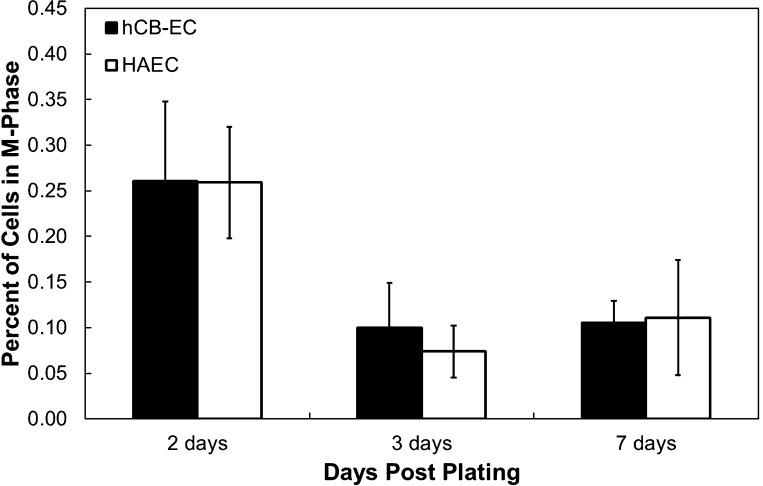
Fig. 4.
Similar M phase mitosis rates in hCB-ECs that underwent <31 population doublings and HAECs that underwent <35 population doublings. The percentage of cells in the M phase was determined for HAECs and hCB-ECs at 2, 3, and 7 days postplating. n = 3.
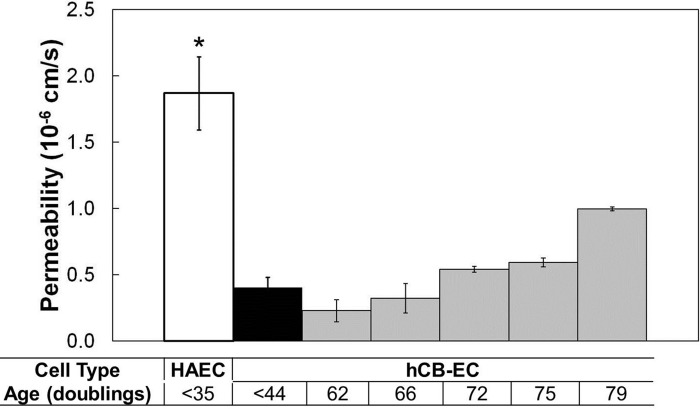
Fig. 6.
Albumin permeability of cells increases with increasing population doublings. Age was defined as population doublings since passage 1. The permeability of hCB-ECs from passage 1 isolation increased with increasing population doublings. The permeability approached that of HAECs. n = 3. *P < 0.05 compared with hCB-EC (<31 doublings); #P < 0.01 compared with hCB-EC (<31 doublings).
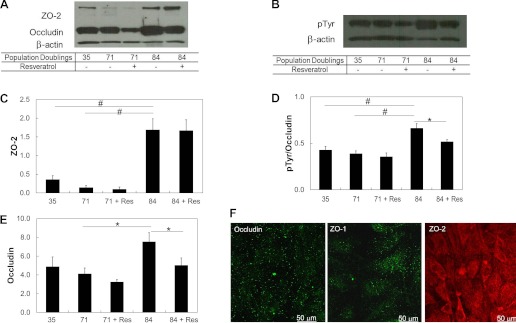
Fig. 7.
Changes in occludin, ZO-2, and phosphorylated occludin with increasing cell age. The increase was mitigated with the addition of 2 μM resveratrol for 48 h. A: Western blot analysis of tight junction proteins probed with antibodies for occludin and ZO-2. B: Western blot analysis of pTyr with control β-actin. C: quantitative analysis of ZO-2. Values are band intensities normalized by the intensity of the β-actin band. D: quantitative analysis of phosphorylated occludin. E: quantitative analysis of occludin. F: immunofluorescence of the tight junction proteins occludin, ZO-1, and ZO-2 in hCB-ECS after 44 population doublings. Data are means ± SE; n = 3. *P < 0.05; #P < 0.01.
Table 2.
Percentage of hCB-ECs in S-phase with increasing population doublings
| Population Doublings Since Passage 1 | Percentage of Cells in the S Phase |
|---|---|
| 44 | 9.6 ± 2.6 |
| 58 | 6.4 ± 1.2 |
| 71* | 4.5 ± 1.8 |
Data are means ± SE; n = 3. Percentages of hCB-ECs in the S phase with increasing population doublings are shown. The percentage of hCB-ECs in the S phase decreased as the population doublings increased. *P < 0.05 compared with 44 population doublings since passage 1.
Table 3.
Resveratrol, 8-pCPT-2′-O-Me-cAMP, and rolipram decrease hCB-EC permeability
| Treatment | Permeability Percent Decrease |
|---|---|
| Resveratrol | |
| 20 μM | 28 ± 7* |
| 2 μM | 41 ± 5* |
| 8-pCPT-2′-O-Me-cAMP | |
| 100 μM | 26 ± 5† |
| Rolipram | |
| 25 μM | 30 ± 1† |
Data are means ± SE; n = 3. *P < 0.05 and †P < 0.01 relative to the untreated control.
RESULTS
Characterization of hCB-ECs.
Flow cytometry showed that hCB-ECs and hPB-ECs were positive for endothelium-specific CD31 and CD34 and negative for CD14, CD45, and CD115, which are found on monocytes or hematopoietic cells. Immunofluorescence indicated that CD31, platelet-EC adhesion molecule, was present at the cell borders of hCB-ECs, HAECs, and hPB-ECs. We previously characterized hCB-ECs and found that they expressed von Willebrand factor, CD31, and VE-cadherin (7). After exposure to 15 dyn/cm2 for 24 or 48 h, hCB-ECs aligned with the direction of flow (7, 13), increased nitric oxide production, and increased mRNA for EC-specific genes sensitive to flow: Kruppel-like factor 2, nitric oxide synthase III, cyclooxygenase 2, and thrombomodulin (7). The level and organization of actin filaments were similar in hCB-ECs and HAECs, as were the associated values of cell stiffness (12).
Low-passage hCB-ECs have lower permeability than HAECs.
The in vitro permeability of HAECs to BSA 7 days postplating (2.4 ± 0.6 × 10−6 cm/s, n = 3) agreed with published values of 3.2 ± 0.4 × 10−6 cm/s for bovine aortic ECs (11) and 1.9 × 10−6 cm/s for human umbilical vein ECs (14). For hCB-ECs that underwent <44 population doublings since passage 1, the average permeability 7 days postplating (3.1 ± 0.5 × 10−7 cm/s, n = 3) was significantly less than the value obtained with HAECs that underwent <35 population doublings (P < 0.01; Fig. 2A). For hPB-ECs (44 population doublings), the average permeability (5.3 ± 0.2 × 10−7 cm/s, n = 2) 7 days postplating was between values for hCB-ECs and HAECs. Differences in permeability between EC types were significant for each day (P < 0.01), but, for each cell type, the permeability did not change significantly over time and was not dependent on donor (P < 0.60).
Expression of occludin and ZO-2 in low-passage hCB-ECs and HAECs does not explain the difference in permeability.
Elevated occludin levels have been correlated with reduced permeability to [14C]sucrose in bovine brain microvascular ECs (16). To assess the degree of tight junction protein expression in low-passage hCB-ECs and HAECs, Western blots were performed for the tight junction proteins ZO-2 (molecular mass: 160 kDa) (4) and occludin (molecular mass: 66 kDa) (3). β-Actin (molecular mass: 37kDa) served as a control (Fig. 2B). The relative intensity for ZO-2 was virtually identical for hCB-ECs (0.3 ± 0.2) and HAECs (0.4 ± 0.2, n = 4). Surprisingly, the relative intensity of occludin was less for hCB-ECs (1.4 ± 0.1) than HAECs (2.5 ± 0.6, n = 4, P < 0.05).
Less phosphorylated occludin in low-passage hCB-ECs than HAECs.
Although the amount of functional occludin is important in maintaining tight junction integrity, the phosphorylation of occludin causes a disruption of tight junctions (17). Therefore, cells with a lower permeability will have less phosphorylated occludin. Western blot analysis and associated densitometry showed that the relative intensity of antiphosphotyrosine antibody colocalized to occludin was 25% lower for hCB-ECs than for the HAECs (P < 0.05; Fig. 2C). Consistent with the lower level of occludin tyrosine phosphorylation in hCB-ECs than HAECs, immunofluorescence showed greater occludin localization at the periphery of hCB-ECs than HAECs (Fig. 2D). Although ZO-1 staining was not intense enough to permit quantification via densitometry, we could visualize ZO-1 by immunofluorescence. The greater localization of ZO-1 to the periphery of hCB-ECs relative to HAECs is consistent with the role of occludin and ZO-1 at EC junctions in contributing to the reduced permeability of hCB-ECs (Fig. 2E). In contrast, ZO-2 was not localized to the periphery in either hCB-ECs and HAECs, suggesting that additional mechanisms are involved in the regulation of permeability (Fig. 2F).
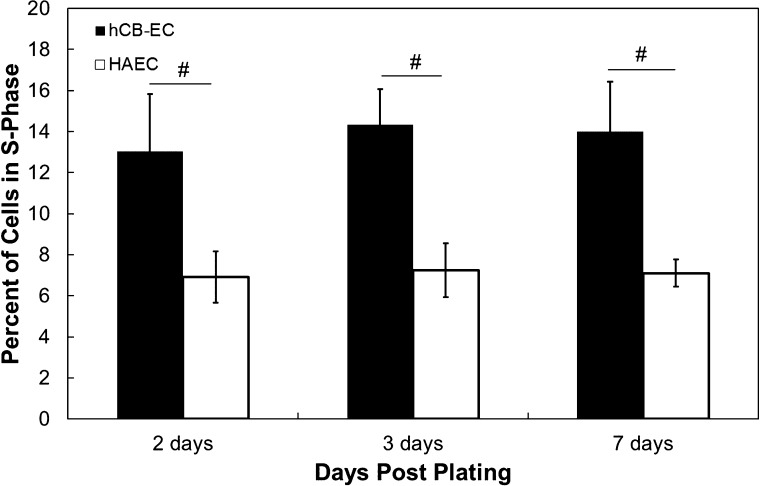
Low-passage hCB-ECs proliferate more than HAECs.
Since cells must break their junctions to divide (43), we hypothesized that a monolayer with a higher permeability would have more cells dividing at any one time. However, on days 2, 3, and 7 postplating, hCB-ECs that underwent <44 population doublings had a higher percentage of cells in the S phase of mitosis than did HAECs that underwent <35 population doublings (P < 0.017; Fig. 3). Differences in the percentage of cells in the S phase between cell types were statistically significant for each day, but for each cell type, the percentage of cells in the S phase did not change significantly over time.
Fig. 3.
Percentage of hCB-ECs that underwent <44 population doublings and HAECs that underwent <35 population doublings in the S phase at 2, 3, and 7 days postplating. hCB-ECs proliferated at a higher rate than HAECs. n = 6–8. #P < 0.017.
While the percentage of cells in the S phase provides some indication of cell turnover, cells divide during the M phase of mitosis, creating leaky junctions and enhancing permeability (32). The percentage of cells in the M phase significantly decreased over time after plating for low-passage hCB-ECs and HAECs (P < 0.05), but on each day, there was no difference between each cell type in the fraction of cells in the M phase (Fig. 4). The higher percentage of hCB-ECs in the S phase can be attributed to the shorter doubling time of hCB-ECs relative to HAECs (7). The low mitotic frequency of mitosis relative to cells in the S phase reflects the duration of labeling and the relative amounts of time in the S and M phases. Because there were similar numbers of hCB-ECs and HAECs dividing, cell mitosis cannot explain the difference in permeability between the two cell types.
Changes in hCB-EC proliferation and permeability with cell aging.
To characterize the aging of hCB-ECs and HAECs, preliminary experiments were performed to measure the amount of telomerase in cells at different numbers of population doublings after the first passage. Telomerase is a specialized reverse transcriptase that is necessary for the synthesis of telomeric DNA at the ends of chromosomes (22). A lack of telomerase hinders the ability of the DNA polymerase to replicate the end of the lagging strand, causing the DNA to shorten by 25–100 bp with each round of cell division. Thus, younger cells exhibit higher concentrations of telomerase (22). In general, the amount of telomerase decreased with increasing cell age in hCB-ECs (Table 1). The amount of telomerase in HAECs at 35 population doublings was equivalent to the value of hCB-ECs at later passages, suggesting that HAECs were older than any of the hCB-ECs tested.
Table 1.
Telomerase activity decreases with increasing population doublings in culture
| Donor | Population Doublings | Telomerase, aM/106 cells |
|---|---|---|
| hCB-ECs | ||
| Donor 1 | 31 | 1.200 |
| 44 | ≤0.004 | |
| 71 | ≤0.004 | |
| Donor 2 | 31 | 0.220 |
| 44 | 0.0280 | |
| 71 | 0.0194 | |
| HAECs | ||
| 35 | ≤0.004 | |
Average telomerase levels generally decreased with increasing population doublings. Human aortic endothelial cells (HAECs) exhibited less telomerase than any of the human cord blood-derived endothelial cells (hCB-ECs) tested, indicating that their relative age was higher than that of the hCB-ECs. Samples were run in triplicate.
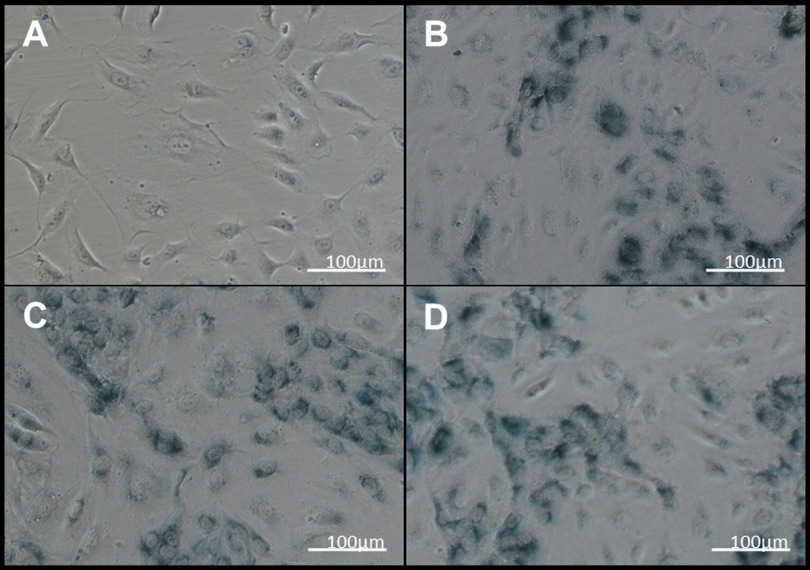
Next, we examined the effect of aging on hCB-EC permeability. Proliferation experiments done in parallel with permeability measurements indicated that the percentage of cells in the S phase decreased with increasing cell age (Table 2), a result expected as cells become senescent (36). Consistent with the lower level of proliferation of older cells, β-galactosidase staining was greater in hCB-ECs that underwent 71 doublings since passage 1 than in hCB-ECs that underwent fewer doublings (Fig. 5, A and B). For hCB-ECs that had undergone 84 population doublings, permeability was 1.5 ± 0.9 × 10−6 cm/s (mean ± SE, n = 3 donors), almost five times the value at a lower passage (Fig. 2). In an experiment with hCB-ECs from an additional donor, the permeability of hCB-ECs increased monotonically with increasing age (Fig. 6). The mean value for late passage hCB-ECs was lower than values obtained with lower-passage HAECs.
Fig. 5.
Senescence-associated-β-galactosidase staining of aging hCB-ECs. A: hCB-ECs at 18 doublings since passage 1. B: hCB-ECs at 71 doublings since passage 1. C: hCB-ECs at 71 doublings since passage 1 with 20 μM resveratrol. D: hCB-ECs at 71 doublings since passage 1 with 2 μM resveratrol.
Resveratrol treatment decreases hCB-EC permeability.
SIRT1 deacetylates proteins involved in regulating antioxidant activity (15). SIRT1 levels decline with aging, whereas elevation of SIRT1 improves EC function (18, 45). To test the effect of SIRT1 levels on endothelial permeability in aging cells, we treated ECs with resveratrol, which increases the expression levels of SIRT1 indirectly (18, 45).
Whereas porcine aortic ECs (53) and human umbilical vein ECs (15) tolerated a dose of 100 μM resveratrol for 16 h, this dose was toxic to hCB-ECs after 48 h. We found that hCB-ECs could tolerate doses ≤20 μM for 48 h. The addition of 20 μM resveratrol for 2 days before measurement decreased the permeability of hCB-ECs that underwent at least 57 population doublings by ∼28% (P < 0.05), whereas incubation of hCB-EC monolayers with 2 μM resveratrol decreased the permeability of hCB-ECs by ∼41% (P < 0.05; Table 3). HAECs incubated with 2 μM resveratrol decreased permeability by ∼22%. Doses of 2 and 20 μM resveratrol had no significant effect on the percentage of cells in the S phase or on β-galactosidase staining (Fig. 5, C and D).
Activation of the Epac1-Rap1 pathway reduces permeability in late passage cells.
The compound 8-pCPT-2′-O-Me-cAMP is a membrane-permanent activator of the exchange factor activated by cAMP, Epac1, which exerts the same effect on cell function as resveratrol (40). Treatment of cells with 100 μM 8-pCPT-2′-O-Me-cAMP during the permeability experiment decreased the permeability of hCB-ECs that underwent at least 57 population doublings by ∼26% (P < 0.01; Table 3).
To confirm that the change in permeability is due to PDE4, which has been proposed as the mode of action of resveratrol, we examined the permeability of hCB-ECs after treatment with rolipram, a specific PDE4 inhibitor that increases cAMP levels at the membrane, which then activates the Epac1-Rap1 pathway. Consistent with the role of resveratrol as a PDE4 inhibitor (40), treatment of hCB-ECs that underwent at least 57 population doublings with 25 μM rolipram caused permeability to decrease by ∼30% (Table 3). Taken together, these results suggest that resveratrol alters permeability in aging cells by increasing membrane-associated cAMP.
Increased expression of ZO-2 and phosphorylated occludin in aging hCB-ECs.
Because the addition of resveratrol decreased the permeability of aged hCB-ECs (Table 3), we examined the tight junction protein expression and localization of cells treated with 2 μM resveratrol for 48 h. Both occludin and phosphorylated occludin levels decreased significantly as hCB-ECs went from 35 to 71 population doublings but then increased between 71 and 84 population doubling (P < 0.01; Fig. 7, A and B). Cells that underwent 84 population doublings and were treated with 2 μM resveratrol had decreased levels of occludin and phosphorylated occludin (P < 0.05; Fig. 7, D and E). The change in phosphorylated occludin was consistent with the increase in hCB-EC permeability with increasing cell age and the reduced permeability of hCB-ECs after treatment with resveratrol.
Like occludin, ZO-2 levels increased for hCB-EC between 71 and 84 population doublings (P < 0.01; Fig. 7C). Treatment with resveratrol did not significantly affect levels of ZO-2 expression (Fig. 7C). From these results, we conclude that higher levels of tyrosine phosphorylation of occludin are associated with the elevated permeability of aging hCB-ECs and that resveratrol decreases permeability, in part, by reducing the levels of tyrosine-phosphorylated occludin.
Unlike early passage hCB-ECs (Fig. 2, D–F), localization of occludin, ZO-1, and ZO-2 in hCB-ECs that underwent 57 population doublings since passage 1 was not at cell junctions (Fig. 7F). The tight junction proteins were diffusely distributed in the cells, and the staining resembled that of HAECs (Fig. 2). In contrast, hCB-ECs that underwent <44 population doublings exhibited localization of occludin and ZO-1 to cell junctions (Fig. 2D). The results indicate that there was a change in the localization of tight junction proteins in hCB-ECs of increasing age, which correlated with the increase in permeability with increasing cell age.
DISCUSSION
To our knowledge, this study demonstrates, for the first time, that the permeability of low-passage hCB-ECs is significantly lower than that of HAECs and that the permeability of hCB-ECs increases with increasing population doublings due to an elevation of tyrosine phosphorylation of occludin and alteration of Epac1 activity. Additionally, early passage hCB-EC permeability values were close to those previously reported for large-vessel endothelium permeability to serum albumin in vivo (5, 6). For instance, permeability of the porcine abdominal aorta to albumin ranges from 8.6 × 10−7 to 27 × 10−7 cm/s (5), and permeability of the rabbit aorta to albumin has been reported as 0.6 × 10−7 cm/s (6) and 0.8 × 10−7 cm/s (35). This is the first type of cultured EC for which in vitro permeability to macromolecules is close to in vivo values for large vessels.
The permeability values measured in this study were obtained under diffusive conditions, whereas in vivo values represent the combined effect of diffusion and convection. Albumin is transported across the endothelium by a vesicular pathway, through endothelial junctions, and across the leaky endothelium. In vitro, ∼80% of water transport occurs through junctions and ∼20% across leaky junctions (11). For transport through the junctions and across the leaky endothelium, convection and diffusion modify the endothelial permeability through each pathway (Pe), as follows (11):
| (3) |
where Po is the diffusive permeability, Jv is the fluid flux, σ is the osmotic reflection coefficient, and Pe is the Peclet number, Pe = Jv(1 − σ)/Po. Convective transport is dependent on Jv and (1 − σ), which represents the drag exerted on the macromolecule by interactions with the junction proteins. For a given macromolecule, the value of σ is sensitive to the radius and volume fraction of junction fibers. A value of Jv = 1.8 × 10−6 cm/s was measured in the rabbit aorta (35). For this value of Jv and an in vitro value of σ = 0.49 for albumin (11), Pe = 2.6 for early passage hCB-ECs and 0.6 for late passage hCB-ECs. Convection would have a more significant effect on Pe for early passage hCB-ECs than for late passage hCB-ECs, although Pe is smaller for early passage ECs (9.9 × 10−7 cm/s) than late passage ECs (2.0 × 10−6 cm/s). Future studies will need to examine the relative importance of each of these pathways in early and late passage hCB-ECs and the manner in which transport is affected by applied pressures across the endothelium.
We examined several possible reasons for the lower permeability of hCB-ECs: increased levels and tyrosine phosphorylation of tight junction proteins, reduced levels of cell mitosis, and alterations in Epac1 activity. Occludin, when linked to ZO-1 and ZO-2, regulates transport through EC tight junctions (43). While we found that the expression of ZO-2 and occludin was not reduced in hCB-ECs relative to HAECs, occludin exhibited reduced levels of tyrosine phosphorylation and was redistributed to cell junctions in hCB-ECs. Mitosis levels also did not correlate with the reduced permeability of hCB-ECs, which is consistent with published results and indicates that cell mitosis is less important than other factors, such as apoptosis, in raising EC permeability (12).
The amount of functional occludin, in the form of nonphosphorylated occludin, as well as its localization to junctions between ECs are important to maintain the tight junction barrier in hCB-ECs (17). Ser490 phosphorylation and ubiquination of occludin are associated with increased permeability of primary bovine retinal ECs due to a loss of occludin from EC-EC junctions and its movement toward lysosomes (37). Elevated albumin permeability was associated with increased occludin tyrosine phosphorylation, and the elevation in permeability was blocked with tyrosine phosphatase and protein kinase inhibitors (17). This finding was further supported by the result that the activation of c-Src by oxidative stress increased the phosphorylation of tyrosine residues 398 and 402, which prevented occludin from assembling in the tight junctions and led to an increase in permeability (21). Treatment of human umbilical vein ECs with tyrosine phosphatase inhibitors led to selective occludin proteolysis and redistribution and increased permeability (47). When bovine brain microvascular ECs were exposed to shear stress for just a few hours, hydraulic permeability and the phosphorylation of tyrosine sites in occludin increased (49). Correspondingly, exposure of confluent monolayers to shear stress for 24 h led to a decrease in tyrosine phosphorylation and an associated decrease in permeability (49). Incubation of ECs with the protein tyrosine phosphatase activity inhibitor dephostatin led to smaller reductions in endothelial permeability after exposure to shear stress (49). In the present study, we found that there was significantly less phosphorylated occludin in hCB-ECs compared with HAECs, which is consistent with the reduced permeability of hCB-ECs. While our results are consistent with published studies that showed that increased occludin tyrosine phosphorylation causes permeability to increase, the phosphorylation of serine and threonine residues on occludin and other junction proteins could also affect permeability (20).
The amount of phosphorylated occludin increased as hCB-ECs underwent more population doublings, and this was associated with an increase in hCB-EC permeability. Treatment with resveratrol decreased hCB-EC permeability and produced a decrease in the expression of phosphorylated occludin. However, significant increases in occludin tyrosine phosphorylation were not observed until hCB-ECs underwent 71 population doublings (Fig. 7D), whereas permeability increased at earlier passages (Fig. 6). Because the changes in permeability preceded the changes in phosphorylated occludin, additional pathways, such as transport through leaky junctions and vesicular transport (11, 34), are likely to influence the permeability of hCB-ECs.
Other factors, in addition to occludin and ZO-1 localization and the extent of phosphorylated occludin in hCB-ECs, likely contribute to the lower permeability of hCB-ECs. Apoptosis is known to be elevated in senescent cells (33) and would produce an increase in the number of leaky junctions and increase permeability (12). The glycocalyx is composed of several membrane-bound macromolecules on the surface of ECs and may regulate the transport of water and macromolecules. Actin-myosin contraction can cause the breakdown of intercellular junctions, leading to elevations of permeability (34).
Aging at the cellular level is associated with replicative senescence or stress-induced senescence. For replicative senescence, after a certain number of population doublings, cells cease to replicate (10). A leading theory for the cause of replicative senescence is telomere shortening. After the telomere is shortened to a certain degree, the cell becomes senescent and does not divide any further to protect against mutation of its genetic code (22). Similarly, stress-induced senescence causes cells to cease to divide. It is primarily caused by environmental stresses, such as intracellular oxidative stress, DNA damage by radiation, or chromatin decondensation. Our finding that hCB-EC permeability increased with increasing population doublings indicates that cell aging and cell senescence play an important role in the regulation of albumin transport.
While ECs divide more rapidly in vitro than in vivo, there is evidence that ECs at lesion-prone sites have undergone replicative senescence by their size, shape, and telemere length (9, 39). The use of 44 or more population doublings to show an effect of cell replication on albumin is not an absolute number, but the data present a general trend at which cells become more susceptible to aging.
Resveratrol has been used to reduce age-associated metabolic phenotypes (40). We found that the addition of 2 μM resveratrol for 48 h was more effective than 20 μM resveratrol at decreasing permeability. Relative to 60 μM, resveratrol at 1 μM was more effective at promoting adhesion and migration of early outgrowth EPCs and low doses of resveratrol promoted reendothelization (25). Incubation of early outgrowth EPCs with resveratrol doses between 10 and 50 μM for 3 days reduced β-galactosidase staining and increased migration, proliferation, and telomerase activity, suggesting a reversal of senescence, and a dose of 100 μM increased apoptosis (50). While ECs can tolerate 100 μM resveratrol for short periods of time, our results suggest that lower doses of resveratrol can reduce permeability without necessarily reducing senescence.
Resveratrol acts upon SIRT1 via competitive inhibition of PDE4, which causes elevated cAMP levels (40). Park et al. (40) showed that the resulting elevation of cAMP activates the effector protein Epac1, sequentially increases Rap1 and intracellular Ca2+, and activates the Ca2+/calmodulin-dependent kinase kinase-β-AMP-activated protein kinase pathway to activate SIRT1. Interestingly, elevated cAMP levels cause a decrease in endothelial permeability by the activation of PKA, which then inactivates myosin light chain kinase (34), or through Epac-mediated Rap1 activation (24). Furthermore, membrane-associated cAMP bound to Epac1 interacts with PDE4 to bind to VE-cadherin (41) and regulates endothelial permeability by redistributing tight junction molecules to cell junctions (19). Thus, in addition to regulating occludin phosphorylation, resveratrol may influence permeability through Epac1-Rap1 interactions with VE-cadherin. We showed that 8-pCPT-2′-O-Me-cAMP, a cAMP analog that specifically activates the Epac1-Rap1 pathway, and rolipram, a specific PDE4 inhibitor, both decrease hCB-EC permeability in a manner similar to resveratrol. These results, along with the results of Park et al., indicate that resveratrol acts as a PDE4 inhibitor, which activates membrane-bound cAMP, which stimulates the Epac1-Rap1 pathway, which ultimately upregulates SIRT1 levels.
The incidence of cerebral vascular disease increases with increasing age and can ultimately lead to stroke or dementia (23). Increases in blood-brain barrier permeability play an important role in the pathophysiology of the diseases due to leakage of blood components into the white matter and vessel wall (44). These age-related white matter changes are associated with an increased incidence of stroke (31). Levels of circulating EPCs are decreased in patients with these age-related white matter changes. Taken together, these results indicate that increases in age-associated permeability can lead to the development of cerebral vascular disease.
Using EPCs in vitro as a model of cell aging may be a useful tool for studying the development and progression of atherosclerosis and cerebrovascular diseases. We (48) have found that when ECs are cultured on quiescent vascular smooth muscle cells that EC proliferation is greatly reduced. This coculture system could then be used to mimic vessel wall structure and reduce hCB-EC proliferation toward in vivo values.
The results of the present study suggest that cell age may be a key biological mechanism for the observed permeability differences between hCB-ECs and HAECs. The study also showed that hCB-ECs may be a suitable model for cell aging, as they can be used to represent very young cells after isolation and they can be passaged a significant number of times until they can be used as a model for older, senescent cells. This is important as the permeability of the endothelium is higher in regions prone to the initiation of atherosclerosis. Additionally, senescent cells are known to be present in areas with atherosclerosis. ECs in these regions undergo more cycles of replication and, due to the local hemodynamics, are more sensitive to extracellular stresses. These environmental conditions can ultimately lead to senescence that increases permeability, either due to alterations in signaling pathways within individual ECs or increased apoptosis leading to leaky junctions.
GRANTS
The work was supported by National Heart, Lung, and Blood Institute Grant HL-88825 (to G. A. Truskey) and by a National Science Foundation Graduate Research Fellowship (to T. M. Cheung).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.M.C. and G.A.T. conception and design of research; T.M.C., M.P.G., and E.B.P. performed experiments; T.M.C., M.P.G., and E.B.P. analyzed data; T.M.C., M.P.G., and G.A.T. interpreted results of experiments; T.M.C. and M.P.G. prepared figures; T.M.C. drafted manuscript; T.M.C., M.P.G., and G.A.T. edited and revised manuscript; T.M.C., M.P.G., E.B.P., and G.A.T. approved final version of manuscript.
REFERENCES
- 1. Ahmann KA, Johnson SL, Hebbel RP, Tranquillo RT. Shear stress responses of adult blood outgrowth endothelial cells seeded on bioartifical tissue. Tissue Eng 17: 2511–2521, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, Levine EM. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol 64: 308–322, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Balda MS, Gonzalez-Mariscal L, Matter K, Cereijido M, Anderson JM. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol 123: 293–302, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beatch M, Jesaitis LA, Gallin WJ, Goodenough DA, Stevenson BR. The tight junction protein ZO-2 contains three PDZ (PSD-95/discs-large/ZO-1) domains and an alternatively spliced region. J Biol Chem 271: 25723–25726, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bell FP, Adamson IL, Schwartz CJ. Aortic endothelial permeability to albumin: focal and regional patterns of uptake and transmural distribution of 131I-albumin in the young pig. Exp Mol Pathol 20: 57–68, 1974 [DOI] [PubMed] [Google Scholar]
- 6. Bratzler R, Chisolm G, Colton C, Smith K, Zilversmit D, Lees R. The distribution of labeled albumin across the rabbit thoracic aorta in vivo. Circ Res 40: 182–190, 1977 [DOI] [PubMed] [Google Scholar]
- 7. Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng 15: 3575–3587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown MA, Zhang L, Levering VW, Wu JH, Satterwhite LL, Brian L, Freedman NJ, Truskey GA. Human umbilical cord blood-derived endothelial cells reendothelialize vein grafts and prevent thrombosis. Arterioscler Thromb Vasc Biol 30: 2150–2155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burrig K. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb Vasc Biol 11: 1678–1689, 1991 [PubMed] [Google Scholar]
- 10. Campisi J. Replicative senescence: an old lives' tale? Cell 84: 497–500, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Cancel LM, Fitting A, Tarbell JM. In vitro study of LDL transport under pressurized (convective) conditions. Am J Physiol Heart Circ Physiol 293: H126–H132, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Cancel LM, Tarbell JM. The role of apoptosis in LDL transport through cultured endothelial cell monolayers. Atherosclerosis 208: 335–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao L, Wu A, Truskey GA. Biomechanical effects of flow and coculture on human aortic and cord blood-derived endothelial cells. J Biomech 44: 2150–2157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang YS, Munn LL, Hillsley MV, Dull RO, Yuan J, Lakshminarayanan S, Gardner TW, Jain RK, Tarbell JM. Effect of vascular endothelial growth factor on cultured endothelial cell monolayer rransport properties. Microvasc Res 59: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Chen Z, Peng IC, Cuia X, Li YS, Chien S, Shyy JYJ. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107: 10268–10273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, Cummins PM. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 292: H3190–H3197, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, Meade G, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1. Arterioscler Thromb Vasc Biol 26: 62–68, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005 [DOI] [PubMed] [Google Scholar]
- 20. DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA. Shear stress regulates occludin content and phosphorylation. Am J Physiol Heart Circ Physiol 281: H105–H113, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 284: 1559–1569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erusalimsky JD, Skene C. Mechanisms of endothelial senescence. Exp Physiol 94: 299–304, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease–systematic review and meta-analysis. Neurobiol Aging 30: 337–352, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu J, Wang C, Fan H, Ding H, Xie X, Xu Y, Wang B, Huang D. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J Cardiovasc Pharmacol 47: 711–721, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation 121: 898–905, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Herrmann RA, Malinauskas RA, Truskey GA. Characterization of sites of elevated low density lipoprotein at the intercostal, celiac, and iliac branches of the rabbit aorta. Arterioscler Thromb 14: 313–323, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28: 1584–1595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105: 2783–2786, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104: 2752–2760, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Jickling G, Salam A, Mohammad A, Hussain MS, Scozzafava J, Nasser AM, Jeerakathil T, Shuaib A, Camicioli R. Circulating endothelial progenitor cells and age-related white matter changes. Stroke 40: 3191–3196, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Lin SJ, Jan KM, Schuessler G, Weinbaum S, Chien S. Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis 73: 223–232, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Mammone T, Gan D, Foyouzi-Youssefi R. Apoptotic cell death increases with senescence in normal human dermal fibroblast cultures. Cell Biol Int 30: 903–909, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Meyer G, Merval R, Tedgui A. Effects of pressure-induced stretch and convection on low-density lipoprotein and albumin uptake in the rabbit aortic wall. Circ Res 79: 532–540, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis. Circulation 105: 1541–1544, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem 284: 21036–21046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nielsen LB, Nordestgaard BG, Stender S, Kjeldsen K. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 12: 1402–1409, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24: 546–550, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown Alexandra L, Kim Myung K, Beaven Michael A, Burgin Alex B, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting camp phosphodiesterases. Cell 148: 421–433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rampersad SN, Ovens JD, Huston E, Umana MB, Wilson LS, Netherton SJ, Lynch MJ, Baillie GS, Houslay MD, Maurice DH. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem 285: 33614–33622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 108: 457–463, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 87: 320–330, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 81: 192–197, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated β-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exp Cell Res 241: 309–315, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci 112: 4347–4356, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng 35: 375–386, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Walsh TG, Murphy RP, Fitzpatrick P, Rochfort KD, Guinan AF, Murphy A, Cummins PM. Stabilization of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signaling leading to modulation of pTyr-occludin levels. J Cell Physiol 226: 3053–3063, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, Zeng CL, Zhang FR, Chen JZ. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br J Pharmacol 155: 387–394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res 93: e76–e86, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol 24: 470–476, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Zu Y, Liu L, Lee MYK, Xu C, Liang Y, Man RY, Vanhoutte PM, Wang Y. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res 106: 1384–1393, 2010 [DOI] [PubMed] [Google Scholar]