Abstract
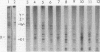
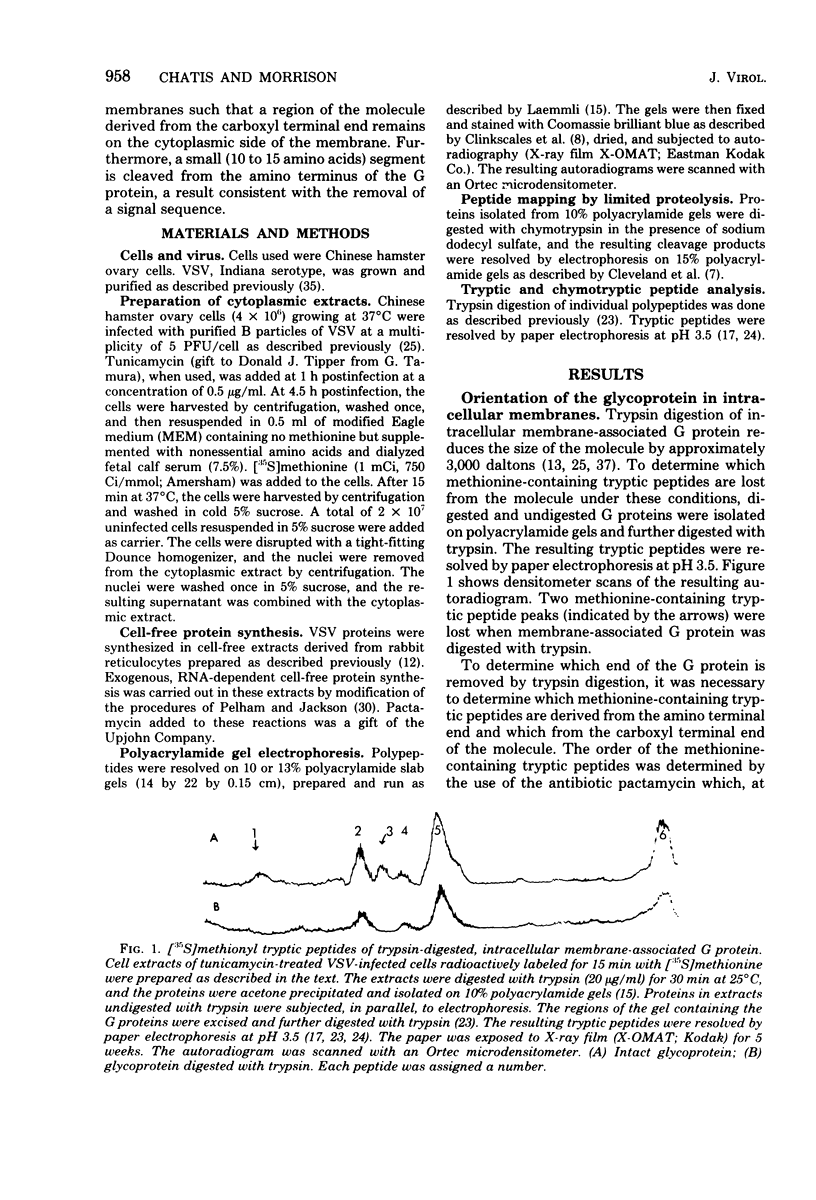
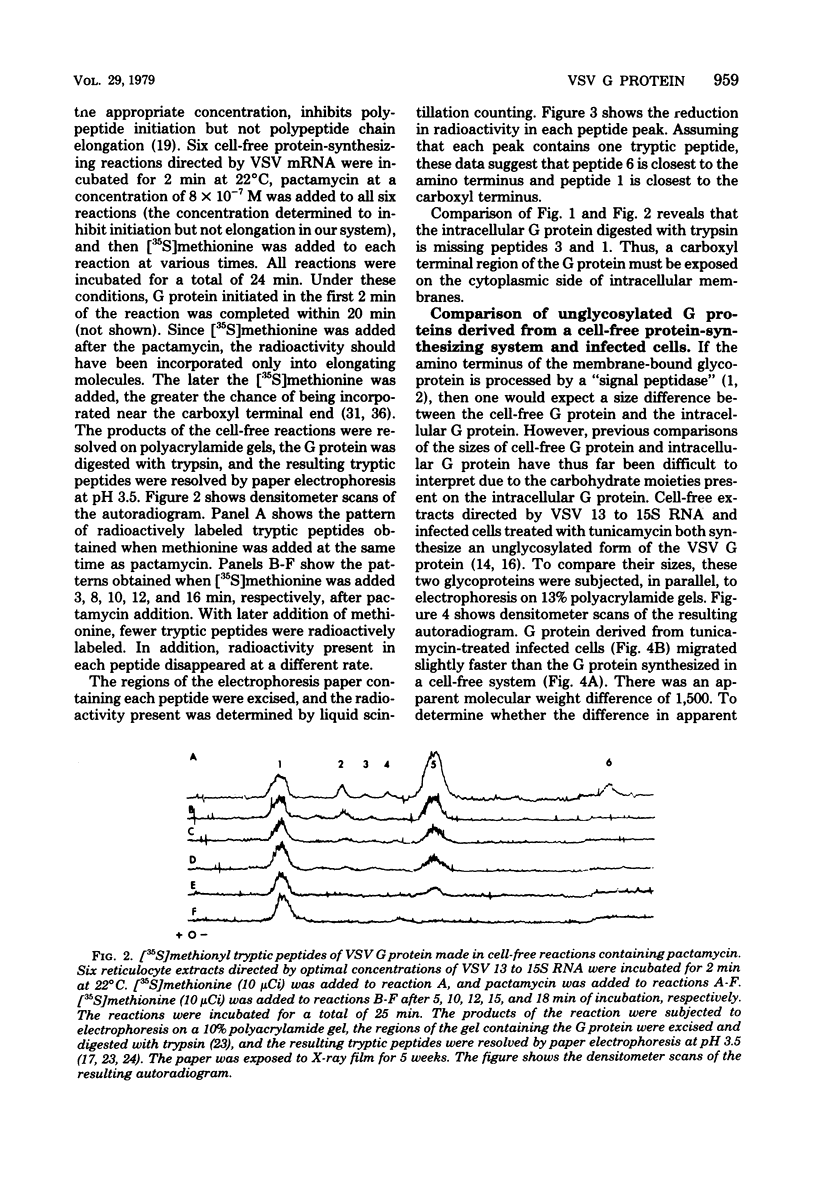
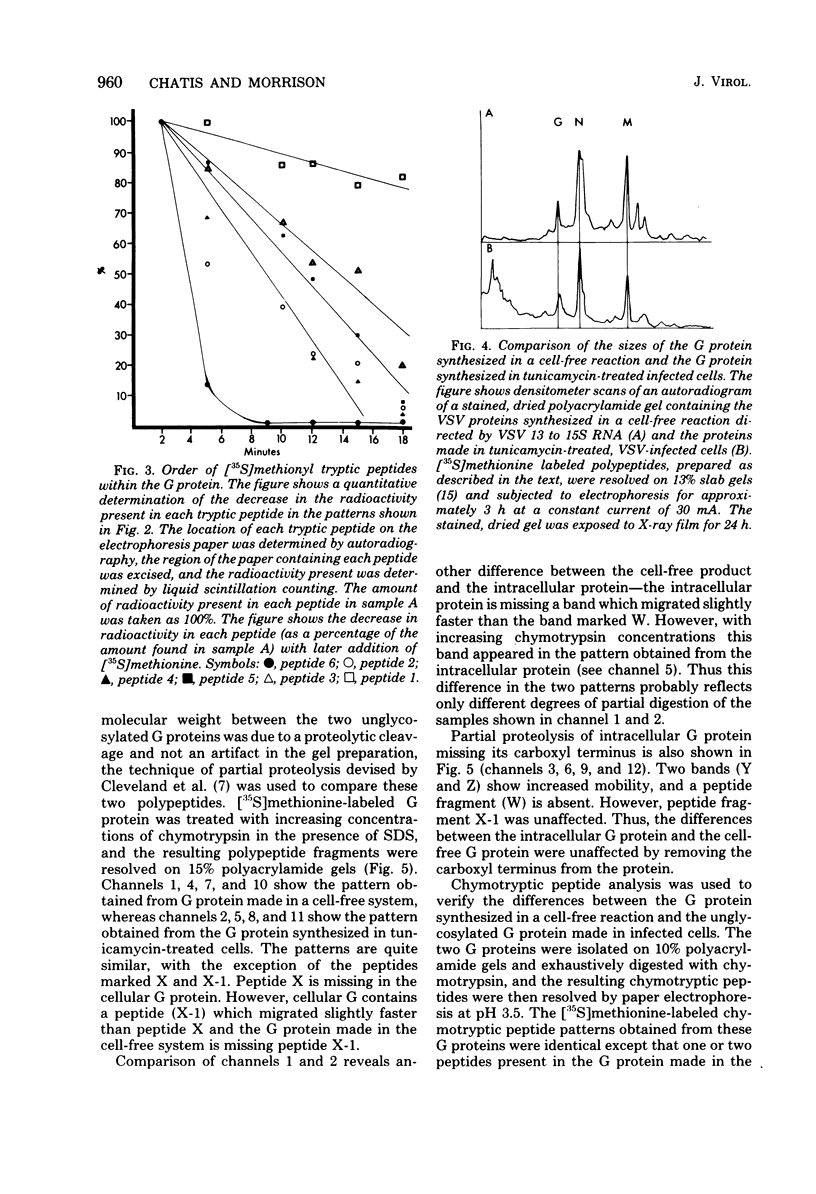
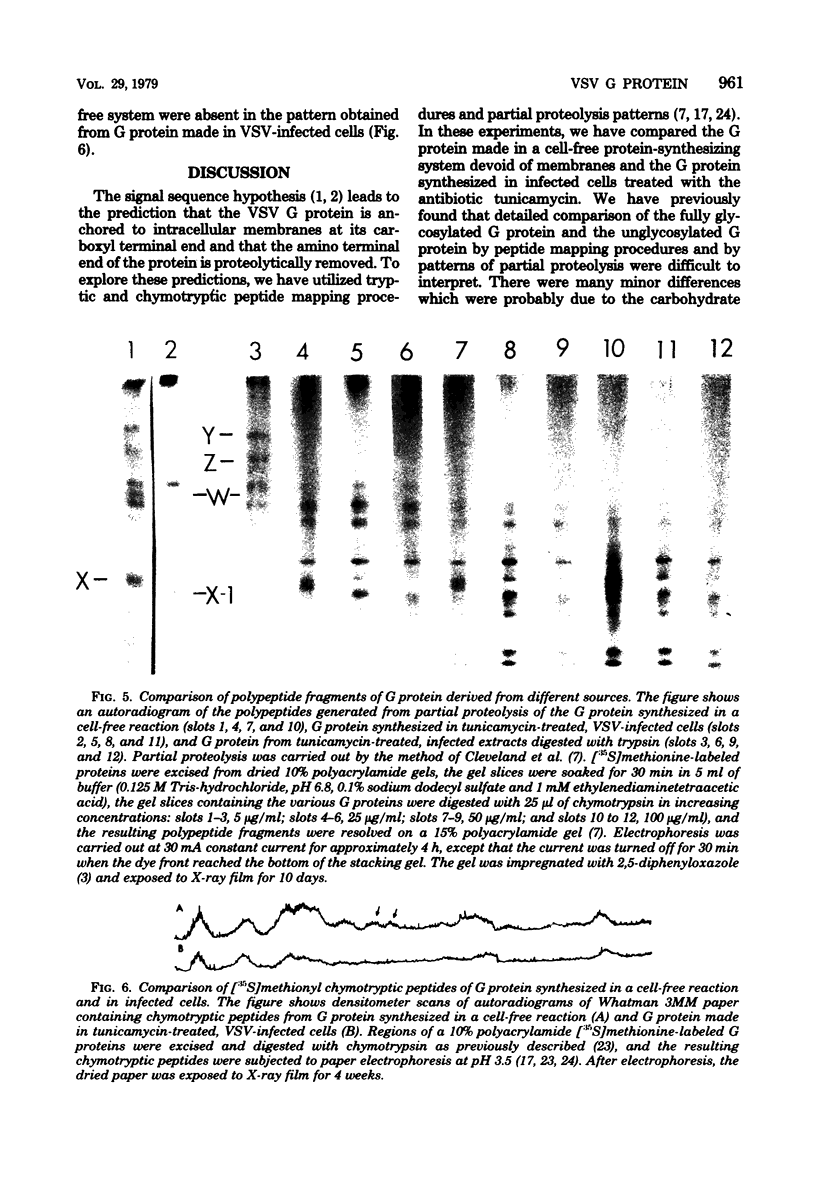
The intracellular vesicular stomatitis virus glycoprotein (G) is inserted into membranes such that a small portion of one end of the molecule is exposed on the cytoplasmic surface of the endoplasmic reticulum and is susceptible to proteolytic digestion (T.G. Morrison, C.O. McQuain, and D. Simpson, J. Virol. 28:368-374). We have determined that this region of the G protein contains two methionyl tryptic peptides. The methionyl tryptic peptides of the G protein have been ordered by the use of the antibiotic pactamycin, and the two methionyl tryptic peptides removed by proteolytic digestion of intracellular G protein have been shown to be derived from the carboxyl terminal end of the protein. In addition, we have found that the unglycosylated G protein synthesized in a reticulocyte cell-free reaction migrates on polyacrylamide gels slightly slower than the unglycosylated G protein synthesized in tunicamycin-treated infected cells. We have also compared these G proteins derived from different sources by partial proteolysis (D.W. Cleveland, S.G. Fischer, M.W. Kirschner, and V.K. Laemmli, J. Biol. Chem. 252:1102-1106) and by chymotryptic peptide analysis. We have found minor differences between the two proteins consistent with the removal of 10 to 15 amino acids from the amino terminus of the intracellular G protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinkscales C. W., Bratt M. A., Morrison T. G. Synthesis of Newcastle disease virus polypeptides in a wheat germ cell-free system. J Virol. 1977 Apr;22(1):97–101. doi: 10.1128/jvi.22.1.97-101.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger R., Compans R. W., Rifkin D. B. The organization of the proteins of vesicular stomatitis virions: labeling with pyridoxal phosphate. Virology. 1975 Aug;66(2):610–615. doi: 10.1016/0042-6822(75)90233-0. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Moyer S. A., Banerjee A. K., Ehrenfeld E. Sub-cellular localization of vesicular stomatitis virus messenger RNAs. Biochem Biophys Res Commun. 1975 Feb 3;62(3):531–538. doi: 10.1016/0006-291x(75)90431-3. [DOI] [PubMed] [Google Scholar]
- Howatson A. F. Vesicular stomatitis and related viruses. Adv Virus Res. 1970;16:195–256. doi: 10.1016/s0065-3527(08)60024-x. [DOI] [PubMed] [Google Scholar]
- Hunt T., Jackson R. J. The rabbit reticulocyte lysate as a system for studying mRNA. Hamatol Bluttransfus. 1974;14:300–307. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Binding of viral glycoprotein mRNA to endoplasmic reticulum membranes is disrupted by puromycin. J Cell Biol. 1977 Aug;74(2):358–364. doi: 10.1083/jcb.74.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Kelley J. M., Wagner R. R. Envelope proteins of vesicular stomatitis virions: accessibility to iodination. Virology. 1974 Sep;61(1):292–296. doi: 10.1016/0042-6822(74)90264-5. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Lodish H. F. Recognition of protein synthesis initiation signals on bacteriophage ribonucleic acid by mammalian ribosomes. J Biol Chem. 1974 Sep 25;249(18):5860–5866. [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. O. Assembly of viral membranes: nature of the association of vesicular stomatitis virus proteins to membranes. J Virol. 1978 Apr;26(1):115–125. doi: 10.1128/jvi.26.1.115-125.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. O., Simpson D. Assembly of viral membranes: maturation of the vesicular stomatitis virus glycoprotein in the presence of tunicamycin. J Virol. 1978 Oct;28(1):368–374. doi: 10.1128/jvi.28.1.368-374.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Morrison T., Stampfer M., Baltimore D., Lodish H. F. Translation of vesicular stomatitis messenger RNA by extracts from mammalian and plant cells. J Virol. 1974 Jan;13(1):62–72. doi: 10.1128/jvi.13.1.62-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A. Glycoprotein fragment associated with vesicular stomatitis virus after proteolytic digestion. Virology. 1974 Dec;62(2):573–577. doi: 10.1016/0042-6822(74)90419-x. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rekosh D. Gene order of the poliovirus capsid proteins. J Virol. 1972 Mar;9(3):479–487. doi: 10.1128/jvi.9.3.479-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. In vitro synthesis of vesicular stomatitis virus membrane glycoprotein and insertion into membranes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):715–719. doi: 10.1073/pnas.75.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]