Abstract
Patients with Alzheimer’s disease (AD), the most prevalent neurodegenerative dementia, are usually elderly; however, ~4–5% develop early-onset AD (EOAD) with onset before age 65. Most EOAD is sporadic, but about 5% of patients with EOAD have an autosomal dominant mutation such as Presenilin 1, Presenilin 2, or alterations in the Amyloid Precursor Protein gene. Although most Alzheimer’s research has concentrated on older, late-onset AD (LOAD), there is much recent interest and research in EOAD. These recent studies indicate that EOAD is a heterogeneous disorder with significant differences from LOAD. From 22–64% of EOAD patients have a predominant nonamnestic syndrome presenting with deficits in language, visuospatial abilities, praxis, or other non-memory cognition. These nonamnestic patients may differ in several ways from the usual memory or amnestic patients. Patients with nonamnestic EOAD compared to typical amnestic AD have a more aggressive course, lack the apolipoprotein E ε4 (APOE ε4) susceptibility gene for AD, and have a focus and early involvement of non-hippocampal areas of brain, particularly parietal neocortex. These differences in the EOAD subtypes indicate differences in the underlying amyloid cascade, the prevailing pathophysiological theory for the development of AD. Together the results of recent studies suggest that nonamnestic subtypes of EOAD constitute a Type 2 AD distinct from the usual, typical disorder. In sum, the study of EOAD can reveal much about the clinical heterogeneity, predisposing factors, and neurobiology of this disease.
Introduction
In 1907, Alois Alzheimer described a 51-year-old patient who developed dementia with predominant language and behavioral changes (1). This patient, Auguste Deter, proved to have the amyloid plaques and neurofibrillary tangles (NFTs) that have come to define the neuropathology of AD. For the next 70 years, early-onset dementia was the embodiment of AD (EOAD), with the much more common late-onset disorder (LOAD) with onset after age 65, considered senile dementia from normal aging. In 1968, however, Blessed and colleagues showed that the brains of patients with senile dementia had plaques and tangles that were qualitatively the same as early-onset forms (2). The consolidation of these two age-related forms of the disease was completed when a 1976 editorial by Robert Katzman emphasized that AD and senile dementia were a single disease (3). By the 1984 NINCDS-ADRDA criteria for AD, the definition of AD was transformed to the usual progressive memory deficit presentation seen in LOAD, with the range of AD spanning onsets from 40–90 years of age (4). From this point on, many clinicians and investigators forgot the example of Auguste Deter and the lessons to be learned from the nonamnestic subtypes of EOAD. Recently, however, many investigators have rediscovered EOAD and have focused on understanding what AD that begins at or before age 65 years can reveal about the clinical manifestations and pathophysiology of this disease.
EOAD is an important clinical problem. Alzheimer’s disease (AD), which is the most common neurodegenerative dementia (5), is particularly devastating when it occurs at a young age. Beyond the psychological and medical toll, EOAD disproportionately impacts individuals during their most productive years, and the cost of treating patients with EOAD is significant. The few epidemiological studies on EOAD indicate an incidence rate of about 6.3/100,000 (6) and a prevalence rate of about 24.2/100,000 in the 45- to 64-year-old age group (7) or between 220,000 and 640,000 people in the United States (8). This compares to a 10–20 times greater incidence and prevalence rates for LOAD. In some specific populations such as U.S. veterans, the frequency of EOAD may be even higher (9). Finally, although the cut-off age of 65 years for EOAD is arbitrary and harkens back to the original AD vs. senile dementia distinction, it remains useful for distinguishing different AD syndromes. Many EOAD patients have nonamnestic syndromes infrequently present among those with LOAD.
Genetic and Predisposing Factors
Not only is there an increased family history of dementia among persons with EOAD, compared to those with LOAD (10), but there are genes that increase the risk of developing EOAD. The most important are the apolipoprotein E (APOE) ε4 alleles. APOEε4 is a susceptibility gene that decreases the age of onset for typical AD (11, 12), and it correlates with the presence of memory loss and hippocampal atrophy in AD (13, 14). The APOEε4 allele may be responsible for those EOAD patients with a typical amnestic presentation (11, 15–17). In contrast, EOAD patients with atypical nonamnestic presentations tend to be APOEε4 negative (11, 15, 16).
Autosomal dominant familial forms of AD account for only ~1% of all patients with AD, but they usually occur at a young age and have EOAD. The most common cause for familial EOAD is a mutation in the Presenilin 1 (PSEN1) gene, followed by Presenilin 2 (PSEN2) and the Amyloid Precursor Protein (APP) gene (18, 19). Patients with familial EOAD are less likely to present with nonamnestic deficits than those with sporadic EOAD (See Table 1) (20). In addition to a much younger age of onset (41.8 ± 5.2 years for PSEN1 vs. 55.9 ± 4.8 for sporadic EOAD), clinicians may be able to distinguish familial EOAD patients with PSEN1 mutations from those with sporadic EOAD by the presence of neurological abnormalities including a history of headaches and pseudobulbar affect, as well as myoclonus and gait abnormality on examination (20). Detailed family history is indicated as part of the evaluation of EOAD because patients with AD genes usually have a positive family history of autosomal dominant transmission (21). EOAD may be present if there are at least three affected family members in two or more generations, some of whom are the patient’s first-degree relatives (21). Some patients with familial EOAD, however, may lack a known family history or may have incomplete penetrance (22). For these reasons, it is important to consider evaluating EOAD patients with a very early age of onset or abnormal neurological findings for genetic factors. Actual genetic testing, however, should only occur with the participation of someone who has expertise in the area of genetic counseling (21).
Table 1.
Presenilin-1 (PSEN1) familial EOAD vs. sporadic (non-familial) EOAD: presenting symptoms on initial clinic visit
| Symptom | PSEN1 familial EOAD (n = 32) |
Non-familial EOAD (n = 81) |
Significance, p |
|---|---|---|---|
| Memory | 27 (84.37%) | 47 (58.02%) | χ2 = 5.91; p <0.05 |
| Visuospatial | 1 (3.12%) | 14 (17.28%) | χ2 = 2.86; ns |
| Language | 3 (9.38%) | 14 (17.28%) | χ2 = 1.48; ns |
| Other | 1 (3.12%)a | 6 (7.4%)b | χ2 = 0.18; ns |
Apathy in PSEN-1 familial EOAD patient.
Personality change (3); writing impairment (2); paranoia (1).
ns, nonsignficant.
There may be additional predisposing factors for EOAD. Susceptibility genes other than APOEε4, such as the single nucleotide polymorphisms CR1, CLU, BIN, and PICALM, or tau gene H1/H1 haplotypes, or even prion gene polymorphisms, could increase the risk of EOAD, but only by a modest degree (23). One study, which used the analysis of parent-child concordance rates to calculate heritability among those with EOAD, proposes an autosomal recessive pattern of inheritance for EOAD, possibly from unknown genes (24–26). Prior psychological trauma may also predispose to EOAD, and there are reports linking an increased incidence of dementia with post-traumatic stress disorder (27, 28). In addition, there is an increased risk of dementia among individuals who have had a traumatic brain injury (29), and dementia pugilistica in boxers or exposure to blast or other closed head injury may lower the age of onset of dementia (30).
EOAD Clinical Subtypes
About 22–64% of cases of EOAD may have an atypical or nonamnestic presentation (31–34). Experts have developed the new National Institute on Aging and the Alzheimer’s Association (NIA-AA) criteria for AD, in part, to take into account the nonamnestic presentations of EOAD (35). These nonamnestic EOAD subtypes differ from typical amnestic AD not only in their non-memory presentations (11, 15, 16, 32, 34), but in having a more aggressive rate of progression with a shorter duration of disease (16, 36, 37),
The EOAD nonamnestic subtypes currently constitute a confusing list of syndromes and classifications (Table 2) (33, 34, 38). Similar to Alois Alzheimer’ original patient, the most common may be a language-impaired or aphasic subtype (32), with recent indications that progressive logopenic aphasia (PLA) may be the same as this nonamnestic EOAD (39, 40). Others suggest that a biparietal subtype, with visuospatial deficits and ideomotor apraxia, is the most nonamnestic EOAD (33). Many investigators describe a posterior cortical atrophy (PCA) subtype of EOAD that has prominent visuospatial or visuoperceptual deficits (41, 42). Some include the biparietal subtype as PCA and divide PCA into temporo-occipital forms and a basic visual variant (32, 43). Still others describe a nonamnestic EOAD subtype with predominant executive deficits (34). Patients with the corticobasal syndrome, characterized by progressive limb apraxia as well as motor changes, have AD in about half of their autopsies (44), and may constitute an additional nonamnestic EOAD subtype. Different combinations of aphasia, apraxia, and agnosia occur as well, and there is no consensus on how to group these nonamnestic subtypes.
Table 2.
Studies with nonamnestic EOAD
| Potential localization | Koedam et al. 2010 n = 87 | Alladi et al. 2007 n = 34 | Stopford et al. 2007 n = 17 |
|---|---|---|---|
| Left parietal | Apraxia/visuospatial (12%) | Corticobasal syndrome (17.5%) | Praxis 23.5% |
| Left parietal | Language (9%) | Language (56%): | Language 23.5% |
| Left temporal-occipital | Aphasia-apraxia-agnosia (8%) | (nonfluent 35%, semantic 6%, mixed 15%) | |
| Dorsolateral frontal | Dysexecutive (2%) | Beh. variant fronto- temporal dem. (6%) | Dysexecutive (41.2%) |
| Right parietal | Posterior cortical atrophy (1%) | Posterior cortical atrophy (20.5%) | Perceptuospatial (11.8%) |
| Right temporal-occipital |
In a 10-year retrospective study, our group divided 125 patients with EOAD into four main subgroups: typical amnestic EOAD, a language group consistent with PLA, a visuospatial group consistent with PCA, and a smaller limb apraxia group not meeting motor criteria for corticobasal syndrome, and termed progressive ideomotor apraxia (PIA) (Table 3) (31). On comparison with 56 patients with LOAD, 80 (64%) of our EOAD patients had a nonamnestic presentation, compared to only 7 (12.5%) of the LOAD patients. On magnetic resonance imaging (MRI), similar to typical AD, the amnestic EOAD patients showed more hippocampal atrophy, but, different from typical AD, the nonamnestic EOAD patients with language presentations had more left parietal changes and the nonamnestic EOAD patients with visuospatial presentations had more right parietal-occipital changes. In sum, the cohort of EOAD contains nonamnestic subtypes whose disease focuses not in the usual memory-hippocampal system, but in posterior neocortex.
Table 3.
EOAD subgroups: demographic features and presenting symptoms
| Amnestic- aEOAD, n = 45 |
Language n = 33 |
Visuospatial n = 35 |
Limb praxis n = 12 |
Significance 4 groups |
|
|---|---|---|---|---|---|
| Age pres. | 55.6 (5.34) | 59.73 (5.02) | 60.91 (4.6) | 58.92 (8.7) | F = 7.07, p <0.001 |
| Age onset | 52.52 (5.76) | 55.91 (5.02) | 56.82 (4.89) | 55.83 (5.62) | F = 4.99, p <0.01 |
| Sex-M/F | 21/24 | 17/16 | 19/16 | 5/7 | χ2 = 0.81, ns |
| 1° FHx | 13 (28.9%) | 18 (54.5%) | 12 (34.3%) | 4 (33.3%) | χ2 = 7.18, ns |
| MMSE | 22.18 (6.02) | 18.36 (7.33) | 21.22 (6.07) | 22.1 (3.41) | F = 2.64, ns |
|
Areas of difficulty on presentation |
45 memory |
16 fluency- effortful, halting, 13 word-finding 8 reading or spelling, 2 auditory comprehension |
16 localization 9 visual perception, 8 visual reading, 6 env. orientation, 4 dressing ability, 4 object recognition, 2 driving ability |
6 mechanics of writing, 2 manual dexterity, 4 complex motor (2 acalculia; 9 aphasia) |
|
From Mendez et al. 2012.
Investigators have proposed specific clinical criteria for PLA and PCA, two of these nonamnestic EOAD subtypes. PLA criteria include an insidious onset and progression of both of the following (40): 1) impaired single-word retrieval in spontaneous speech and naming (anomia) and 2) impaired repetition of sentences and phrases. In addition, there are at least three of the following other features: Speech (phonologic) errors, spared single-word comprehension and object knowledge, spared motor speech, and absence of frank agrammatism. In addition, PLA results in reading difficulty due to phonological alexia. PCA criteria include an insidious onset and progression of all of the following (42): 1) visual complaints with intact primary visual functions, except for possible visual field deficits; 2) evidence of predominant complex visual disorder, such as oculomotor apraxia, optic ataxia, environmental disorientation (header, topographic, landmark), dressing apraxia, abnormal anti-saccades, neglect, constructional difficulty, simultanagnosia, visual agnosia, prosopagnosia; and 3) proportionally less impaired deficits in memory and verbal fluency. In addition, PCA most commonly affects the dorsal visual stream involving spatial localization rather than the ventral visual stream involving object identification (41).
Neuropsychological studies have also shown a higher prevalence of non-memory symptoms as the initial presentation of EOAD (33). Among EOAD patients compared to LOAD patients, most recent studies find worse visuospatial functions, including spatial localization and hand-eye coordination, with relative sparing of memory (45–48). After age correction, EOAD patients also have worse motor praxis when compared to LOAD patients (45). In one neuropsychological comparison of 81 EOAD and 91 LOAD, the EOAD patients had relative sparing of memory and worse visuospatial functions, attention, and executive functions, whereas the LOAD patients tended to have worse memory (38). In contrast, different investigators found worse frontal-executive performance among LOAD patients rather than among EOAD patients (49). This discrepancy may be due to the presence of decreased working memory in EOAD, not from frontal-executive dysfunction but from an impaired capacity-dependent, episodic buffer located in the parietal lobe (50).
Neuroimaging Results
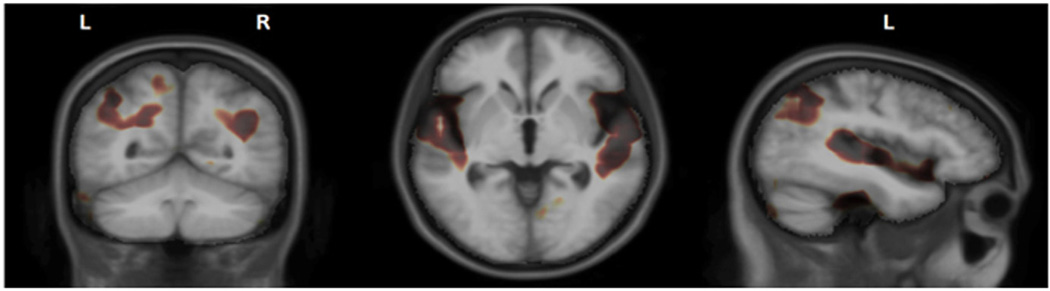
Patients with EOAD, in general, have a different structural neuroimaging pattern than patients with LOAD, probably due to the presence of the nonamnestic subtypes (51–53). For nonamnestic EOAD, investigators have reported differences in grade and distribution of gray matter atrophy in posterior parts of the brains compared to more medial temporal regions in LOAD (48, 54–57). In EOAD (with embedded variants), voxel based morphometry (VBM) studies have shown more widespread changes and less atrophy in anterior hippocampus and amygdala compared to LOAD (58). In another VBM study of EOAD, cortical atrophy maps showed changes affecting all lobes, whereas they are more confined in LOAD (48). Moreover, we used tensor based morphometry to create 3D Jacobian maps of local differences in tissue volume, and showed that EOAD subjects had significantly smaller volume in superior temporal and posterior parietal regions, compared to LOAD subjects (Figure 1).
Figure 1.
Regions where EOAD had significantly smaller volume compared to LOAD.
Magnetic resonance imaging (MRI) shows temporoparietal atrophy, even in the presence of hippocampal sparing, in nonamnestic EOAD subtypes (59). Analysis of cortical thickness patterns in EOAD subtypes suggests that different clinical presentations of AD represent points in a phenotypic spectrum of neuroanatomic variation (60). These investigators found overlapping temporoparietal areas on cortical thickness measures among 15 LPA, 25 PCA, and 14 amnestic EOAD patients compared to 30 normal controls. Areas of involvement in PLA and PCA are regionally distributed in posterior neocortex with involvement of corresponding white matter tracts (39, 61, 62), and there may be greater parietal precuneus involvement in EOAD vs. LOAD (63). Others have found that there are bilateral overlapping areas of parietal and posterior temporal involvement in PLA and PCA (61). However, in PLA, there is thinning of left posterior temporal, inferior parietal, medial temporal, and posterior cingulate (64), and in PCA there is greater involvement of the right parietal and bilateral occipital lobes (31, 61) . In sum, despite overlap, in parietal neocortex, there are clear differences among subtypes with more involvement in superior temporal lobe in LPA, bilateral occipital-parietal areas in PCA, and bilateral medial temporal and posterior parietal areas in typical amnestic EOAD.
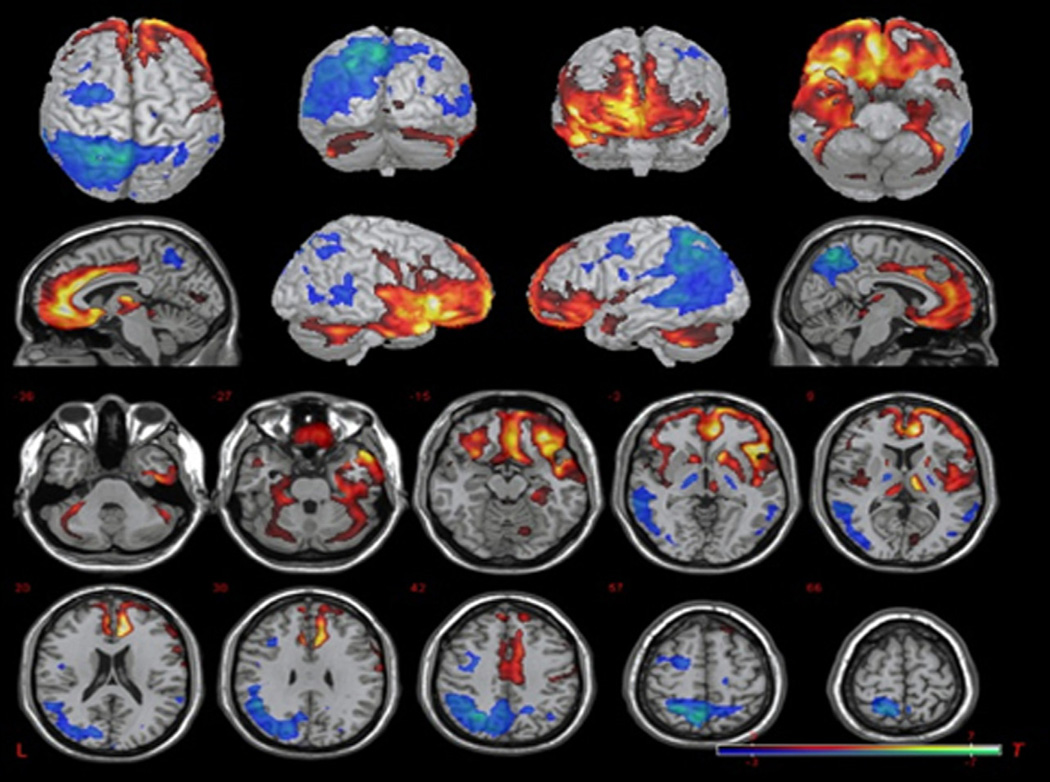
Patients with EOAD not only show more severe cortical atrophy, but also have more glucose hypometabolism than patients with LOAD (48, 55, 57, 63, 65–69). On 18fluoro-deoxyglucose positron emission tomography (FDG-PET) studies, comparably demented patients with EOAD (with embedded variants) showed greater regional hypometabolism, particularly in parietal areas, than normal controls and those with LOAD (67, 68, 70, 71–74). In our study, EOAD patients, compared to LOAD patients, also had greater focal hypometabolism in the parietal regions (left worse than right), whereas LOAD patients, compared to the EOAD patients, had greater hypometabolism in the bilateral inferior temporal regions and inferior frontal regions (right worse than left) (Figure 2) (Kaiser, in press). In a recent study, younger patients with EOAD (<62 years) had decreased FDG in the parietal cortex associated with more severe impairment in visuospatial fuctions, attention, and executive function vs. decreased posterior cingulate uptake and impairment in memory in LOAD (75).
Figure 2.
Base comparison of EOAD relative to LOAD. Two-sample t-test comparison shows relative hypometabolism in the EOAD group (blue) and relative hypometabolism in the LOAD group (red) (uncorrected p = 0.01, T = 2.43, kc = 10 voxels). Picture is in neurological convention; brain’s left is your left. From Kaiser et al., in press.
In addition to FDG-PET, nuclear imaging probes can detect amyloid-β deposits in the brain with [11C]6-OH-BTA-1 Pittsburgh Compound B (PIB) and other forms of amyloid PET imaging (76). [11C]PiB is a radioactive diagnostic agent tagged with a radioisotope that binds to fibrillar amyloid plaques and has been sensitive to the detection of typical AD (77). Patients with EOAD, like those with LOAD, have increased global [11C]PIB retention without specific differences in distribution (78), and some, but not all, studies have found greater amyloid burden on [11C]PiB imaging in EOAD compared to LOAD (78, 79). With the exception of increased PIB-PET signal in the neostriatum in familial EOAD, distinct distributions of amyloid load have been absent in patients with PLA (79, 80) or PCA (81, 82), and there have not been regional differences in amyloid burden when EOAD was compared to LOAD (57). One recent study, however, has succeeded in showing that patients with EOAD have increased [11C]PIB binding in parietal areas associated with decreased visuospatial functions when compared to LOAD (75). Finally, there are greater regional changes on [18F]FDG-PET than on [11C]PIB-PET in nonamnestic EOAD syndromes (82), suggesting that not amyloid plaque deposition but metabolic impairment drives the clinical presentation of the nonamnestic EOAD subtypes.
Neurobiological Differences from Load
Nonamnestic EOAD is different from typical amnestic AD in having greater initial pathology in posterior parietal and adjacent areas rather than in the more typical entorhinal-hippocampus region (58, 61, 83). Postmortem, there are higher burdens of amyloid plaques and NFTs in EOAD than in LOAD (84), and these appear concentrated in posterior neocortical areas. There are relatively greater focal neocortical NFTs and less hippocampal atrophy in PCA, PLA, and PIA when compared to typical amnestic AD (43, 85–87). In comparison, typical AD is associated with early volume loss in the hippocampus and medial temporal regions, as well as the temporoparietal cortex, posterior cingulate, and precuneus (88, 89). In further support of this, neuropathological findings report a hippocampal sparing subgroup of AD that is younger at death and has higher than expected NFTs in inferior parietal, superior temporal, and middle frontal cortices (83).
Although there are no consistent differences in cerebrospinal fluid (CSF) biomarkers across the EOAD subtypes, they do point to a specific sequence of pathological changes. Amyloid-β1–42 and hyperphosphorylated tau levels in CSF do not differ according to age at onset (90). Compared to amnestic EOAD, however, patients with nonamnestic EOAD tend to have higher tau levels in the CSF but do not differ in levels of amyloid-β (91). These findings suggest a faster course with greater NFT density in nonamnestic vs. amnestic EOAD. This is further examplifyed in those at risk for autosommal dominant AD due to PSEN1, PSEN2, and APOE (92). Because the age at clinical onset is similar among generations with an autosomal dominant form of AD, this allows for predicting the age of onset of AD and for demontrating the sequence of pathological changes (92). Preceding dementia, there is decreased CSF amyloid-β 25 years before; positive [11C]PIB-PET, increased CSF tau, and increased brain atrophy 15 years before;, and decreased FDG metabolism and memory 10 years before (92, 93). Higher CSF tau levles in nonamnestic AD is, therefore, consistent with a faster rate of disease.
Type 2 AD
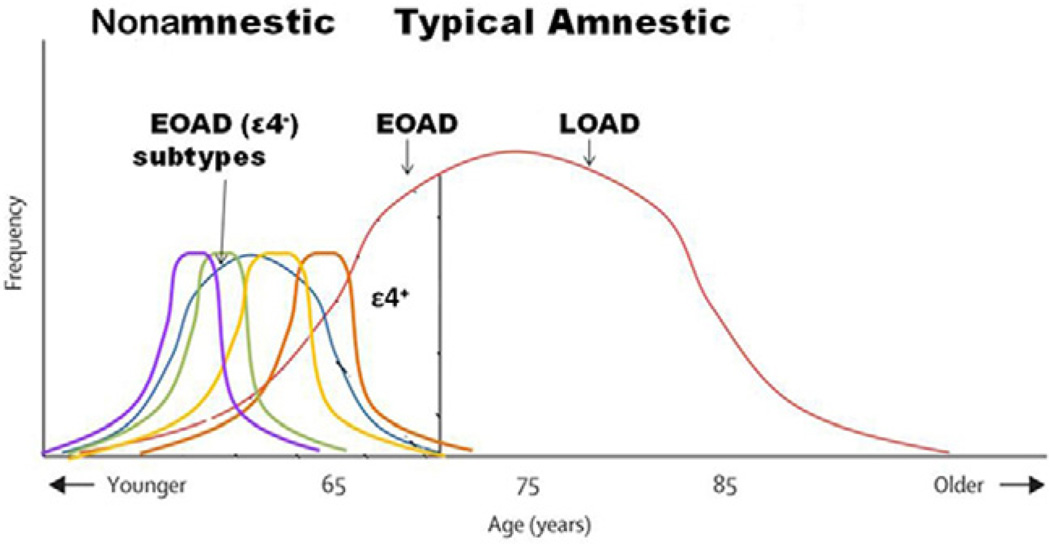
The clinical and neuropathological differences of nonamnestic EOAD from typical AD suggests that nonamnestic EOAD is a Type 2 AD with overlapping nonamnestic presentations and a relatively aggressive disease course not affected by the age-lowering effects of APOEε4 (Figure 3). Parietal lobe dysfunction is an early characteristic of nonamnestic EOAD. Specifically, Type 2 AD may be made up of clinically overlapping visuospatial, language, or praxis syndromes which differ, as a group, from typical amnestic AD of early or late onset. Defining Type 2 AD challenges the dominant clinical view of AD as necessarily an insidious memory decline in the elderly.
Figure 3.
Nonamnestic (Type 2 AD) vs. typical, amnestic EOAD. This graph also contains postulated differences in APOEε4. Adapted and modified from Van der Flier et al. 2011.
Type 2 AD also challenges the prevailing sequence of initial amyloid-β1–42 deposition followed by NFT formation in the entorhinal-hippocampal cortex and suggests an alternate cascade in the expression of Alzheimer pathophysiology. The prevailing Alzheimer cascade theory indicates a specific sequence of neuropathological changes. First, there is accumulation of amyloid-β in the brain which drives the neurodegeneration. Amyloid-β levels in CSF decrease early and reach plateau levels long before dementia develops whereas tau levels, which reflect neurodegeneration, continue to increase as cognition declines. Investigators postulate that typical AD begins with amyloid-β deposition followed by NFTs in the entorhinal-hippocampal region (94, 95). In nonamnestic EOAD, there is selective vulnerability of posterior neocortical areas and relative hippocampal sparing (83, 95). Whereas typical AD, which includes amnestic EOAD as the younger end of the age distribution, follows the proposed Alzheimer cascade (94), nonamnestic EOAD represents a continuum of a common early deposition of NFTs in posterior parietal-precuneus/posterior cingulate with involvement of adjacent neocortex and white matter tracts. The alternate cascade in Type 2 AD still begins with amyloid-β deposition but proceeds to earlier and more prominent NFTs in posterior neocortex rather than in the more typical hippocampal-posterior cingulate system. Type 2 AD is composed of overlapping syndromes with a similar or proximate hub of onset in posterior parietal-precuneus/posterior cingulate and adjacent gray and white matter (34, 61, 96). In nonamnestic EOAD, the origin of an apparent predilection for early amyloid-β deposits and neurodegeneration in parietal and related areas is unclear, but there are several possible considerations. Typical amnestic AD impairs the default mode neural network disrupting hippocampal-cortical memory systems, and nonamnestic EOAD may impair other neural networks early in the course such as the frontoparietal control network (97) leading to early parietal amyloid-β deposition. Type 2 AD may also affect temporoparietal connectivity as compensation to counteract a parietal-posterior cingulate gyrus disconnection (98). There may be different amyloid-β oligomer profiles in EOAD vs. LOAD (99). These oligomers, rather than the amyloid-β insoluble fibrils, may the the molecular pathogens that trigger synaptic dysfunction in EOAD and negatively affect the number of nicotinic acetylcholinesterase receptors (99). Moreover, levels of anti-amyloid-β IgG (IgG1 and IgG3), which may help control the development of amyloid plaques, are lower in PCA vs. typical AD (100). These serum anti-amyloid-β antibodies may differentially react to amyloid-β oligomers in nonamnestic EOAD. This work is clearly preliminary, but it indicates how researchers are beginning to explore the underlying reasons for Type 2 AD. In conclusion, EOAD, in comparison to LOAD, reveals insights regarding the underlying pathophysiology of AD. EOAD has many non-memory presentations (language, visuospatial, executive) which tend to be APOEε4 negative, lack a determinative Alzheimer mutation, and have distinct focal gray and white matter changes and regional hypometabolism. We propose a Type 2 AD, which results from early, regional vulnerability to amyloid deposition in posterior neocortex. At onset, nonamnestic EOAD subtypes involve parietal and associated neocortex whereas typical amnestic AD involves the hippocampi and related structures. Although amyloid β1–42 deposition may initiate the cascade in both forms of AD, the effects result in an initial impact of neurodegeneration and NFTs in neocortex in nonamnestic EOAD as opposed to the usual Braak and Braak staging with onset in transentorhinal cortex in typical amnestic AD. Researchers are just beginning to explore the underlying pathophysiological factors for this selective vulnerability in Type 2 AD. Finally, clinicians need to recognize patients with nonamnestic EOAD because of implications for clinical care, clinical trials, developing AD diagnostic criteria, genetic testing, and quality-of-life factors. Dementia devastates at any age, but when it affects someone pre-retirement and with financial and family responsibilities, prompt diagnosis and mobilization of resources is absolutely vital (101).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer A, Stelzmann RA, Schnitzlein HN, et al. An English translation of Alzheimer’s 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin Anat. 1995;8:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 3.Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- 4.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 5.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 6.Bickel H, Burger K, Hampel H, et al. Presenile dementia in memory clinics--incidence rates and clinical features. Nervenarzt. 2006;77:1079–1085. doi: 10.1007/s00115-005-1949-y. [DOI] [PubMed] [Google Scholar]
- 7.Renvoize E, Hanson M, Dale M. Prevalence and causes of young onset dementia in an English health district. Int J Geriatr Psychiatry. 2011;26:106–107. doi: 10.1002/gps.2456. [DOI] [PubMed] [Google Scholar]
- 8.Alzheimer’s A. Early-Onset Dementia: A National Challenge, A Future Crisis. Washington, DC: Alzheimer’s Association; 2006. [Google Scholar]
- 9.McMurtray A, Clark DG, Christine D, et al. Early-onset dementia: frequency and causes compared to late-onset dementia. Dement Geriatr Cogn Disord. 2006;21:59–64. doi: 10.1159/000089546. [DOI] [PubMed] [Google Scholar]
- 10.McMurtray AM, Ringman J, Chao SZ, et al. Family history of dementia in early-onset versus very late-onset Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:597–598. doi: 10.1002/gps.1540. [DOI] [PubMed] [Google Scholar]
- 11.van der Flier WM, Pijnenburg YA, Fox NC, et al. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE varepsilon4 allele. Lancet Neurol. 2011;10:280–288. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 12.van der Flier WM, Schoonenboom SN, Pijnenburg YA, et al. The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology. 2006;67:526–527. doi: 10.1212/01.wnl.0000228222.17111.2a. [DOI] [PubMed] [Google Scholar]
- 13.Lind J, Larsson A, Persson J, et al. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson Y, Gibbons L, Pritchard A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;23:60–66. doi: 10.1159/000097038. [DOI] [PubMed] [Google Scholar]
- 16.Schott JM, Ridha BH, Crutch SJ, et al. Apolipoprotein e genotype modifies the phenotype of Alzheimer disease. Arch Neurol. 2006;63:155–156. doi: 10.1001/archneur.63.1.155. [DOI] [PubMed] [Google Scholar]
- 17.Rogalski EJ, Rademaker A, Harrison TM, et al. ApoE E4 is a susceptibility factor in amnestic but not aphasic dementias. Alzheimer Dis Assoc Disord. 2011;25:159–163. doi: 10.1097/WAD.0b013e318201f249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasa M, Gelpi E, Antonell A, et al. Clinical features and APOE genotype of pathologically proven early-onset Alzheimer disease. Neurology. 2011;76:1720–1725. doi: 10.1212/WNL.0b013e31821a44dd. [DOI] [PubMed] [Google Scholar]
- 19.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J Neurol. 2006;253:139–158. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 20.Joshi A, Ringman JM, Lee AS, et al. Comparison of clinical characteristics between familial and non-familial early onset Alzheimer’s disease. J Neurol. 2012 doi: 10.1007/s00415-012-6481-y. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman JS, Hahn SE, Catania JW, et al. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13:597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llado A, Fortea J, Ojea T, et al. A novel PSEN1 mutation (K239N) associated with Alzheimer’s disease with wide range age of onset and slow progression. Eur J Neurol. 2010;17:994–996. doi: 10.1111/j.1468-1331.2010.02949.x. [DOI] [PubMed] [Google Scholar]
- 23.Bertram L. Alzheimer’s genetics in the GWAS era: a continuing story of replications and refutations. Curr Neurol Neurosci Rep. 2011;11:246–253. doi: 10.1007/s11910-011-0193-z. [DOI] [PubMed] [Google Scholar]
- 24.La Rue A, O’Hara R, Matsuyama SS, et al. Cognitive changes in young-old adults: effect of family history of dementia. J Clin Exp Neuropsychol. 1995;17:65–70. doi: 10.1080/13803399508406582. [DOI] [PubMed] [Google Scholar]
- 25.Wingo TS, Lah JJ, Levey AI, et al. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch Neurol. 2012;69:59–64. doi: 10.1001/archneurol.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brickell KL, Steinbart EJ, Rumbaugh M, et al. Early-onset Alzheimer disease in families with late-onset Alzheimer disease: a potential important subtype of familial Alzheimer disease. Arch Neurol. 2006;63:1307–1311. doi: 10.1001/archneur.63.9.1307. [DOI] [PubMed] [Google Scholar]
- 27.Sorrell JM, Durham S. Meeting the mental health needs of the aging veteran population. J Psychosoc Nurs Ment Health Serv. 2011;49:22–25. doi: 10.3928/02793695-20101207-01. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 31.Mendez MF, Lee AS, Joshi A, et al. Nonamnestic presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012 doi: 10.1177/1533317512454711. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 33.Koedam EL, Lauffer V, van der Vlies AE, et al. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19:1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- 34.Stopford CL, Snowden JS, Thompson JC, et al. Variability in cognitive presentation of Alzheimer’s disease. Cortex. 2008;44:185–195. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koedam EL, Pijnenburg YA, Deeg DJ, et al. Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord. 2008;26:147–152. doi: 10.1159/000149585. [DOI] [PubMed] [Google Scholar]
- 37.Rogaeva E. The solved and unsolved mysteries of the genetics of early-onset Alzheimer’s disease. Neuromolecular Med. 2002;2:1–10. doi: 10.1385/NMM:2:1:01. [DOI] [PubMed] [Google Scholar]
- 38.Smits LL, Pijnenburg YA, Koedam EL, et al. Early onset Alzheimer’s disease is associated with a distinct neuropsychological profile. J Alzheimers Dis. 2012;30:101–108. doi: 10.3233/JAD-2012-111934. [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio R, Agosta F, Toba MN, et al. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex. 2011 doi: 10.1016/j.cortex.2011.10.002. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai PH, Teng E, Liu C, et al. Posterior cortical atrophy: evidence for discrete syndromes of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2011;26:413–418. doi: 10.1177/1533317511418955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14:33–40. doi: 10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 43.Tang-Wai D, Mapstone M. What are we seeing? Is posterior cortical atrophy just Alzheimer disease? Neurology. 2006;66:300–301. doi: 10.1212/01.wnl.0000202093.81603.d8. [DOI] [PubMed] [Google Scholar]
- 44.Mathew R, Bak TH, Hodges JR. Diagnostic criteria for corticobasal syndrome: a comparative study. J Neurol Neurosurg Psychiatry. 2012;83:405–410. doi: 10.1136/jnnp-2011-300875. [DOI] [PubMed] [Google Scholar]
- 45.Sa F, Pinto P, Cunha C, et al. Differences between early and late-onset Alzheimer’s disease in neuropsychological tests. Front Neurol. 2012;3:81. doi: 10.3389/fneur.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koss E, Edland S, Fillenbaum G, et al. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer’s disease: a CERAD analysis, Part XII. Neurology. 1996;46:136–141. doi: 10.1212/wnl.46.1.136. [DOI] [PubMed] [Google Scholar]
- 47.Mendez MF. The accurate diagnosis of early-onset dementia. Int J Psychiatry Med. 2006;36:401–412. doi: 10.2190/Q6J4-R143-P630-KW41. [DOI] [PubMed] [Google Scholar]
- 48.Frisoni GB, Pievani M, Testa C, et al. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain. 2007;130:720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- 49.Licht EA, McMurtray AM, Saul RE, et al. Cognitive differences between early- and late-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2007;22:218–222. doi: 10.1177/1533317506299156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stopford CL, Thompson JC, Neary D, et al. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex. 2012;48:429–446. doi: 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Lee GJ, Lu PH, Medina LD, et al. Regional brain volume differences in symptomatic and pre-symptomatic carriers of familial Alzheimer’s disease mutations. J Neurol Neurosurg Psychiatry. 2012 doi: 10.1136/jnnp-2011-302087. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kemppainen NM, Aalto S, Wilson IA, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67:1575–1580. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- 53.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 54.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26:333–340. [PMC free article] [PubMed] [Google Scholar]
- 55.Kemp PM, Holmes C, Hoffmann SM, et al. Alzheimer’s disease: differences in technetium-99m HMPAO SPECT scan findings between early onset and late onset dementia. J Neurol Neurosurg Psychiatry. 2003;74:715–719. doi: 10.1136/jnnp.74.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in "probable" Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain. 2010;133:512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiino A, Watanabe T, Maeda K, et al. Four subgroups of Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage. 2006;33:17–26. doi: 10.1016/j.neuroimage.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Whitwell JL, Jack CR, Jr, Przybelski SA, et al. Temporoparietal atrophy: a marker of AD pathology independent of clinical diagnosis. Neurobiol Aging. 2011;32:1531–1541. doi: 10.1016/j.neurobiolaging.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ridgway GR, Lehmann M, Barnes J, et al. Early-onset Alzheimer disease clinical variants: multivariate analyses of cortical thickness. Neurology. 2012;79:80–84. doi: 10.1212/WNL.0b013e31825dce28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–1578. doi: 10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Migliaccio R, Agosta F, Scola E, et al. Ventral and dorsal visual streams in posterior cortical atrophy: a DT MRI study. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.12.025. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 64.Rohrer JD, Rossor MN, Warren JD. Alzheimer’s pathology in primary progressive aphasia. Neurobiol Aging. 2012;33:744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Waal H, Stam CJ, Blankenstein MA, et al. EEG abnormalities in early and late onset Alzheimer’s disease: understanding heterogeneity. J Neurol Neurosurg Psychiatry. 2011;82:67–71. doi: 10.1136/jnnp.2010.216432. [DOI] [PubMed] [Google Scholar]
- 66.Jagust WJ, Reed BR, Seab JP, et al. Alzheimer’s disease. Age at onset and single-photon emission computed tomographic patterns of regional cerebral blood flow. Arch Neurol. 1990;47:628–633. doi: 10.1001/archneur.1990.00530060036013. [DOI] [PubMed] [Google Scholar]
- 67.Kim EJ, Cho SS, Jeong Y, et al. Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain. 2005;128:1790–1801. doi: 10.1093/brain/awh539. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto S, Ishii K, Sasaki M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci. 2002;200:27–32. doi: 10.1016/s0022-510x(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 69.Yasuno F, Imamura T, Hirono N, et al. Age at onset and regional cerebral glucose metabolism in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:63–67. doi: 10.1159/000017024. [DOI] [PubMed] [Google Scholar]
- 70.Mielke R, Herholz K, Grond M, et al. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging. 1992;13:93–98. doi: 10.1016/0197-4580(92)90015-p. [DOI] [PubMed] [Google Scholar]
- 71.McMurtray AM, Licht E, Yeo T, et al. Positron emission tomography facilitates diagnosis of early-onset Alzheimer’s disease. Eur Neurol. 2008;59:31–37. doi: 10.1159/000109258. [DOI] [PubMed] [Google Scholar]
- 72.Lantos PL, Luthert PJ, Hanger D, et al. Familial Alzheimer’s disease with the amyloid precursor protein position 717 mutation and sporadic Alzheimer’s disease have the same cytoskeletal pathology. Neurosci Lett. 1992;137:221–214. doi: 10.1016/0304-3940(92)90408-y. [DOI] [PubMed] [Google Scholar]
- 73.Mann UM, Mohr E, Gearing M, et al. Heterogeneity in Alzheimer’s disease: progression rate segregated by distinct neuropsychological and cerebral metabolic profiles. J Neurol Neurosurg Psychiatry. 1992;55:956–959. doi: 10.1136/jnnp.55.10.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SH, Seo SW, Yoon DS, et al. Comparison of neuropsychological and FDG-PET findings between early- versus late-onset mild cognitive impairment: a five year longitudinal study. Dement Geriatr Cogn Disord. 2010;29:213–223. doi: 10.1159/000278422. [DOI] [PubMed] [Google Scholar]
- 75.Ossenkoppele R, Zwan MD, Tolboom N, et al. Amyloid burden and metabolic function in early-onset Alzheimer’s disease: parietal lobe involvement. Brain. 2012;135:2115–2125. doi: 10.1093/brain/aws113. [DOI] [PubMed] [Google Scholar]
- 76.Barrio JR, Satyamurthy N, Huang SC, et al. Dissecting molecular mechanisms in the living brain of dementia patients. Acc Chem Res. 2009;42:842–850. doi: 10.1021/ar800189x. [DOI] [PubMed] [Google Scholar]
- 77.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choo IH, Lee DY, Kim JW, et al. Relationship of amyloid-beta burden with age-at-onset in Alzheimer disease. Am J Geriatr Psychiatry. 2011;19:627–634. doi: 10.1097/JGP.0b013e318202bf3a. [DOI] [PubMed] [Google Scholar]
- 79.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leyton CE, Villemagne VL, Savage S, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 81.de Souza LC, Corlier F, Habert MO, et al. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer’s disease. Brain. 2011;134:2036–2043. doi: 10.1093/brain/awr130. [DOI] [PubMed] [Google Scholar]
- 82.Rosenbloom MH, Alkalay A, Agarwal N, et al. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76:1789–1796. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marshall GA, Fairbanks LA, Tekin S, et al. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J Geriatr Psychiatry Neurol. 2007;20:29–33. doi: 10.1177/0891988706297086. [DOI] [PubMed] [Google Scholar]
- 85.Gefen T, Gasho K, Rademaker A, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135:1554–1565. doi: 10.1093/brain/aws076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 87.Malkani RG, Dickson DW, Simuni T. Hippocampal-sparing Alzheimer’s disease presenting as corticobasal syndrome. Parkinsonism Relat Disord. 2011 doi: 10.1016/j.parkreldis.2011.11.022. (epub). [DOI] [PubMed] [Google Scholar]
- 88.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davatzikos C, Bhatt P, Shaw LM, et al. Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.023. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouwman FH, Schoonenboom NS, Verwey NA, et al. CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiol Aging. 2009;30:1895–1901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Koric L, Felician O, Guedj E, et al. Could clinical profile influence CSF biomarkers in early-onset Alzheimer disease? Alzheimer Dis Assoc Disord. 2010;24:278–283. doi: 10.1097/WAD.0b013e3181d712d9. [DOI] [PubMed] [Google Scholar]
- 92.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buchhave P, Minthon L, Zetterberg H, et al. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 94.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kas A, de Souza LC, Samri D, et al. Neural correlates of cognitive impairment in posterior cortical atrophy. Brain. 2011;134:1464–1478. doi: 10.1093/brain/awr055. [DOI] [PubMed] [Google Scholar]
- 97.Vincent JL, Kahn I, Snyder AZ, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacobs HI, Van Boxtel MP, Heinecke A, et al. Functional integration of parietal lobe activity in early Alzheimer disease. Neurology. 2012;78:352–360. doi: 10.1212/WNL.0b013e318245287d. [DOI] [PubMed] [Google Scholar]
- 99.Bao F, Wicklund L, Lacor PN, et al. Different beta-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging. 2012;33:825, e1–e13. doi: 10.1016/j.neurobiolaging.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Dorothee G, Bottlaender M, Moukari E, et al. Distinct patterns of antiamyloid-beta antibodies in typical and atypical Alzheimer disease: distinct patterns of anti-abeta antibodies and AD. Arch Neurol. 2012:1–5. doi: 10.1001/archneurol.2012.604. [DOI] [PubMed] [Google Scholar]
- 101.Hunt DC. Young-onset dementia: a review of the literature and what it means for clinicians. J Psychosoc Nurs Ment Health Serv. 2011;49:28–33. doi: 10.3928/02793695-20110302-05. [DOI] [PubMed] [Google Scholar]