Abstract
Secretagogues acting at a variety of receptor types activate electrogenic K+ secretion in guinea pig distal colon, often accompanied by Cl− secretion. Distinct blockers of KCa1.1 (BK, Kcnma1), iberiotoxin (IbTx), and paxilline inhibited the negative short-circuit current (Isc) associated with K+ secretion. Mucosal addition of IbTx inhibited epinephrine-activated Isc (epiIsc) and transepithelial conductance (epiGt) consistent with K+ secretion occurring via apical membrane KCa1.1. The concentration dependence of IbTx inhibition of epiIsc yielded an IC50 of 193 nM, with a maximal inhibition of 51%. Similarly, IbTx inhibited epiGt with an IC50 of 220 nM and maximal inhibition of 48%. Mucosally added paxilline (10 μM) inhibited epiIsc and epiGt by ∼50%. IbTx and paxilline also inhibited Isc activated by mucosal ATP, supporting apical KCa1.1 as a requirement for this K+ secretagogue. Responses to IbTx and paxilline indicated that a component of K+ secretion occurred during activation of Cl− secretion by prostaglandin-E2 and cholinergic stimulation. Analysis of KCa1.1α mRNA expression in distal colonic epithelial cells indicated the presence of the ZERO splice variant and three splice variants for the COOH terminus. The presence of the regulatory β-subunits KCaβ1 and KCaβ4 also was demonstrated. Immunolocalization supported the presence of KCa1.1α in apical and basolateral membranes of surface and crypt cells. Together these results support a cellular mechanism for electrogenic K+ secretion involving apical membrane KCa1.1 during activation by several secretagogue types, but the observed K+ secretion likely required the activity of additional K+ channel types in the apical membrane.
Keywords: iberiotoxin, paxilline, epinephrine, ATP, prostaglandin E2, cholinergic
electrogenic K+ secretion by colonic epithelial cells contributes to the high K+ concentration in the lumen (30, 82). Various secretagogues stimulate the rate of this K+ secretion while Na+ absorption diminishes the luminal Na+ content (2, 64). In this way the colonic epithelium alters the fluid arriving from the small intestine into a composition with high K+ concentration and low Na+ concentration. Those epithelial cells responsible for producing this K+ secretion use the active Cl− secretory mechanism (16, 17) with the inclusion of apical membrane K+ channels (21). The extent of secretagogue activation determines the final colonic fluid and electrolyte balance and emanates from several sources including the enteric and sympathetic nervous systems as well as paracrine systems in the mucosa (8, 58, 87).
Excessive rates of colonic fluid secretion often lead to hypokalemia due to the attendant K+ secretion. Massive losses occur during invasion by virulent bacteria as with cholera (12, 63, 78) or with some types of villous adenomas (11, 46), but subtler imbalances over time also can lead to severe hypokalemia. Central to the regulation of luminal composition is the range of relative K+ and Cl− secretory rates possible with various secretagogues (64, 87). The profound enteric nervous system discharge created during cholera (44) produces the ultimate synergistic fluid secretion with Cl− secretion predominant over K+ secretion during this high fluid flow. In comparison, the sympathetic/parasympathetic imbalance occurring with acute colonic pseudo-obstruction results in luminal K+ concentrations near 150 mM (69, 80), likely due to the β-adrenergic activation of K+ secretion without active Cl− secretion (64, 86). In contrast to the catastrophic K+ losses exhibited in these pathological regulatory conditions, a well-managed K+ loss by stimulating increased colonic K+ secretion might aid patients during end-stage renal disease (ESRD) such that total body K+ balance could be maintained (27, 48, 67).
Transepithelial fluid flows occur as the result of osmotic gradients developed by net solute absorption or secretion with the Na+/K+-ATPase in the basolateral membrane generally setting the electrochemical gradients (2, 30). Simply opening apical membrane K+ channels allows these fluid-transporting cells to also secrete K+ by a mechanism involving pump uptake and channel exit. Regulatory control of these apical K+ channels provides the means to match K+ secretory rates to any physiological process, such that the range of K+ channel proteins available allows each cell type to use those channels that fit best into the cellular signaling initiating fluid flow.
Although numerous studies support the general cellular mechanism for electrogenic K+ secretion (30), the molecular identity of the apical membrane K+ channels required during secretagogue activation is not definitively established, with both KCa1.1 (BK, Kcnma1) and KCa3.1 (Kcnn4) implicated in apical K+ exit during colonic K+ secretion (34, 49, 72, 74). In particular, distal colon from KCa1.1-null mice exhibits attenuated electrogenic K+ secretion (70, 75, 76). For these KCa1.1-null mice, the P2Y2/P2Y4-receptor-mediated K+ secretory response is eliminated (70) and responses to both β-adrenergic and aldosterone stimulation are substantially reduced (75, 76). Together these results support KCa1.1 as an important component of the apical membrane K+ conductance responsible for K+ exit into the colonic lumen during K+ secretion.
Activation of KCa1.1 depends on both intracellular Ca2+ and membrane voltage, and also can be altered via phosphorylation including that by protein kinase A (PKA) (3, 10, 32, 81). Specifically, membrane depolarization opens KCa1.1, with Ca2+ increases shifting this voltage sensitivity to more negative membrane voltages. Splicing of KCa1.1 proteins to include the alternative exon STREX converts the PKA enhancement of activation into inhibition (5, 79). Combining Ca2+ and PKA sensitivity likely provides the means for opening KCa1.1 during secretagogue activation. However, the typical voltage sensitivity is such that cytosolic Ca2+ would have to reach values near 10 μM for KCa1.1 to activate given the apical membrane voltage present in colonic epithelial cells (42). Since KCa1.1 forms channel complexes with auxillary subunits KCaβ (Kcnmb), the ability of KCaβ to alter activation kinetics (3, 9, 40, 60) may be responsible in part for producing the channel activity necessary to support K+ secretion. The experiments in this study examine involvement of KCa1.1 using specific inhibitors during stimulation with physiological secretagogues, as well as KCa1.1 localization within the colonic epithelium.
METHODS
Male guinea pigs (500–700 g body wt, Hartley strain, Hilltop Lab Animals, Scottdale, PA) received standard chow and water ad libitum and were housed on site at least 2 wk before experiments. Guinea pigs were euthanized with an animal decapitator (Harvard Apparatus, Holliston, MA) in accordance with a protocol approved by the Wright State University Laboratory Animal Care and Use Committee. Colonic mucosa was isolated as described previously (86). These isolated colonic mucosal sheets were used for measurement of transepithelial electrical parameters, mRNA detection by RT-PCR, protein detection by immunoblot, and confocal immunolocalization.
Transepithelial current measurement.
Isolated mucosal sheets were used for measurement of transepithelial current and conductance as described previously (39). Briefly, mucosal sheets were mounted in Ussing chambers (0.64-cm2 aperture), supported on the serosal face by nuclepore filters (∼10-μm thick, 5-μm pore diameter; Whatman, Clifton, NJ). Bathing solutions (10 ml) were circulated by gas-lift through water-jacketed reservoirs (38°C). Standard Ringer's solution contained (in mM) 145 Na+, 5.0 K+, 1.4 Ca2+, 1.2 Mg2+, 125 Cl−, 25 HCO3−, 4.0 H(3-X)PO4X−, and 10 d-glucose. Solutions were continually gassed with 95% O2-5% CO2, which maintained solution pH at 7.4. Automatic voltage clamps (Physiologic Instruments, San Diego, CA) permitted measurement of short-circuit current (Isc) and calculation of transepithelial conductance (Gt) from current responses to voltage pulses imposed across the mucosa ( ± 5 mV, 3-s duration, 60-s intervals). Isc was referred to as positive for cation flow across the epithelium from mucosal to serosal side (K+ secretion, negative; Cl− secretion, positive).
Mucosal responses to physiological secretagogues and to inhibitors were examined after producing a quiescent basal condition by suppressing neural and paracrine activators persisting in the isolated colonic mucosa (87). Removal of all muscle layers limited influences from nerves in this mucosal preparation. Compounds released from the mucosa into the bathing solutions were reduced in concentration (∼8,000-fold) by replacing the solutions three times after mounting the mucosa. Prostanoid production within the isolated mucosa was suppressed by the COx-1 inhibitor SC560 (1 μM) and the COx-2 inhibitor CAY10404 (1 μM) added to both bathing solutions. The action of peptide-YY and neuropeptide-Y released from mucosal cells was inhibited using the neuropeptide-receptor-Y2 antagonist BIIE0246 (1 μM) added to the serosal bath. Amiloride (10 μM) added to the mucosal bath inhibited electrogenic Na+ absorption. Sequential addition of the secretagogues epinephrine, prostaglandin E2 (PGE2), and carbachol (CCh) allowed examination of the full range in secretory responses from modulatory to flushing to synergistic. Prior addition of epinephrine does not alter subsequent PGE2 responses (23, 24, 39), and combined addition of PGE2 and CCh produces the synergistic mode of secretion (87).
Drugs were added in small volumes from concentrated stock solutions. CAY10404, prostaglandin E2, and SC560 were obtained from Cayman Chemical (Ann Arbor, MI); charybdotoxin (ChTx) from Abcam Biochemicals (Cambridge, MA); epinephrine from Hospira (Lake Forest, IL); and BIIE0246, iberiotoxin (IbTx), ICI-118551, and paxilline from Tocris Bioscience (Ellisville, MO). All other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Detection of mRNA and proteins.
Total RNA was extracted by RNeasy-Mini-Kit (Qiagen, Valencia, CA) from isolated mucosa and EDTA-released epithelial cells (38). Individual crypts were dissected under 40× magnification with Dumont no. 5 forceps (22). For isolated crypts, a guanidinium thiocyanate-phenol-chloroform extraction (TRIzol) method was used to obtain total RNA. Briefly (86), after reverse transcription of mRNA, cDNA was amplified by PCR. Primers specific for KCa1.1α (Kcnma1) and KCaβ (Kcnmb) (Table 1) were based on previous design (33, 52) and alignment of nucleotide sequences for human, mouse, and rat: initial denaturing 95°C (10 min), 40 cycles denaturation 94°C (1 min), annealing 55°C (1 min), extension 72°C (1 min), final extension 72°C (7 min). Primers for Kcnma1 amplified the exon 16–20 segment, which included ZERO/STREX splicing, as well as the alternative COOH-termini from the exon 26–27 segment and the exon 26–28 segment. Since multiple exons were transcribed for Kcnma1 and for Kcnmb, contamination with genomic DNA would have resulted in much larger products than those predicted and obtained. Splicing variants were verified by inspection of sequencing chromatograms. The two primer sets used for Kcnmb3 (61, 68) were forward 5′-ggt-ttg-cca-tga-tgg-gct-tct-c-3′ with reverse 5′-aca-gac-atc-tga-agg-cca-gca-c-3′ and forward 5′-cat-cgc-cat-gat-ggc-ctc-ct-3′ with reverse 5′-tca-gag-cgc-ctc-cca-gca-at-3′, but neither generated a product with homology to Kcnmb3.
Table 1.
Primers for KCa1.1α and KCaβ
| Forward | Reverse | ||
|---|---|---|---|
| KCa1.1α Kcnma1 | ex16–ex20 (STREX) | 5′-agc-cat-tga-gta-caa-gtc-tg-3′ | 5′-gga-gtc-cat-gtt-gtc-aat-ct-3′ |
| ex26–ex27 | 5′-tgg-tga-tct-gtt-ctg-caa-agc-3′ | B 5′-agc-cgc-tct-tcc-tgc-acg-tac-ttc-3′ | |
| ex26–ex28 ↓ | ↓ | E 5′-ctg-ctt-gtg-gat-tta-tat-tgg-3′ | |
| F 5′-tcc-tgg-gag-tca-aca-ttc-atc-3′ | |||
| G 5′-tag-ttc-tgg-tct-cct-ggg-agt-3′ | |||
| H 5′-tcc-acc-agt-gat-tca-gca-tgt-3′ | |||
| KCaβ1 Kcnmb1 | 5′-tgt-gct-gtc-atc-acc-tac-t-3′ | 5′-cat-ggc-aat-aat-gag-gag-3′ | |
| KCaβ2 Kcnmb2 | 5′-gac-tgg-cta-tga-tgg-tgt-g-3′ | 5′-ggt-cag-aat-agc-agg-aga-ag-3′ | |
| KCaβ4 Kcnmb4 | 5′-cgt-gtc-gct-ctt-cat-ctt-3′ | 5′-caa-tgc-agg-agg-aca-atc-3′ |
Reverse primers in exon 27 (B) and exon 28 (E, F, G, H) were used with the forward primer positioned in exon 26. (numbering based on constitutive exons for KCa1.1α, 15).
Proteins were isolated from colonic epithelial cells (86). Briefly, after disruption by sonication in a buffered solution containing protease inhibitors, samples were centrifuged to obtain a membrane sample. Following SDS-PAGE and transfer to polyvinylidene difluoride membranes, incubation with KCa1.1 (Kcnma1) specific primary antibody (1:1,000, NeuroMab clone L6/60 of mouse slo1, 75–022; UC Davis/NIH NeuroMab Facility, Davis, CA), and then with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) allowed detection of protein.
Tissue fixation and immunolocalizaton.
Colonic tissues were fixed after isolation, as described previously (24). Briefly, isolated mucosal sheets were chemically fixed followed by dehydration, sectioning, and mounting on gelatin-coated slides. Sections were permeabilized, blocked, and then incubated for 48 h (4°C) with primary antibody. Antibodies for KCa1.1 (Kcnma1) were obtained from UC Davis/NIH NeuroMab Facility (Davis, CA) [NeuroMab clone L6/60 (75–022), COOH terminus of mouse slo1; 1:50] and Santa Cruz Biotechnology (Santa Cruz, CA) [polyclonal rabbit-anti-MaxiKα (H300), COOH-terminal residues 937–1236 of human Kcnma1; 1:50]. Antiserum for Kcnma1 (53) was generously provided by A. J. Hudspeth at the Howard Hughes Medical Institute and Laboratory of Sensory Neuroscience, The Rockefeller University, New York, NY (1:200; rabbit antiserum-KCa1.1, residues KYVQEDRL). Secondary antibodies to detect immunoreactivity (2 h, room temp) were obtained from Invitrogen (Carlsbad, CA): donkey-anti-mouse IgG antibody, conjugated to AlexaFluor488 (4 ng/μl) and donkey-anti-rabbit IgG antibody, conjugated to AlexaFluor488 (4 ng/μl). Labeling of actin was obtained with phalloidin conjugated to AlexaFluor568 (0.005 units/μl). Sections were washed and mounted in Vectashield (Vector Labs, Burlingame, CA). Fluorescence of double-labeled sections was visualized with an Olympus FluoView FV300 confocal microscope in the Microscopy Core Facility of the WSU and PHP Neuroscience Institute. Images were acquired using identical confocal aperture, background, and gain settings.
Data analysis.
Responses of Isc and Gt to secretagogues and inhibitors were obtained from adjacent mucosae in each colon to permit direct comparisons. Recordings of Isc were digitized at 10-s intervals to examine the secretory time course. Concentration responses of Isc and Gt were fit by Henri-Michaelis-Menten binding curves using a nonlinear least-squares procedure. Results were reported as means and standard error of the mean (SE) with the number of animals (n) indicated. Statistical comparisons were made using two-tailed Student's t-test for paired responses, with significant difference accepted at P < 0.05.
RESULTS
The guinea pig distal colon produces high rates of electrogenic K+ secretion in response to many physiological neurotransmitters, hormones, and paracrine factors (23, 64, 87). Involvement of KCa1.1 during these agonist responses was examined by sensitivity to selective inhibitors.
KCa1.1 blockers inhibit β-adrenergic K+ secretion.
Basal Isc was negative, consistent with a low rate of ongoing K+ secretion in the ex vivo distal colonic mucosa (23). Addition of iberiotoxin (IbTx), an α-K channel toxin peptide blocker of KCa1.1 (18, 56), to the mucosal solution during this basal condition significantly reduced Isc toward zero (Fig. 1A, Table 2) and decreased Gt by 0.60 ± 0.15 mS/cm2 (n = 5, P = 0.017), supporting a contribution of KCa1.1 to apical membrane conductance during basal K+ secretion. Paxilline, an alkaloid blocker of KCa1.1 (56, 66, 73), also reduced basal Isc toward zero (Table 2) but with a slower time course.
Fig. 1.
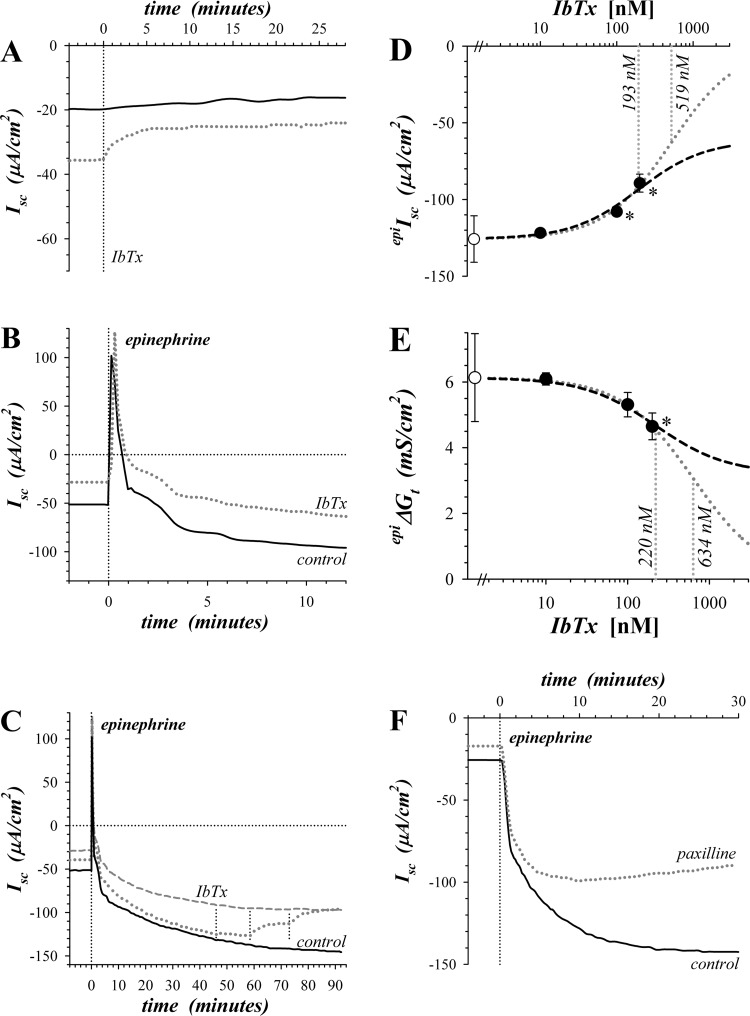
Sensitivity of epinephrine activated secretion to KCa1.1 blockers. Isolated mucosae were stimulated by epinephrine addition to the serosal solution from the standard basal condition with electrogenic secretion monitored by short-circuit current (Isc) (see methods). A: Isc was measured in adjacent mucosae during basal conditions with addition of IbTx (300 nM) to the mucosal solution of one (dotted line). B: adjacent mucosae were stimulated by epinephrine (5 μM) with one pretreated by mucosally added IbTx (200 nM) 60 min prior to epinephrine (dotted line). C: a third mucosa adjacent to those shown in panel B was treated with three successive concentrations of IbTx (10, 100, and 200 nM) beginning after epinephrine stimulation (dotted line), control (solid line), and IbTx pretreatment (dashed line). D and E: responses of epinephrine-activated Isc (epiIsc) and transepithelial conductance (epiΔGt) to 3 concentrations of IbTx were measured with an adjacent mucosa as time control. Control epiIsc and epiΔGt were −125.8 ± 15.2 μA/cm2 and +6.13 ± 1.34 mS/cm2 (○, n = 3), respectively. Fractional inhibition by IbTx was corrected for any time-dependent changes, averaged, and scaled to the control epiIsc or epiΔGt (●). Values significantly different from control (P < 0.05) are indicated by an asterisk (*). A fit of Henri-Michaelis-Menten kinetics with a single binding site was made to the concentration dependence from each mucosa and the average (n = 3) shown as a dashed line: epiIsc, IbTxIC50 = 193 ± 41 nM, maximal fractional inhibition = 0.513 ± 0.121; epiΔGt, IbTxIC50 = 220 ± 77 nM, maximal fractional inhibition = 0.476 ± 0.040. An additional fit was made assuming that IbTx could inhibit epiIsc or epiΔGt completely (dotted line). F: adjacent mucosae were stimulated by epinephrine (1.0 μM) with one pretreated by paxilline (1.0 μM) addition to both mucosal and serosal solutions 35 min prior to epinephrine (dotted line).
Table 2.
Action of KCa1.1 channel inhibitors on K+ secretion
| IbTx |
Paxilline |
|||||
|---|---|---|---|---|---|---|
| IbTxΔΔIsc, μA/cm2 | IbTxΔΔIsc/secΔIsc | n | paxΔΔIsc, μA/cm2 | paxΔΔIsc/secΔIsc | n | |
| Basal | +9.9 ± 0.6* (0.0001) | −0.258 ± 0.033* (0.001) | 5 | +4.1 ± 1.2* (0.017) | −0.145 ± 0.059* (0.043) | 8 |
| Epinephrine | +20.8 ± 5.1* (0.027) | −0.281 ± 0.047* (0.010) | 5 | +16.4 ± 5.0* (0.013) | −0.216 ± 0.054* (0.005) | 8 |
| ATP(transient) | +11.6 ± 2.6* (0.047) | −0.654 ± 0.209 (0.089) | 3 | +9.6 ± 1.4* (0.002) | −0.454 ± 0.038* (0.0003) | 5 |
| ATP(sustained) | +3.1 ± 0.9 (0.074) | −0.293 ± 0.069* (0.052) | 3 | +3.9 ± 1.9 (0.104) | −0.317 ± 0.102* (0.036) | 5 |
Values are means ± SE (P values). Secretagogue-activated short-circuit current (secΔIsc = secretagogue − control) was measured for epinephrine (Fig. 1) and ATP (Fig. 3), and inhibitor-induced differences in secΔIsc (inhibΔΔIsc = inhibitor − control) were compared as well as the fractional inhibition (inhibΔΔIsc/secΔIsc). Inhibitors were added prior to secretagogue activation: paxilline (mucosal and serosal) 1.0 μM; IbTx (mucosal) 300 nM for basal and epinephrine, 200 nM for ATP. Significant differences from zero are indicated by asterisks (P values).
Epinephrine activates a transient Cl− secretory response followed by sustained K+ secretion (epiIsc, 25). Mucosally added IbTx inhibited the negative Isc resulting from epinephrine activation of K+ secretion (Fig. 1B, Table 2) without altering the Cl− secretory Isc transient. Inhibition of the epinephrine-activated Gt (epiΔGt) by 1.21 ± 0.30 mS/cm2 (n = 5, P = 0.015) during IbTx treatment further supported a contribution by apical membrane KCa1.1 to epinephrine-activated electrogenic K+ secretion.
Sensitivity of epiIsc to IbTx was quantified further by consecutive addition of increasing concentration to the mucosal solution during ongoing epinephrine stimulation (Fig. 1C). IbTx inhibited ∼50% of epiIsc with an IC50 of 193 nM (Fig. 1D). Similarly, IbTx inhibited ∼50% of epiΔGt with an IC50 of 220 nM (Fig. 1E). This low sensitivity of electrogenic K+ secretion to IbTx supported involvement of β-subunits, KCaβ (Kcnmb), which would interfere with toxin binding (40, 51, 60). Use of higher IbTx concentrations to further substantiate the proportion of block would be confounded by increasing nonspecificity, because IbTx blocks other K+ channels at concentrations above 300 nM, particularly KV1.3, which is present in colonocytes (18, 20).
Paxilline (1.0 μM) also inhibited epiIsc (Fig. 1F, Table 2) and epiΔGt (−1.32 ± 0.40 mS/cm2, n = 5, P = 0.014) consistent with a contribution of KCa1.1 to producing electrogenic K+ secretion. Paxilline at concentrations above 1.0 μM also can inhibit Ca2+ ATPase and the inositol 1,4,5-trisphosphate receptor (4, 43). The extent of paxilline action on KCa1.1 was examined by restricting addition to the mucosal bathing solution and focusing on the early portion of the epinephrine response to obviate nonspecific actions. Addition of paxilline to only the mucosal solution (1.0 μM) produced a similar inhibition of epiIsc (Table 3) and epiΔGt (−1.27 ± 0.36 mS/cm2, n = 10, P = 0.006) as two sided addition, supporting an apical location for the KCa1.1 involved. Increasing the mucosal paxilline concentration to 10 μM enhanced inhibition of epiIsc (Table 3) with a fractional inhibition of ∼50%, similar to the maximal value estimated for IbTx (Fig. 1D). Further inhibition at longer incubation may be confounded by inhibition of other proteins involved in Ca2+ signaling.
Table 3.
Action of mucosal paxilline on epinephrine-activated K+ secretion
| Paxilline (1.0 μM) |
Paxilline (10 μM) |
|||||
|---|---|---|---|---|---|---|
| pax-1ΔΔIsc, μA/cm2 | pax-1ΔΔIsc/secΔIsc | n | pax-10ΔΔIsc, μA/cm2 | pax-10ΔΔIsc/secΔIsc | n | |
| Basal | +1.4 ± 1.4 (0.33) | −0.020 ± 0.033 (0.57) | 10 | +6.7 ± 1.1* (0.0001) | −0.155 ± 0.059* (0.0001) | 12 |
| Epinephrine | +16.0 ± 4.5* (0.006) | −0.170 ± 0.043* (0.004) | 10 | +43.3 ± 3.9* (0.0001) | −0.495 ± 0.042* (0.0001) | 12 |
Values are means ± SE (P values). Secretagogue-activated Isc (secΔIsc = secretagogue − control) was measured for epinephrine (Fig. 1), and inhibitor induced differences in secΔIsc (inhibΔΔIsc = inhibitor − control) were compared as well as the fractional inhibition (inhibΔΔIsc/secΔIsc). Paxilline (mucosal) was added prior to secretagogue activation. Significant differences from zero are indicated by asterisks (P values).
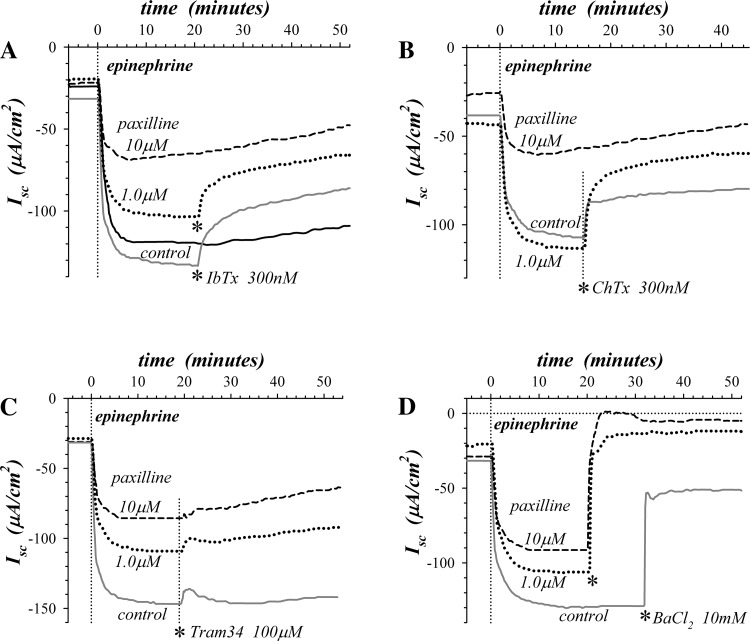
Since paxilline promotes binding of IbTx and ChTx to KCa1.1 (26, 35, 55), epiIsc was measured in the combined presence of paxilline and toxin. Treatment with the β2-adrenergic receptor antagonist ICI-118551 suppressed the Cl− secretory transient (25, 87) which revealed the full K+ secretory time course. IbTx (300 nM) added during epinephrine stimulation produced greater inhibition of epiIsc with paxilline (1.0 μM) present, and approached the fractional inhibition produced by 10 μM paxilline alone (Fig. 2A). ChTx also exhibited a similar synergistic inhibition of epiIsc (Fig. 2B). These interactions between KCa1.1 inhibitors that bind to distinct sites in the channel (60, 88) provided additional support for the involvement of KCa1.1 in epinephrine-activated K+ secretion.
Fig. 2.
Sensitivity of epinephrine-activated K+ secretion to combined K+ channel blockers. Adjacent mucosae were pretreated with the β2-adrenergic receptor antagonist ICI-118551 (1.0 μM) added to the serosal solution 30 min prior to epinephrine (5 μM) stimulation. A: 2 of 4 mucosae were pretreated with mucosally added paxilline 55 min prior to epinephrine, at either 1.0 μM (dotted line) or 10 μM (dashed line). After maximal activation, IbTx (300 nM) was added mucosally to control (gray solid line) and 1.0 μM paxilline pretreated mucosae (asterisks). A control mucosa without channel inhibitors is shown (black solid line). Combined IbTx/pax fractional inhibition was 0.77 compared with maximal inhibition (10 μM paxilline), exceeding the expectation of 0.67 for independent inhibitor actions. B: 2 of 3 mucosae were pretreated mucosally with paxilline 60 min prior to epinephrine, at either 1.0 μM (dotted line) or 10 μM (dashed line). After maximal activation ChTx (300 nM) was added mucosally to control (solid line) and 1.0 μM paxilline-pretreated mucosae (asterisk). Combined ChTx/pax fractional inhibition was 0.74 compared with 10 μM paxilline, exceeding the expectation of 0.49 for independent inhibitor actions. C and D: 2 of 3 mucosae were pretreated with paxilline 50 min prior to epinephrine, at either 1.0 μM mucosal and serosal (dotted line) or 10 μM mucosal (dashed line). After maximal activation, either Tram34 (100 μM) or BaCl2 (10 mM) was added mucosally to control (solid line) and paxilline-pretreated mucosae (asterisks).
The contribution of KCa3.1 (Kcnn4) to apical membrane K+ conductance was examined by sensitivity of epiIsc to Tram34 (1, 24). The minor Tram34 inhibition observed in the presence of paxilline (Fig. 2C) indicated that the remaining epiIsc was not due to a large involvement of KCa3.1 or the other K+ channels blocked by this high concentration of Tram34 (83). The relatively nonselective K+ channel blocker Ba2+ inhibited epiIsc in the presence of paxilline (Fig. 2D), illustrating further the reliance of K+ secretion on apical membrane K+ channels (21, 30, 64). The incomplete inhibition of control epiIsc by Ba2+ suggested that KCa1.1 was relatively resistant to this Ba2+ block.
KCa1.1 blockers inhibit purinergic K+ secretion.
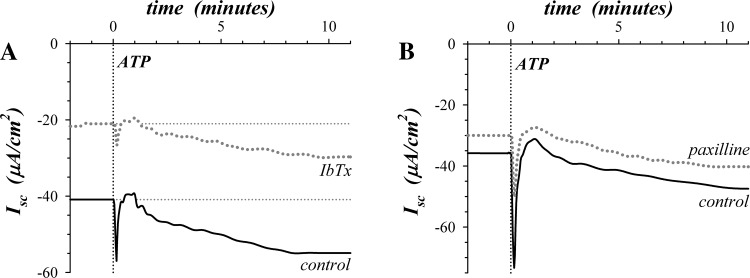
ATP activates a transient electrogenic K+ secretory response followed by slower activating sustained K+ secretion (87). IbTx and paxilline inhibited both the transient and sustained components of purinergic K+ secretion (Fig. 3, Table 2). The fractional inhibition of the sustained response was similar to that for epinephrine activated K+ secretion, suggesting a ∼50% contribution by KCa1.1 to the apical membrane K+ conductance required for secretion. In contrast, the transient ATP response appeared to require KCa1.1 as the sole apical K+ channel, although the fractional inhibition was not statistically different from that for the sustained response.
Fig. 3.
Sensitivity of ATP-activated K+ secretion to KCa1.1 blockers. Isolated mucosae were stimulated by ATP (100 μM) addition to the mucosal solution from the standard basal condition with electrogenic secretion monitored by Isc. A: adjacent mucosae were stimulated by ATP with one pretreated by IbTx (200 nM) addition to the mucosal solution 25 min prior to ATP (dotted line). B: adjacent mucosae were stimulated by ATP with one pretreated by paxilline (1.0 μM) addition to both mucosal and serosal solutions 20 min prior to ATP (dotted line).
KCa1.1 blockers inhibit K+ secretion during Cl− secretory activation.
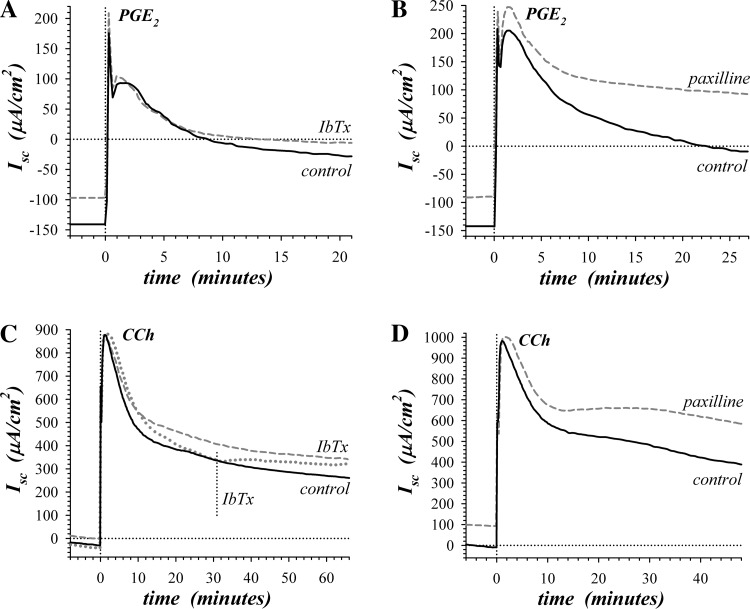
Electrogenic K+ secretion also accompanies the Cl− secretion stimulated by prostaglandin E2 (PGE2) and carbachol (CCh) (64, 87). During these secretory modes, the rate of Cl− secretion exceeds that for K+ such that the resulting Isc is positive. As might have been anticipated, PGE2Isc (Fig. 4, A and B) was more positive in the presence of paxilline (+31.8 ± 8.4 μA/cm2, n = 4, P = 0.032); however, PGE2Isc was not consistently altered with IbTx (−9.4 ± 18.0 μA/cm2, n = 5, P = 0.63). By comparing the PGE2-induced difference in Isc from the prior steady-state epinephrine activation, the change in Isc (PGE2ΔIsc) represented increases in Cl− secretory rate together with any differences in K+ secretion between the epinephrine condition and the PGE2 condition. In the presence of paxilline, PGE2ΔIsc was essentially identical with control PGE2ΔIsc (Table 4), indicating that the transition to this secretory mode by PGE2 occurred primarily via increasing Cl− secretion and likely leaving the K+ secretory rate similar to the rate activated by epinephrine. The significantly lower PGE2ΔIsc in the presence of IbTx (Table 4) could result from decreased Cl− secretion; but more likely given the result with paxilline, K+ secretion became more resistant to IbTx during PGE2 activation.
Fig. 4.
Sensitivity of prostanoid and synergistic secretion to KCa1.1 blockers. Isolated mucosae were stimulated consecutively by serosal addition of epinephrine (5 μM), PGE2 (3 μM), and CCh (10 μM). A: during the prostanoid secretion initiated by PGE2, Isc was measured in adjacent mucosae with one pretreated by IbTx (300 nM) addition to the mucosal solution 170 min prior to PGE2 (dashed line). B: during PGE2-initiated secretion, Isc was measured in adjacent mucosae with one pretreated by paxilline (1.0 μM) addition to both mucosal and serosal solutions 60 min prior to PGE2 (dashed line). C: during the synergistic secretion stimulated by CCh, Isc was measured in adjacent mucosae with one pretreated mucosally by IbTx (100 nM) addition 170 min prior to CCh (dashed line). A third adjacent mucosa was treated mucosally with IbTx (100 nM) during CCh activation (dotted line). D: during CCh-stimulated synergistic secretion, Isc was measured in adjacent mucosae with one pretreated by paxilline (1.0 μM) addition to both mucosal and serosal solutions 80 min prior to CCh (dashed line).
Table 4.
Action of KCa1.1 channel inhibitors on Cl− and K+ secretion
| IbTx |
Paxilline |
|||||
|---|---|---|---|---|---|---|
| IbTxΔΔIsc, μA/cm2 | IbTxΔΔIsc/secΔIsc | n | paxΔΔIsc, μA/cm2 | paxΔΔIsc/secΔIsc | n | |
| PGE2 | −45.0 ± 15.3* (0.042) | −0.218 ± 0.058* (0.020) | 5 | +0.4 ± 25.1 (0.99) | +0.055 ± 0.127 (0.69) | 4 |
| CCh/PGE2 | +90.4 ± 25.1* (0.023) | +0.499 ± 0.222 (0.088) | 5 | +98.3 ± 19.0* (0.014) | +0.467 ± 0.206 (0.108) | 4 |
Values are means ± SE (P values). Secretagogue-activated Isc (secΔIsc = secretagogue − control) was measured for PGE2 and CCh/PGE2 (Fig. 4), and inhibitor-induced differences in secΔIsc (inhibΔΔIsc = inhibitor − control) were compared as well as the fractional inhibition (inhibΔΔIsc/secΔIsc). PGE2 (3 μM) was added during steady-state epinephrine activation and CCh (10 μM) was added after PGE2. Inhibitors were added prior to secretagogue activation: paxilline (mucosal and serosal) 1.0 μM; IbTx (mucosal) 300 nM. Significant differences from zero are indicated by asterisks (P values).
Activation of the synergistic secretory mode by stimulating with CCh in the presence of PGE2 (25, 87) produced a large positive Isc consistent with a high Cl− secretory rate (Fig. 4, C and D). Inhibition of KCa1.1 led to a more positive CChIsc with either IbTx (+81.0 ± 19.4 μA/cm2, n = 5, P = 0.014) or paxilline (+138.8 ± 27.0 μA/cm2, n = 4, P = 0.014). Addition of IbTx during the synergistic secretory response quickly led to a more positive Isc consistent with inhibiting a synergistic component of K+ secretion. Comparing CChΔIsc in the presence and absence of inhibitors indicated that IbTx and paxilline had similar actions on the synergistic mode (Table 4), likely suppressing the K+ secretory component of the response. The response to 10 μM paxilline added mucosally was not significantly different from that to 1 μM added to both mucosal and serosal baths (data not shown).
Distal colonic epithelial cells express KCa1.1.
Immunoblotting with a monoclonal antibody allowed detection of protein expression for KCa1.1α in colonic epithelial cells (Fig. 5). RT-PCR of mRNA from colonic epithelial cells using three primer sets (Fig. 6A) confirmed the presence of KCa1.1α and demonstrated splicing events. Sequences for guinea pig KCa1.1α have been deposited with GenBank (accession numbers JQ241358, JQ241359, JQ241360). The product from the exon-16 to exon-20 region indicated the absence of the insertion exon STREX, which is termed the ZERO variant (84). Inclusion of STREX would have resulted in a larger product. Primers for detecting the alternative terminations for KCa1.1α yielded products (Fig. 6A) consistent with termination in exon-27 or an early termination in exon-28 (Fig. 6B). The termination in exon-27 resulted in an amino acid sequence ending of QEERL, whereas the termination in exon-28 resulted in an amino acid sequence ending of EMVYR (15). The splicing of exon-28 occurred with two variants that differed by an omission of 3 bp at the beginning of exon-28. Insertions and omissions expected for shifts in the reading frame that lead to the other two known endings (53, 65, 85) were not detected in the PCR products.
Fig. 5.
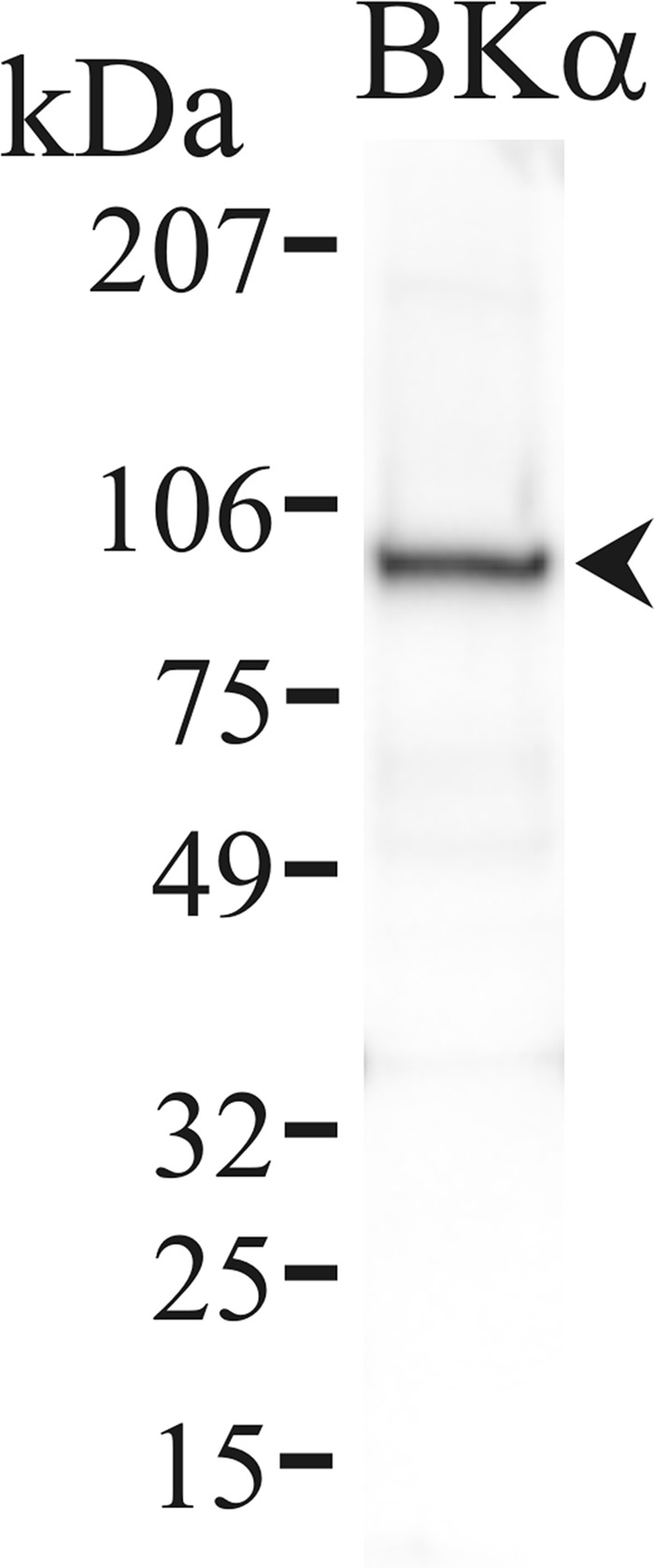
Detection of KCa1.1α in colonic epithelial cells. Protein isolated from the membrane fraction of distal colonic epithelial cells was immunoblotted with an antibody against the KCa1.1 protein (Kcnma1) NeuroMab clone L6/60. An immunoreactive band consistent with KCa1.1 occurred at ∼100 kDa (arrowhead) using this monoclonal antibody. Use of the secondary antibody alone eliminated all bands (data not shown), indicating that the primary antibody was necessary for the observed result.
Fig. 6.
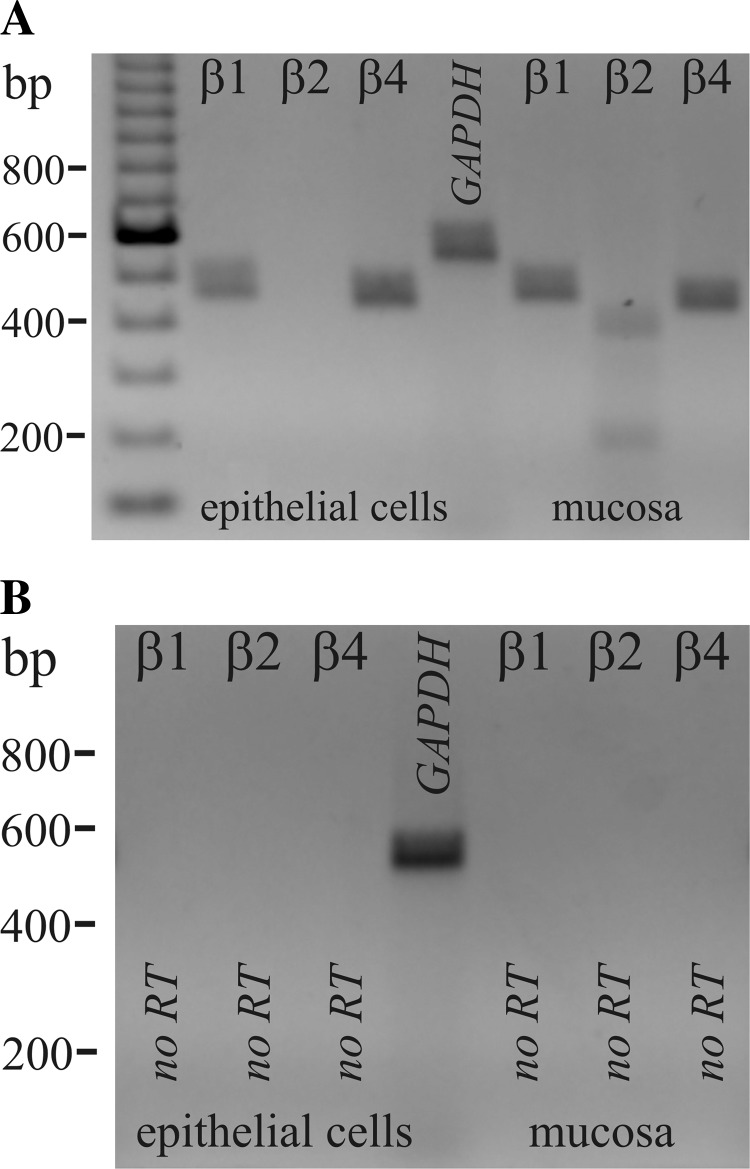
Expression of mRNA for KCa1.1α. A: RT-PCR of colonic epithelial cell mRNA amplified KCa1.1(Kcnma1) products with sizes predicted by the position of the primers, 357 base pairs for the segment from exon-16 to exon-20 (534 bp including the insertion exon STREX), 351 base pairs for exon-26 to exon-27, and 445 base pairs for exon-26 to exon-28 (numbering based on constitutive exons, 15). Sequencing of products confirmed identity with KCa1.1 (homology with human 95%, 95%, 97%). Amplification of a GAPDH product (555 bp) served as positive control for RNA isolation. Absence of product when not including reverse transcriptase (RT) indicated further the lack of genomic DNA contamination. B: sequences for rat, mouse, human, and guinea pig are shown (differences from human indicated by shading) together with the splice site for the alternative COOH termini of KCa1.1 (alternative reading frames illustrated by the distinct amino acid sequences). The beginning of exon-28 is indicated (◇) as well as amino acid variations resulting from sequence differences (=). QEERL ending: [27] exon-27(stop), human NM_001161352, mouse NM_001253364; EMVYR endings: [28a] exon-28(stop), human NM_001014797, mouse NM_010610; [28a*] exon-28(skip 1st 3 bp; stop), mouse NM_001253363, rat NM_031828. VEDEC endings: [28b] insert(1 bp) + exon-28(skip 1st 2 bp; stop), rabbit AB009312; [28b*] insert(3n + 2 bp) (n = 9, 3′-end exon-27) + exon-28(stop), mouse NM_001253358; FKSSP endings: insert(3n + 1 bp) + exon-28(stop); [28c] n = 0 rat U93052 or [28c*] n = 15 rat U55995. The transcripts in guinea pig distal colonic epithelial cells included three alternate sequences: QEERL[27], EMVYR[28a], and EMVYR[28a*].
The β-subunit for KCa1.1, KCaβ (Kcnmb), modifies activation kinetics and inhibitor sensitivity (3, 9, 40, 51, 60). RT-PCR of mRNA from colonic epithelial cells indicated the presence of KCaβ1 and KCaβ4 (Fig. 7). Expression of KCaβ2 mRNA was detected in colonic mucosal samples, serving as a positive control for the apparent absence of KCaβ2 in colonic epithelial cells and indicating expression in an interstitial cell type only. Sequences for guinea pig KCaβ1, KCaβ2, and KCaβ4 have been deposited with GenBank (accession numbers JQ241361, JQ241362, JQ241363). Primers designed from either the human or rat sequence (61, 68) failed to result in products consistent with KCaβ3, leaving the presence of KCaβ3 in guinea pig distal colonic epithelial cells unresolved.
Fig. 7.
Expression of mRNA for KCaβ. A: RT-PCR of colonic epithelial cell mRNA and colonic mucosal mRNA amplified Kcnmb products with sizes predicted by the position of the primers, 477 base pairs for Kcnmb1 (KCaβ1), 405 base pairs for Kcnmb2 (KCaβ2), and 456 base pairs for Kcnmb4 (KCaβ4). Sequencing of products confirmed identity with Kcnmb (homology with human 90%, 95%, 95%). Amplification of a GAPDH product (555 bp) served as positive control for RNA isolation. B: absence of product when not including reverse transcriptase indicated the lack of genomic DNA contamination.
Localization of KCa1.1 in distal colonic epithelial cells.
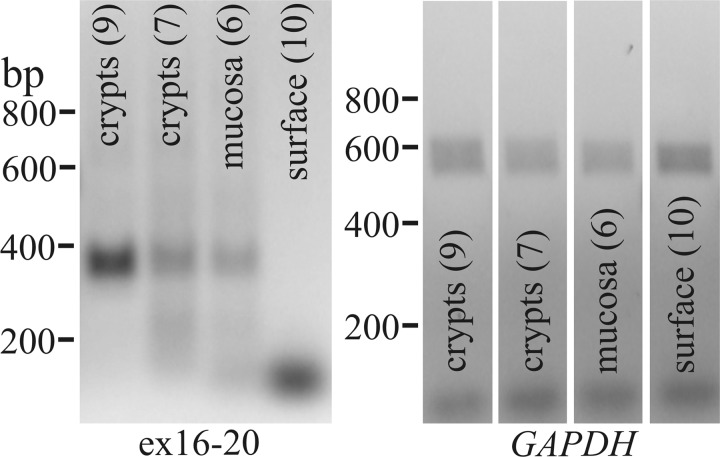
Numerous studies have indicated the presence of KCa1.1 in colonic epithelial cells within the apical and basolateral membranes at both surface and crypt locations (14, 28, 47, 70, 71, 75, 77). Expression of KCa1.1α mRNA was examined by RT-PCR for samples from crypts isolated by manual dissection. Crypts were sectioned to remove any surface cells and included only the lower two-thirds to one-half of each crypt. Portions of intact mucosa were processed as well as portions of mucosa after the crypts had been removed entirely. A product consistent with that found in larger mucosal samples (Fig. 6) was detected in crypt samples but not from surface epithelium (Fig. 8).
Fig. 8.
Distribution of KCa1.1α in the colonic epithelium. RT-PCR of colonic crypt mRNA amplified a 357 base pair KCa1.1 product for the segment from exon-16 to exon-20. Samples included 9 crypts, 7 crypts, mucosal piece containing 6 crypts, and surface epithelium after complete removal of 10 crypts from a mucosal piece. GAPDH products indicated isolation of RNA from each sample.
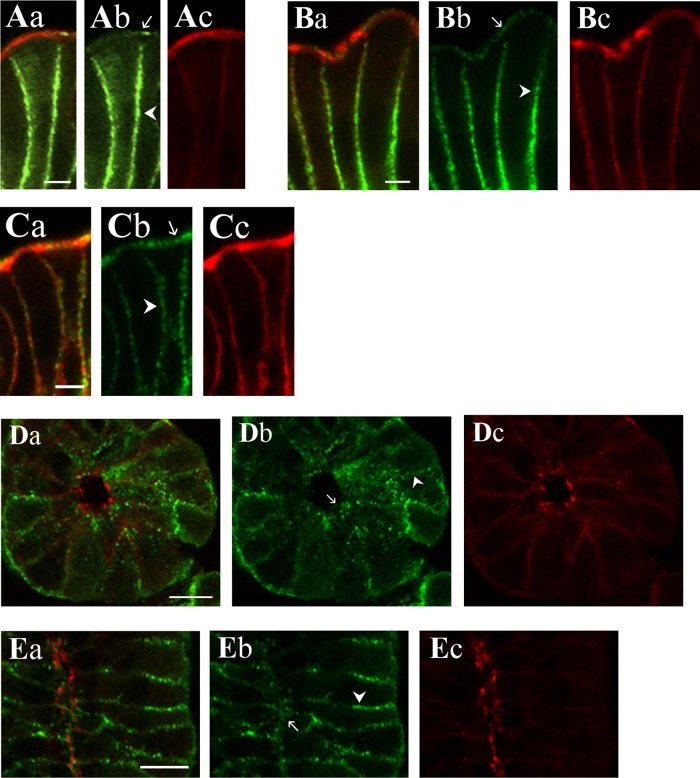
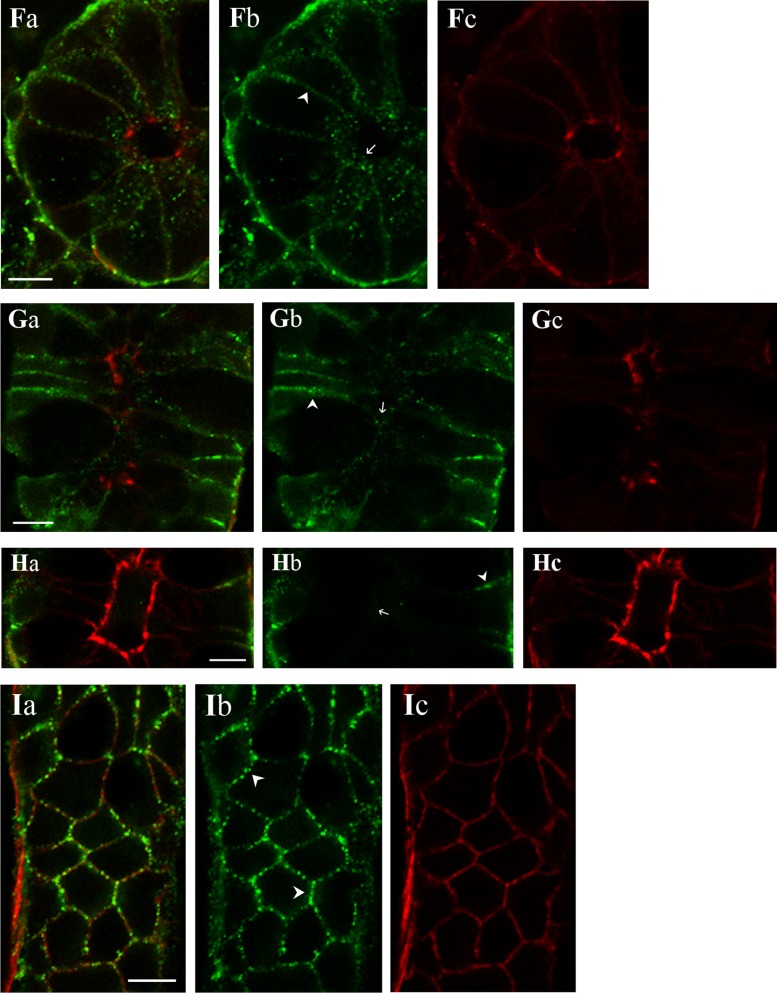
Immunolocalization (Fig. 9) exhibited KCa1.1α immunoreactivity (KCa1.1αIR) in colonic epithelial cells from both the surface and crypt regions. The use of three antibodies validated in several cell types (6, 53, 54, 59) supported the identity of this localization as KCa1.1α. For both surface and crypt cells, KCa1.1αIR occurred as puncta along the cell margins consistent with a presence in lateral membranes (Fig. 9). Apical localization of KCa1.1α was apparent in the brush border of surface cells (Fig. 9, A and B) and in the apical pole of crypt cells (Fig. 9, D–G). The termination in exon-27 results in a protein ending with the amino acids, QEERL. Using an anti-serum specific for the chicken COOH-terminal sequence (QEDRL), KCa1.1αIR was detected in the brush border and lateral membranes of surface cells (Fig. 9C). KCa1.1αIR-(QEDRL) was not apparent in the apical pole of crypt cells, but rather only in cell margins close to the base of crypt cells (Fig. 9H). A longitudinal profile along the edge of a crypt showed this KCa1.1αIR-(QEDRL) as the outline of cell margins (Fig. 9I). Perhaps an alternate COOH terminus such as EMVYR allowed trafficking to the apical location within crypt cells.
Fig. 9.
KCa1.1α localization in the colonic epithelium. KCa1.1α was detected in distal colonic mucosa by immunoreactivity to 3 antibodies (KCa1.1αIR; green; b). Actin detected with labeled phalloidin revealed the morphology of the epithelium (red; c) for cellular localization of KCa1.1αIR (a). Prominent KCa1.1αIR was apparent in surface cells along the lateral cell margins (arrowhead) and the brush-border membrane (arrow). Labeling was similar with each antibody, anti-KCa1.1α-L6/60 (A), anti-KCa1.1α-H300 (B), and anti-KCa1.1α-QEDRL (C). Scale bars, 5 μm. Crypt cross-section and longitudinal profiles are shown (D–I). Prominent KCa1.1αIR was apparent in crypt cells along the lateral cell margins (arrowhead). Apical poles also exhibited punctate KCa1.1αIR (arrow). Labeling was similar with anti-KCa1.1α-L6/60 (D and E) and anti-KCa1.1α-H300 (F and G) antibodies. Anti-KCa1.1α-QEDRL (H and I) exhibited KCa1.1αIR only along the lateral margins at the basal end of the crypt cells (arrowhead); luminal margin is indicated by an arrow. Scale bars, 10 μm. Use of the secondary antibody alone led to loss of labeling (data not shown), indicating that the primary antibodies were necessary for the labeling observed.
DISCUSSION
Sympathetic activation of K+ secretion.
β-Adrenergic activation of electrogenic K+ secretion conforms to the cellular model for Cl− secretion with the inclusion of apical membrane K+ channels (21, 29). Sensitivity to IbTx and paxilline supported a reliance on KCa1.1 for ∼50% of apical membrane K+ exit (Fig. 1, D and E, Table 3). The relatively high IC50 for IbTx (∼200 nM) may relate to the involvement of KCaβ subunits that reduce blocker affinity. A splice variant of KCa3.1 (Kcnn4) with low sensitivity to Tram34 occurs in the apical membrane of rat colon (1), but the only minor inhibition by Tram34 at high concentration (24, Fig. 2C) indicates that KCa3.1 was likely not responsible for the remaining apical K+ exit during epinephrine activated K+ secretion.
Previous studies comparing epinephrine responses in distal colon from KCa1.1-null mice and wild-type mice clearly demonstrate a requirement for the KCa1.1 channel in producing electrogenic K+ secretion (76). However, because of the calculations necessary to account for the concomitant Cl− secretion during the early time course examined, the exact proportion of the β-adrenergic K+ secretion dependent on KCa1.1 was not determined definitively. The higher rate of β-adrenergic K+ secretion in guinea pig distal colon (∼5-fold) made the determination of the contribution by KCa1.1 more precise (Fig. 1D), but it may be that the guinea pig generated a higher K+ secretion than mouse simply by using additional types of K+ channels in the apical membrane. If these K+ channel types activate with distinct time courses, then sensitivity during the early and sustained phases would differ. Overall, KCa1.1 provided a significant if not sole contribution to the apical membrane K+ conductance activated by epinephrine in mammalian distal colon.
Purinergic activation of K+ secretion.
Addition of ATP to the luminal side of the distal colon produces a small transient electrogenic K+ secretion that is absent in KCa1.1-null mice (70). This absolute dependence on KCa1.1 was supported by the inhibitor sensitivity of this transient ATP response in guinea pig distal colon (Fig. 3, Table 2). The sustained component of the ATP response involving adenosine (87) had an inhibitor sensitivity supporting a smaller contribution by KCa1.1 during adenosine receptor stimulation, similar to that for epinephrine-activated K+ secretion (Table 2). Thus each K+ secretagogue activates a specific set of K+ channels with KCa1.1 taking a smaller or larger role depending on the character of the physiological response.
Cl− secretagogues.
Activation of Cl− secretion in colon generally includes an attendant electrogenic K+ secretion (21, 34, 64). The relative proportion of K+ to Cl− secretion varies with secretagogue type and time after activation. Inhibition of apical membrane K+ channels increases the measured Isc (as K+ secretion diminishes) whereas inhibition of basolateral membrane K+ channels decreases Isc (as Cl− secretion diminishes). The generally positive Isc during Cl− secretagogue activation in the presence of KCa1.1 blockers (Fig. 4, B and D), or in colonic mucosa from KCa1.1-null mice (50), suggested that KCa1.1 present in the basolateral membrane were of minor importance for sustaining Cl− secretion. Instead, apical membrane KCa1.1 contributed to K+ secretion during prostanoid (PGE2) and synergistic (PGE2/CCh) stimulation of Cl− secretion.
Apical membrane K+ channels provide a route for K+ exit during K+ secretion, but also contribute to setting the membrane electrical potential difference and thereby to defining the electrochemical gradients for ion flow. Together with basolateral K+ channels, apical K+ channels maintain a negative membrane potential that drives Cl− exit. As presented for rat distal colon (36), apical K+ channels could ensure apical Cl− exit and perhaps have the largest influence during periods of similar K+ and Cl− secretory rates when apical K+ conductance would be substantial. Inhibition of the PGE2 response by mucosally added IbTx (Table 4) seemed to support this concept of apical K+ channels maintaining apical Cl− exit, except that block with paxilline left the PGE2 response unaltered (Table 4). This discrepancy of action for the two KCa1.1 blockers supported an alternative proposal in which PGE2 increased K+ secretion above the epinephrine-stimulated rate by activating KCa1.1 even more resistant to IbTx, possibly by recruiting KCa1.1α with different KCaβ's. The similarity of blocker sensitivity with PGE2/CCh stimulation (Table 4) supported a return of moderate IbTx sensitivity during synergistic activation.
Epithelial localization of KCa1.1.
An epithelial presence for KCa1.1 permits a contribution to various transepithelial flows. Apical localization provides for K+ exit into the lumen in support of K+ secretion, while basolateral localization supports other flows dependent on the basolateral Na+/K+-ATPase (2, 30). Both surface and crypt cells of guinea pig distal colon exhibited KCa1.1αIR in apical and basolateral membranes (Fig. 9). KCa1.1αIR in crypts suggested localization in apical vesicles perhaps representing a pool of channels ready for insertion. Previous studies support a more restrictive KCa1.1α localization. Immunolabeling and IbTx binding indicate a predominant presence in apical and basolateral membranes of surface cells but with an increase in crypt labeling during various stresses (14, 28, 47, 68, 71). Other studies localize KCa1.1αIR only to crypt apical membranes (70, 75). KCa1.1α mRNA distribution also supported the presence in colonic crypts (77, Fig. 8). The source of these discrepancies in localization may include species differences, physiological state of the animals, and sensitivity of detection methods. The high K+ secretory rate exhibited by guinea pig distal colon indicated a normal physiological state with a large contingent of apical membrane K+ channels, thus making detection easier.
Alternative splicing of KCa1.1α mRNA alters many properties of the resulting channels including regulation and membrane trafficking (3, 10). Inclusion of the STREX exon converts KCa1.1 sensitivity from cAMP activation to inhibition (5, 79). The mouse colon expresses both the ZERO and STREX variants (13, 76) with stimulation by aldosterone making the ZERO variant predominant (76) thereby strengthening cAMP activation. The apparently exclusive expression of the ZERO variant in guinea pig distal colon (Fig. 6A) presumably contributed to the large capacity for K+ secretion by favoring KCa1.1 activation.
Trafficking of KCa1.1 to apical and basolateral membranes determines its role either as a component of the K+ secretory path or as a supporting element for Cl− secretion or Na+ absorption. COOH-terminal splicing of KCa1.1 alters its membrane expression with QEERL and EMVYR variants trafficking to the plasma membrane (7, 45). The lack of KCa1.1αIR for the QEERL variant in the crypt apical pole (Fig. 9H) suggested that the COOH terminus contributes to the routing signals for the apical and basolateral membranes (37). Since surface cell apical membranes exhibited KCa1.1αIR for the QEERL variant (Fig. 9C), this apical membrane targeting distinction appeared to be specific for crypt cells, which would require the EMVYR variant.
Epithelial regeneration in the colon occurs via stem cells in the base of the crypt producing cells that move along the crypt structure to positions in the surface epithelium (30). During this progression KCa1.1αIR appeared in both apical and basolateral membranes (Fig. 9), such that subunit composition of apically located KCa1.1 changed as cells moved from crypt to surface (Fig. 9, C and H). KCa1.1α mRNA expression also decreases in the transition from crypt to surface of mouse colon (77). The apparent lack of mRNA for KCa1.1α in guinea pig surface cells supported this finding (Fig. 8) despite the surface presence of KCa1.1αIR (Fig. 9, A–C). Presumably KCa1.1α persisted during the ∼1 day duration of surface cells without replenishment by protein synthesis, suggesting a relatively long life-time for the KCa1.1α protein.
Auxillary KCa1.1 subunits.
The KCaβ subunits (Kcnmb) contribute to cell type-specific activity of KCa1.1 (3, 9, 40, 60). Of the behaviors promoted by these subunits, KCaβ1 would best suit sustained K+ secretion by its increasing of Ca2+ sensitivity and shifting of voltage activation toward negative values. The slower activation and lower Ca2+ sensitivity produced with KCaβ4 also might serve K+ secretory requirements, but the rapid inactivation conferred by KCaβ2 and some splice variants of KCaβ3 would limit the utility of KCa1.1 for sustained K+ secretion. Previous studies indicate expression of KCaβ mRNA in colonic epithelium with conflicting results. In mouse colon, KCaβ1, KCaβ2, and KCaβ4 were detected (14, 62), but another study detected only KCaβ2 (75). Human sigmoid colon expresses all four KCaβ's (68). Perhaps reflecting species differences, guinea pig distal colonic epithelial cells expressed only KCaβ1 and KCaβ4 (Fig. 7).
KCaβ subunits also confer differential sensitivities to the toxin blockers IbTx and ChTx (51, 60) permitting a functional test of which KCaβ's combine with KCa1.1 to facilitate K+ secretion. Interaction with KCaβ1 or KCaβ4 lowers sensitivity of KCa1.1 to IbTx, whereas KCaβ2 or KCaβ4 lowers sensitivity to ChTx. The low sensitivity of K+ secretion in guinea pig distal colon to both IbTx and ChTx (Figs. 1D and 2B, Table 2) supported the involvement of KCaβ4 together with KCa1.1 in a secretory K+ channel complex. Since KCa1.1 and KCaβ combine in a 1:1 manner, the tetrameric KCa1.1 channel complex would include four individual KCaβ subunits. The extremely low sensitivity for IbTx conferred by KCaβ4 (57) suggested that the observed K+ secretory sensitivity to IbTx resulted from a KCa1.1 complex including both KCaβ1 and KCaβ4 such that ChTx sensitivity was reduced.
K+ secretory activation.
A variety of secretagogues stimulate colonic K+ secretion apparently via cells capable of Cl− secretion (29, 64) with an output ranging from primarily K+ secretion (Figs. 1 and 3) to mostly Cl− secretion (Fig. 4). Salivary glands also secrete fluid driven by electrogenic Cl− secretion that includes K+ secretion. The apical K+ exit involves KCa1.1α together with KCaβ1 and KCaβ4 (57), similar to guinea pig distal colonic K+ secretion. Recent studies demonstrate that flow-dependent K+ secretion in the kidney emanates from intercalated cells using apical membrane KCa1.1 and basolateral Na+:K+:2Cl−-cotransporters (41). Localization of KCaβ's in the kidney indicates that KCaβ4 occurs in the intercalated cells, and KCaβ1 segregates to neighboring principal cells (19). Thus intercalated cells secrete K+ via KCa1.1/KCaβ4, together with acid secretion, whereas principal cells secrete K+ via KCa1.1/KCaβ1, together with Na+ absorption (31). A common theme for K+ secretory cells is the matching of K+ channel complexes to the physiological demands of the organ; and distal colonic epithelium will use KCa1.1/KCaβ complexes that best serve the physiological requirements of this distal-most gastrointestinal site.
GRANTS
This study was supported by a grant from the National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases (DK-65845).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z., S.T.H., and D.R.H. conception and design of research; J.Z., S.T.H., and D.R.H. performed experiments; J.Z., S.T.H., and D.R.H. analyzed data; J.Z., S.T.H., and D.R.H. interpreted results of experiments; J.Z. and D.R.H. drafted manuscript; S.T.H. and D.R.H. edited and revised manuscript; D.R.H. prepared figures; D.R.H. approved final version of manuscript.
REFERENCES
- 1. Barmeyer C, Rahner C, Yang Y, Sigworth FJ, Binder HJ, Rajendran VM. Cloning and identification of tissue-specific expression of KCNN4 splice variants in rat colon. Am J Physiol Cell Physiol 299: C251–C263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrett KE, Keely SJ. Integrative physiology and pathophysiology of intestinal electrolyte transport. Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 2006, p. 1931–1951 [Google Scholar]
- 3. Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bilmen JG, Wootton LL, Michelangeli F. The mechanism of inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase by paxilline. Arch Biochem Biophys 406: 55–64, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cheron G, Sausbier M, Sausbier U, Neuhuber W, Ruth P, Dan B, Servais L. BK channels control cerebellar Purkinje and Golgi cell rhythmicity in vivo. PLoS One 4(11): e7991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chiu YH, Alvarez-Baron C, Kim EY, Dryer SE. Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of an Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol 77: 497–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooke HJ, Christofi FL. Enteric neural regulation of mucosal secretion. Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 2006, p. 737–762 [Google Scholar]
- 9. Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol 116: 411–432, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci 66: 852–875, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duthie HL, Atwell JD. The absorption of water, sodium, and potassium in the large intestine with particular reference to the effects of villous papillomas. Gut 4: 373–377, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flores CA, Cid LP, Sepúlveda FV. Strain-dependent differences in electrogenic secretion of electrolytes across mouse colon epithelium. Exp Physiol 95: 686–698, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Flores CA, Melvin JE, Figueroa CD, Sepúlveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol 583: 705–717, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fodor AA, Aldrich RW. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu Rev Physiol 71: 19–36, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Frizzell RA, Field M, Schultz SG. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol Renal Fluid Electrolyte Physiol 236: F1–F8, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med 2(6): a009563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giangiacomo KM, Ceralde Y, Mullmann TJ. Molecular basis of α-KTx specificity. Toxicon 43: 877–886, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Grunnet M, Rasmussen HB, Hay-Schmidt A, Klærke DA. The voltage-gated potassium channel subunit, Kv1.3, is expressed in epithelia. Biochim Biophys Acta 1616: 85–94, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Halm DR, Frizzell RA. Active K+ transport across rabbit distal colon: relation to Na+ absorption and Cl− secretion. Am J Physiol Cell Physiol 251: C252–C267, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 277: C501–C522, 1999. [Corrigenda 278: C212–C233, 1999] [DOI] [PubMed] [Google Scholar]
- 23. Halm DR, Halm ST. Prostanoids stimulate K+ secretion and Cl− secretion in guinea pig distal colon via distinct pathways. Am J Physiol Gastrointest Liver Physiol 281: G984–G996, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Halm ST, Zhang J, Halm DR. β-adrenergic activation of K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol 299: G81–G95, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanner M, Schmalhofer WA, Munujos P, Knaus HG, Kaczorowski GJ, Garcia ML. The β subunit of the high-conductance calcium-activated potassium channel contributes to the high-affinity receptor for charybdotoxin. Proc Natl Acad Sci USA 94: 2853–2858, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayes CP, Jr, McLeod ME, Robinson RR. An extrarenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 80: 207–216, 1967 [PubMed] [Google Scholar]
- 28. Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, Klærke DA. Localization of Ca2+ -activated big-conductance K+ channels in rabbit distal colon. Pflügers Arch 446: 61–68, 2003 [DOI] [PubMed] [Google Scholar]
- 29. He Q, Halm ST, Zhang J, Halm DR. Activation of the basolateral membrane Cl− conductance essential for electrogenic K+ secretion suppresses electrogenic Cl− secretion. Exp Physiol 96: 305–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens 20: 512–517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology 24: 26–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jamali K, Naylor BR, Kelly MJ, Rønnekleiv OK. Effect of 17β-estradiol on mRNA expression of large- conductance, voltage-dependent, and calcium-activated potassium channel α and β subunits in guinea pig. Endocr J 20: 227–237, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol 285: G185–G196, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, Sanchez M, Giangiacomo K, Reuben JP, Smith AB, 3rd, Kaczorowski GJ, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry 33: 5819–5828, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Kumar NSN, Singh SK, Rajendran VM. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am J Physiol Gastrointest Liver Physiol 299: G707–G714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci USA 101: 15237–15242, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Halm DR. Secretory modulation of basolateral membrane inwardly rectified K+ channel in guinea pig distal colonic crypts. Am J Physiol Cell Physiol 282: C719–C735, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Liao T, Wang L, Halm ST, Lu L, Fyffe REW, Halm DR. The K+ channel KVLQT (Kcnq1) located in the basolateral membrane of distal colonic epithelium is not essential for activating Cl− secretion. Am J Physiol Cell Physiol 289: C564–C575, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Lippiat JD, Standen NB, Harrow ID, Phillips SC, Davies NW. Properties of BKCa channels formed by bicistronic expression of hSloα and β1–4 subunits in HEK293 cells. J Membr Biol 192: 141–148, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lohrmann E, Greger R. The effect of secretagogues on ion conductances of in vitro perfused, isolated rabbit colonic crypts. Pflügers Arch 429: 494–502, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Longland CL, Dyer JL, Michelangeli F. The mycotoxin paxilline inhibits the cerebellar inositol 1,4,5-trisphosphate receptor. Eur J Pharmacol 408: 219–225, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol 90: 109–120, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581: 1000–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macrae FA, Young GP. Neoplastic and nonneoplastic polyps of the colon and rectum. Textbook of Gastroenterology (5th ed), edited by Yamada T. Hoboken, NJ: Blackwell, 2009, p. 1611–1639 [Google Scholar]
- 47. Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Mathialahan T, Sandle GI. Dietary potassium and laxatives as regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant 18: 341–347, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol 564: 269–279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa1.1 (BK), KCa3.1 (SK4) and KV7.1 (KCNQ1) channels in mouse colonic Cl566 secretion. Acta Physiol (Oxf) 189: 251–258, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meyer G, Bazzini C, Bottà G, Garavaglia ML, Simona R, Manfredi R, Sironi C, De Biasi S, Paulmichl M. K+ channel cAMP activated in guinea pig gallbladder epithelial cells. Biochem Biophys Res Commun 290: 1564–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Miranda-Rottmann S, Kozlov AS, Hudspeth AJ. Highly specific alternative splicing of transcripts encoding BK channels in the chicken's cochlea is a minor determinant of the tonotopic gradient. Mol Cell Biol 30: 3646–3660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol 496: 289–302, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Molinari EJ, Sullivan JP, Wan Y, Brioni JD, Gopalakrishnan M. Characterization and modulation of [125I]iberiotoxin-D19Y/Y36F binding in the guinea-pig urinary bladder. Eur J Pharmacol 388: 155–161, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Nardi A, Olesen SP. BK channel modulators: a comprehensive overview. Curr Med Chem 15: 1126–1146, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284: C535–C546, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Onaga T, Zabielski R, Kato S. Multiple regulation of peptide YY secretion in the digestive tract. Peptides 23: 279–290, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Park SY, Lee JH, Kim CD, Lee WS, Park WS, Han J, Kwak YG, Kim KY, Hong KW. Cilostazol suppresses superoxide production and expression of adhesion molecules in human endothelial cells via mediation of cAMP-dependent protein kinase-mediated maxi-K channel activation. J Pharmacol Exp Ther 317: 1238–1245, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 90: 755–796, 2010 [DOI] [PubMed] [Google Scholar]
- 61. Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klærke DA. Differential expression of BK channel isoforms and β-subunits in rat neuro-vascular tissues. Biochim Biophys Acta 1788: 380–389, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, Kunzelmann K. Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19: 77–88, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Rabbani GH, Greenough Cholera WB., III Textbook of Secretory Diarrhea, edited by Lebenthal E, Duffy M. New York: Raven, 1990, p. 233–253 [Google Scholar]
- 64. Rechkemmer G, Frizzell RA, Halm DR. Active K+ transport across guinea pig distal colon: action of secretagogues. J Physiol 493: 485–502, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem 272: 11710–11717, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Sanchez M, McManus OB. Paxilline inhibition of the α-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 67. Sandle GI, Gaiger E, Tapster S, Goodship TH. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 71: 393–401, 1986 [DOI] [PubMed] [Google Scholar]
- 68. Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M, MacLennan KA. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol 212: 66–73, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Saunders MD, Kimmey MB. Systematic review: acute colonic pseudo-obstruction. Aliment Pharmacol Ther 22: 917–925, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Simon M, Duong JP, Mallet V, Jian R, MacLennan KA, Sandle GI, Marteau P. Over-expression of colonic K+ channels associated with severe potassium secretory diarrhoea after haemorrhagic shock. Nephrol Dial Transplant 23: 3350–3352, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Singh SK, O'Hara B, Talukder JR, Rajendran VM. Aldosterone induces active K+ secretion by enhancing mucosal expression of Kcnn4c and Kcnma1 channels in rat distal colon. Am J Physiol Cell Physiol 302: C1353–C1360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sings H, Singh S. Tremorgenic and nontremorgenic 2,3-fused indole diterpenoids. Alkaloids Chem Biol 60: 51–163, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Sørensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflügers Arch 459: 645–656, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Sørensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger Aldosterone increases KCa1 J.1. (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sørensen MV, Sausbier M, Ruth P, Seidler U, Riederer B, Praetorius HA, Leipziger J. Adrenaline-induced colonic K+ secretion is mediated by KCa1.1 (BK) channels. J Physiol 588: 1763–1777, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sørensen MV, Strandsby AB, Larsen CK, Praetorius HA, Leipziger J. The secretory KCa1.1 channel localises to crypts of distal mouse colon: functional and molecular evidence. Pflügers Arch 462: 745–752, 2011 [DOI] [PubMed] [Google Scholar]
- 78. Speelman P, Butler T, Kabir I, Ali A, Banwell J. Colonic dysfunction during cholera infection. Gastroenterology 91: 1164–1170, 1986 [DOI] [PubMed] [Google Scholar]
- 79. Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA 101: 11897–11902, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Dinter TG, Jr, Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005 [DOI] [PubMed] [Google Scholar]
- 82. Wrong OM, Edmonds CJ, Chadwick VS. The Large Intestine: Its Role in Mammalian Nutrition and Homeostasis. Lancaster, UK: MTP Press, 1981 [Google Scholar]
- 83. Wulff H, Miller MJ, Hänsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca++-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Nat Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446, 1998 [DOI] [PubMed] [Google Scholar]
- 85. Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics 7: 2188–2198, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of β1- and β2-adrenergic receptors. Am J Physiol Gastrointest Liver Physiol 297: G269–G277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang J, Halm ST, Halm DR. Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: desensitization via the Y2-neuropeptide receptor. Am J Physiol Gastrointest Liver Physiol 297: G278–G291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhou Y, Tang QY, Xia XM, Lingle CJ. Glycine311, a determinant of paxilline block in BK channels: a novel bend in the BK S6 helix. J Gen Physiol 135: 481–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]