Abstract
The gut microbiota is essential for the maintenance of intestinal immune homeostasis and is responsible for breaking down dietary fiber into short-chain fatty acids (SCFAs). Butyrate, the most abundant bioactive SCFA in the gut, is a histone deacetylase inhibitor (HDACi), a class of drug that has potent immunomodulatory properties. This characteristic of butyrate, along with our previous discovery that conventional dendritic cells (DCs) are required for the development of experimental colitis, led us to speculate that butyrate may modulate DC function to regulate gut mucosal homeostasis. We found that butyrate, in addition to suppressing LPS-induced bone marrow-derived DC maturation and inhibiting DC IL-12 production, significantly induced IL-23 expression. The upregulation of mRNA subunit IL-23p19 at the pretranslational level was consistent with the role of HDACi on the epigenetic modification of gene expression. Furthermore, the mechanism of IL-23p19 upregulation was independent of Stat3 and ZBP89. Coculture of splenocytes with LPS-stimulated DCs pretreated with or without butyrate was performed and showed a significant induction of IL-17 and IL-10. We demonstrated further the effect of butyrate in vivo using dextran sulfate sodium (DSS)-induced colitis and found that the addition of butyrate in the drinking water of mice worsened DSS-colitis. This is in contrast to the daily intraperitoneal butyrate injection of DSS-treated mice, which mildly improved disease severity. Our study highlights a novel effect of butyrate in upregulating IL-23 production of activated DCs and demonstrates a difference in the host response to the oral vs. systemic route of butyrate administration.
Keywords: short-chain fatty acids, immunoregulation, mucosal immunology, inflammatory bowel disease basic research
the human gut microbiota is a highly complex and abundant biomass that is critical for the maintenance of intestinal immune homeostasis. Mice lacking luminal bacteria (i.e., mice raised in a germ-free environment) are highly susceptible to opportunistic pathogens (9) and have delayed gut mucosal leukocyte development (24). Perturbations of the normal human gut microbiota are associated with many inflammatory conditions including atopic dermatitis, eczema, food allergy, inflammatory bowel disease (IBD), pouchitis, and vaginitis (15). Reductions in probiotics such as bifidobacteria and lactobacilli have been reported in patients with pouchitis (18). The exact mechanism of how the human microbiota affect intestinal immune homeostasis is unknown.
Gut luminal bacteria have an important symbiotic role with their host through their ability to break down otherwise indigestible carbohydrates into short-chain fatty acids (SCFAs), such as acetate, proprionate, and butyrate, which can then be metabolized by the intestinal mucosa. Of these SCFAs, butyrate is the most biologically active (17). Butyrate is a trophic factor for the small intestinal epithelia and an important fuel for colonocytes, which use butyrate to maintain growth when nutrients are diverted by a proximal stoma (1, 13). In contrast to these growth-promoting effects in normal cells, butyrate has also been shown to induce cell cycle arrest and apoptosis in colon carcinoma cells (8). Butyrate has also been shown to inhibit NF-κB activity in the human colon cancer HT-29 cell line (10). However, the effect of butyrate on the mucosal immune system is not well defined.
It is known that butyrate inhibits the production of IL-12 by dendritic cells (DCs) and prevents the development of Th1 cells (19, 23). Because production of IL-23 by DCs is important for the development of Th17 cells, the primary objective of this study was to gain an understanding of the effect of butyrate on the IL-23/IL-17 axis through its effect on DCs. Using murine bone marrow-derived DCs (BM-DCs), we showed that butyrate inhibited DC maturation and downregulated the production of IL-12 but increased the production of IL-23. An increase in IL-23p19 mRNA expression suggested that the IL-23 increase is a pretranslational event. We further showed that, when cocultured with splenocytes in a mixed leukocyte reaction, butyrate-treated lipopolysaccharide (LPS)-stimulated DCs induced T cells to produce more IL-17 compared with the amount produced by phosphate-buffered saline (PBS)-treated LPS-stimulated DCs. In vivo, mice treated with oral butyrate developed more severe colitis with dextran sulfate sodium (DSS) treatment, whereas systemic butyrate treatment resulted in less severe colitis. Therefore, our results indicate that butyrate induces DC IL-23 production and modulates chemically induced colitis. Our study describes a novel mechanism of butyrate in gut immune homeostasis.
MATERIALS AND METHODS
Mice.
Specific pathogen-free female wild-type C57BL/6 and ZBP-89 delta-Nter mice (14) aged 8–10 wk were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in the Unit for Laboratory Animal Medicine at the University of Michigan Health System. Experiments were conducted on mice aged 10–14 wk. All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Media, cytokines, and reagents.
Complete medium (CM) consisted of RPMI-1640 medium with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The recombinant cytokines, mouse granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/ml), and mouse IL-4 (10 ng/ml) (R&D Systems, Minneapolis, MN), were diluted in CM. Sodium butyrate was purchased from Sigma-Aldrich (St. Louis, MO). DSS (average molecular weight, 36–50 kDa) was purchased from MP Biomedicals (Aurora, OH).
Generation of BM-DCs.
Erythrocyte-depleted murine bone marrow cells were cultured in CM with GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) at 1 × 106 cells/ml (11). On day 6, nonadherent BM-DCs were harvested by vigorous pipetting and enriched by gradient centrifugation using OptiPrep density gradient medium (Sigma-Aldrich). The low-density interface containing BM-DCs was collected by gentle aspiration. The recovered BM-DCs were washed twice with RPMI-1640 and cultured in CM with GM-CSF (10 ng/ml).
Flow cytometric analysis.
BM-DCs were washed twice with ice-cold PBS containing 0.5% BSA and sodium azide. After a 30-min incubation with Fc block (1 μg/100 μl; BD Biosciences PharMingen, San Diego, CA), the cells were incubated with FITC, PE, or PerCP-conjugated antibodies or isotype control antibodies (1:100 dilution). These cells were washed, resuspended in ice-cold 2% paraformaldehyde, and analyzed using a Coulter XL Flow Cytometer (Hileah, FL). For intracellular cytokine staining, cells were permeabilized with Perm/Fix Solution (BD Biosciences PharMingen) before staining. The percentage of CD4+CD25+Foxp3+ Treg was measured using the Mouse Regulatory T Cell Staining Kit (eBioscience, San Diego, CA). Dot plots and histograms were obtained using FlowJo version 4.3 (Tree Star, Ashland, OR).
Animal studies.
To induce chronic colitis, C57BL/6 mice received three 5-day cycles of 3% DSS in drinking water (20). Each 5-day cycle was alternated with a 6-day cycle of untreated drinking water. These mice also received daily intraperitoneal injections of butyrate (1 g/kg dissolved in PBS) or PBS as a control, throughout the entire course. Weight measurements and Hemoccult (Beckman Coulter, Fullerton, CA) fecal occult blood tests were performed daily. The mice were killed on day 34. In a separate experiment, butyrate was added to drinking water (final concentration: 8 gm/200 ml or 4% butyrate) in addition to 3% DSS. Weight measurements and Hemoccult (Beckman Coulter, Fullerton, CA) fecal occult blood tests were performed daily. Lamina propria single cells were isolated (4) from the large intestine of mice and analyzed by fluorescence-activated cell sorting (FACS) analysis.
Chromatin immunoprecipitation assay.
The chromatin immunoprecipitation ChIP kit (Millipore, Temecula, CA) was used to perform assays on mouse DCs. Briefly, 1 × 106 DCs were treated with 1% (vol/vol) formaldehyde for 10 min at room temperature to cross-link DNA with the associated proteins. Cross-linking was stopped with glycine. Cells were harvested, lysed, and then subjected to sonication. Lysates were precleared with protein A agarose/salmon sperm DNA (50% slurry), and 1% aliquots were collected to use as “input” DNA. The precleared chromatin samples were incubated overnight at 4°C with monoclonal mouse anti-Stat3 antibody (Cell Signaling Technology, Danvers, MA) or normal mouse immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, CA). The antibody-chromatin complex was mixed with protein A agarose/salmon sperm DNA (50% slurry) for 1 h and then centrifuged. The precipitated immune complex was washed, and the cross-linked protein-genomic DNA was eluted with freshly prepared elution buffer (1% sodium dodecyl sulfate and 0.1 M NaHCO3). The cross-linked products were reversed with an overnight incubation at 65°C and then treated with proteinase K for 1 h at 45°C. The DNA released was purified by QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and submitted for PCR analysis. The primers for mouse IL-23p19 were as follows: forward: 5′ TGCAGTCTTCTGTGTTCCTGGAGA 3′; reverse: 5′ TTTAAGGTCCCTGCACTGTAAGGC 3′.
PCR array.
PCR array plates were designed by SuperArray (Frederick, MD) to screen for DC tolerogenic mechanisms (34 genes) (25). BM-DCs were treated with butyrate with or without LPS; PBS-pulsed BM-DCs served as the control. DC mRNA was harvested, and RT-PCR analysis was performed using the SuperArray plate. Quantitative RT-PCR was also performed using the following primers: mouse IL-23p19: forward: 5′ AAGTTCTCTCCTCTTCCCGTCGC 3′, reverse: 5′ TCTTGTGGAGCAGCAGATGTGAG 3′; mouse IL-12p40: forward: 5′ CAATCAGGGCTTCGTAGGTA 3′, reverse: 5′ GGCCCTGGTTTCTTATCAAT 3′; mouse IL-12p35: forward: 5′ GAGGACTTGAAGATGTACCAG 3′, reverse: 5′ TTCTATCTGTGTGAGGAGGGC 3′; mouse IL-6: forward: 5′ CTACCCCAATTTCCAATGCT3′, reverse: 5′ ACCACAGTGAGGAATGTCCA 3′; GAPDH: forward: 5′ TTGAAGGGTGGAGCCAAAAGG 3′, reverse: 5′ TTGCTGACAATCTTGAGTGAGTTG 3′.
Stat3 Western blot analysis.
DCs were collected, washed, and lysed with a solution containing protease and phosphatase inhibitors (Cell Signaling Technology). Protein was then run through SDS-PAGE, transferred to PVDF membranes, and blotted with primary and secondary antibodies (Cell Signaling Technology). Protein detection was performed by chemiluminescence. GAPDH was used as an endogenous housekeeping reference protein.
Statistical analysis.
Statistical significance was determined by unpaired Student's t-test, ANOVA, and survival analysis using commercially available software (PRISM; GraphPad, San Diego, CA). Values of P < 0.05 were considered significant. All data are presented as means ± SE.
RESULTS
Butyrate suppressed LPS-induced DC maturation.
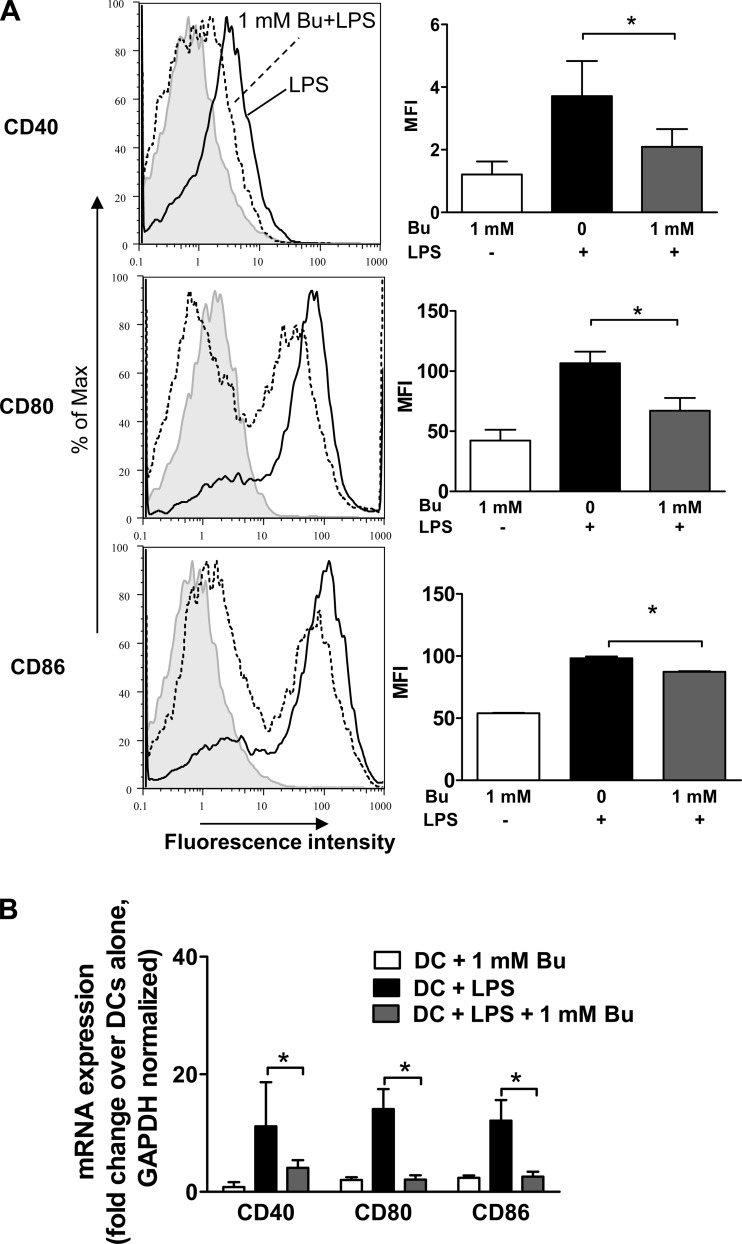
To investigate the effect of the immunomodulatory properties of butyrate at the cellular level, we focused on the effects of butyrate treatment on BM-DCs in vitro. Mature DCs, such as those stimulated by LPS, express CD40, CD80, and CD86. To determine whether butyrate alters the maturation of LPS-induced DCs, we stimulated naïve BM-DCs with LPS and butyrate for 18 h. CD40, CD80, and CD86 expression were measured by FACS analysis. We observed diminished expression of all three DC markers after the administration of butyrate (Fig. 1A). To confirm this observation, we measured the mRNA expression of these markers using quantitative RT-PCR array analysis. The addition of butyrate to LPS led to diminished levels of CD40, CD80, and CD86 mRNA expression compared with the treatment of BM-DCs with LPS alone (Fig. 1B). These data suggest that butyrate treatment either suppresses the ability of LPS to induce a mature phenotype in immature BM-DCs or maintains these cells in a semimatured state.
Fig. 1.
Butyrate (Bu) suppressed lipopolysaccharide (LPS)-induced dendritic cell (DC) maturation. A: surface expression of semimature DCs. C57BL/6 bone marrow-derived DCs (BM-DCs) were treated for 18 h with LPS alone (black line) or LPS (1 μg/ml) and butyrate (1 mM) (dashed line) and analyzed by fluorescence-activated cell sorting (FACS). BM-DCs were stained with phycoerythrin (PE)-conjugated CD40, CD80, or CD86 and compared with isotype controls (gray line). The averages of 3 separate experiments were computed as mean fluorescence intensity (MFI). B: mRNA was collected from BM-DCs treated with LPS alone or with LPS and butyrate for 18 h and analyzed using quantitative RT-PCR array. Data are representative of 3 independent experiments. *P < 0.05.
Butyrate altered LPS-induced DC cytokine production.
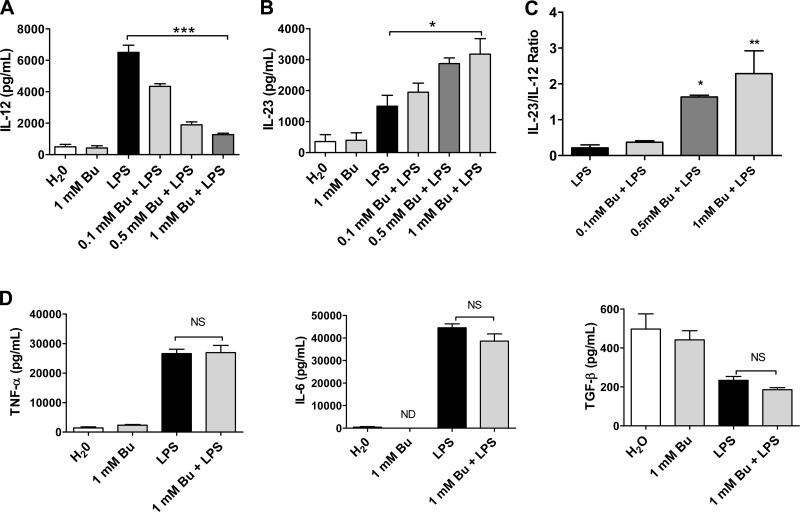
In addition to measuring the expression of maturation markers, we investigated the effects of butyrate treatment on cytokine production by BM-DCs. BM-DCs were treated with LPS plus increasing concentrations of butyrate for 18 h. The mRNA from the BM-DCs was analyzed by PCR array for inflammation-related gene expression. We observed that butyrate treatment, in a dose-dependent manner, inhibited the production of IL-12 compared with baseline LPS treatment, whereas the production IL-23 was significantly increased (Fig. 2, A and B). The ratio of IL-23 to IL-12 production was significantly increased by butyrate (Fig. 2C). There was no significant change in the levels of TNF-α, IL-6, or TGF-β between the LPS and the LPS/butyrate treatments, implying that these cytokines are regulated by different mechanisms (Fig. 2D).
Fig. 2.
Butyrate altered LPS-induced DC cytokine production. A and B: unstimulated BM-DCs (106/ml) were treated with LPS (1 μg/ml) plus increasing concentrations of butyrate in vitro for 18 h. Supernatants were collected, and IL-12 and IL-23 cytokine production was analyzed using ELISA. C: ratio of IL-23 to IL-12. D: cytokine production of helper T cells Th1 and Th2 was analyzed using ELISA. Data are a compilation of 3 independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001). ND, not detected; NS, not significant.
Pretranslational regulation of IL-12/IL-23 dendritic cell expression by butyrate.
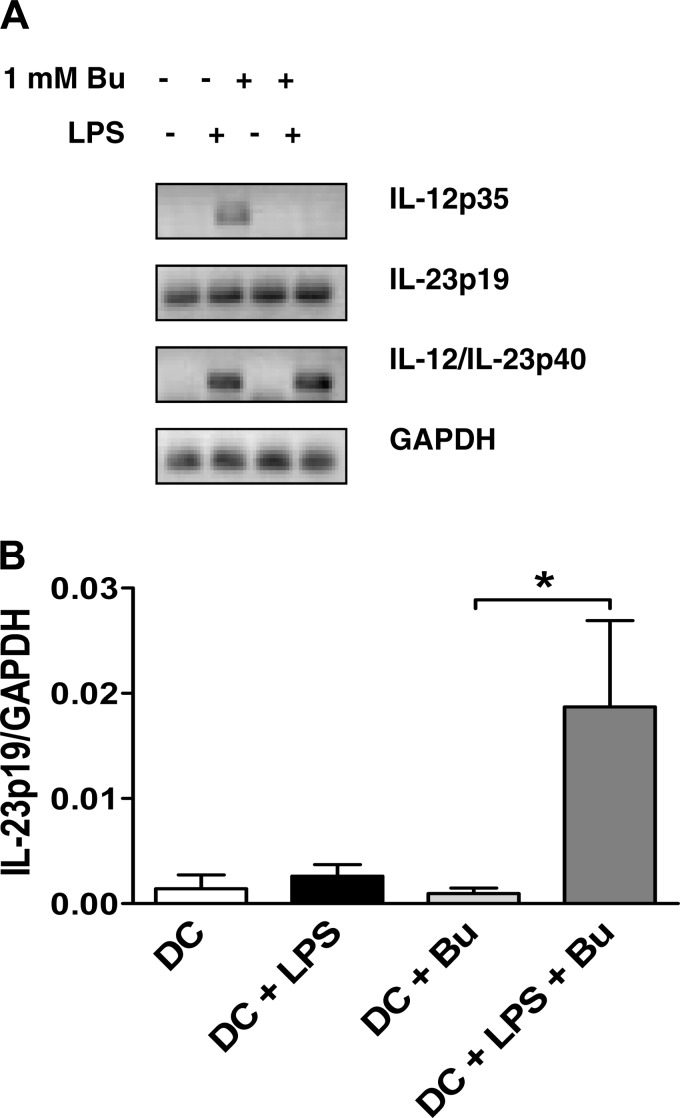
To further elucidate the effect of butyrate on IL-12 and IL-23 production, we analyzed mRNA expression in LPS/butyrate-treated BM-DCs using semi-quantitative RT-PCR. IL-12p35 mRNA expression was increased in LPS-treated cells but not detected in LPS/butyrate-treated cells (Fig. 3A). mRNA expression of the shared subunit, IL-12p40, was not affected. IL-23p19 expression was increased in cells treated with LPS and butyrate compared with LPS alone. These findings were confirmed by quantitative RT-PCR analysis using a custom RT-PCR array plate (Fig. 3B).
Fig. 3.
Pretranslational regulation of IL-12/IL-23 DC expression by butyrate. A: BM-DCs were treated with butyrate (1 mM) and LPS (1 μg/ml) for 18 h, and mRNA expressions of IL-12p35, IL-23p19, IL-12/IL-23p40, and GAPDH were compared with the use of semiquantitative RT-PCR. B: quantitative RT-PCR array was used to quantify IL-23p19 mRNA expression. Data are a compilation of 3 independent experiments. *P < 0.05.
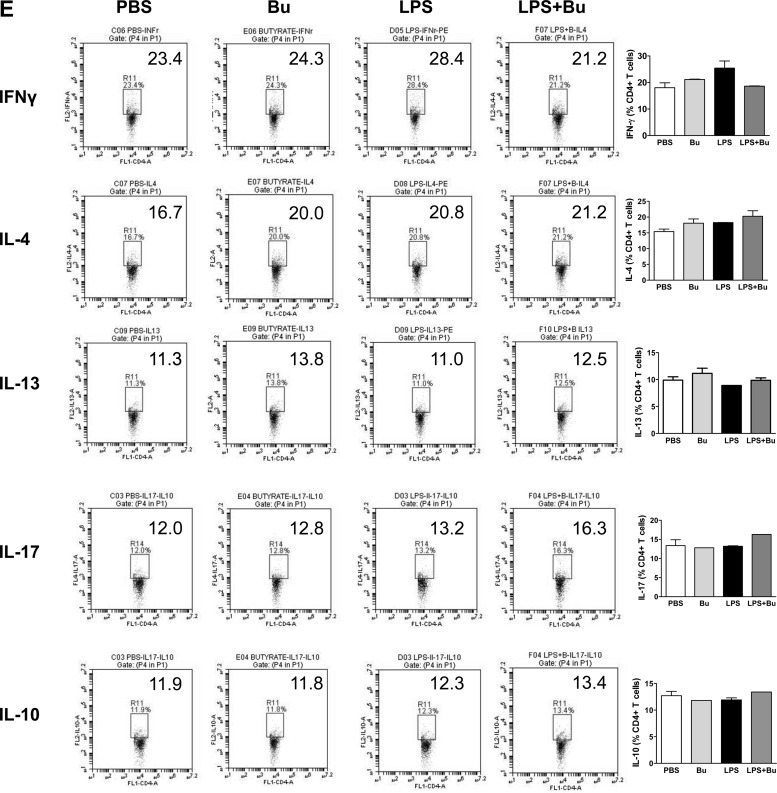
Butyrate-treated LPS-stimulated DCs increased IL-17 and IL-10 production by T cells.
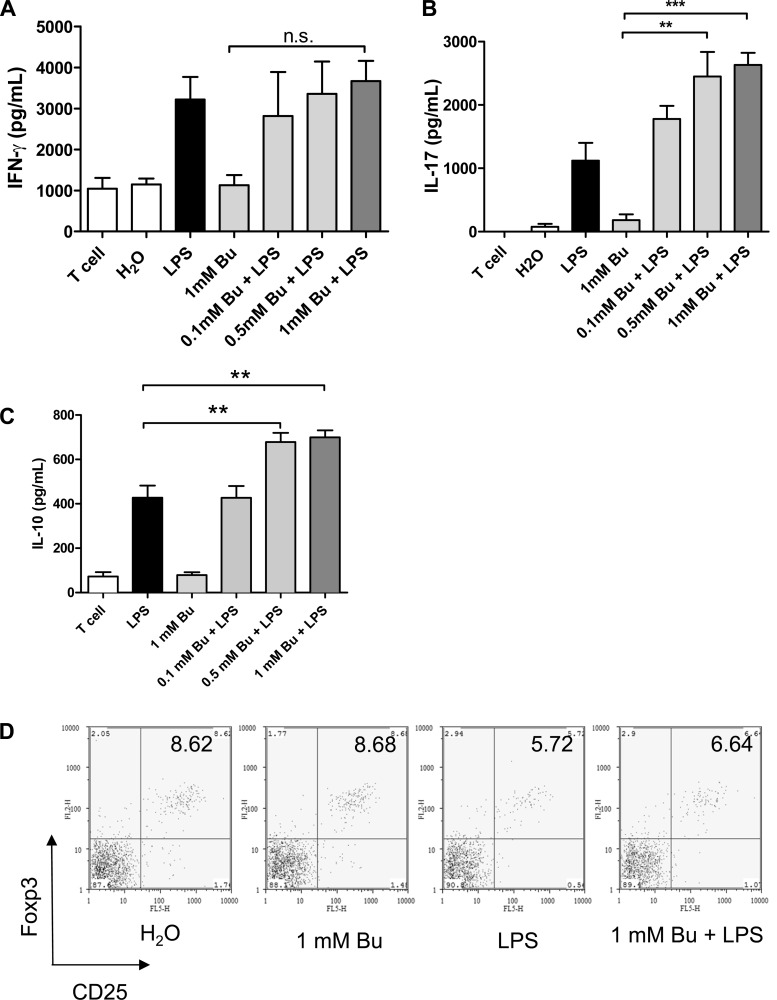
To confirm that the changes in DC function induced by butyrate had physiological significance, we cocultured LPS/butyrate-stimulated BM-DCs with naïve splenocytes. After an 18-h incubation with LPS and butyrate, the BM-DCs were washed and co-cultured in a mixed-splenocyte reaction for 3 days. Analysis of the cellular supernatant and intracellular cytokine expression showed an increase in IL-17 and IL-10 production by cocultured splenocytes in the butyrate-treated group, but not IFN-γ, IL-4, or IL-13 (Fig. 4, A–C, and E). However, unlike the known effect of butyrate on the induction of regulatory T cells (Tregs)(2), butyrate did not enhance DC-mediated Treg induction (Fig. 4D).
Fig. 4.
Butyrate-induced IL-23 upregulation by LPS-stimulated DCs is capable of priming IL-17 and IL-10 production by T cells. A–C: BM-DCs treated with butyrate and LPS for 18 h were washed and then cocultured with naïve splenocytes at a ratio of 1 to 10 for 72 h. The production of IFN-γ, IL-17, and IL-10 by cocultured T cells was analyzed using ELISA. Data are a compilation of 3 independent experiments. D: T cells were fixed and stained with CD4, CD25, and Foxp3 for FACS. CD4+ cells were gated, and the proportions of CD4+CD25+Foxp3+ cells were calculated. Data are representative of 3 independent experiments. (**P < 0.01, ***P < 0.001) NS, not significant. E: production of intracellular cytokines by cocultured T cells was analyzed using FACS. Data shown are representative of 2 separate experiments.
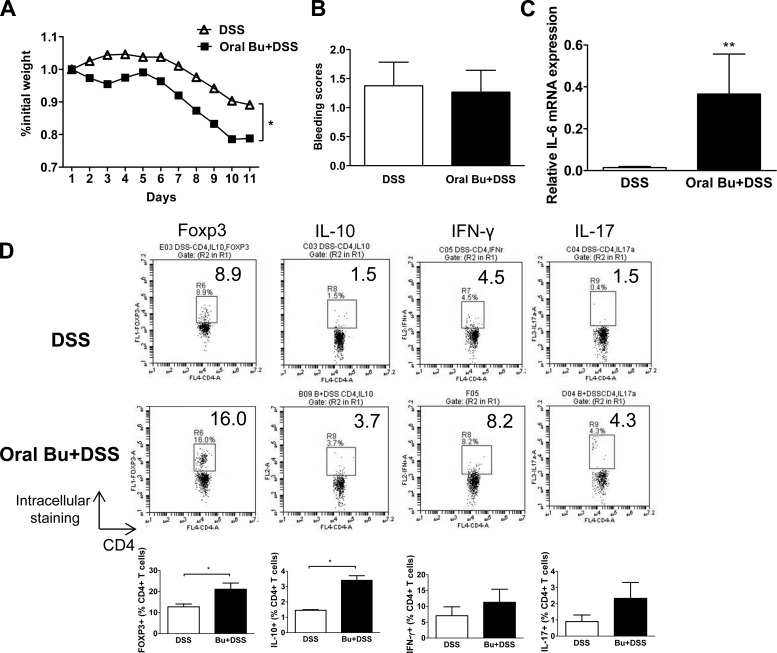
Oral butyrate exacerbated DSS-induced colitis in a murine model.
To assess the physiological effects of butyrate administration in a murine model of colitis, we administered butyrate by adding butyrate to the drinking water of mice while they received 3% DSS treatment. Butyrate-treated mice exhibited increased weight loss after one cycle of DSS treatment but no increase in bleeding scores (Fig. 5, A and C). The mice also appeared morbid and were killed per protocol, and the analysis of the mouse colon showed an increased colonic IL-6 mRNA expression compared with results from DSS treatment alone (Fig. 5B). Intracellular cytokine analysis of lamina propria CD4+ T cells showed increased Foxp3 and IL-10 as well as a trend toward higher IFN-γ and IL-17 (Fig. 5D). These findings indicate that oral delivery of butyrate exacerbates chemically induced colitis.
Fig. 5.
Oral butyrate exacerbated dextran sodium sulfate (DSS)-induced colitis. C57BL/6 mice (n = 5) were given butyrate in drinking water (4% butyrate). These mice also received 3% DSS in drinking water and were analyzed on day 11. A and B: percentage of daily weight change and bleeding scores were tabulated. Bleeding was scored as follows: 0 = no bleeding; 1 = positive Hemoccult blood test (minimal color change to green); 2 = positive Hemoccult blood test (maximal color change to blue); 3 = blood visible in the stool and no clotting on the anus; and 4 = gross bleeding from the anus with clotting. C: colonic tissue IL-6 mRNA was measured using quantitative PCR (*P < 0.05; **P < 0.01). Data normalized to GAPDH expression. D: colonic lamina propria cells were stained to analyze the intracellular cytokine profile of helper T cell by FACS. Dot plots and mean % CD4+ T cell were shown.
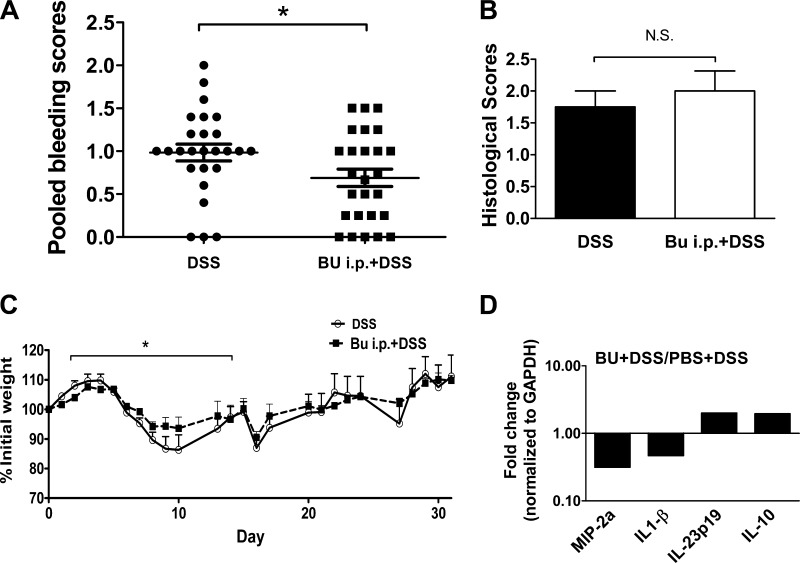
Systemic butyrate attenuated DSS-induced colitis in a murine model.
To determine whether systemic butyrate treatment yields different treatment outcome, mice also received butyrate through intraperitoneal injection. In this experiment, butyrate-treated mice had a lower bleeding score and a trend toward lower weight loss after one cycle of DSS treatment compared with mice receiving daily PBS injections (Fig. 6, A and C). Thus these mice received a total of three cycles of DSS treatment. No significant difference was found in histological scores (Fig. 6B). The effect of the butyrate treatment was most profound during the acute phase of DSS-induced colitis. Measurement of colonic tissue-derived cytokine mRNA expression by RT-PCR array analysis showed differences in the cytokine production between the treatment groups. In the mice treated with systemic butyrate, the upregulation of regulatory pathways (e.g., IL-10) was evident, compared with the downregulation of inflammatory chemokine macrophage inflammatory protein-2α and cytokine IL-1β (Fig. 6D). These findings revealed that systemic treatment of butyrate yields different outcome compared with oral delivery of butyrate.
Fig. 6.
Intraperitoneally injected butyrate attenuated DSS-induced colitis. C57BL/6 mice (n = 5) were given daily intraperitoneal injections of 1 g/kg butyrate or PBS. These mice also received three 5-day cycles of 3% DSS in drinking water, each 5-day cycle alternating with a 6-day cycle of untreated drinking water. A and B: pooled bleeding and histological scores. C: percentage of daily weight change and bleeding scores were tabulated. D: colonic tissue cytokine mRNA expression was analyzed using quantitative PCR normalized to the expression of GAPDH (*P < 0.05).
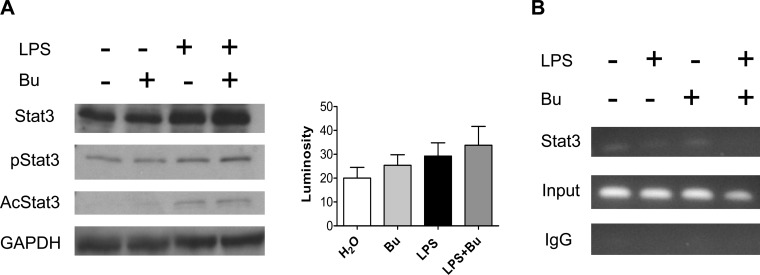
Butyrate-enhanced IL-23p19 expression is independent of ZBP-89 or Stat3.
To further examine the mechanism of butyrate-induced IL-23p19 expression, we first determined whether ZBP-89 protein was involved, as we had recently shown that the binding of butyrate to ZBP-89 mediated the effect of butyrate on intestinal cell growth (3). We found that the effect of butyrate on IL-23 induction was similar using BM-DCs from wild-type or ZBP-89 delta-Nter mutant mice (data not shown). We next turned our attention to Stat3, as Stat3 upregulation in tumor cells was found to induce IL-23 expression (12). We found that butyrate did not increase phosphorylation of Stat3, nor did it increase acetylation of Stat3 (Fig. 7A). Chromatin immunoprecipitation assay further confirms this by showing no increased Stat3 binding to the IL-23p19 promoter, indicating that the regulation of IL-23 expression is mediated by butyrate independent of Stat3 (Fig. 7B).
Fig. 7.
Butyrate-enhanced IL-23p19 expression was independent of Stat3. BM-DCs treated with LPS alone or with LPS and 1 mM butyrate for 18 h were processed for Western blot analysis of total Stat3 (A), phosphorylated Stat3 (pStat3) and acetylated Stat3 (AcStat3), and chromatin immunoprecipitation (ChIP) assay (B). Mean luminosity of pStat3 bands was measured by Photoshop. Treatment with butyrate did not increase Stat3 activation and binding to the IL-23p19 promoter.
DISCUSSION
The human gut microbiota plays an important role in modulating intestinal immune homeostasis. However, the exact mechanism of how gut microbes contribute to the immunomodulation is unknown. Because butyrate is highly produced in the gut by the microbiota, we studied immunomodulatory of butyrate in the gut and showed the effect of butyrate on DCs in altering the balance of IL-12/IL-23 cytokine production after LPS stimulation. We observed that butyrate treatment inhibits IL-12 and enhances IL-23 production by LPS-stimulated DCs through a pretranslational modification independent of ZBP-89 and Stat3. The increase in IL-23 production induced by butyrate treatment resulted in increased IL-17 and IL-10 production. Using a model of chemical-induced colitis, we showed the immunomodulatory property of butyrate in vivo, and the outcome depends on the route of delivery (i.e., oral delivery yields exacerbation and systemic delivery yields amelioration of colitis). These findings indicate a novel mechanism by which butyrate-induced IL-23 production by DCs contributed to the regulation of intestinal immune responses.
One issue that arises in implicating the effect of butyrate on DCs is that of the ability of butyrate to penetrate the tight junction of the intestinal epithelium. It has been shown that the primary transporter of butyrate, monocarboxylate transporter 1, is expressed on intestinal epithelial cells (5), which would allow butyrate to influence lamina propria DCs in the mucosa. Alternatively, mucosal DCs are capable of extending their cytoplasmic projections into the gut lumen (16); therefore, they could be conditioned by the presence of butyrate in the lumen.
Studies using human DCs have also shown that butyrate decreases the expression of costimulatory molecules and IL-12 production by LPS-stimulated DCs (19, 23). We showed that T cells stimulated by butyrate-treated LPS-stimulated DCs produced higher levels of IL-17 and IL-10 than control LPS-stimulated DCs without affecting Foxp3 differentiation. Given that human cancer cells upregulate Stat3, which inhibits IL-12 and increases IL-23 production by tumor-infiltrating DCs leading to increased IL-10 production by T cells (12), we investigated the involvement of Stat3 and found no significant signaling through the Stat3 pathway. Butyrate may regulate IL-23 transcription through its action as a histone deacetylase inhibitor. As IL-23R polymorphism has been associated with IBD (6), an increase in IL-23 expression may impact the risk of developing IBD. In fact, it has been shown that patients with active IBD have higher levels of butyrate in their stool (21).
Another interesting observation from our study is the different response to oral vs. systemic butyrate treatment. Because butyrate has been shown to induce T cell energy (7), systemic delivery may primarily affect the peripheral priming of T cell immunity. Oral delivery of 4% butyrate is likely to have a greater influence on the intestinal mucosal compartment upregulating the IL-23 production of lamina propria DCs, leading to exacerbated immune response to DSS treatment. It is worth noting that Vieira et al. (22) showed that lower oral dose (0.5%) of butyrate attenuated acute DSS colitis. Thus it is important to avoid overdosing butyrate in clinical practice, as it may exacerbate rather than ameliorate colitis. Future studies are needed to compare the efficacy of low- vs. high-dose butyrate oral therapy.
In summary, our findings demonstrate a novel property of butyrate in altering the response of DCs to bacterial antigens. We found that butyrate upregulates the DC IL-23 production through a Stat3-independent pathway. We also showed that the in vivo response to butyrate depends on the route of delivery (i.e., oral route exacerbated colitis, whereas systemic route ameliorated colitis). Our study provides additional insight into the potential therapeutic benefit of systemic butyrate in the management of IBD.
GRANTS
This publication was made possible by the following grants: R21AI087869 (G. Huffnagle, J. Kao) from National Institutes of Allergy and Infectious Diseases, U19AI090871 (G. Huffnagle, J. Kao), from the National Institutes of Allergy and Infectious Diseases, R01 DK087708-01 (J. Kao), from the National Institutes of Digestive, Diabetes, and Kidney Diseases, and the Pilot Feasibility Grant from the Foundation of Digestive Health and Nutrition (J. Kao). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.E.B., J.L.M., G.B.H., and J.Y.K. conception and design of research; B.E.B., M.Z., S.Y.O., T.S.C., T.W.W., J.L., N.A.V., C.-c.C., and J.Y.K. performed experiments; B.E.B., M.Z., S.Y.O., T.S.C., T.W.W., J.L., N.A.V., C.-c.C., and J.Y.K. analyzed data; B.E.B., M.Z., S.Y.O., T.S.C., T.W.W., J.L., N.A.V., C.-c.C., and J.Y.K. interpreted results of experiments; B.E.B., M.Z., and J.Y.K. prepared figures; B.E.B. and J.Y.K. drafted manuscript; B.E.B. and J.Y.K. edited and revised manuscript; B.E.B., M.Z., S.Y.O., T.S.C., T.W.W., J.L., N.A.V., J.L.M., C.-c.C., G.B.H., and J.Y.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Current address for T. Wang: Section of Computational Biomedicine, Department of Medicine and Pulmonary Center, Boston University Medical Center, Boston University, Boston, MA 02118.
REFERENCES
- 1.Agarwal VP, Schimmel EM. Diversion colitis: a nutritional deficiency syndrome? Nutr Rev 47: 257–261, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, Hancock WW. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol 136: 348–363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai L, Kao JY, Law DJ, Merchant JL. Recruitment of ataxia-telangiectasia mutated to the p21(waf1) promoter by ZBP-89 plays a role in mucosal protection. Gastroenterology 131: 841–852, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol 179: 6255–6262, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Daly K, Cuff MA, Fung F, Shirazi-Beechey SP. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem Soc Trans 33: 733–735, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314: 1461–1463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontenelle B, Gilbert KM. n-Butyrate anergized effector CD4(+) T cells independent of regulatory T cell generation or activity. Scand J Immunol 75: 457–463, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Freeman HJ. Effects of differing concentrations of sodium butyrate on 1,2-dimethylhydrazine-induced rat intestinal neoplasia. Gastroenterology 91: 596–602, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Gordon HA, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev 35: 390–429, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118: 724–734, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol 170: 3806–3811, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15: 114–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koruda MJ, Rolandelli RH, Bliss DZ, Hastings J, Rombeau JL, Settle RG. Parenteral nutrition supplemented with short-chain fatty acids: effect on the small-bowel mucosa in normal rats. Am J Clin Nutr 51: 685–689, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Law DJ, Labut EM, Adams RD, Merchant JL. An isoform of ZBP-89 predisposes the colon to colitis. Nucleic Acids Res 34: 1342–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason KL, Huffnagle GB, Noverr MC, Kao JY. Overview of gut immunology. Adv Exp Med Biol 635: 1–14, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2: 361–367, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 21: 351–366, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Ruseler-van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance? Gut 35: 658–664, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saemann MD, Parolini O, Bohmig GA, Kelemen P, Krieger PM, Neumuller J, Knarr K, Kammlander W, Horl WH, Diakos C, Stuhlmeier K, Zlabinger GJ. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol 71: 238–246, 2002 [PubMed] [Google Scholar]
- 20.Sun X, Yamada H, Shibata K, Muta H, Tani K, Podack ER, Iwakura Y, Yoshikai Y. CD30 ligand is a target for a novel biological therapy against colitis associated with Th17 responses. J Immunol 185: 7671–7680, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Treem WR, Ahsan N, Shoup M, Hyams JS. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 18: 159–164, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM, Gomes-Santos AC, Faria AM, Peluzio MC, Cara DC, Alvarez-Leite JI. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 23: 430–436, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Morinobu A, Horiuchi M, Liu J, Kumagai S. Butyrate inhibits functional differentiation of human monocyte-derived dendritic cells. Cell Immunol 253: 54–58, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Williams AM, Probert CS, Stepankova R, Tlaskalova-Hogenova H, Phillips A, Bland PW. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 119: 470–478, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Liu M, Luther J, Kao JY. Helicobacter pylori directs tolerogenic programming of dendritic cells. Gut Microbes 1: 325–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]