Abstract
Vitamin D deficiency affects more that 1 billion people worldwide and is associated with an increased risk of developing a number of inflammatory/autoimmune diseases, including inflammatory bowel disease (IBD). At present, the basis for the impact of vitamin D on IBD and mucosal immune responses is unclear; however, IBD is known to reflect exaggerated immune responses to luminal bacteria, and vitamin D has been shown to play a role in regulating bacteria-host interactions. Therefore, to test the effect of active vitamin D on host responses to enteric bacteria, we gave 1,25(OH)2D3 to mice infected with the bacterial pathogen Citrobacter rodentium, an extracellular microbe that causes acute colitis characterized by a strong Th1/Th17 immune response. 1,25(OH)2D3 treatment of infected mice led to increased pathogen burdens and exaggerated tissue pathology. In association with their increased susceptibility, 1,25(OH)2D3-treated mice showed substantially reduced numbers of Th17 T cells within their infected colons, whereas only modest differences were noted in Th1 and Treg numbers. In accordance with the impaired Th17 responses, 1,25(OH)2D3-treated mice showed defects in their production of the antimicrobial peptide REG3γ. Taken together, these studies show that 1,25(OH)2D3 suppresses Th17 T-cell responses in vivo and impairs mucosal host defense against an enteric bacterial pathogen.
Keywords: inflammatory bowel disease, dextran sodium sulfate, crohn's disease, Citrobacter rodentium
the active form of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3 or calcitriol], is a hormone that plays a critical role in many different cellular processes, including cell proliferation, apoptosis, and immune modulation (30). In fact, epidemiological studies have implicated vitamin D insufficiency in the pathogenesis of T-cell mediated, inflammation-driven diseases, including the inflammatory bowel diseases (IBD), Crohn's disease (CD), and ulcerative colitis (27). At present, it is unclear exactly how vitamin D levels impact the development of IBD; however, research has shown that vitamin D deficiency is associated with higher disease activity and longer disease duration in IBD patients (6, 36, 45, 53). Furthermore, CD patients supplemented with vitamin D3 had lower relapse rates compared with placebo-treated patients (35), while a pilot study in CD patients demonstrated a short-term beneficial effect of a 1,25(OH)2D3 analog on disease activity over a 1-yr course (50). Animal models of colitis, including the dextran sodium sulfate (DSS) model, demonstrate that vitamin D deficiency exacerbates disease, while 1,25(OH)2D3 supplementation ameliorates colitis (8, 14, 21, 69), indicating that lack of vitamin D plays a role (albeit poorly defined) in the pathogenesis of IBD. Although the protective mechanisms are unclear, 1,25(OH)2D3 and its analogs have been demonstrated in vitro to inhibit cell proliferation, induce apoptosis, and suppress inflammatory mediators, including TNF-α, IFN-γ, IL-6, and IL-12 in peripheral blood mononuclear cells from patients with IBD (2, 3, 43, 47, 59). Hence, vitamin D therapy may be a novel treatment option for patients with IBD; however, further research is required to understand the underlying mechanisms and potential limitations of treatment with the active compound.1
IBD is thought to result from inappropriate immune responses to commensal bacteria found within the gastrointestinal (GI) tract, resulting from a complex interplay among genetic, immunological, and environmental factors. In general, CD is characterized by increased production of Th1/Th17-related cytokines, and evidence suggests that environmental factors, including childhood infections, diet, and sunlight exposure (i.e., vitamin D) may also affect the pathogenesis of CD (40). Although the basis for the beneficial actions of vitamin D during colitis is unclear, studies have suggested that 1,25(OH)2D3 signaling through the vitamin D receptor (VDR) can alter inflammatory responses in the host through a number of mechanisms, including suppressing Toll-like receptor (TLR) expression (55), blocking NF-κB signaling (65), targeting MAPK phosphatase-1 (66), modulating dendritic cell behavior (20), and/or skewing T-cell responses toward a regulatory phenotype (33, 39). In vitro, 1,25(OH)2D3 acts directly on T cells to inhibit proliferation and production of inflammatory cytokines, including IL-2, IFN-γ, TNF-α, and IL-17 (7). As such, 1,25(OH)2D3 can be considered a potent immunosuppressive agent, and we, therefore, wondered whether the immunosuppressive actions of 1,25(OH)2D3 treatment could potentially impair host defenses against enteric microbes, since Th1/Th17 responses are critical for the clearance of many bacterial infections (5).
Pathogenic strains of Escherichia coli, including enterohemorrhagic E. coli and enteropathogenic E. coli (EPEC) are important causes of infectious diarrhea, with EPEC contributing to as many as 1 million infant deaths per year in developing nations (44). Mucosa-associated E. coli has been observed in greater numbers in patients with IBD compared with healthy controls, and these microbes have been shown to play a role in driving inflammation in the intestine (reviewed in Ref. 51). Since pathogenic strains of E. coli do not colonize mice, researchers rely on the related but mouse-specific attaching and effacing bacterial pathogen Citrobacter rodentium. Following infection, C. rodentium intimately attaches to epithelial cells lining the cecum and colon, resulting in barrier disruption, crypt hyperplasia, loss of goblet cells, mucosal infiltration with immune cells, and a strong Th1/Th17 response (18). The effect of vitamin D levels on host responses to C. rodentium infection have not yet been addressed. To test this, we treated mice undergoing either DSS or C. rodentium-induced colitis with 1,25(OH)2D3 or with vehicle. Interestingly, while 1,25(OH)2D3-treated mice were strongly protected against chemically induced colitis, they were significantly more susceptible to bacteria-induced colitis, and suffered widespread mucosal ulceration and increased pathogen burdens. Through assessment of the host immune response, we found that 1,25(OH)2D3 treatment led to a selective suppression of Th17 T cells and the associated antimicrobial peptide REG3γ. Thus, these studies report, for the first time, that 1,25(OH)2D3 treatment suppresses mucosal Th17 responses, thereby increasing host susceptibility to an enteric bacterial pathogen (9a).
MATERIALS AND METHODS
Mice.
Six- to eight-week-old male C57BL/6 mice were obtained from the Centre for Disease Modeling at the University of British Columbia. Mice were maintained in sterilized, filter-topped cages, handled in tissue culture hoods, and fed autoclaved food (PicoLab Rodent Diet 20 no. 5053; Lab Diet, Brentwood, MO) and water under specific pathogen-free conditions in the animal facility at the Child and Family Research Institute. Sentinel animals were routinely tested for common pathogens. The protocols used were approved by the University of British Columbia's Animal Care Committee and in direct accordance with guidelines drafted by the Canadian Council on the Use of Laboratory Animals.
1,25(OH)2D3 treatment.
Crystalline 1,25(OH)2D3 (SAFC Pharma, Madison, WI) was dissolved in RNA-grade ethanol at 0.025 mg/ml and stored at −20°C. The 1,25(OH)2D3 stock was diluted in sterile PBS immediately prior to injection. Mice were administered either vehicle (PBS with 0.12% ethanol) or 10 ng 1,25(OH)2D3 (0.5 μg/kg) via intraperitoneal injection daily for up to 11 days. The 1,25(OH)2D3 dose was chosen on the basis of previous in vivo studies with mice (32). For additional dose-response studies, mice were administered 5 ng or 15 ng 1,25(OH)2D3 daily.
Induction of DSS colitis.
For DSS studies, colitis was induced by adding dextran sodium sulfate (36,000–55,000 kDa, no. 160110; MP Biomedicals, Solon, OH) to sterile drinking water at a concentration of 3% (wt/vol). Animals were treated with DSS for 7 days and then allowed to recover by removing the DSS from their drinking water for an additional 2 days (9 days total). Mice were weighed daily and monitored for signs of distress, including stool consistency and rectal bleeding.
Bacterial strains and infection of mice.
Mice were infected by oral gavage with 0.1 ml of an overnight culture of Luria broth (LB) containing ∼2.5 × 108 CFU of wild-type or ΔespF streptomycin-resistant C. rodentium (formerly C. freundii biotype 4280, strain DBS100). Mice were weighed daily and monitored for signs of distress.
FITC-dextran intestinal permeability assay.
The FITC-dextran assay was performed as previously described (23). Briefly, mice were orally gavaged with 150 μl of 80 mg/ml 4 kDa FITC-dextran (Sigma, St. Louis, MO; FD4) in PBS 4 h prior to death. Mice were anesthetized and blood was collected by cardiac puncture, which was added immediately to a final concentration of 3% acid-citrate dextrose (20 mM citric acid, 100 nM sodium citrate, 5 mM dextrose) (Harald Schulze, Shivdasani Laboratory, DFCI). Plasma was collected and fluorescence was quantified using a Wallac Victor fluorimeter (Perkin-Elmer Life Sciences, Boston, MA) at excitation wavelengths of 485 nm, emission wavelengths of 530 nm for 0.1 s.
Tissue collection.
For DSS studies, mice were killed at day 9 after administration. For C. rodentium studies, mice were killed at day 6 or day 10 postinfection. Mice were anesthetized with halothane and killed by cervical dislocation; tissues were then collected for further analysis. The large intestine was resected and divided into four sections, i.e., cecum, proximal colon, midcolon, and distal colon for further analysis. Tissues were immediately placed in 10% neutral buffered formalin (Fisher) (48 h, 4°C) for histological studies, or placed in RNAlater (Qiagen, Gaithersburg, MD) and stored at −80°C for subsequent RNA extraction. For certain experiments, the colon was opened longitudinally, stool was gently removed, and the tissue was rolled (Swiss roll) from distal to proximal end, fixed with a needle, and placed in 10% neutral buffered formalin (Fisher) (48 h, 4°C) for histological processing.
Serum calcium.
Mice were anesthetized with halothane, and blood was collected by cardiac puncture. Blood was allowed to clot naturally at room temperature, and cells were removed by centrifugation. Plasma was collected and stored at −80°C until analysis with the calcium colorimetric assay kit (BioVision Research, Mountain View, CA; cat. no. K380–250). This assay uses the chromogenic complex, which forms between calcium ions and O-cresolphthalein. Samples were quantified at OD 575 nm using a Wallac Victor fluorimeter (Perkin-Elmer Life Sciences, Boston, MA) and compared with the calcium standard provided with the kit.
C. rodentium enumeration.
For enumeration of C. rodentium, tissues were prepared as previously described (4). Briefly, whole tissues were collected, weighed, and homogenized in a MixerMill 301 bead miller (Retche). Tissue homogenates were serially diluted in PBS and plated onto Luria-Bertani agar plates containing 100 mg/ml strep, incubated overnight at 37°C, and C. rodentium colonies were enumerated the following day, normalizing them to the tissue or stool weight (per gram). For fecal bacterial burden analysis, stool pellets were collected from live mice at various time points postinfection and processed, as described above.
Immunofluorescence staining.
Immunofluorescence staining of colonic tissues for Ki67 and Tir was performed as previously described (4, 23). Briefly, paraffin-embedded sections were deparaffinized and then rehydrated, followed by antigen retrieval using 0.1 M citric acid monohydrate (Sigma) with 0.05% Tween 20 (pH 6.0) and steam for 45 min. Slides were blocked in PBS with 2% normal goat serum, 1% BSA, 0.1% Triton X-100 and 0.05% Tween 20. Primary antibodies used were rabbit antisera generated against Ki67 (1:200; Abcam) or rat antisera generated against C. rodentium specific Tir (1:5K; gift from W. Deng). This was followed by secondary Alexa Fluor 568-conjugated goat anti-rabbit or anti-rat IgG antibodies (Molecular Probes, Eugene, OR) and Prolong Gold antifade reagent containing 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL; Roche, Toronto, Ontario, Canada) was performed as described by the manufacturer (Roche Diagnostics, Mannheim, Germany). Sections were viewed at 350 and 594 nm on a Zeiss AxioImager microscope. Images were obtained using a Zeiss AxioImager microscope equipped with an AxioCam HRm camera operating through AxioVision software (version 4.4).
Histopathological scoring.
To assess tissue pathology, paraffin-embedded colonic tissue sections (5 μm) were stained with hematoxylin and eosin and were examined by two blinded observers. For DSS colitis, tissue sections were assessed for inflammatory cell infiltration (0 = none; 1 = mild; 2 = moderate; 3 = severe) and overall tissue damage (0 = none; 1 = up to 50% loss of crypts; 2 = more than 50% loss of crypts; 3 = total disruption of crypts and loss of epithelial cells), as previously described (42). The maximum score that could be obtained with this system is 6 points. For C. rodentium, tissue sections were assessed for submucosal edema (0 = no change; 1 = mild; 2 = moderate; 3 = profound), epithelial hyperplasia (scored based on percentage above the height of the control where 0 = no change; 1 = 1–50%; 2 = 51–100%; 3 = >100%), epithelial integrity (0 = no change; 1 = <10 epithelial cells shedding per lesion; 2 = 11–20 epithelial cells shedding per lesion; 3 = epithelial ulceration; 4 = epithelial ulceration with severe crypt destruction) and neutrophil and mononuclear cell infiltration (0 = none; 1 = mild; 2 = moderate; 3 = severe), as previously described (4). The maximum score that could be obtained with this system is 13 points.
RNA extraction and quantitative RT-PCR.
Colon tissues were collected and stored in RNAlater (Qiagen) at −80°C, and total RNA was extracted using the Qiagen RNeasy kit, as previously described (4). Total RNA was quantified using a NanoDrop spectrophotometer (ND1000). RNA was reverse-transcribed using a Qiagen Omniscript RT kit (Qiagen), according to manufacturer's instructions. Quantitative PCR was carried out using a Bio-Rad Miniopticon or Opticon2, as previously described (4). Melting point analysis confirmed the specificity for each of the PCR reactions. Quantitation was performed using GeneEx Macro OM 3.0 software. The primer sequences and reaction conditions for β-actin, TNF-α, iNOS, IFN-γ, IL-17A, IL-17F, IL-22, IL-23, and RegIIIγ have previously been described (4).
Lamina propria mononuclear cell isolation.
Lamina propria mononuclear cells (LPMC) were isolated as previously described (28). Briefly, colons and ceca were collected, washed, and placed in a shaking incubator at 37°C in RPMI 1640 containing 10% heat-inactivated FCS and 5 mM EDTA. Tissues were then cut into 1-mm pieces followed by digestion with 0.2 mg/ml type VIII collagenase (Sigma Aldrich, Oakville, ON, Canada). Samples were then layered on a Percoll gradient (Amersham Biosciences, Uppsala, Sweden), and the LPMC collected from the 40–75% Percoll interface were used in subsequent assays.
Antibody staining and flow cytometry.
Surface Ab staining was performed at 4°C in PBS/2% FCS with 0.1% sodium azide (FACS buffer) with fluorescently labeled CD4, CD25, and FoxP3 Ab (BD Pharma and eBiosciences, San Diego, CA). For intracellular cytokine staining, 0.5 × 106 cells were resuspended in culture medium (RPMI 1640 containing 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 5 μM b-mercaptoethanol) and stimulated with 50 ng/ml phorbol myristate acetate (Calbiochem, Mississauga, ON, Canada) and 1 μg/ml ionomycin (Sigma Aldrich) for 6 h, with the addition of 10 μg/ml brefeldin A (Sigma Aldrich) after 1 h. Cells were subsequently fixed with 4% formaldehyde in FACS buffer (fixation buffer) for 10 min and incubated with fluorescently labeled IFN-γ and IL-17 Abs (BD Biosciences, San Jose, CA) in FACS buffer containing 1% saponin (permeabilization buffer). Unbound Abs were washed away using the permeabilization buffer, and cells were resuspended in FACS buffer. Samples were read on a FACSCanto (BD Biosciences) and analyzed using FlowJo software, version 8.7.
Statistical analysis.
Statistical significance was calculated by using either a two-tailed Student's t-test or the Mann-Whitney U-test unless otherwise indicated, with assistance from GraphPad Prism Software, version 4.00 (GraphPad Software, San Diego, CA, www.graphpad.com). A P value of ≤0.05 was considered significant. The results are expressed as the means ± SE.
RESULTS
1,25(OH)2D3 administered intraperitoneally protects mice against acute DSS-induced colitis.
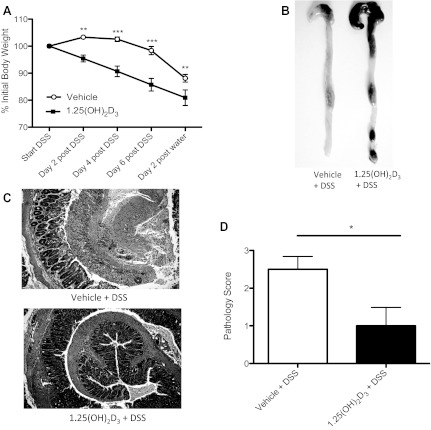
Prior to testing the potential effect of 1,25(OH)2D3 on the host response to C. rodentium infection, we first sought to verify previously published studies showing that 1,25(OH)2D3 and its analogs can ameliorate DSS-induced colitis in C57BL/6 mice. These earlier studies administered 1,25(OH)2D3 either orally or intrarectally at high doses (∼50 ng/day) (21, 43). Although the luminal delivery of 1,25(OH)2D3 was protective, the high doses used, as well as the variation in the results obtained, led us to test the effect of 1,25(OH)2D3 using lower doses and an alternative route of delivery that offers less variation. Mice were administered either vehicle or 10 ng of 1,25(OH)2D3 via intraperitoneal injection 1 day prior to being exposed to 3% DSS in their drinking water and then every day thereafter until death. Mice treated with 1,25(OH)2D3 lost more body weight during DSS challenge compared with vehicle-treated mice (20% vs. 12% of initial body weight, respectively) (Fig. 1A). However, control mice treated daily with 1,25(OH)2D3 lost 10% of their initial body weight compared with vehicle-treated mice (data not shown). This result suggests that the heightened weight loss seen in mice given both DSS and 1,25(OH)2D3 reflects weight loss triggered by 1,25(OH)2D3 rather than increased susceptibility to DSS colitis. 1,25(OH)2D3-induced weight loss may be a sign of hypercalcemia, which is defined as serum calcium levels higher than 10 mg/dl (31). As expected, 1,25(OH)2D3-treated control mice had higher serum calcium levels compared with vehicle-treated control mice [10.89 ± 0.55 mg/dl vs. 7.54 ± 0.66 mg/dl (P < 0.05; n = 8 per group), respectively] following 10 days of treatment. Despite their increased weight loss, 1,25(OH)2D3-treated mice showed no clinical signs of morbidity (i.e., hunched posture, ruffled fur) and appeared active and healthy. There were no observed differences in stool consistency or rectal bleeding between vehicle and 1,25(OH)2D3-treated mice.
Fig. 1.
1,25(OH)2D3-treated mice are protected against DSS-induced damage. A: body weight. Mice were treated with vehicle (○) or 1,25(OH)2D3 (■) intraperitoneally daily throughout 3% DSS challenge. Each data point represents the average body weight pooled from 8 mice and is expressed as the percentage of the initial body weight ± SE. Results are representative of 2 independent experiments. *P<0.05; **P<0.01; ***P<0.001. Mann-Whitney U-test). B: representative digital macroscopic image of the lower GI tract from mice administered 3% DSS. Vehicle-treated mice (left) had shrunken ceca and the lower GI tract was nearly devoid of contents, compared with 1,25(OH)2D3 (right) treated mice, which appeared more normal. C: formalin-fixed, “Swiss-rolled” tissues were stained with hematoxylin and eosin. Focus is on distal colon. Top: vehicle. Bottom: 1,25(OH)2D3. Original magnification = 200×. D: pathology score. n = 6–7 per group. Results are representative of 2 independent experiments. *P < 0.05, Mann-Whitney U-test).
Upon death, the ceca and colons of 1,25(OH)2D3-treated mice appeared healthy, whereas the ceca of vehicle-treated mice were shrunken and their colons appeared inflamed and contained no formed stool (Fig. 1B). Histologically, vehicle-treated mice had severe mucosal damage in the distal colon, characterized by a loss of crypts, severe ulceration, and infiltration of inflammatory cells (Fig. 1C). In contrast, the distal colons of 1,25(OH)2D3-treated mice showed minimal pathology with histopathological scoring, revealing significantly less tissue damage than vehicle-treated mice, scoring 1.0 ± 0.5 vs. 2.5 ± 0.3 (P < 0.05; n = 6–7 per group) (Fig. 1D). Taken together, our assessments confirm that 1,25(OH)2D3 dramatically protects the mammalian GI tract during DSS colitis.
1,25(OH)2D3-treated mice infected with C. rodentium develop macroscopic erosions in the colon and cecum at day 10 postinfection.
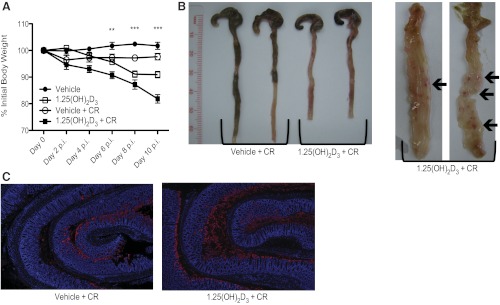
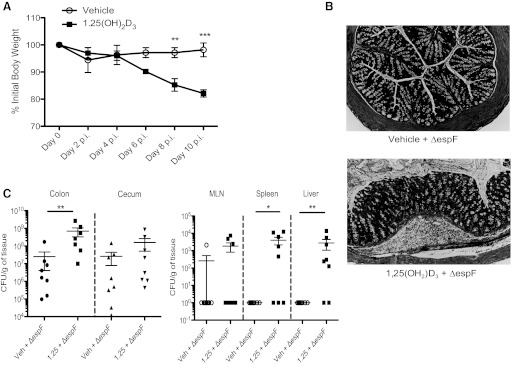
To determine the role of 1,25(OH)2D3 during infection with C. rodentium, mice were administered 10 ng of 1,25(OH)2D3 1 day prior to infection and then every day thereafter for the duration of the study. As shown in Fig. 2A, infected vehicle-treated mice displayed a slight drop in body weight at day 2 postinfection, followed by full recovery by day 10 postinfection. In contrast, infected 1,25(OH)2D3-treated mice steadily lost weight as their infection progressed and by day 10 postinfection, they had lost 18% of their initial body weight. Serum calcium levels were higher in 1,25(OH)2D3-treated mice compared with vehicle-treated mice at day 10 postinfection [10.16 ± 0.40 mg/dl vs. 6.46 ± 0.27 mg/dl SE (P < 0.01; n = 8 per group)]. There was, however, no difference in serum calcium levels between C. rodentium infected vs. noninfected mice. There were no observed differences in stool consistency or rectal bleeding between infected mice given vehicle or 1,25(OH)2D3-treated mice; however, the 1,25(OH)2D3-treated mice had shortened colons, compared with vehicle-treated mice [5.96 ± 0.18 cm vs. 6.67 ± 0.11 cm, respectively (P < 0.01; n = 18 per group)], and 70% of these mice displayed macroscopic mucosal damage and small ulcers in their middle and distal colons at day 10 postinfection, whereas similar pathology was only seen in 20% of vehicle-treated mice [68.75 ± 11.96% vs. 18.75 ± 10.07%, respectively (P < 0.01; n = 16 per group)] (Fig. 2B).
Fig. 2.
1,25(OH)2D3-treated mice develop colonic ulcerations and have greater bacterial burdens at day 10 postinfection. A: body weight. Mice were treated with vehicle or 1,25(OH)2D3 intraperitoneally daily throughout infection. Each data point represents the average body weight pooled from 8–16 mice and is expressed as the percentage of the initial body weight with SE. Results are representative of 2–4 independent experiments. **P < 0.01; ***P < 0.001. Two-way ANOVA. B, right: representative digital image of the lower GI tract from mice at day 10 postinfection. Vehicle-treated mice (left) appeared normal, compared with 1,25(OH)2D3-treated mice (right), which had shortened, inflamed colons nearly devoid of contents. Left: representative digital image of the lower GI tract, opened longitudinally from proximal (top) to distal (bottom) from 1,25(OH)2D3-treated mice at day 10 postinfection. Arrows are pointing to erosion/ulcerated regions in middle and distal colon. C: representative images of formalin-fixed, “Swiss-rolled” colon tissues at day 10 postinfection. Blue denotes DAPI; red dentoes Tir. Original magnification = ×50.
1,25(OH)2D3-treated mice carry increased C. rodentium burdens.
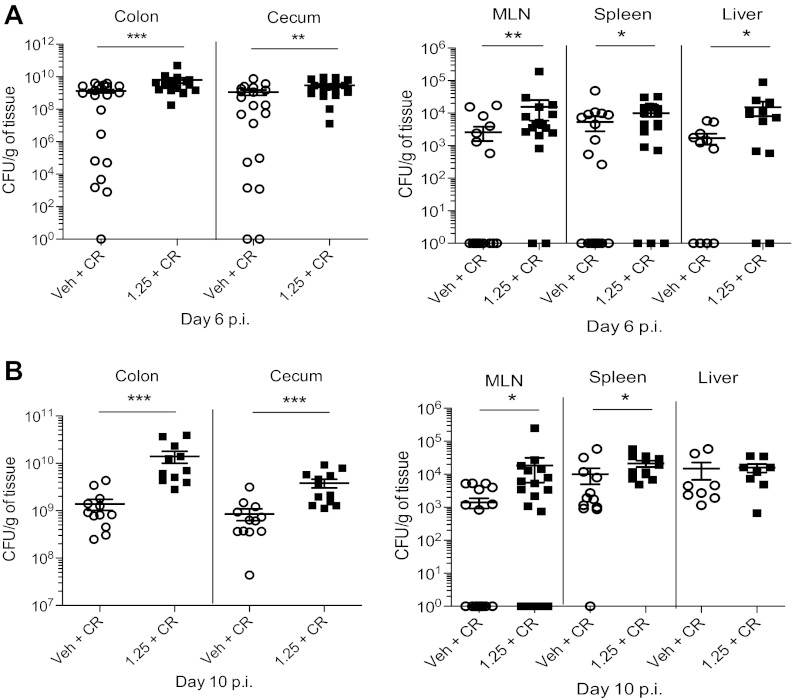
To address whether the exaggerated mucosal damage suffered by the 1,25(OH)2D3-treated mice was associated with increased pathogen burdens, colons were Swiss-rolled and immunostained for C. rodentium-derived translocated intimin receptor (Tir) (16). As expected, C. rodentium were localized to the distal colon in vehicle-treated mice at day 10 postinfection. In contrast, 1,25(OH)2D3-treated mice had strong Tir staining throughout the entire colon, with significant staining found in the midcolon and even reaching the proximal colon (Fig. 2C). To quantify bacteria, tissues were homogenized and plated: at both day 6 and day 10 postinfection, 1,25(OH)2D3-treated mice carried significantly higher C. rodentium burdens in their colons and ceca, compared with vehicle-treated mice (Fig. 3). C. rodentium is a noninvasive pathogen, and bacterial loads are primarily limited to the intestinal lumen and mucosal surface of WT mice. Interestingly, 1,25(OH)2D3-treated mice carried more culturable C. rodentium from extra-intestinal tissues, including mesenteric lymph node (MLN), spleen, and liver at day 6 postinfection and day 10 postinfection, compared with vehicle-treated mice, indicating greater bacterial translocation to these systemic sites (Fig. 3). These data show that 1,25(OH)2D3 treatment increases pathogen burdens during the course of infection.
Fig. 3.
1,25(OH)2D3-treated mice have greater pathogen burdens during infection with C. rodentium. A: whole tissues were homogenized and plated on Luria broth/strep-treated plates to enumerate C. rodentium burdens. Day 10 postinfection. For colon and ceca, n = 12 per group; for MLN, n = 19 per group; for spleen, n = 12 per group; for liver, n = 8 per group. Results are representative of 3–5 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. Mann-Whitney U-test). B: day 6 postinfection. For colon and ceca, n = 20 per group; for MLN, n = 20 per group; for spleen, n = 20 per group; for liver, n = 12 per group. Results are representative of 3–5 independent experiments. *P < 0.05; ***P < 0.001. Mann-Whitney U-test).
1,25(OH)2D3-treated mice have worsened histological damage during C. rodentium infection.
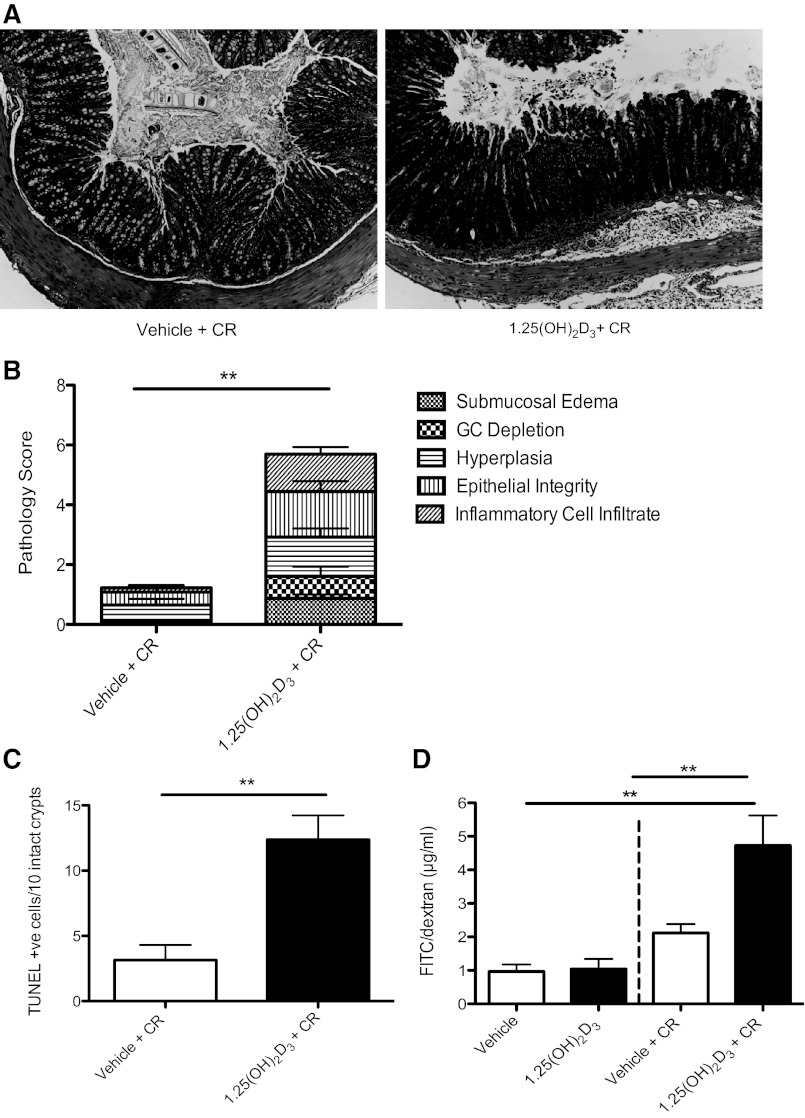
C. rodentium initially colonizes the cecum and then spreads to the distal colon of WT mice, resulting in characteristic histological damage to these regions, including goblet cell depletion, crypt hyperplasia, loss of epithelial integrity, and inflammatory cell infiltration. At day 10 postinfection, 1,25(OH)2D3-treated mice had significantly increased submucosal edema [1.42 ± 0.13 vs. vehicle: 0.58 ± 0.80 (P < 0.01; n = 6–7 per group)] and a higher number of mucosal ulcers [0.71 ± 0.28 vs. vehicle: 0 ± 0 (P < 0.05; n = 6–7 per group)] throughout the entire colon, as determined by examining Swiss-rolled sections. Tissue cross sections were also examined, and although there was a trend for more damage in the 1,25(OH)2D3-treated mice in the distal colon and cecum between groups at day 10 postinfection, the differences did not reach statistical significance. In contrast, the midcolon of 1,25(OH)2D3-treated mice was the site of the greatest damage in the 1,25(OH)2D3-treated mice with significantly elevated scores for edema, goblet cell depletion, hyperplasia, and infiltrating inflammatory cells, compared with vehicle-treated mice (Fig. 4B). Note, that 1,25(OH)2D3 had no effect on histology scores in uninfected mice (data not shown).
Fig. 4.
1,25(OH)2D3-treated mice have worsened histological damage at day 10 postinfection with C. rodentium. A: representative image of cross section of the midcolon at day 10 postinfection. Original magnification = ×200. B: midcolon was assessed for histological damage by scoring system for C. rodentium described in materials and methods; n = 7 per group. **P < 0.01. Results are representative of two independent experiments. Mann-Whitney U-test. C: midcolon was assessed for number of TUNEL-positive cells in the lumen; n = 8 per group. Results are representative of 3 independent experiments. **P < 0.01, Mann-Whitney U-test. D: FITC/dextran. Mice were treated with vehicle or 1,25(OH)2D3 for 7 days. Uninfected vehicle-treated, n = 3; uninfected 1,25(OH)2D3-treated n = 4; Vehicle and 1,25(OH)2D3-treated at day 6 postinfection; n = 12 per group. Results are representative of 3 independent experiments. **P < 0.01, Mann-Whitney U-test.
1,25(OH)2D3-treated mice show altered epithelial responses during C. rodentium infection.
To address the mechanisms underlying the mucosal damage that developed in the infected 1,25(OH)2D3-treated mice, intestinal epithelial cell proliferation and cell death were determined by Ki67 staining and the TUNEL assay, respectively. As previously shown (23, 62), infected mice displayed more Ki67-positive and TUNEL-positive cells in their colons, compared with uninfected mice at day 10 postinfection (data not shown). However, in the midcolon, infected 1,25(OH)2D3-treated mice had more TUNEL-positive epithelial cells in the midcolon, compared with vehicle-treated mice at day 10 postinfection (Fig. 4C). Furthermore, 1,25(OH)2D3-treated mice had large numbers of TUNEL-positive cells in both the cecal and colonic lumen at day 10 postinfection, indicating greater epithelial cell sloughing within these mice. These results are in agreement with previous work indicating that 1,25(OH)2D3 can induce apoptosis in human colonic epithelial cells in vitro (reviewed by Ref. 56). However, these changes were only seen during infection, as there were no obvious effects of 1,25(OH)2D3 on cell proliferation or apoptosis between uninfected groups. We did not find any significant difference in Ki67-positive cells in the midcolon between vehicle and 1,25(OH)2D3-treated mice at day 10 postinfection.
To determine whether the observed changes in apoptosis coincided with altered barrier integrity in vivo, mice were orally gavaged with FD4 at day 6 postinfection, and their sera was assessed for levels of translocated FD4. As expected, vehicle-treated mice infected with C. rodentium had higher levels of detectible FD4 in the serum, compared with control mice at day 6 postinfection (Fig. 4D). However, 1,25(OH)2D3-treated mice had significantly higher levels of FD4 in their serum, compared with vehicle-treated mice [4.72 ± 0.89 μg/ml vs. 2.11 ± 0.26 μg/ml respectively (P < 0.05)], indicating that 1,25(OH)2D3 treatment leads to exaggerated barrier disruption during infection with C. rodentium (Fig. 4D).
1,25(OH)2D3-treated mice suffer increased bacterial burdens and ulceration in response to attenuated C. rodentium.
To determine whether the observed epithelial pathology that develops in infected mice during 1,25(OH)2D3 treatment reflected exaggerated bacteria-driven pathology, or alternatively, an abnormal host response to C. rodentium, we challenged mice with a C. rodentium mutant lacking the translocated bacterial effector protein EspF (52). Previous studies have shown that the EspF protein plays a critical role in the disruption of tight junctions and the induction of apoptosis in infected colonic epithelial cells (12, 49). Furthermore, this effector has been shown to play a critical role in causing mucosal damage and ulcerations in susceptible mouse strains (24). As expected, vehicle-treated mice challenged with the ΔespF mutant suffered little in the way of weight loss or mucosal damage. In contrast, infected 1,25(OH)2D3-treated mice steadily lost weight, as their infection progressed and by day 10 postinfection, they had lost ∼18% of their initial body weight (Fig. 5A). Furthermore, 1,25(OH)2D3-treated mice developed macroscopic erosions in their middle and distal colons at day 10 postinfection. Histologically, the midcolon of 1,25(OH)2D3-treated mice showed significant edema and inflammatory cell infiltrate, with areas of ulceration (Fig. 5B). In agreement with this increased damage, 1,25(OH)2D3-treated mice had 20-fold higher pathogen burdens in their colons, compared with vehicle-treated mice, indicating greater colonization. 1,25(OH)2D3-treated mice also carried more culturable C. rodentium mutant from extraintestinal tissues at day 10 postinfection, compared with vehicle-treated mice, indicating greater bacterial translocation to these systemic sites (Fig. 5C).
Fig. 5.
1,25(OH)2D3-treated mice are more susceptible to infection with ΔespF C. rodentium mutant. A: body weight. Mice were treated with vehicle or 1,25(OH)2D3 intraperitoneally daily throughout infection. Each data point represents the average body weight pooled from eight mice and is expressed as the percentage of the initial body weight ± SE. Results are representative of two independent experiments. **P < 0.01; ***P < 0.001, two-way ANOVA. B: representative image of cross section of the midcolon at day 10 postinfection. Original magnification = ×200. C: ΔespF C. rodentium burden day 10 postinfection; n = 8 per group. Results are representative of 3–5 independent experiments. *P < 0.05; **P < 0.01. Mann-Whitney U-test).
1,25(OH)2D3 treatment suppresses colonic cytokine mRNA levels during infection.
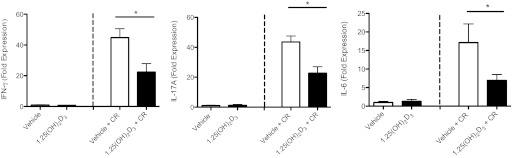
To determine whether the increased susceptibility of 1,25(OH)2D3-treated mice to C. rodentium infection could reflect modulation of the host immune response, we assessed the distal colon for expression of genes encoding cytokines that have been shown to influence susceptibility to C. rodentium infection. The distal colon was chosen for analysis, since there was comparable histological damage between both groups at this site. The inflammatory mediators TNF-α and iNOS have previously been shown to play an important role in controlling C. rodentium pathogen load (26, 61). Surprisingly, there were no changes in the mRNA levels of these genes between vehicle and 1,25(OH)2D3-treated mice at day 10 postinfection (data not shown). As expected, the expression of Th1- (IFN-γ) and Th17-related cytokines (IL-6, IL-17A) were upregulated in the distal colon of mice challenged with C. rodentium compared with uninfected controls, as previously shown (15, 29, 68). However, at day 10 postinfection, 1,25(OH)2D3 treatment strikingly suppressed the elevated expression of IFN-γ, IL-6, and IL-17A compared with vehicle-treated mice (Fig. 6). There was also a trend for reduced expression of IL-17F in the distal colon of 1,25(OH)2D3-treated mice at day 10 postinfection, compared with vehicle (0.82 ± 0.08- vs. 1.65 ± 0.33-fold expression, respectively); however, this did not reach statistical significance (P = 0.09). We also measured gene expression of other cytokines and growth factors and found no differences in the colonic expression of the anti-inflammatory cytokines IL-10 or TGF-β or other Th17-related cytokines (IL-22, IL-23) between vehicle and 1,25(OH)2D3-treated mice at day 10 postinfection (data not shown).
Fig. 6.
1,25(OH)2D3-treated mice have suppressed colonic cytokine mRNA levels during infection with C. rodentium. Expression of IFN-γ, IL-17A, and IL-6 in distal colon at baseline and day 10 postinfection as assessed by RT qPCR; n = 8 per group. Results are representative of three independent experiments. *P < 0.05. Mann-Whitney U-test).
1,25(OH)2D3-treated mice have fewer CD4+IL-17A+ cells in the colon at day 10 postinfection.
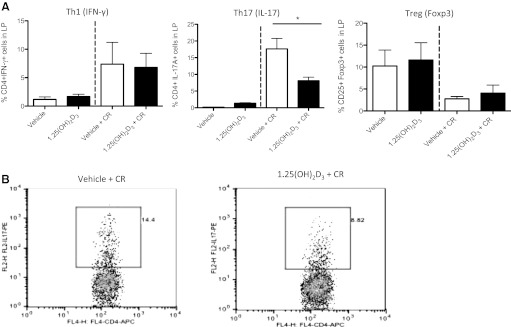
On the basis of the reduced expression of IFN-γ and IL-17A, we next asked whether 1,25(OH)2D3 treatment led to alterations in immune cell populations in the colon and in the spleen. Therefore, we isolated lymphocytes from the spleen and colonic lamina propria and assessed intracellular cytokine expression by FACS analysis. Effector CD4+ T cells can be classified on the basis of the signature cytokines that they produce. For instance, Th1 cells produce IFN-γ, while Th17 cells are characterized by IL-17A (13). CD4+ T cells can also develop into regulatory cells (Treg) that express FoxP3. As expected, there were more CD4+IFN-γ+ and CD4+IL-17A+ cells in the lamina propria of C. rodentium-challenged mice at day 10 postinfection, compared with uninfected mice (Fig. 7). Although 1,25(OH)2D3 treatment had no overt impact on CD4+IFN-γ+ cell numbers in the colons of C. rodentium-infected mice, it did lead to significantly fewer colonic CD4+IL-17A+ cells compared with vehicle-treated mice at day 10 postinfection. This suppression seemed to be specific to the intestine, since there was no difference in CD4+IL-17A+ populations in the spleens of 1,25(OH)2D3-treated vs. vehicle-treated mice at day 10 postinfection (not shown). The lamina propria of C. rodentium-infected mice contained fewer CD4+CD25+FoxP3+ cells at day 10 postinfection compared with uninfected mice (Fig. 7A). Although there was a trend for 1,25(OH)2D3-treated mice to have more CD4+CD25+FoxP3+ cells in their colonic lamina propria at day 10 postinfection, compared with vehicle-treated mice, these changes did not reach significance.
Fig. 7.
1,25(OH)2D3-treated mice have fewer CD4+IL-17A+ cells in the colon at day 10 postinfection. A: FACS analysis. Lamina propria (colon + cecum with cecal patch removed) from 2–4 mice were pooled together for each data point. Results are representative of three independent experiments. *P < 0.05, Mann-Whitney U-test). B: representative FACS plot of proportion of CD4+IL-17+ cells in lamina propria at day 10 postinfection (PBS + CR 14.4% vs. Cal + CR 8.82%).
1,25(OH)2D3-treated mice have reduced expression of the antimicrobial peptide RegIIIγ.
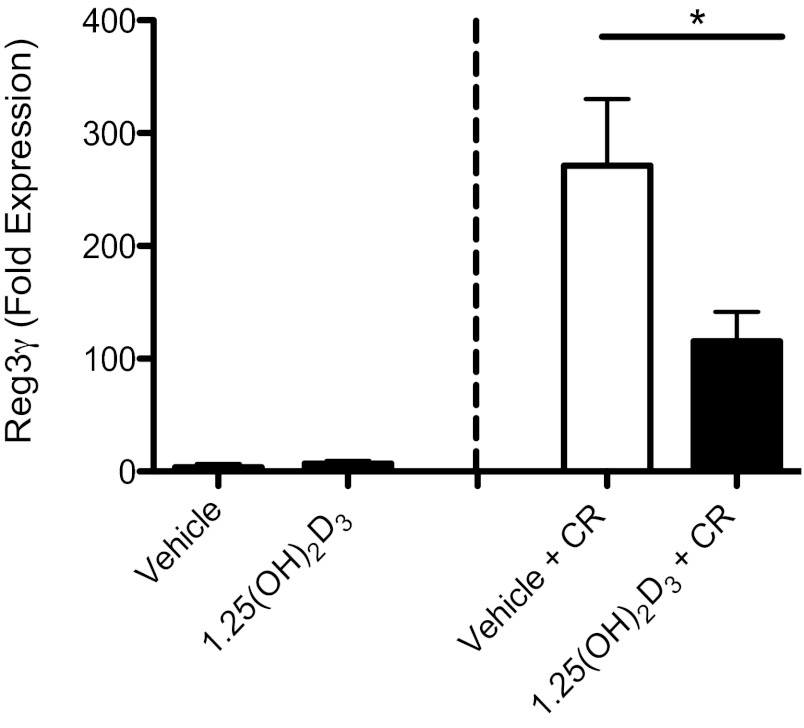
Th17-associated cytokines exert strong host-protective roles during C. rodentium infection, although the basis for their protective effects is poorly understood. Considering that a major feature of the 1,25(OH)2D3-treated mice was increased C. rodentium burdens, we decided to assess gene expression for several antimicrobial factors previously linked to host defense against C. rodentium, since these could be the effector molecules through which Th17 cells exert their effects. As previously shown, the expression of RegIIIγ was upregulated in the distal colon of mice challenged with C. rodentium compared with uninfected controls (68). Interestingly, 1,25(OH)2D3-treated mice had reduced expression of RegIIIγ in the distal colon at day 10 postinfection, compared with vehicle-treated mice (Fig. 8). There were no differences in the expression of RegIIIβ, S100A8, or S100A9 between vehicle and 1,25(OH)2D3-treated mice at day 10 postinfection, indicating a selective effect of 1,25(OH)2D3 on RegIIIγ.
Fig. 8.
1,25(OH)2D3-treated mice have reduced expression of the antimicrobial peptide RegIIIγ. Expression of Reg3γ in distal colon at baseline and day 10 postinfection as assessed by RT qPCR; n = 8 per group. Results are representative of three independent experiments. *P < 0.05. Mann-Whitney U-test).
DISCUSSION
Our work confirms that 1,25(OH)2D3 treatment can protect mice against DSS-induced colitis; however, this is the first study to show that providing exogenous and active vitamin D [i.e., 1,25(OH)2D3] increases host susceptibility to an enteric bacterial pathogen. It has previously been shown that mice treated with high-dose 1,25(OH)2D3 (75 ng, 3 times/wk) developed exaggerated crypt hyperplasia at day 12 postinfection with C. rodentium; however, other aspects of disease were not reported (54). We found that 1,25(OH)2D3-treated infected mice suffered from increased C. rodentium burdens and exaggerated colonic tissue damage, compared with vehicle-treated mice. In agreement with these findings, 1,25(OH)2D3-treated mice showed defects in their expression of colonic IFN-γ, IL-17A, and IL-6 at day 10 postinfection. These cytokines have been shown to play important roles in regulating pathogen burdens, as well as mucosal damage during C. rodentium infection (15, 57, 58). In association with their increased susceptibility, 1,25(OH)2D3-treated infected mice showed substantially reduced numbers of Th17 T cells in their colons during C. rodentium infection, whereas only modest changes were noted in Th1- and Treg T-cell numbers. In agreement with a suppression of Th17 responses, 1,25(OH)2D3-treated mice showed defects in their production of the Th17-associated antimicrobial peptide REG3γ, which has been shown to play a role in mucosal repair and host defense during infection with C. rodentium (68).
As previously shown, mice treated with 1,25(OH)2D3 were protected against DSS-induced colitis (21, 43). Vehicle-treated mice developed severe mucosal damage in the colon post-DSS, characterized by a loss of crypts and infiltration of inflammatory cells, whereas the colons of 1,25(OH)2D3-treated mice appeared relatively undamaged. It is currently unclear how 1,25(OH)2D3 ameliorates DSS-induced colitis. Previous studies have indicated that 1,25(OH)2D3 treatment can suppress several inflammatory mediators in mice challenged with DSS, including TNF-α and IFN-γ (67); however, the underlying mechanism is unknown. We have not investigated the Th17 response in DSS-challenged mice, since the effect of 1,25(OH)2D3 treatment is so dramatic, it is likely that all host inflammatory/immune markers are suppressed. It is also unknown whether Th17 responses play a role during DSS challenge, since T and B cells are dispensable in the acute DSS colitis model (17). However, Alex et al. (1) showed that mice administered 3% DSS for 7 days had increased colonic expression of IL-12 and IL-17, while IL-17A-/- mice were protected against DSS challenge, indicating the IL-17A may play an important role in the pathogenesis of DSS colitis (34).
The newly described Th17 cells are abundant at mucosal surfaces and characterized by the production of IL-17A, a proinflammatory cytokine associated with increased severity of various inflammatory diseases, including Type 1 diabetes, multiple sclerosis, and IBD (reviewed by Ref. 11). As such, strategies to block and/or regulate Th17 responses are currently being investigated, including 1,25(OH)2D3 treatment. Indeed, it was recently shown that 1,25(OH)2D3 can directly suppress IL-17A by dissociation of histone acetylase activity from the IL-17A promoter and recruitment of histone deacetylase and VDR/RXR binding to NFAT sites (37). Furthermore, recent studies have found that 1,25(OH)2D3 can suppress the development of Th17 cells and production of associated cytokines, such as IL-6, IL-17, IL-22, and IL-23, in vitro (9, 10, 33, 37, 60). However, most of these studies have focused on in vitro regulation, whereas in vivo studies are needed to better clarify the potential therapeutic use of modifying vitamin D levels to alter Th17 responses. Our findings indicate that 1,25(OH)2D3 does, indeed, suppress Th17 responses in vivo, and while such suppression may prove protective against autoimmune diseases, it can also impair antimicrobial defenses in the GI tract. In fact, IL-17A has been shown to induce the expression of antimicrobial peptides, stimulate the production of proinflammatory cytokines and induce granulopoietic factors and chemokines to recruit neutrophils in response to infection (reviewed by Ref. 13). As such, Th17 responses are critical for the clearance of extracellular bacterial infections (5). In agreement with the suppressed colonic Th17 response, we found that 1,25(OH)2D3-treated mice carried significantly higher C. rodentium burdens in their colons and ceca compared with vehicle-treated mice at day 10 postinfection. Furthermore, 1,25(OH)2D3-treated mice suffered C. rodentium colonization throughout their entire colon, as determined by Tir staining on infected epithelial cells, including the middle and proximal regions of the colon, areas not normally colonized by C. rodentium in WT mice. It is currently unknown why 1,25(OH)2D3-treatment altered the typical infection pattern; however, it can be assumed that 1,25(OH)2D3 treatment modified the host response to C. rodentium, rather than having a direct effect on the bacteria, since previous work has shown that 1,25(OH)2D3 does not directly alter bacterial growth or activity, in vitro (63).
Interestingly, we found that 1,25(OH)2D3-treated mice developed worsened histological damage, particularly in the midcolon, an area not typically damaged by C. rodentium in WT mice. In association with the mucosal damage, we found that 1,25(OH)2D3 treatment selectively suppressed IL-6 mRNA levels within the colon. IL-6 is a cytokine shown to play a key role in mucosal protection during challenge with C. rodentium (15). Furthermore, our group has found that mice deficient in TLR2 develop ulceration in the midcolon during C. rodentium challenge, which was attributed to a defect in IL-6 production within the colonic mucosa (24). Normally, C. rodentium colonizes the cecum and then spreads to the distal colon of WT mice, resulting in histological damage to these regions. At first thought, it seems that the exaggerated damage in the midcolon of 1,25(OH)2D3-treated mice was simply caused by higher bacterial burdens in the colon, or perhaps, the immunosuppressive effects of 1,25(OH)2D3 allowed the pathogenic bacteria to colonize areas of the gut it does not normally inhabit. However, we also found similar results when 1,25(OH)2D3-treated mice were challenged with a C. rodentium strain lacking the translocated effector espF (ΔespF), a strain that does not typically cause mucosal damage in WT mice. These findings indicate that the observed erosions and epithelial damage in 1,25(OH)2D3-treated mice are most likely due to host-driven changes in the epithelial response to C. rodentium challenge, since C. rodentium typically requires EspF to induce mucosal pathology.
The intestinal surface is lined by a single layer of epithelial cells, which function as a barrier to separate the bacteria-rich lumen from underlying host cells. Previous work has shown that vitamin D plays an important role in maintaining barrier integrity in the intestine (21, 38, 41, 67); however, the role of 1,25(OH)2D3 on barrier integrity is less clear. Our results show that 1,25(OH)2D3-treated mice develop worsened intestinal epithelial barrier dysfunction, compared with vehicle-treated mice during infection with C. rodentium, as determined by the FITC/dextran assay. Correspondingly, 1,25(OH)2D3-treated mice suffered from increased bacterial translocation out of the GI tract and to the MLN, spleen, and liver at day 6 and day 10 postinfection, compared with vehicle-treated mice. We also found that 1,25(OH)2D3-treated mice had altered epithelial responses at day 10 postinfection, including increased cell death, as determined by TUNEL staining. Our results are in agreement with previous work demonstrating that 1,25(OH)2D3 can induce apoptosis in human colonic epithelial cells in vitro (reviewed in Ref. 56). However, it is currently unknown whether the defects in barrier integrity are primary or secondary to the immune mediated effects of 1,25(OH)2D3.
Our current understanding of the potential for vitamin D levels to impact on host susceptibility to pathogens is limited. There is an established link between vitamin D deficiency and tuberculosis (22, 64) and viral respiratory infections (25, 48). Moreover, low-vitamin D intake has also been correlated with differences in fecal microbial composition, including elevated levels of Bacteroides in vitamin D-deficient volunteers (46). However, the role of vitamin D during enteric infections is currently unclear. Recently, Edrington et al. (19) found that supplementing cattle with vitamin D3 had no effect of fecal shedding of E. coli 0157:H7; however, the researchers also looked at seasonal variation and found that the proportion of cattle shedding E. coli 0157:H7 was higher in the summer months (16.7% in the summer vs. 6.7% in the winter, P = 0.08), which correlated with higher serum vitamin D levels. Together with our results, these findings raise the question of whether there may be an unexpected benefit to vitamin D deficiency, i.e., potentially, the development of stronger Th17 responses against specific bacterial pathogens. Correspondingly, while vitamin D supplementation has been shown to protect against autoimmune/inflammatory conditions (27), it is currently unknown whether such immune suppression could increase the susceptibility of a host to specific types of infection. It is tempting to speculate that human migration away from the equator and to more northerly climate and its associated risk for vitamin D insufficiency may have yielded both beneficial, as well as detrimental effects on our immune system, leaving individuals at greater risk of autoimmune disease, but more resistant to enteric infections. Future research is necessary to answer these questions and to help clarify the role of vitamin D in maintaining mucosal homeostasis during enteric infections and other inflammatory challenges. Taken together, these studies show that 1,25(OH)2D3 is a potent, yet selective, immunosuppressive agent, and as such, 1,25(OH)2D3 treatment may protect against Th17 T-cell driven damage during experimental, as well as potentially clinical colitis; however, caution should be advised, since Th17 responses are also critical in providing host defense against extracellular bacteria, including those pathogenic strains of E. coli implicated in the pathogenesis of IBD.
ACKNOWLEDGMENTS
We thank the Animal Care Facility staff at Child and Family Research Institute (CFRI) for their help with animal care. We also acknowledge Dr. Wanyin Deng for providing the anti-C. rodentium Tir antisera. This work was supported by two Grants in Aid awarded by the Crohn's and Colitis Foundation of Canada to B. A. Vallance and K. Jacobson, and an operating grant to B. A. Vallance from the Canadian Institutes for Health Research (CIHR). N. R. Ryz was supported by a Vanier Canada Graduate Scholarship Doctoral Research Award, and a Four-Year Doctoral Fellowship from the University of British Columbia. H. P. Sham was funded by a CFRI studentship and J. Chan was funded by a CIHR Masters award. M. K. Levings holds a Canada Research Chair in Transplantation. S. J. Patterson holds a CIHR/Canadian Association of Gastroenterology postdoctoral fellowship. B. A. Vallance is the Children with Intestinal and Liver Disorders (CHILD) Foundation Research Scholar, a Michael Smith Foundation for Health Research Scholar, and the Canada Research Chair in Pediatric Gastroenterology. K. Jacobson is a CHILD Foundation Clinical Investigator.
Footnotes
This article is the topic of an Editorial Focus by Sylvia Christakos (9a).
REFERENCES
- 1. Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 15: 341–352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardizzone S, Cassinotti A, Trabattoni D, Manzionna G, Rainone V, Bevilacqua M, Massari A, Manes G, Maconi G, Clerici M, Bianchi Porro G. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on Th1/Th2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol 22: 63–71, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bartels LE, Jørgensen SP, Agnholt J, Kelsen J, Hvas CL, Dahlerup JF. 1,25-dihydroxyvitamin D3 and dexamethasone increase interleukin-10 production in CD4+ T cells from patients with Crohn's disease. Int Immunopharmacol 15: 1755–1764, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic. PLoS Pathog 13: e1000902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol 30: 196–203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bours PH, Wielders JP, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25 hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int 22: 2857–2867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol 23: 519–528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130: 2648–2652, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLos One 23: e12925, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Christakos S. Vitamin D deficiency: protective against enteric infection? Focus on “Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses.” Am J Physiol Gastrointest Liver Physiol (November 1, 2012). doi:10.1152/ajpgi.00320-2012.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, van Leeuwen JP, Lubberts E. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum 62: 132–142, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Costa VS, Mattana TC, da Silva ME. Unregulated IL-23/IL-17 immune response in autoimmune disease. Diabetes Res Clin Pract 88: 222–226, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Crane JK, McNamara BP, Donnenberg MS. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell Microbiol 3: 197–211, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol 159: 109–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324: 23–33, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ, Hanson E, Eckmann L. IL-6 dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol 180: 6816–6826, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deng W, Vallance BA, Li Y, Puente JL, Finlay BB. Citrobacter rodentium translocate intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol Microbiol 48: 95–115, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107: 1643–1652, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann NY Acad Sci 1072: 28–38, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Edrington TS, Farrow RL, Mackinnon KM, Callaway TR, Anderson RC, Nisbet DJ. Influence of vitamin D on fecal shedding of Escherichia coli 0157:H7 in naturally colonized cattle. J Food Prot 75: 314–319, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Ferreira GB, van Etten E, Verstuyf A, Waer M, Overbergh L, Gysemans C, Mathieu C. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behavior in vitro and in vivo. Diabetes Metab Res Rev 27: 933–941, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, Biggs BA. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis 46: 443–446, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. MyD88 signaling plays a critical role in host defense by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol 10: 618–631, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, Vallance BA. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol 10: 388–403, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169: 384–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonçalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of luminal pathogenic bacteria and immunopathology in infectious colitis. Infect Immun 69: 6651–6659, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine 77: 552–557, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Hardenberg G, Yao Y, Piccirillo CA, Levings MK, Steiner TS. Toll-like receptor 5 deficiency protects from wasting disease in a T cell transfer colitis model in T cell receptor-β deficient mice. Inflamm Bowel Dis 18: 85–93, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun 67: 3031–3039, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 12: 4–18, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Polek TC, Murthy S, Blutt SE, Boehm MF, Zou A, Weigel NL, Allegretto EA. Novel nonsecosteroidal vitamin D receptor modulator inhibits the growth of LNCaP xenograft tumors in athymic mice without increased serum calcium. Prostate 49: 224–233, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) mouse. Cancer Res 62: 741–746, 2002 [PubMed] [Google Scholar]
- 33. Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, Nishimura T. 1alpha,25-dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett 134: 7–16, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun 377: 12–16, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Jørgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther 32: 377–383, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Joseph AJ, George B, Pulimood AB, Seshadri MS, Chacko A. 25 (OH) vitamin D level in Crohn's disease: Association with sun exposure and disease activity. Indian J Med Res 130: 133–137, 2009 [PubMed] [Google Scholar]
- 37. Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, Youssef S. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 31: 3653–3669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kallay E, Bareis P, Bajna E, Kriwanek S, Bonner E, Toyokuni S, Cross HS. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol 40: 1191–1196, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Khoo AL, Joosten I, Michels M, Woestenenk R, Preijers F, He XH, Netea MG, van der Ven AJ, Koenen HJ. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology 134: 459–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 474: 307–317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 151: 2423–2432, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, Adorini L. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett 131: 49–58, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Lebeis SL, Sherman MA, Kalman D. Protective and destructive innate immune responses to enteropathogenic Escherichia coli and related A/E pathogens. Future Microbiol 3: 315–328, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: The Manitoba IBD cohort study. Am J Gastroenterol 103: 1451–1459, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J 8: 49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinesi M, Treves C, d'Albasio G, Bagnoli S, Bonanomi AG, Stio M. Vitamin D derivatives induce apoptosis and downregulate ICAM-1 levels in peripheral blood mononuclear cells of inflammatory bowel disease patients. Inflamm Bowel Dis 14: 597–604, 2008 [DOI] [PubMed] [Google Scholar]
- 48. McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 44: 981–988, 2009 [DOI] [PubMed] [Google Scholar]
- 49. McNamara BP, Koutsouris A, O'Connell CB, Nougayréde JP, Donnenberg MS, Hecht G. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J Clin Invest 107: 621–629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszenyi L, Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn's disease patients. Inflamm Bowel Dis 15: 1656–1662, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do proteobacteria play? Nat Rev Gastroenterol Hepatol 9: 219–230, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Nagai T, Abe A, Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J Biol Chem 280: 2998–3011, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics 118: 1950–1961, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peleg S, Sellin JH, Wang Y, Freeman MR, Umar S. Suppression of aberrant transient receptor potential cation channel, subfamily V, member 6 expression in hyperproliferative colonic crypts by dietary calcium. Am J Physiol Gastrointest Liver Physiol 299: G593–G601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36: 361–370, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutr Rev 10 Suppl 2: S116–S24, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Simmons CP, Goncalves NS, Ghaem-Maghami M, Bajaj-Elliott M, Clare S, Neves B, Frankel G, Dougan G, MacDonald TT. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-γ. J Immunol 168: 1804–1812, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect Immun 78: 2653–2666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stio M, Bonanomi AG, d'Albasio G, Treves C. Suppressive effects of 1,25-dihydroxyvitamin D3 and its analogues EB 1089 and KH 1060 on T lymphocyte proliferation in active ulcerative colitis. Biochem Pharmacol 61: 365–371, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, Su SB, Chan CC, Adorini L, Caspi RR. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol 182: 4624–4632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vallance BA, Deng W, De Grado M, Chan C, Jacobson K, Finlay BB. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen Citrobacter rodentium in infected mice. Infect Immun 70: 6424–6435, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun 71: 3443–3453, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173: 2909–2912, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Williams B, Williams A, Anderson S. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J 27: 941–942, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-κB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 92: 10990–10994, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127–2135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 12: 57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14: 282–289, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-α pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 35: 217–224, 2005 [DOI] [PubMed] [Google Scholar]