Abstract
Sonic hedgehog (Shh) is a morphogen critically involved in development that is reexpressed in atherosclerotic lesions. It also stimulates proliferation of vascular smooth muscle cells (SMCs). Autophagy in vascular SMCs is known to promote SMC survival and increase plaque stability. The aim of this study was to investigate whether Shh induces autophagy of vascular SMCs. Our study showed that both Shh protein and microtubule-associated protein 1 light chain 3 (LC3)-II were increased in SMCs within neointimal lesions of mouse common carotid arteries. In cultured mouse aortic SMCs, recombinant mouse Shh stimulated LC3-II levels. Overexpression of wild-type mouse Shh through the tetracycline-regulated expression-inducible system in human aortic SMCs time-dependently increased the levels of LC3-II and also stimulated protein kinase B (AKT) phosphorylation. Pretreatment with AKT inhibitor IV (AKTI IV) inhibited AKT phosphorylation and the increase in LC3-II. Shh-induced autophagy was further confirmed by the formation of autophagosomes as detected by immunostaining and transmission electron microscopy, which was inhibited by AKTI IV. Shh further increased SMC LC3-II in the presence of bafilomycin A1, (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester, and pepstatin A or siRNA for the autophagy-related gene 7 (ATG7). In addition, Shh induced SMC proliferation, which was inhibited not only by AKTI IV but also by cyclopamine, an inhibitor of Shh receptor. Inhibition of autophagy with 3-methyladenine (3-MA), bafilomycin A1, or ATG7 siRNA resulted in inhibition of cell proliferation. Treatment with 3-MA, AKTI IV, or cyclopamine inhibited neointima formation in mouse common carotid arteries. Taken together, our results have shown that Shh induces autophagy of vascular SMCs involving AKT activation, suggesting a role of autophagy in Shh-induced cellular responses.
Keywords: smooth muscle cells, autophagy, sonic hedgehog, protein kinase B
sonic hedgehog (Shh), a 20-kDa secreted protein, has a critical role in the development of many organs (13), including the cardiovascular system (2). Before hedgehog (Hh) proteins are secreted and act through autocrine and/or paracrine mechanisms, they undergo autocatalytic processing and cholesterol modification (22, 23). The receptor for Shh is composed of two multispan transmembrane proteins Patched (Ptc) and Smoothened (Smo). Shh first binds to Ptc to release the inhibition of Ptc on Smo. Activated Smo stimulates the transcriptional activity of its target genes, including Ptc in the Hh signaling pathway (8), by binding the glioma-associated transcription factor.
Shh also has a potential role in the pathogenesis of vascular disease. In response to hypoxia, Shh signaling was reactivated to promote the growth of human pulmonary arterial smooth muscle cells (SMCs) (44). In addition, Shh signaling plays critical roles in angiogenesis, either in development (5, 19) or in ischemic adult tissues (3, 31). Mice genetically deficient in Hh proteins have a smaller and less-organized capillary plexus (4), further supporting a role of Shh in the growth of vascular pericytes. However, it remains unknown how Shh regulates vascular SMC functions.
Autophagy is an intracellular degradation process of long-lived proteins and excess or dysfunctional organelles that can be identified by electron microscopy (27). During autophagy, the cytosolic form of microtubule-associated protein 1 light chain 3–1 (LC3-I) is converted to the phosphatidylethanolamine-conjugated form of LC3 (LC3-II) to promote autophagosome formation (1). Punctate LC3 and autophagosome formation can be detected by immunofluorescence microscopy and electron microscopy (11, 25, 42, 43). Therefore, the increase of expression and turnover of LC3-II has been widely used to indicate activation of autophagy (18). Autophagy, which promotes cell survival (7), occurs in vascular SMCs (14). It plays a protective role in free-cholesterol-overload-induced death of SMCs (47).
Both Shh and autophagy are important for survival, but it remains unknown whether Shh stimulates autophagy of vascular SMCs. Given that Shh is increased in the neointima of vein grafts obtained from mice undergoing restenosis (21), and neointima formation results from proliferation of vascular SMCs, the current study was designed to examine whether the expression of Shh coexists with autophagy in neointimal lesions and investigate if Shh activates autophagy in vascular SMCs.
MATERIALS AND METHODS
Materials.
The amino-terminal peptide of recombinant mouse sonic hedgehog (N-Shh) and antibody for N-Shh (1:1,000, AF464) were purchased from R&D Systems (Minneapolis, MN). Cyclopamine was purchased from LC Laboratories (Woburn, MA). Bafilomycin A1 was purchased from Abcam (Cambridge, MA). 4′,6-Diamidino-2-phenylindole (DAPI), protein kinase B (AKT) inhibitor IV (AKTI IV), rapamycin, (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (E64d), pepstatin A, 3-methyladenine (3-MA), antibodies for LC3 (1:1,000, L7543), smooth muscle α-actin (SM α-actin, 1:1,000, A2547), β-actin (1:5,000, A5316), and α-tubulin (1:1,000, T5168) were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for c-Myc (1:500, sc-40), phospho (p)-S6K (1:1,000, sc-8416), S6K (1:1,000, sc-8418), proliferating cell nuclear antigen (PCNA) (1:1,000, sc-7907), and p27 (1:1,000, sc-528) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for p-AKT (1:1,000, no. 4051), AKT (1:1,000, no. 9272), p-4E-binding protein (BP) 1 (1:1,000, no. 9451), and 4E-BP1 (1:1,000, no. 9644) were from Cell Signaling Technology (Danvers, MA). Antibody for p62 (1:2,000, 610832) was purchased from BD Transduction Laboratories (San Diego, CA). Peroxidase-conjugated AffiniPure anti-mouse, goat, and rabbit IgGs (1:5,000) were purchased from Molecular Probes (Eugene, OR). Secondary antibodies labeled with Alex Fluor-488 (goat anti-mouse IgG, green), Alex Fluor-568 (goat anti-rabbit IgG, red), or Alexa Fluor 594 (donkey anti-goat IgG, red), Dulbecco's modified Eagle's medium (DMEM), DMEM-F-12, heat-inactivated FBS, and Pluronic F-127 gels (PLF-127 gels) were purchased from Invitrogen (Carlsbad, CA).
Neointima formation through ligation of mouse common carotid arteries.
All animal studies were approved by the animal care and use committee at Nankai University and were performed according to the Guidelines for the Care and Use of Laboratory Animals at Nankai University (A5521-01), which strictly conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication no. 85-23, revised 1996). C57BL/6 male mice (8–10 wk, 20–25 g) were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences of China. The mice were anesthetized by intraperitoneal injection of 8% chloral hydrate (4 ml/kg body wt). The left common carotid arteries were dissected and ligated near the carotid bifurcation, as we previously described (20, 49). After ligation (14 days), both left and right (to serve as a negative control) common carotid arteries were sampled for examination of vascular wall morphologies using hematoxylin and eosin (H&E) staining and for protein extraction.
In the pharmacological studies, dimethyl sulfoxide (DMSO) was used to dissolve various inhibitors to achieve different concentrations of 3-MA (25 mM), AKTI IV (50 μM), and cyclopamine (1, 10 μM) and subsequently applied around the ligation site through 25% PLF-127 gel. Control animals received DMSO and 25% PLF-127 gel only. Mice were then killed 21 days after carotid artery ligation, followed by isolation of the left and right carotid arteries for morphological studies and protein extraction.
Histology and immunohistochemistry.
After quick excision of both left and right common carotid arteries, the vessels were immersion-fixed in 4% paraformaldehyde overnight. The vessel segments were embedded in paraffin, and serial sections (5 μm) were cut for H&E staining and analysis of vascular wall morphologies as previously described (49). Five sections spanning the vessel segment from each mouse were analyzed.
Cell culture and drug treatment.
Mouse aortic SMCs (CRL-2797; ATCC) were maintained in DMEM, containing 10% FBS as recommended. When cells reached about 80% confluence, the medium containing 10% FBS was removed, followed by treatment with N-Shh (1 μg/ml), rapamycin (0.1 μM), or cyclopamine (40 μM) for 24 h. Cells were then harvested for extraction of protein and mRNA for further analyses. Human aortic SMCs (ATCC, CRL-199) were maintained in DMEM-F-12 containing 10% FBS and 1% penicillin-streptomycin. At about 80% confluence, cells were washed twice with PBS and recultured in serum-free medium containing various drugs, such as doxycycline (Dox, 1 μg/ml), rapamycin (0.1 μM), AKTI IV (5 μM), E64d (10 μg/ml) plus pepstatin A (10 μg/ml), 3-MA (2.5 mM), bafilomycin A1 (20 nM), and cyclopamine (40 μM). After 24 h treatment, cells were harvested for the corresponding experiments.
Expression of Shh via the tetracycline-regulated expression system.
The tetracycline-regulated expression (T-Rex)-inducible system for overexpression of mouse wild-type Shh (Shh-TR cells) and myocardin (myocardin-TR cells) was established in human aortic SMCs containing TR (tetracycline-repression) protein (TR cells), as we previously described for the myocardin gene (41). Briefly, the mouse wild-type Shh sequence from Addgene Plasmid 13999:pBS mShh (Addgene, Cambridge, MA) was cloned into plasmid pcDNA4/TO/myc-His A (Invitrogen) with the following primer sequences: forward GAATTCATGCTGCTGCTGCTGG and reverse CTCGAGGCTGGACTTGACCGCC. After transfection, cells harboring both TR and mouse wild-type Shh were selected with Zeocin (100 μg/ml) and blasticidin (10 μg/ml). After drug selection, expression of Shh through the T-REx system was confirmed by Western blot with anti-N-Shh and anti-c-myc monoclonal antibodies. In the time course experiments of Shh induction, cells were treated with Dox for 0, 3, 5, 8, 12, 24, and 48 h, as indicated in results.
Small-interfering RNA and plasmid transfection.
SMCs were seeded in a 6-well plate or 96-well plate at 60% confluence in DMEM-F-12 medium containing 10% FBS. After 24 h, the cells were transfected with a mixture of autophagy-related gene 7 (ATG7) small-interfering RNA (siRNA) or negative-control siRNA (NC siRNA) (RIBOBIO, Guangzhou, China) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen). Transfection effectiveness was determined by Western blot detection of LC3-II expression and real-time quantitative PCR (RT-qPCR) detection of ATG7 mRNA levels. After transfection for 36 h, cells were serum-starved for 24 h, followed by various treatments for an additional 24 h.
Water-soluble tetrazolium salt assay.
Cell proliferation was measured using the water-soluble tetrazolium salt (Wst-1) assay (12, 30). Briefly, cells were seeded in a 96-well plate (2 × 103 cells/well) in culture medium containing 10% FBS for 24 h. Cells were then serum-starved for 24 h, followed by drug treatment for an additional 24 h. In the ATG7 siRNA experiment, cells were seeded in a 96-well plate (1 × 103 cells/well) with culture medium containing 10% FBS for 24 h, followed by transfection with NC siRNA or ATG7 siRNA for 36 h. Cells were then serum-starved for 24 h and treated with N-Shh for an additional 24 h. At the end of treatment, cells were incubated with 10 μl Wst-1 reagent (Beyotime Institute of Biotechnology, Haimen, China) for 2 h, followed by measurement of absorbance at 450 nm using a spectrophotometer.
Immunostaining and fluorescence microscopy.
To determine the formation of autophagosomes, cells were first cultured on glass cover slips in six-well plates with various treatments for 24 h and fixed for immunostaining with LC3 antibody and Alexa Fluor 568-conjugated goat anti-rabbit IgG (red). The nuclei were counterstained with DAPI (1 μg/ml, 5 min), followed by analysis using Olympus BX51 fluorescence microscopy (Olympus, Tokyo, Japan).
To determine the expression of Shh and LC3 in SMCs of neointimal lesions in mouse common carotid arteries, both left and right carotid arteries were isolated, immersion-fixed in 4% paraformaldehyde for at least 2–8 h, and then embedded in paraffin for serial sections (5 μm). Primary and secondary antibodies for immunostaining were as follows: antibody for SM α-actin and Alexa Fluor 488-conjugated goat anti-mouse IgG (green), antibody for N-Shh and Alexa Fluor 594-conjugated donkey anti-goat IgG (red), and antibody for LC3 and Alexa Fluor 568-conjugated goat anti-rabbit IgG (red). The nuclei were counterstained with DAPI (1 μg/ml). Analysis was performed using Olympus BX51 fluorescence microscopy (Olympus).
RNA preparation and RT-qPCR analysis.
Total RNA was isolated from cultured SMCs with TRIzol Reagent (Takara Biotechnology, DaLian, China). RNA (1 μg) was used for first-strand cDNA synthesis using the kit according to the manufacturer's recommendations. To determine the effectiveness of ATG7 siRNA transfection, RT-qPCR was performed to detect ATG7 mRNA levels using the following primers (Shanghai Bioengineering, Shanghai, China): human ATG7, 5′-CTGCCAGCTCGCTTAACATTG-3′ (forward), 5′-GTGGGAGCACTCATGTCAAAA-3′ (reverse) and human β-actin, 5′-CGGGAAATCGTCCGTGACATTAAG-3′ (forward), 5′-TGATCTCCTTCTGCATCCTGTCGG-3′ (reverse). Conditions for the RT-qPCR reactions were as follows: 10 min at 95°C followed by 40 cycles of 15 s at 94°C, 15 s at 60°C, 15 s at 72°C, and a final extension of 10 min at 72°C.
Transmission electron microscopy.
Autophagosomes were further confirmed using transmission electron microscopy (TEM). Briefly, cultured cells (≥106 cells), after various treatments, were washed with ice-cold PBS and fixed with glutaraldehyde (2.5% in 0.1 M phosphate buffer, pH 7.4) for 2 h. Cells were then postfixed in 1% osmium tetroxide and embedded in Epon 812. Sections (50 nm) were stained with uranyl acetate/lead citrate (Sigma-Aldrich, St. Louis, MO) and examined under a Hitachi H-600 TEM at the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Tianjin, China).
Western blot analysis.
Protein samples from cells with various treatments were prepared in ice-cold radioimmunoprecipitation assay lysis buffer. Equal amounts of protein were separated by SDS-PAGE. Proteins were then transferred onto nitrocellulose membrane (Immobilon-P; Millipore, Bedford, MA), followed by blocking with 5% nonfat milk for 2 h. The membranes were then incubated with primary antibodies for 2 h and horseradish peroxidase-conjugated secondary antibodies for 1.5 h. The blot signals were visualized using the West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, IL). The images were detected and analyzed by a Molecular Imager Chemidoc XRS System (Bio-Rad Laboratories, Hercules, CA). Signals from the Western blots were quantified by densitometry. Data were normalized to a loading control (e.g., β-actin) and expressed as the fold increase of the control value.
Statistical analysis.
All experiments were repeated at least three times. The data are presented as means ± SE. Statistical differences were analyzed using the unpaired Student t-test between two groups. Differences were evaluated using the Student's t-test or one-way ANOVA with Instat3.0 (Graphpad Software, La Jolla, CA). Differences with a P value <0.05 were considered statistically significant.
RESULTS
Upregulation of Shh expression correlates with enhanced expression of LC3-II in neointimal lesions of mouse common carotid arteries.
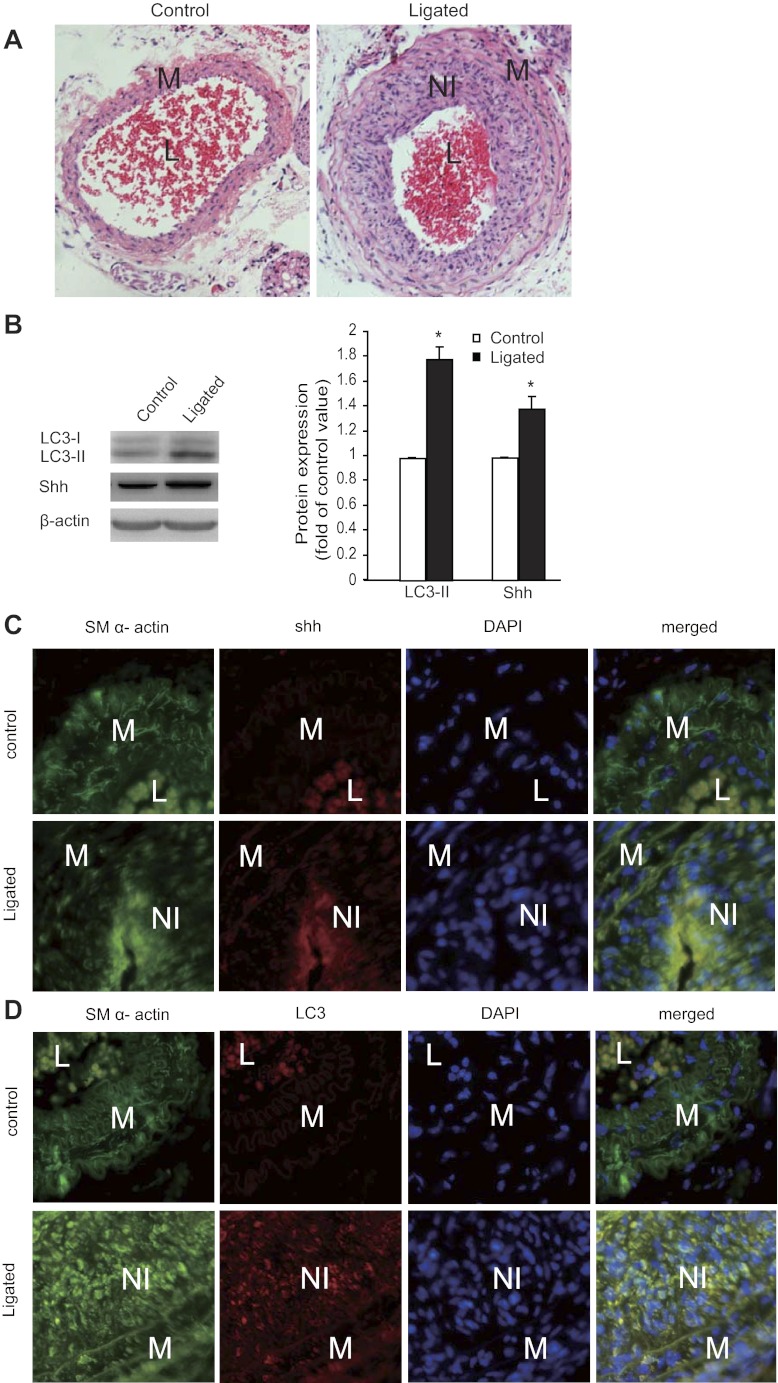
Our results showed that significant neointimal lesions were formed after 14-day ligation (Fig. 1A). Our results revealed a marked increase in Shh expression (Fig. 1B), consistent with a previous report (21). In addition, the conversion of LC3-I to LC3-II in ligated tissues was significantly increased compared with normal control tissues, as detected using Western blot (Fig. 1B), suggesting the presence of enhanced autophagy. To further localize the expression of Shh and LC3 in SMCs, we performed double staining for SM α-actin and Shh or LC3 in tissue sections. Our results (Fig. 1C) showed significant immunostaining signals for Shh in the neointimal lesion area, which overlapped with SM α-actin staining. Our results for LC3 staining (Fig. 1D) showed an increase for LC3 expression in those cells positively stained for SM α-actin. As shown in Fig. 1A, erythrocytes were left within the lumina of control vessels. The positive signals for SM α-actin and Shh within the lumina of these vessels (Fig. 1, C and D) likely resulted from the autofluorescence of hemoglobin (6). The inner surface of the cell membrane of erythrocytes can also nonspecifically take up both fluorescein and fluorescein conjugates (24). Nevertheless, our results promoted us to further investigate Shh-induced autophagy of SMCs in vitro.
Fig. 1.
Expression of sonic hedgehog (Shh) correlates with autophagy in neointimal lesions of mouse common carotid arteries. Neointimal lesions were created by ligation of mouse common carotid arteries as described in the methods. A: representative micrographs of hematoxylin and eosin (H&E) staining of tissue sections from control (left) and ligated (right) arteries. B: representative Western blots (left) and quantitative data normalized to β-actin (right, *P < 0.05, n = 3) showed the expression levels of light chain 3 (LC3)-I (18 kDa), LC3-II (16 kDa), and Shh in the control and neointimal lesions. NI, neointima; L, lumen; M, media. C and D: tissue sections were stained for Shh (red, C), LC3 (red, D), and smooth muscle (SM) α-actin (green, C and D). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue; C and D; original magnification, ×200).
Shh induces autophagy in cultured SMCs.
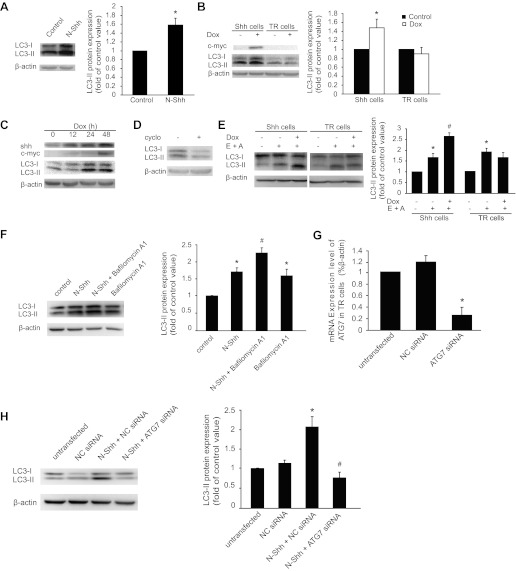
We determined whether exogenous Shh induces autophagy in cultured mouse aortic SMCs. To do so, cultured SMCs were treated with 1 μg/ml N-Shh for 24 h according to previous reports (21, 28), followed by detection of autophagy through examining the conversion of LC3-I to LC3-II. Our results showed that incubation of SMCs with N-Shh resulted in increased LC3-II conversion (Fig. 2A).
Fig. 2.
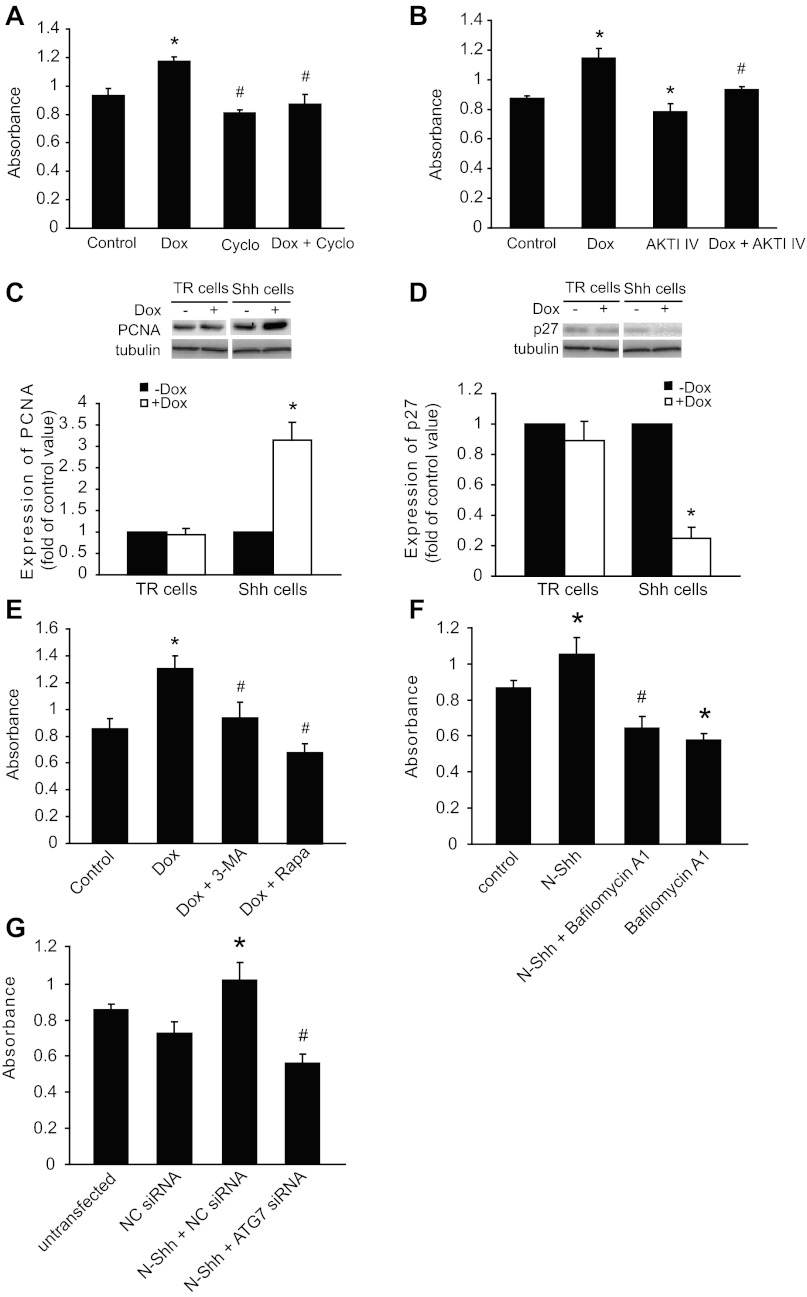
Exogenous Shh treatment or Shh induction stimulates LC3-II expression. A: representative Western blots (left) showing the levels of LC3-I and -II protein in mouse aortic smooth muscle cells (SMCs) treated with or without (control) the recombinant amino-terminal peptide of mouse sonic hedgehog (N-Shh). The quantitative results normalized to β-actin are shown in the bar graph (right, *P < 0.01, n = 3). B: Shh expression was induced by addition of doxycycline (Dox) through the inducible tetracycline-regulated expression (T-Rex) system, as described in methods. Human vascular SMCs harboring Shh-T-REx (Shh cells) or tetracycline-repression (TR) cells were treated with or without Dox for 24 h. Representative Western blots (left) showing the expression of Shh (detected with anti-c-myc antibody) and LC3-I and LC3-II. Densitometry of LC3-II Western blot signals was normalized to β-actin in the bar graph (right, *P < 0.05, n = 3). C: representative Western blots showing a time-dependent effect of LC3-I and LC3-II upregulation upon Shh induction. Shh cells were treated with Dox for 0, 12, 24, and 48 h. D: representative Western blots showing the levels of LC3-I and -II in the presence of Shh in response to cyclopamine (cyclo) treatment for 24 h. E: representative Western blots (left) showing the effects of (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methylbutane ethyl ester (E64d, E) and pepstatin A (A) treatment (24 h) on the levels of LC3-I and -II in Shh and TR cells. The quantitative results normalized to β-actin are shown in the bar graph (right, *P < 0.01 vs. control and #P < 0.05 vs. E64d + pepstatin A treated, n = 3). F: representative Western blots (left) showing the effects of N-Shh and bafilomycin A1 treatment (24 h) on the levels of LC3-I and -II in TR cells. The quantitative results normalized to β-actin are shown in the bar graph (right, *P < 0.01 vs. control and #P < 0.05 vs. N-Shh treated, n = 3). G: real-time quantitative PCR showing the mRNA levels of autophagy-related gene 7 (ATG7) in TR cells transfected with negative-control (NC) small-interfering RNA (siRNA) or ATG7 siRNA for 48 h (*P < 0.05 vs. NC siRNA control, n = 3). H: representative Western blots (left) showing the effects of N-Shh treatment (24 h) on the levels of LC3-I and -II in TR cells transfected with NC siRNA or ATG7 siRNA for 48 h. The quantitative results normalized to β-actin are shown in the bar graph (right, *P < 0.01 vs. NC siRNA control, #P < 0.05 vs. N-Shh + NC siRNA control, n = 3).
To extend our findings, we examined the effects of Shh on autophagy in human aortic SMCs. Given that intracellular Shh undergoes various modifications before secretion, we used the T-Rex-inducible system to overexpress mouse Shh in human aortic SMCs, as previously described (41). As shown in Fig. 2B, addition of Dox resulted in the expression of Shh, as detected using anti-c-myc antibody. The induction of Shh was accompanied by an increase in LC3-II conversion compared with TR control cells (Fig. 2B). Time-dependent induction of Shh in human aortic SMCs resulted in a corresponding increase in LC3-II (Fig. 2C). We also observed an increase in LC3-I protein (Fig. 2C) but not its mRNA (data not shown).
To eliminate the possibility that any protein expression through the T-REx system would induce autophagy, we examined LC3-II conversion in response to overexpression of myocardin, as we described in our previous studies (41). Our Western blot results showed that overexpression of myocardin even decreased LC3-II conversion (data not shown). In addition, Shh-induced LC3-II conversion was also inhibited by cyclopamine, an Smo inhibitor (Fig. 2D).
It is well known that LC3-II itself will be degraded in autolysosomes during autophagy (26). Therefore, treatment with lysosomal protease inhibitors will enhance the abundance of LC3-II if Shh indeed increases autophagic flux. As expected, Shh-induced LC3-II conversion at 24 h was further increased by pretreatment with E64d and pepstatin A, inhibitors of proteolytic degradation (Fig. 2E). Additionally, treatment with either E64d or pepstatin A alone increased LC3-II, thus indicating that lysosomal degradation of LC3-II was present in SMC autophagy (data not shown). Moreover, increased LC3-II levels due to treatment with N-Shh for 24 h was further enhanced by pretreatment with bafilomycin A1, an inhibitor of autophagosome-lysosome fusion and subsequent acidification (17, 48) (Fig. 2F).
We wished to determine the role of p62 in Shh-induced autophagy in SMCs. The expression of p62 was detected in the positive control HeLa cells, but no p62 was detected in the SMCs we tested, such as mouse aortic SMCs, Shh cells, or TR cells (data not shown). We then determined whether ATG7 plays a role in Shh-induced autophagy. To do so, we transfected cells with ATG7 siRNA and then measured Shh-induced LC3-II conversion. As shown in Fig. 2G, transfection with ATG7 siRNA, but not NC siRNA, downregulated the mRNA levels of ATG7 and significantly inhibited LC3-II conversion induced by N-Shh (Fig. 2H).
Shh activates the mammalian target of rapamycin pathway.
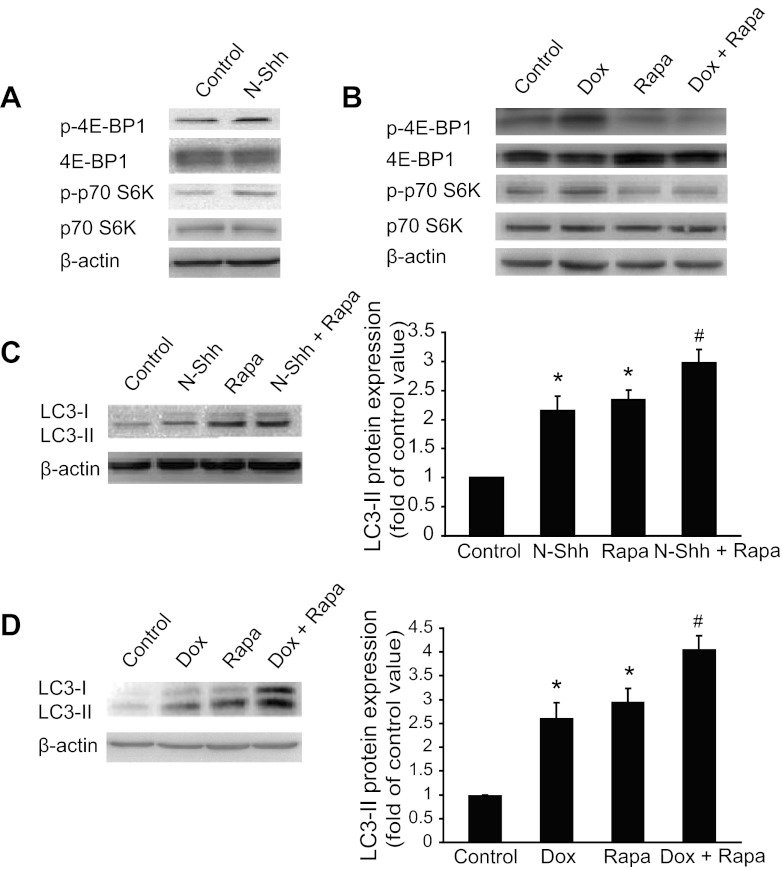
Inhibition of the mammalian target of rapamycin (mTOR) pathway is known to induce autophagy (16, 32). We examined the effects of exogenous Shh on phosphorylation of 4E-BP1 (p-4E-BP1) and S6K (p-S6K), two downstream targets of mTOR. Unexpectedly, our results showed that addition of Shh increased the levels of p-4E-BP1 and p-S6K in cultured mouse aortic SMCs (Fig. 3A). In human aortic SMCs, induction of Shh by Dox also increased p-4E-BP1 and p-S6K levels, which were inhibited by pretreatment with rapamycin, an mTOR inhibitor (Fig. 3B). Addition of Dox did not increase p-4E-BP1 and p-S6K levels in TR control cells (data not shown). Treatment with rapamycin increased LC3-II conversion (Fig. 3C). Notably, Shh more potently stimulated LC3-II conversion in the presence of rapamycin than either N-Shh or rapamycin alone (Fig. 3C). In human SMCs harboring the Shh-T-Rex-inducible system, addition of Dox and rapamycin together also more significantly increased LC3-II levels (Fig. 3D).
Fig. 3.
Shh-induced autophagy is independent of the mammalian target of rapamycin (mTOR) pathway. A: Western blot detection of phospho (p)-4E-binding protein 1 (BP1), 4E-BP1, p-p70 S6K, and p70 S6K in mouse aortic SMCs treated with or without (control) N-Shh for 24 h. B: Western blot results of p-4E-BP1, 4E-BP1, p-p70 S6K, and p70 S6K in Shh cells treated with or without Dox and rapamycin (Rapa) for 24 h. C: Western blot detection (left) and quantified data (right) of LC3-I and LC3-II in mouse aortic SMCs treated without or with N-Shh in the presence or absence of rapamycin. *P < 0.01 vs. control and #P < 0.05 vs. Dox treated, n = 3. D: representative Western blot results (left) and summarized data (right) showing the effects of rapamycin on the expression of LC3-I and -II for 24 h in Shh cells. *P < 0.01. vs. control and #P < 0.05 vs. Dox treated, n = 3.
Shh-induced autophagy involves activation of AKT.
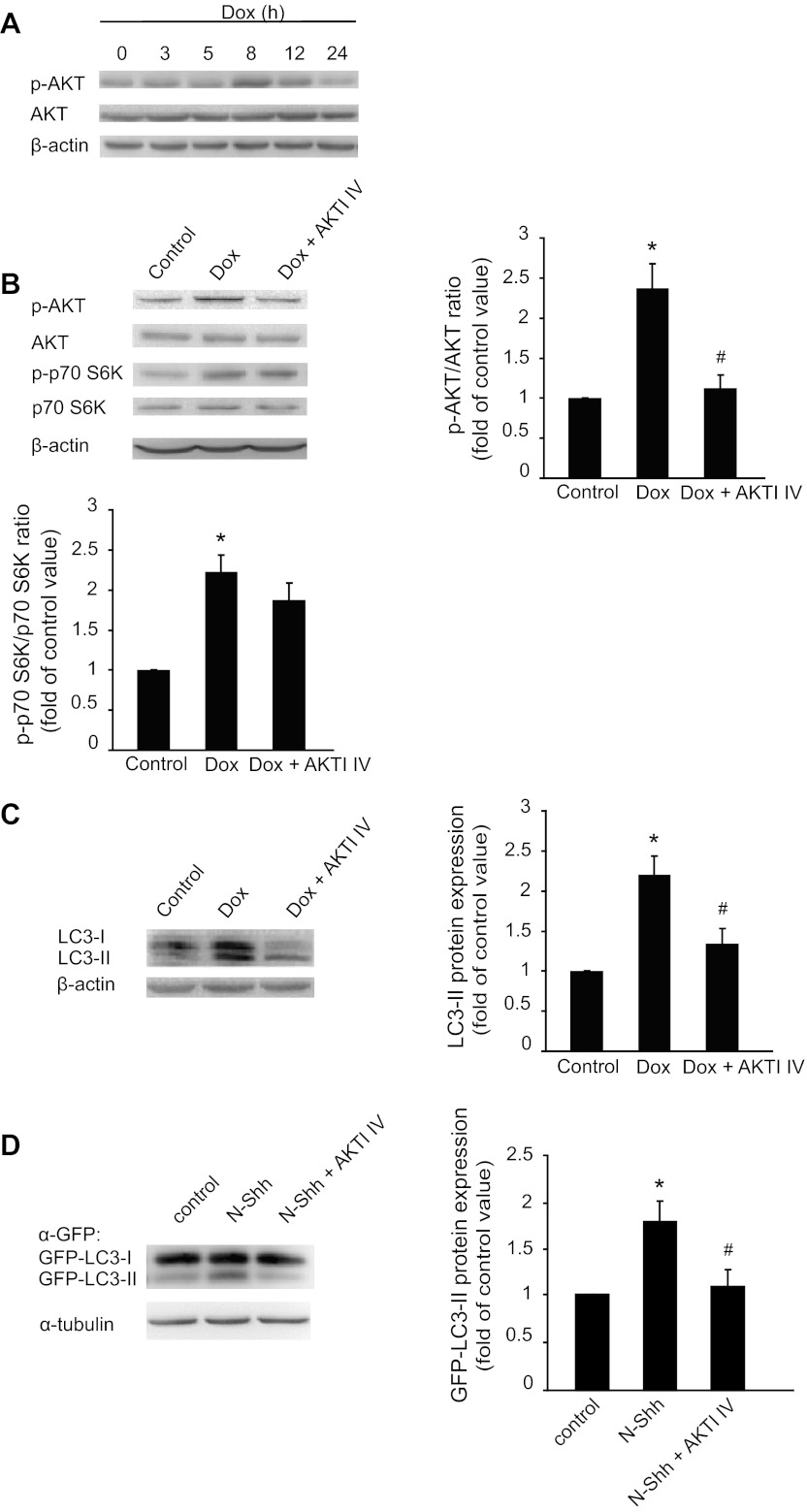
Evidence suggests that other signaling pathways, such as AKT (37), may lead to the autophagic process. We further examined the involvement of AKT in Shh-induced autophagy. Our results showed that addition of N-Shh increased the levels of p-AKT in cultured mouse aortic SMCs at 15 min (data not shown). Dox-induced expression of Shh time-dependently increased phosphorylation of AKT in human aortic SMCs, which reached the maximal level at 8 h and returned to the basal level after 24 h (Fig. 4A). In TR cells, Dox did not increase AKT phosphorylation (data not shown). Pretreatment with AKT inhibitor, AKTI IV, inhibited Shh-induced phosphorylation of AKT at 8 h (Fig. 4B) and LC3-II conversion at 24 h (Fig. 4C). However, AKTI IV did not inhibit Shh-induced phosphorylation of p70 S6K at 8 h (Fig. 4B). After characterizing the LC3-II conversion of endogenous LC3, we examined whether Shh treatment induced the LC3-II conversion of overexpressed green fluorescent protein (GFP)-LC3-II in SMCs. As expected, our results showed that addition of N-Shh increased GFP-LC3-II conversion, which was inhibited by AKTI IV (Fig. 4D).
Fig. 4.
Inhibition of protein kinase B (AKT) inhibits Shh-induced LC3-II conversion. A: expression of p-AKT and AKT in Shh cells in the presence or absence of Shh induction (Dox) for different times (3, 5, 8, 12, and 24 h). B: effect of AKT inhibitor IV (AKTI IV, 10 μM) on the level of p-AKT and p-p70 S6K with or without Shh induction (Dox) for 8 h. *P < 0.01 vs. control and #P < 0.05 vs. Dox treated, n = 3. C: effect of AKTI IV on LC3-II conversion in cells with or without Shh induction (Dox). *P < 0.01 vs. control and #P < 0.05 vs. Dox treated, n = 3. D: effect of AKTI IV on green fluorescent protein (GFP)-LC3-II conversion in cells with and without Shh treatment. *P < 0.01 vs. control and #P < 0.05 vs. Shh treated, n = 3.
Inhibition of AKT prevents Shh-induced formation of autophagosomes.
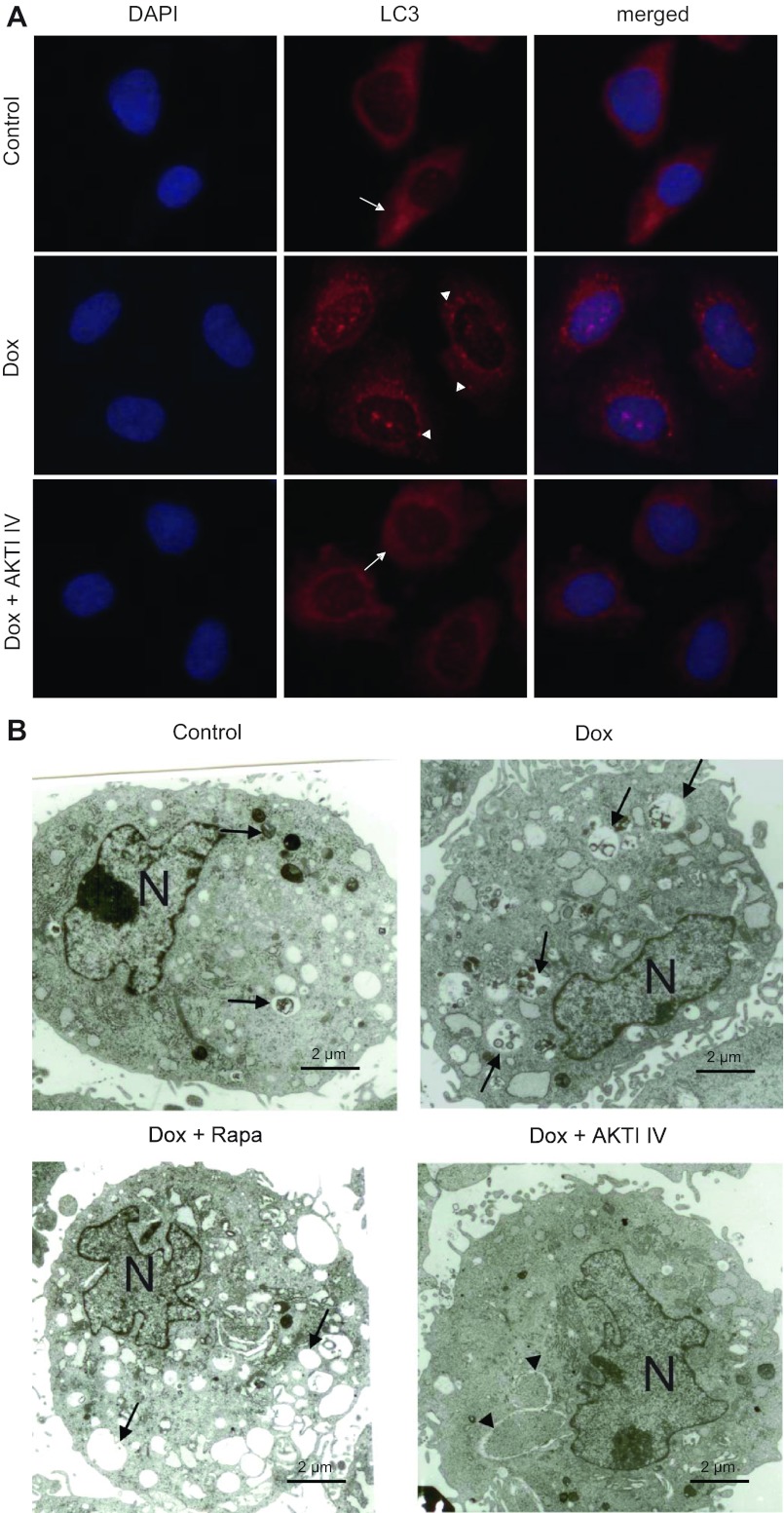
To further establish the role of AKT in Shh-induced autophagy, we examined whether inhibition of AKT by AKTI IV inhibits Shh-induced autophagosome formation. As shown in Fig. 5A, induction of Shh by treatment with Dox caused formation of LC3 puncta resulting from diffuse cytoplasmic forms of LC3, indicating the activation of autophagy. This process was inhibited by pretreatment with AKTI IV. Furthermore, TEM showed that induction of Shh resulted in mild to extensive autophagic vacuolization and phagocytotic/pinocytic body formation, revealing different stages of autophagosome formation, such as early double-membrane vacuoles, myelin figures, and single-membrane vacuoles (Fig. 5B). Pretreatment with AKTI IV prevented the formation of autophagosomes (Fig. 5B).
Fig. 5.
AKTI IV inhibits Shh-induced punctate LC3 and autophagosomes. A: representative images of fluorescence microscopy (original magnification, ×400) of SMCs harboring the Shh-T-REx system treated with or without AKTI IV in the presence or absence of Dox induction for 24 h. Cells were stained with LC3 antibody and Alexa Fluor 568-conjugated goat anti-rabbit IgG (red). The nuclei were counterstained with DAPI (blue). B: transmission electron microscopy images (original magnification, ×15,000) of Shh cells in the presence or absence of Shh induction (Dox) treated with or without rapamycin or AKTI IV for 24 h. N, nucleus. Arrows, autophagic vacuolization with content or myelin figures and single-membrane vacuoles; arrowheads, early double-membrane vacuoles.
Shh stimulates proliferation of vascular SMCs.
We then examined whether induction of Shh by Dox stimulates SMC proliferation. As shown in Fig. 6A, Wst-1 results showed that induction of Shh by Dox significantly increased cell proliferation, which was completely inhibited by the Shh receptor inhibitor cyclopamine. Cell proliferation induced by Shh was also inhibited by pretreatment with AKTI IV (Fig. 6B). Our further studies revealed that induction of Shh by Dox in human aortic SMCs resulted in upregulation of PCNA (Fig. 6C) and downregulation of p27 (Fig. 6D). Moreover, treatment with either 3-MA, an autophagy inhibitor, or rapamycin inhibited proliferation of SMCs with Shh overexpression (Fig. 6E). Because 3-MA was also reported to inhibit the phosphoinositide 3-kinase (PI3K) pathway (15, 45), we further examined the effects of an autophagy inhibitor, bafilomycin A1, and ATG7 siRNA on Shh-induced SMC proliferation. Wst-1 results showed that treatment with bafilomycin A1 (Fig. 6F) or ATG7 siRNA (Fig. 6G) significantly inhibited N-Shh-induced proliferation of SMCs.
Fig. 6.
Proliferative effects of Shh induction on SMCs is inhibited by AKTI IV, cyclopamine, and 3-methyladenine (3-MA). A: Shh cells with or without Dox induction were treated with cyclopamine (Cyclo) for 24 h. Dimethyl sulfoxide (DMSO, control) was the vehicle control for cyclopamine. Cell numbers were determined using the water-soluble tetrazolium salt (Wst-1) assay (*P < 0.05 vs. control and #P < 0.05 vs. Dox treated, n = 4). B: Shh cells with or without Dox induction were incubated with AKTI IV for 24 h, followed by determination of cell numbers (*P < 0.05 vs. control and #P < 0.05 vs. Dox treated, n = 4). C and D: Western blots showing the effects of Shh induction on the expression of proliferating cell nuclear antigen (PCNA, C) and p27 (D) (top: representative Western blots). The expression of PCNA (C) or p27 (D) was normalized to α-tubulin (*P < 0.05 vs. without Dox treated, n = 5). E: Shh cells with or without Dox induction were treated with 3-MA or rapamycin for 24 h, followed by determination of cell numbers (*P < 0.05 vs. control and #P < 0.05 vs. Dox treated, n = 4). F: TR cells were treated with N-Shh, bafilomycin A1, or N-Shh and bafilomycin A1 for 24 h, followed by determination of cell numbers (*P < 0.05 vs. control and #P < 0.05 vs. N-Shh treated, n = 4). G: TR cells were transfected with NC siRNA or ATG7 siRNA in the presence of N-Shh for 24 h, followed by determination of cell numbers (*P < 0.05 vs. NC siRNA control and #P < 0.05 vs. N-Shh + NC siRNA, n = 4).
3-MA, AKTI IV, and cyclopamine inhibit neointima formation.
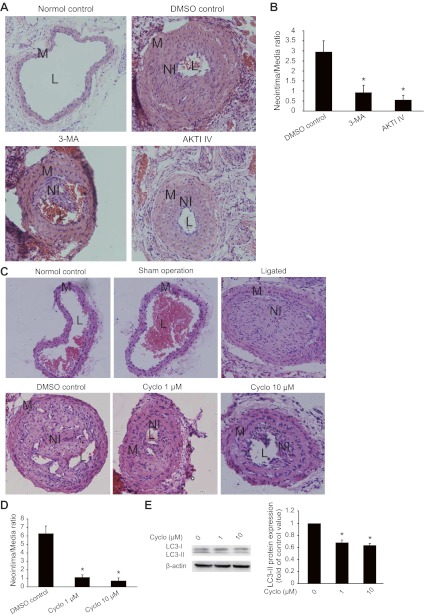
To validate the role of Shh in SMC proliferation in vivo, we examined the effects of 3-MA, AKTI IV, and cyclopamine on neointimal lesion formation. In the neointimal model established by ligation of common carotid arteries, 3-MA, AKTI IV, and cyclopamine were applied to the ligated arteries through mixture with the PLF-127 gel for 3 wk. As shown in Fig. 7, A and B, treatment with 3-MA or AKTI IV significantly inhibited neointima formation. In addition, treatment with cyclopamine inhibited not only neointima formation (Fig. 7, C and D) but also LC3-II conversion (Fig. 7E).
Fig. 7.
Neointima formation of mouse carotid arteries is inhibited by 3-MA, AKTI IV, and cyclopamine. Neointimal lesions were generated through ligation of mouse common carotid arteries as described in methods. 3-MA, AKTI IV, and cyclopamine were applied through PLF-127 gel. DMSO was the vehicle control for cyclopamine. A and B: representative micrographs of H&E staining (A) showing the effects of 3-MA and AKTI IV on the development of neointima (original magnification, ×200). The sizes of the lesions (B) were calculated as the ratio of the neointima area to that of the media (*P < 0.01 vs. DMSO control; n = 3). C and D: representative micrographs of H&E staining showing the effects of cyclopamine on the development of neointima (C; original magnification, ×200). The sizes of neointimal lesions were calculated as the ratio of the area of the neointima to that of the media (D; *P < 0.01 vs. DMSO control, n = 3). E: Western blot results (left) showing the effects of cyclopamine on LC3-II conversion in neointimal lesions. The intensity of bands was quantified and normalized to the DMSO control (right, *P < 0.01, n = 3).
DISCUSSION
Our study has for the first time demonstrated that exogenous or overexpressed Shh induces an increase in LC3-II and autophagosome formation in mouse and human aortic SMCs, indicating activation of autophagy. Given the developmental role of Shh, our study has also implicated a potential role for autophagy in the development of the vascular system.
It was reported that inhibition of mTOR by rapamycin activates autophagy in various cell types (16, 32), including vascular SMCs (14). Although Shh activates mTOR, Shh induced a potent autophagy response, which is independent of the mTOR pathway and not inhibited by mTOR activation. This speculation was also supported by our observation that rapamycin, which alone induced autophagy of SMCs, further increased Shh-induced autophagy. More recently, neuregulin (NRG) was reported to promote autophagy of prostate cancer cells and also activated phosphorylation of S6K and AKT. Interestingly, treatment with NRG and rapamycin resulted in higher levels of LC3-II than from treatment with each alone in human prostate carcinoma cell line LNCaP cells (36). Therefore, our study has revealed a novel model for autophagy with dual regulatory mechanisms. More specifically, our results also showed that addition of rapamycin indeed increased LC3-II levels, suggesting activation of autophagy in cultured SMCs. This finding indicates a role of mTOR in the regulation of autophagy in SMCs, echoing the findings from other studies (16). Taken together, our results strongly suggest that rapamycin and Shh induce autophagy through different signaling mechanisms. Therefore, a stimulatory effect of Shh on LC3-II and autophagosome formation suggests that Shh stimulates another pathway to activate autophagy, independent of the mTOR pathway. The mTOR-independent mechanism for autophagy was previously described in several other cell types (35), such as glucosamine-induced autophagy in HeLa cells (38) and trehalose-induced autophagy in COS-7 cells (34). In addition activation of the mTOR pathway and autophagy were also found to coexist in the rat submandibular gland during ligation-induced atrophy (39).
Our results have demonstrated a role for AKT in Shh-induced autophagy in vascular SMCs. Notably, Shh was reported to stimulate the AKT pathway in several other cells, such as 10T1/2 mesodermal cells (33), chick muscle cells, C2 mouse myogenic cells (9), and bone marrow-derived endothelial progenitor cells (10), further supporting our conclusion that Shh activates AKT in vascular SMCs. The role of AKT in Shh-induced autophagy was suggested by our finding that the inhibition of AKT by a specific AKT inhibitor, AKTI IV, reduces Shh-increased LC3-II and autophagosome formation. Several studies have suggested that Shh may activate PI3K (10, 33, 46) and/or the RAS-mitogen/extracellular signal-regulated kinase (MEK) pathway (40). Our unpublished data have shown that Shh did stimulate the mitogen-activated protein kinase (MAPK) pathway, but inhibition of MEK by PD-98059 failed to reduce Shh-induced autophagy. In addition, we observed that LY-294002, an inhibitor of PI3K, inhibited Shh-induced autophagy (unpublished observation), suggesting a potential role of PI3K in Shh-induced activation of AKT. However, more detailed studies are required to define how Shh activates AKT in SMCs.
We have not revealed the signaling mechanism downstream of AKT. As discussed above, it is unlikely to be through the mTOR pathway, because activation of the mTOR pathway inhibits autophagy and AKTI IV did not inhibit Shh-induced phosphorylation of S6K. In addition, the MAPK pathway was previously reported to play a role in autophagy in vascular SMCs in response to osteopontin treatment (50). Although our unpublished data did show that Shh stimulates phosphorylation of p44/42 MAPK, inhibition of the MEK by PD-98059 did not significantly affect Shh-induced autophagy. Therefore, more research is warranted to define the exact mechanisms underlying Shh-induced autophagy.
Shh-induced cell proliferation has been reported for many cell types, including pancreatic cancer cells (29), muscle satellite cells (9), and SMCs (21, 28). To reveal a potential role of autophagy, we examined the effects of inhibition of autophagy in SMC proliferation. Our results showed that pretreatment with autophagy inhibitors such as 3-MA and bafilomycin A1 prevented Shh-induced proliferation of vascular SMCs. Additionally, inhibition of autophagy with ATG7 siRNA significantly attenuated Shh-induced SMC proliferation, strongly suggesting a role for autophagy in proliferation of vascular SMCs. Notably, treatment with 3-MA, AKTI IV, or cyclopamine significantly reduced neointima formation in mouse carotid arteries in response to ligation. Reduction of LC3-II formation by cyclopamine in neointimal lesions has provided further support to our conclusion.
In summary, our results have revealed that either overexpression of Shh or exogenous Shh induces autophagy of vascular SMCs through activation of AKT, suggesting a potential role of Shh in vascular pathophysiology. Additionally, our data showing a role of Shh-induced autophagy in SMC proliferation implicate a critical role for autophagy in the development of blood vessels and the pathogenesis of proliferative vascular disease. Therefore, Shh may become a therapeutic target for vascular disease.
GRANTS
This research was supported by the Canadian Institutes of Health Research (to X.-L. Zheng), the Natural Science Foundation of China (30772568 to Y. Gui), and the National Basic Research Program of China (2011CB944003). X.-L. Zheng is a recipient of a Senior Scholarship Award from Alberta Innovates-Health Solutions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.L., J.L., Y.L., L.C., L.-j.X., D.L., and Y.W. performed experiments; H.L., J.L., Y.L., L.C., L.-j.X., D.L., Y.W., Z.X., Y.G., and X.-L.Z. analyzed data; H.L., J.L., Y.L., L.C., L.-j.X., D.L., Y.W., Z.X., Y.G., and X.-L.Z. interpreted results of experiments; H.L. prepared figures; H.L., P.S., Y.G., and X.-L.Z. drafted manuscript; H.L., P.S., Z.X., Y.G., and X.-L.Z. edited and revised manuscript; H.L., J.L., Y.L., P.S., L.C., L.-j.X., D.L., Y.W., Z.X., Y.G., and X.-L.Z. approved final version of manuscript; P.S., Z.X., Y.G., and X.-L.Z. conception and design of research.
REFERENCES
- 1. Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature 466: 68–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bijlsma MF, Peppelenbosch MP, Spek CA. Hedgehog morphogen in cardiovascular disease. Circulation 114: 1985–1991, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bijlsma MF, Spek CA. The Hedgehog morphogen in myocardial ischemia-reperfusion injury. Exp Biol Med (Maywood) 235: 447–454, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development 129: 361–372, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med 14: 308–313, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Casella GT, Bunge MB, Wood PM. Improved immunocytochemical identification of neural, endothelial, and inflammatory cell types in paraffin-embedded injured adult rat spinal cord. J Neurosci Methods 139: 1–11, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12, Suppl 2: 1509–1518, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cohen MM., Jr The hedgehog signaling network. Am J Med Genetics 123A: 5–28, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Elia D, Madhala D, Ardon E, Reshef R, Halevy O. Sonic hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1773: 1438–1446, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Fu JR, Liu WL, Zhou JF, Sun HY, Xu HZ, Luo L, Zhang H, Zhou YF. Sonic hedgehog protein promotes bone marrow-derived endothelial progenitor cell proliferation, migration and VEGF production via PI 3-kinase/Akt signaling pathways. Acta Pharmacol Sin 27: 685–693, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Gimenez-Xavier P, Francisco R, Platini F, Perez R, Ambrosio S. LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int J Mol Med 22: 781–785, 2008 [PubMed] [Google Scholar]
- 12. Guertler A, Kraemer A, Roessler U, Hornhardt S, Kulka U, Moertl S, Friedl AA, Illig T, Wichmann E, Gomolka M. The WST survival assay: an easy and reliable method to screen radiation-sensitive individuals. Radiat Prot Dosimetry 143: 487–490, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Heemskerk J, DiNardo S. Drosophila hedgehog acts as a morphogen in cellular patterning. Cell 76: 449–460, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J 410: 525–534, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Ito S, Koshikawa N, Mochizuki S, Takenaga K. 3-Methyladenine suppresses cell migration and invasion of HT1080 fibrosarcoma cells through inhibiting phosphoinositide 3-kinases independently of autophagy inhibition. Int J Oncol 31: 261–268, 2007 [PubMed] [Google Scholar]
- 16. Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 584: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Kawasaki M, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol 300: H2261–H2271, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Klionsky D, Abdalla F, Abeliovich H. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8: 445–544, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolesova H, Roelink H, Grim M. Sonic hedgehog is required for the assembly and remodeling of branchial arch blood vessels. Dev Dyn 237: 1923–1934, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17: 2238–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Li F, Duman-Scheel M, Yang D, Du W, Zhang J, Zhao C, Qin L, Xin S. Sonic hedgehog signaling induces vascular smooth muscle cell proliferation via induction of the G1 cyclin-retinoblastoma axis. Arterioscler Thromb Vasc Biol 30: 1787–1794, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science 304: 1755–1759, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem 73: 891–923, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Massey BW, Klein SJ, Reilly EB. Enhancement of immunofluorescent staining of erythrocytes by saponin hemolysis. Transfusion 5: 434–439, 1965 [DOI] [PubMed] [Google Scholar]
- 25. McLeland CB, Rodriguez J, Stern ST. Autophagy monitoring assay: qualitative analysis of MAP LC3-I to II conversion by immunoblot. Methods Mol Biol 697: 199–206, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542–545, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrow D, Sweeney C, Birney YA, Guha S, Collins N, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am J Physiol Cell Physiol 292: C488–C496, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA 104: 5103–5108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin Chim Acta 293: 157–166, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation 108: 479–485, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36: 585–595, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA 103: 4505–4510, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282: 5641–5652, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16: 46–56, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Schmukler E, Shai B, Ehrlich M, Pinkas-Kramarski R. Neuregulin promotes incomplete autophagy of prostate cancer cells that is independent of mTOR pathway inhibition. PLoS One 7: e36828, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy 3: 635–637, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Shintani T, Yamazaki F, Katoh T, Umekawa M, Matahira Y, Hori S, Kakizuka A, Totani K, Yamamoto K, Ashida H. Glucosamine induces autophagy via an mTOR-independent pathway. Biochem Biophys Res Commun 391: 1775–1779, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Silver N, Proctor G, Arno M, Carpenter GH. Activation of mTOR coincides with autophagy during ligation-induced atrophy in the rat submandibular gland (Abstract). Cell Death Dis 1: e14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA 104: 5895–5900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang RH, Zheng XL, Callis TE, Stansfield WE, He J, Baldwin AS, Wang DZ, Selzman CH. Myocardin inhibits cellular proliferation by inhibiting NF-kappaB(p65)-dependent cell cycle progression. Proc Natl Acad Sci USA 105: 3362–3367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol 445: 77–88, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol 445: 29–76, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Wang G, Zhang Z, Xu Z, Yin H, Bai L, Ma Z, Decoster MA, Qian G, Wu G. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim Biophys Acta 1803: 1359–1367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285: 10850–10861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xia YP, Dai RL, Li YN, Mao L, Xue YM, He QW, Huang M, Huang Y, Mei YW, Hu B. The protective effect of sonic hedgehog is mediated by the phosphoinositide 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience 209: 1–11, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Xu K, Yang Y, Yan M, Zhan J, Fu X, Zheng X. Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res 51: 2581–2590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23: 33–42, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Yin H, Jiang Y, Li H, Li J, Gui Y, Zheng XL. Proteasomal degradation of myocardin is required for its transcriptional activity in vascular smooth muscle cells. J Cell Physiol 226: 1897–1906, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Zheng YH, Tian C, Meng Y, Qin YW, Du YH, Du J, Li HH. Osteopontin stimulates autophagy via integrin/CD44 and p38 MAPK signaling pathways in vascular smooth muscle cells. J Cell Physiol 227: 127–135, 2012 [DOI] [PubMed] [Google Scholar]