Abstract
Alzheimer’s disease (AD), the most devastating chronic neurodegenerative disease in adults, causes dementia and eventually, death of the affected individuals. Clinically, AD is characterized as late-onset, age-dependent cognitive decline due to loss of neurons in cortex and hippocampus. The pathologic corollary of these symptoms is the formation of senile plaques and neurofibrillary tangles. Senile plaques are formed due to accumulation of oligomeric amyloid beta (Aβ) forming fibrillary plaques. This occurs due to the amyloidogenic processing of the amyloid precursor protein (APP) by various secretases. On the other hand, neurofibrillary tangles are formed due to hyperphosphorylation of cytoskeleton proteins like tau and neurofilament. Both are hyperphosphorylated by cyclin-dependent kinase-5 (Cdk5) and are part of the paired helical filament (PHF), an integral part of neurofibrillary tangles. Unlike other cyclin-dependent kinases, Cdk5 plays a very important role in the neuronal development. Cdk5 gets activated by its neuronal activators p35 and p39. Upon stress, p35 and p39 are cleaved by calpain resulting in truncated products as p25 and p29. Association of Cdk5/p25 is longer and uncontrolled causing aberrant hyperphosphorylation of various substrates of Cdk5 like APP, tau and neurofilament, leading to neurodegenerative pathology like AD. Additionally recent evidence has shown increased levels of p25, Aβ, hyperactivity of Cdk5, phosphorylated tau and neurofilament in human AD brains. This review briefly describes the above-mentioned aspects of involvement of Cdk5 in the pathology of AD and at the end summarizes the advances in Cdk5 as a therapeutic target.
Keywords: Cyclin dependent kinase 5 (Cdk5), Hyperphosphorylation, Neurodegeneration, Alzheimer’s disease, Plaques, Tangles
Introduction
Among many neurodegenerative disorders, Alzheimer’s disease (AD) causing dementia affects the elderly population globally. Clinical characterization of AD is described as a late-onset, age-dependent, progressive cognitive decline resulting in irreversible loss of neurons, especially in the cortex and hippocampus. Pathological hallmarks of AD are the presence of extracellular senile plaques and intracellular neurofibrillary tangles (NFTs). Senile plaques are formed due to accumulation of amyloid-beta 42 peptide (Aβ42), a proteolytic cleavage product of the amyloid precursor protein (APP), and NFTs are formed as a result of tau hyperphosphorylation and medium/heavy neurofilament proteins (NF-M/H) (1,2). Usually, most (90%) cases of AD are late-onset and sporadic but there are 5–10% of cases of familial AD (FAD), which occur early in life. FAD occurs due to gene mutations in APP and/or presenilin1 and 2 (PS1, PS2), whereas sporadic AD occurs due to a large number of external and internal insults involving environmental factors and lifestyle stresses.
It is now well established that neuronal stress and toxic factors induce hyperactivation of Cdk5. In addition to the presence of senile plaques and NFTs, it has also been reported that AD brains have p25 due to the proteolytic cleavage of p35, the Cdk5 activator (3,4). Cdk5 is a proline directed serine (Ser)/threonine (Thr) kinase, a member of CDK family due to its close sequence homology to human CDC2 (5,6). Normally, Cdk5 activity is tightly regulated in the nervous system by neuron-specific cyclin-related molecules p35 and p39. Activity of Cdk5/p35 is essential for neuronal development and function. Regulated Cdk5 activity plays an important role in cytoskeletal protein phosphorylation, neurogenesis, synaptic plasticity, cognition, and neuronal survival in embryo and adult (7–10). Under neuronal stress/insults (e.g., oxidative stress, glutamate, Aβ etc), calpain, a Ca2+ activated protease, cleaves p35 into a p10 and p25 fragment. The latter forms a more stable and hyperactive Cdk5-p25 complex, causing aberrant hyperphosphorylation of tau and neurofilament proteins (NF-M/H) and induces neuronal cell death (11–13).
Plaque Formation in AD
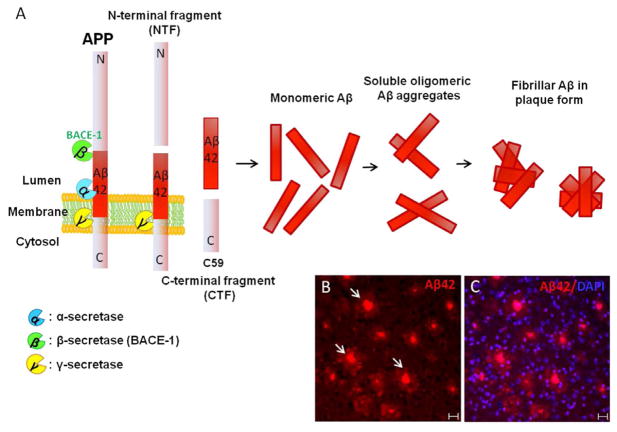
Plaque formation is a pathological hallmark of AD and is usually formed due to accumulation of the Aβ peptide. Under physiological conditions, sequential cleaving of amyloid precursor protein (APP) is initiated by aspartyl proteases α-secretase followed by γ-secretase to yield nonamyloidgenic fragments (14). Due to environmental factors, mutations in APP and presenilin1 and 2 (PS1, PS2), the sequence of cleavage by protease is changed and α-secretase is replaced by β-secretase (BACE1) followed by γ-secretase to yield two principle Aβ peptides, namely, Aβ40 and Aβ42 (15). Aβ peptides are usually the natural products of cleavage and monomers of Aβ40 are much more prevalent than Aβ42 and act as a trophic factor. Among the two, Aβ42 is generated more due to neuronal insults and more easily becomes aggregated and forms insoluble amyloid plaques disrupting the normal homeostasis resulting in hyperactivation of kinases (as shown in Figure 1). Monomeric Aβ42 forms oligomeric units, which are still soluble but at higher concentrations become aggregated form the insoluble amyloid plaque. An imbalance between the production, clearance and aggregation of these peptides causes plaque formation, leading to the initiation factor in AD and is termed as amyloid hypothesis (16,17). During physiological conditions, cleavage by α-secretase is carried out within the Aβ sequence; therefore, it prevents Aβ42 generation. Most of the AD cases are usually late-onset and sporadic but there are 5–10% of cases of familial AD (FAD) occurring as early-onset and in an autosomal-dominant manner. The cause of FAD is due to mutations in APP, either PS1/PS2 or both (14,18). There are 32 APP, 179 PS1 and 14 PS2 gene mutations resulting in early-onset fully penetrant AD. Mainly, APP mutations either cluster around the γ-secretase cleave site or BACE1 cleavage site, causing changes in peptide formation. Presence of either a single mutation or a combination of mutations all result in the increased production of the less soluble and more toxic Aβ42 relative to Aβ40 (19).
Figure 1.
(A) Schematic representation of APP processing by various secretases under physiological and pathological conditions. (B–C) Cortex of a 9-month-old AD model mouse (5XFAD) with APP and PS1 mutations stained with antibody against Aβ42 (in red) and nuclear stain DAPI (in blue). Arrows indicate the presence of amyloid plaques. Bar = 20 μm.
Loss of APP function is not deleterious but accumulation of Aβ is toxic to the cells. Various cell culture studies have shown that Aβ42 causes toxicity, resulting in death of all cells within 24 h exposure (20). The likely mechanism of cell death is via apoptosis triggered by oxidative effects of Aβ (21,22). To better understand the AD mechanism, several transgenic mouse models have been developed with human APPs or PS1 or both mutations. Most of these transgenic mice that overexpress mutant human APPs develop Aβ deposition by 4–6 months and show evidence of subsequent neuronal loss (23). These mice show synaptic dysfunction, abnormalities on spatial memory tests and inflammation (24–27). In parallel with AD, these AD transgenic mice also display activation of multiple caspases including caspases 3, 6, 7, 8 and 9 (28,29).
During a person’s lifetime, a large amount of APP is metabolized into Aβ in the brain but during aging the delicate equilibrium of production and clearance is affected. In the initial stages of AD, the concentration of Aβ42 in the cerebrospinal fluid (CSF) starts to fall, while in the brain concentration of Aβ42 rises in the brain (30,31). This could be due to either change in the Aβ transport from CSF to brain or also could be due to the change in the ratio of Aβ42 to Aβ40 within the brain. Other reasons for this imbalance could be due to age-associated acetylation of the α-secretase gene reducing the nonamyloidogenic process of APP (32). Also, an increase in BACE1 activity has been reported in early AD brain, which would result in increased amyloidogenic processing (33,34).
In addition to the genetics, environmental factors also play an important role in the onset as well as the progression of the disease. Recent studies carried out in Aβ transgenic mice as well as human AD patients have shown that exercising and intellectual stimulation have reduced Aβ thereby showing improvement in memory loss by stimulating synaptic processes and growth factors (35–37). These results are indicative of potential exploration in the area as to cure or delay the onset of AD.
Tangle Formation in AD (Tau and Neurofilament)
The event that follows plaque formation in AD is the formation of NFTs. In contrast to Aβ plaques, NFTs so far have not been associated with mutations but are due to modulations of kinase and phosphatase activities. NFTs are composed of aberrantly hyperphosphorylated cytoskeletal components like tau and neurofilament proteins.
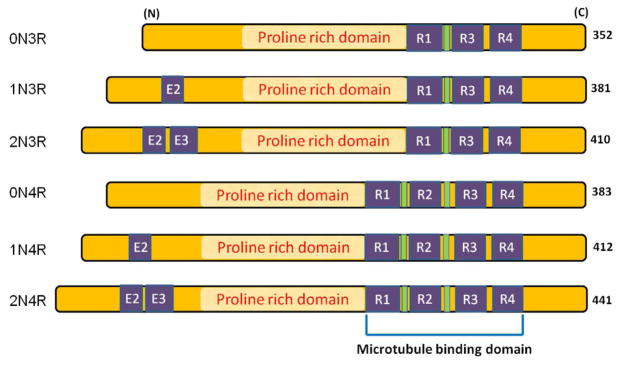
Normally, tau functions to regulate microtubule (MT) assembly and transport. There are six tau isoforms in human brain produced from a single gene through alternative mRNA splicing (38). Based on the number of microtubule-binding repeats, tau can be categorized into two groups, one with three repeats (3R) and the other with four repeats (4R) as shown in Figure 2. In the tau filaments obtained from AD brain, similar to normal human brain, all six isoforms are found. Tau, an abundant soluble protein in axons, normally promotes assembly and stability of microtubules and vesicle transport but, when hyperphosphorylated, becomes insoluble, lacks affinity for microtubules and forms paired helical structures. Like Aβ oligomers, intermediate aggregates of abnormal tau molecules are cytotoxic and impair cognition (39–41). Filamentous tau is also found in other neurodegenerative disorders like corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), Pick’s disease and Parkinson-dementia complex of Guam (42). None of these diseases, unlike AD, lack Aβ pathology. When tau becomes hyperphosphorylated in AD, it dissociates from MT assembly, resulting in destabilizing MTs and impairment of axonal transport. The phospho-tau aggregates form filamentous structures called paired helical filaments (PHFs), which further combine to form the aggregates of insoluble NFTs (43). Under physiological conditions, proteasome assembly cleans up any aggregate that may be potentially toxic to the system. Inhibition of this clean-up process by the proteasome is sufficient to induce neuronal degeneration and death (44). Also, various reports suggest that, at least in some part, formation of highly insoluble NFT is associated with oxidative stress (45,46). Due to hyperphosphorylation of tau, not only the normal function of stabilizing microtubules is hampered, but a gain of toxic function is exhibited due to sequestering of normal tau. Reports have shown that the absence of normal tau results in the disruption of microtubules (47,48). Abnormal hyperphosphorylation of tau is a reflection of both an abnormal activation of kinases as well as decreased phosphatase activity (49). Among others, cyclin-dependent kinase 5 (Cdk5) is one of the major players causing aberrant hyperphosphorylation of tau (discussed later).
Figure 2.
Six isoforms of human tau. Absence of exon 2 and 3 (E2, E3) near the N-terminus of tau gives rise to 0N tau isoforms whereas presence of E2 gives rise to 1N and both E2 and E3 give rise to 2N tau isoforms. Similarly, presence or absence of R1-R4 repeats in the microtubule binding domain gives rise to 3R or 4R tau isoforms. The proline-rich domain is found in between the N and C terminal and is present in all six isoforms. Most of the phosphorylation sites are found in proline-rich domain.
In AD brain, in addition to hyperphosphorylated tau, another cytoskeleton protein, neurofilaments are also known to be aberrantly hyperphosphorylated (1,2,50). Neurofilament preparations from rat and mouse also revealed that Cdk5 can phsophorylate specific KSP repeats in NF-H and NF-M (51). Neurofilaments play an important role in maturation and maintenance of axonal integrity as they are necessary for axon caliber and conduction velocity of the nerve fiber. Based on their molecular weight, neurofilaments are classified as neurofilament heavy, medium and light (NF-H; 200-kDa, NF-M; 150-kDa and NF-L; 68-kDa, respectively). They are the most abundant neuronal cytoskeleton proteins and are normally found phosphorylated in axons but in neurodegenerative diseases like AD and amyotrophic lateral sclerosis (ALS), they are aberrantly hyperphosphorylated in the cell bodies and form aggregates. Neurofilaments may become hyperphosphorylated by the same kinase as tau because most of the phospho-neurofilament antibodies cross-react with phospho-tau antibodies indicative of shared epitopes at various phosphorylated sites (52). Therefore, along with tau, neurofilaments are another target for AD therapeutics. In addition to Cdk5, tau can also be phosphorylated by other kinases like glycogen synthase kinase-3β (GSK 3β), cyclic AMP-dependent protein kinase (PKA), calcium, calmodulin-dependent protein kinase II (CaMKII) and mitogen-activated protein kinase (MAPKs). Some of these kinase share same epitopes on tau for phosphorylation.
Cdk5: An Atypical Cyclin-dependent Kinase
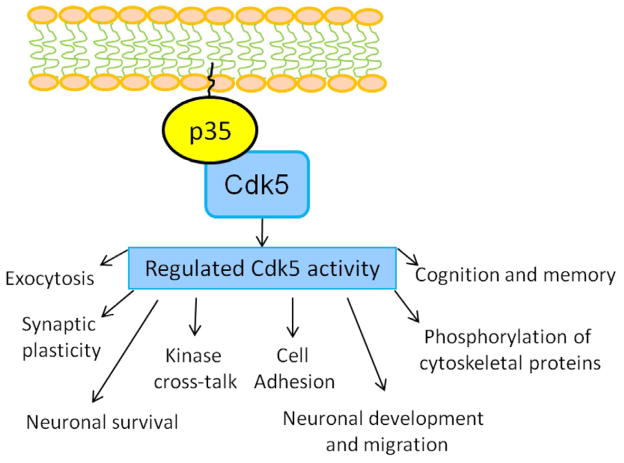
Cyclin dependent kinase 5 (Cdk5), a proline-directed serine/threonine kinase, has a sequence homology to the other cell cycle kinases like Cdk1 and Cdk2. Functionally, Cdk5 does not play any role in cell cycle; on the contrary it has multiple roles in neuron development, neuronal survival, phosphorylation of cytoskeletal proteins and synaptic plasticity (7–10). Like the other cyclin-dependent kinase family members, Cdk5 also requires association with a regulatory protein for its activation. In mammals, p35 and p39 are the two known proteins to activate Cdk5. The active form of Cdk5 is found primarily in the nervous system due to its activator proteins being specifically expressed in neuronal cells (53–55). Activity of Cdk5 is tightly regulated and plays an important role in CNS development by phosphorylating the specific serine or threonine site of numerous substrate proteins closely associated with neuronal migration, synaptogenesis, and synaptic transmission as well as synaptic plasticity as shown in Figure 3, (7–10). Mice lacking Cdk5 or its promoter p35/p39 are either embryonically lethal or are born with severe disruption in the migration and position of the neurons and axonal fiber tracts (7,26,56). Among the two, p35 is the major and most well-studied activator protein of Cdk5 comprised of 307 amino acids with 35-kDa mass; p35 can be separated into two regions—p10 and p25. The N-terminal p10 region is 98 amino acids containing the myristoylated region important for membrane targeting of p35 (4). Also, p10 contains the signal for degradation via ubiquitin-proteosome pathway (57). The C-terminal p25 region is rich in proline stretch and comprises 209 amino acids. p25 has the Cdk5 binding as well as activation domain (58). A large body of evidence shows that both p35 and p39 have a short half-life in vitro and are prone to ubiquitin-mediated proteosome degradation, indicating that Cdk5 activity is short-lived and tightly regulated (57,58).
Figure 3.
Role of Cdk5/p35 in physiology. Under physiological conditions, Cdk5 is tightly regulated due to its neuron-specific promoter p35 and is involved in various biologically important processes as shown.
Deregulation of Cdk5
The tight regulation of Cdk5 is disrupted under many neurotoxic or stress conditions. Various stress like ischemic brain damage, oxidative stress, mitochondrial dysfunctions, excitotoxicity, Aβ exposure, calcium dyshomeostasis and inflammation lead to a rise in the intracellular Ca2+. High Ca2+ concentration activates calpain-mediated cleavage of p35 to p25, forming a more stable Cdk5/p25 complex (12,53,54,60,61). p25 can effectively activate Cdk5 both in vitro and in vivo because it contains the necessary elements for activation (4). Both p35 and p25 have distinct properties as p35 has a very short half life due to its susceptibility to degradation via a ubiquitin-proteosome pathway, whereas p25 has a very long half-life. Also, localization of p35 differs from p25. Due to the p10 myristoylated N-terminal end, p35 is bound to the membrane, whereas lack of p10 makes p25 localize to the cell soma and nucleus. These properties of p25 form a stronger association with Cdk5, leading to the formation of a more stable and hyperactive Cdk5/p25 complex. This hyperactive complex then causes aberrant hyperphosphorylation of various cytoskeletal components such as tau and neurofilaments (medium/heavy, NF-M/H), leading to neurodegeneration and cell death (11–13). Also, deregulated activity of Cdk5/p25 promotes oxidative stress and mitochondrial dysfunction (62). Oxidative stress further inhibits the neurofilament axonal transport in response to neurofilament phosphorylation (63). Also, reactive oxygen species are increased in cell culture studies when cells were exposed to Aβ and glutamate toxicity causing Cdk5 to promote mitochondrial damage and induce p38 activation (62,64). Therefore, hyperactivity of Cdk5 is involved in promoting cell death via a feedback loop mechanism by being an upstream regulator as well as a downstream effector of mitochondrial dysfunction (62). Additionally, AD brains display a significant increase in Cdk5 activity along with an increase in p25 and p38 levels (65,66). In addition to AD, increased Cdk5 activity is also found in a mouse model as well as in patients with ALS and Parkinson’s disease (PD) (67–69). Consequently, Cdk5 has been closely associated with various neurodegenerative diseases such as AD, ALS, PD and Huntington’s disease (HD) (70).
Role of Cdk5 in Plaque Formation
As discussed earlier, senile plaques are formed due to accumulation of fibrillar Aβ aggregates. Aβ is formed due to the sequential cleaving of APP by β-secretase (BACE1) followed by γ-secretase in the transmembrane region liberating Aβ peptides (71). Due to the abundance of extracellular deposition of Aβ peptides, plaques are formed. Hyperactivity of Cdk5 produces Aβ aggregates that induce neurotoxicity resulting in activation of kinases and inhibition of phosphatases preceeding NFT formation (12,21). Upon neuronal insult with either Aβ or glutamate, primary cortical neurons showed enhanced Cdk5 activity. After treatment with inhibitors like roscovitine or Cdk5 inhibitory peptide (CIP), cells displayed reduction in hyperactive Cdk5 (21,72,73).
Various cell culture studies indicated that APP is also a substrate of Cdk5 and phosphorylation at Thr668 has been demonstrated by Cdk5 (74). Thr668 phosphorylation in vitro affects the binding of APP to the cytoplasmic adaptor protein Fe65, indicating that phosphorylation of APP at Thr668 plays an important role in normal functioning (75). Recent evidence indicates upregulation of Thr668-mediated phosphorylation of APP in AD brain tissues where it is enriched in large endocytic vesicles. Interestingly, in cultured neurons inhibition of Thr668 phosphorylation using Cdk inhibitors or expression of the mutant APP (Thr668A) display marked reduction in Aβ peptides (76). Additionally, in p25 transgenic mice increased APP Thr668 phosphorylation has been observed compared to wild-type mice (77). These studies together strongly suggest that Cdk5 hyperactivation may be an early event in AD because Cdk5 is implicated in phosphorylation of APP, making it more prone to Aβ formation.
Role of Cdk5 in Tangle Formation
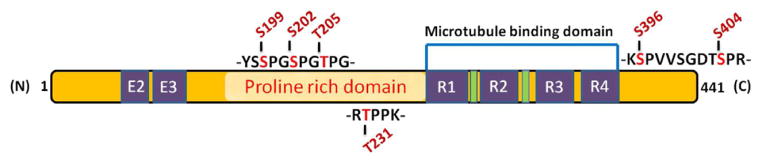
Tangle is another pathological hallmark for AD and is comprised of hyperphosphorylated cytosketal proteins such as tau and neurofilaments. Various studies indicated that Cdk5 and tau can be co-purified from microtubule preparations (78). Using phosphoepitope specific antibodies and mass spectrometric analysis, various phosphorylation sites on tau were revealed (79–81). Figure 4 depicts the various phosphorylation sites on tau mediated by Cdk5. These sites are particularly hyperphosphorylated in AD brains. PHF preparations have shown that tau is phosphorylated at S202, T205, S396 and S404, among others. In human brains, immunohistochemical analysis revealed Cdk5 localization to NFT-bearing neurons (82,83). Additionally, an increase in Cdk5 immunoreactivity has been observed in the pretangle neurons indicating involvement of Cdk5 during the early stage of pathogenesis (84,85). Results from in vitro studies indicated that the kinase activity of Cdk5 in phosphorylating tau is significantly higher in the presence of p25 compared to p35 (86). Similarly, p25 transgenic mice exhibit hyperphosphorylation of tau and neurofilaments (13). Another tau transgenic mice p301L, when crossed with p25 transgenic mouse, exhibits development of NFTs (11). In another transgenic mouse model where expression of p25 can be induced, similar pathology was displayed (77). Recent studies indicated that antibodies against phospho-neurofilaments (like SMI 31) cross-reacts with phospho tau antibodies and vice versa in human AD brain, suggesting the close association of both. Studies carried out using phospho-proteomics technique confirms that the human AD tangle preparation contains phosphorylated NF proteins in addition to tau (87,88). Similar to tau, neurofilaments in culture, when exposed to increased expression of Cdk5/p25, results in hyperphosphorylation and impaired axonal transport (89). Cumulative evidence suggests that the cleavage of p35 into p25 is an extremely important event as it leads to alteration of substrate specificity for Cdk5. Because of its contribution to tau pathology and tangle formation, Cdk5/p25 has been identified as a prime therapeutic target for AD.
Figure 4.
Cdk5-mediated phosphorylation sites detected on tau from Alzheimer brain. The sites are shown in red color and are mostly located in proline-rich domain with a few at the C-terminal.
Cdk5: A Possible Drug Target for AD
During the last two decades multiple approaches have been used to fight against AD starting from the use of Aβ antibody, antioxidants, neurotrophins, statins to the use of hormone replacement and gene therapy (90). Also, multiple drug treatments such as targeting Aβ clearance or as cholinesterase inhibitors have been used but the results have not been very promising (91,92). In order to test these various drug/gene treatments and approaches, various transgenic mouse models have been used. Whether in animal or human trials, the most effective drug treatment or regimen has yet to be determined because all current therapies either have strong side effects or are not very effective.
Involvement of Cdk5/p25 with not only the formation of Aβ plaques but also NFTs, the two important pathological hallmarks of AD, makes it an obvious choice as a therapeutic target. Recent studies carried out in the transgenic AD mouse models using lentiviral or adeno-associated viral vectors to silence Cdk5 via RNA interference (RNAi) show reduction in phosphorylation of tau and decreased number of NFTs in the hippocampus (93). Due to the involvement of Cdk5/p25, kinase inhibitors (such as roscovitine) have been an obvious choice as therapeutic targets. Accordingly, the effect of compounds such as aminothizole, resembling roscovitine, a kinase inhibitor targeting ATP binding sites in Cdk5 and other kinases, have been studied (94–96). However, these compounds are not very specific as they not only inhibit Cdk5/p25 but also Cdk5/p35 and other cyclin-dependent kinases, leading to serious side effects and thereby reducing the therapeutic efficacy.
In recent years our laboratory has attempted to overcome this problem by generating several truncated peptides of p25, based on the structure and kinetics of the Cdk5/p25 complex (21,73,79). Among several others, peptides like Cdk5 inhibitory peptide (CIP) and p5 in cell culture studies have shown specific inhibition of Cdk5/p25 hyperactivity without inhibiting endogenous Cdk5/p35 or other cyclin-dependent kinase activities. In particular, peptide p5 also displayed a reduction in tau hyperphosphorylation in cortical neurons when subjected to Aβ-induced stress along with reduction in neuronal apoptosis. These results indicate the potential of the candidate peptide p5 to be further investigated in animal and human clinical trials.
In conclusion, it may be mentioned that many promising candidates reducing the hyperactivity of Cdk5 are being introduced in the field of AD therapeutics but careful evaluation and testing of these promising candidates need to be carried out on transgenic AD mouse models before proceeding to human clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sternberger NH, Sternberger LA, Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cork LC, Sternberger NH, Sternberger LA, et al. Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer’s disease. J Neuropathol Exp Neurol. 1986;45:56–64. doi: 10.1097/00005072-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Clark AW, Rosales JL, et al. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci Res. 1999;34:21–29. doi: 10.1016/s0168-0102(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 4.Patrick GN, Zukerberg L, Nikolic M, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 5.Meyerson M, Enders GH, Wu CL, et al. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmich MR, Pant HC, Wada E, et al. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohshima T, Ward JM, Huh CG, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolic M, Dudek H, Kwon YT, et al. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 9.Tan TC, Valova VA, Malladi CS, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- 10.Fischer A, Sananbenesi F, Schrick C, et al. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble W, Olm V, Takata K, et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee MS, Kwon YT, Li M, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 13.Ahlijanian MK, Barrezueta NX, Williams RD, et al. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 17.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Maarouf CL, Daugs ID, Spina S, et al. Histopathological and molecular heterogeneity among individuals with dementia associated with presenilin mutations. Mol Neurodegener. 2008;3:20. doi: 10.1186/1750-1326-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci USA. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yankner BA, Dawes LR, Fisher S, et al. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245:417– 420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- 21.Zheng YL, Li BS, Amin ND, et al. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur J Biochem. 2002;269:4427–434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande A, Mina E, Glabe C, et al. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci. 2006;26:6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oakley H, Cole SL, Logan S, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 25.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Chen KS, Knox J, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 27.El Khoury J, Toft M, Hickman SE, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 28.Halawani D, Tessier S, Anzellotti D, et al. Identification of caspase-6-mediated processing of the valosin containing protein (p97) in Alzheimer’s disease: a novel link to dysfunction in ubiquitin proteasome system-mediated protein degradation. J Neurosci. 2010;30:6132–6142. doi: 10.1523/JNEUROSCI.5874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer’s disease: is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2:108–118. [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinerman JR, Irizarry M, Scarmeas N, et al. Distinct pools of beta-amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol. 2008;65:906–912. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donmez G, Wang D, Cohen DE, et al. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Holsinger RM, McLean CA, Beyreuther K, et al. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 34.Yang LB, Lindholm K, Yan R, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 35.Yuede CM, Zimmerman SD, Dong H, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 37.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goedert M, Wischik CM, Crowther RA, et al. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khlistunova I, Biernat J, Wang Y, et al. Inducible expression of Tau repeat domain in cell models of tauopathy: aggregation is toxic to cells but can be reversed by inhibitor drugs. J Biol Chem. 2006;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 40.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oddo S, Vasilevko V, Caccamo A, et al. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 42.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 43.Hanger DP, Betts JC, Loviny TL, et al. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 44.Keck S, Nitsch R, Grune T, et al. Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem. 2003;85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x. [DOI] [PubMed] [Google Scholar]
- 45.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 46.Cras P, Smith MA, Richey PL, et al. Extracellular neurofibrillary tangles reflect neuronal loss and provide further evidence of extensive protein cross-linking in Alzheimer disease. Acta Neuropathol. 1995;89:291–295. doi: 10.1007/BF00309621. [DOI] [PubMed] [Google Scholar]
- 47.Iqbal K, Alonso Adel C, Chen S, et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Iqbal K, Zaidi T, Bancher C, et al. Alzheimer paired helical filaments. Restoration of the biological activity by dephosphorylation. FEBS Lett. 1994;349:104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- 49.Stoothoff WH, Johnson GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta. 2005;1739:280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 50.Rudrabhatla P, Grant P, Jaffe H, et al. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer’s disease by iTRAQ. FASEB J. 2010;24:4396–4407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shetty KT, Link WT, Pant HC. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nukina N, Kosik KS, Selkoe DJ. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci USA. 1987;84:3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749– 759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 54.Amin ND, Albers W, Pant HC. Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35. J Neurosci Res. 2002;67:354–362. doi: 10.1002/jnr.10116. [DOI] [PubMed] [Google Scholar]
- 55.Tsai LH, Delalle I, Caviness VS, Jr, et al. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 56.Ko J, Humbert S, Bronson RT, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patrick GN, Zhou P, Kwon YT, et al. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- 58.Tang D, Chun AC, Zhang M, et al. Cyclin-dependent kinase 5 (Cdk5) activation domain of neuronal Cdk5 activator. Evidence of the existence of cyclin fold in neuronal Cdk5a activator. J Biol Chem. 1997;272:12318–12327. doi: 10.1074/jbc.272.19.12318. [DOI] [PubMed] [Google Scholar]
- 59.Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- 60.Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004;10:452–458. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Quintanilla RA, Orellana DI, Gonzalez-Billault C, et al. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res. 2004;295:245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Sun KH, de Pablo Y, Vincent F, et al. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- 63.Shea TB, Zheng YL, Ortiz D, et al. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J Neurosci Res. 2004;76:795–800. doi: 10.1002/jnr.20099. [DOI] [PubMed] [Google Scholar]
- 64.Chang K-H, de Pablo Y, Lee H-p, et al. Cdk5 is a major regulator of p38 cascade: relevance to neurotoxicity in Alzheimer’s disease. J Neurochem. 2010;113:1221–1229. doi: 10.1111/j.1471-4159.2010.06687.x. [DOI] [PubMed] [Google Scholar]
- 65.Pei JJ, Braak E, Braak H, et al. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- 66.Zhu X, Rottkamp CA, Hartzler A, et al. Activation of MKK6, an upstream activator of p38, in Alzheimer’s disease. J Neurochem. 2001;79:311–318. doi: 10.1046/j.1471-4159.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 68.Alvira D, Ferrer I, Gutierrez-Cuesta J, et al. Activation of the calpain/cdk5/p25 pathway in the girus cinguli in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:309–313. doi: 10.1016/j.parkreldis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Avraham E, Rott R, Liani E, et al. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. doi: 10.1074/jbc.M608243200. [DOI] [PubMed] [Google Scholar]
- 70.Shukla V, Mishra SK, Pant HC. Oxidative stress in neurodegeneration. Adv Pharmacol Sci. 2011;2011:572634. doi: 10.1155/2011/572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 72.Kesavapany S, Zheng YL, Amin N, et al. Peptides derived from Cdk5 activator p35, specifically inhibit deregulated activity of Cdk5. Biotechnol J. 2007;2:978–987. doi: 10.1002/biot.200700057. [DOI] [PubMed] [Google Scholar]
- 73.Zheng YL, Kesavapany S, Gravell M, et al. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2005;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iijima K, Ando K, Takeda S, et al. Neuron-specific phosphorylation of Alzheimer’s beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem. 2000;75:1085–1091. doi: 10.1046/j.1471-4159.2000.0751085.x. [DOI] [PubMed] [Google Scholar]
- 75.Ando K, Iijima KI, Elliott JI, et al. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001;276:40353–40361. doi: 10.1074/jbc.M104059200. [DOI] [PubMed] [Google Scholar]
- 76.Lee MS, Kao SC, Lemere CA, et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz JC, Tseng HC, Goldman JA, et al. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 78.Sobue K, Agarwal-Mawal A, Li W, et al. Interaction of neuronal Cdc2-like protein kinase with microtubule-associated protein tau. J Biol Chem. 2000;275:16673–16680. doi: 10.1074/jbc.M000784200. [DOI] [PubMed] [Google Scholar]
- 79.Flaherty DB, Soria JP, Tomasiewicz HG, et al. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000;62:463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 80.Ishiguro K, Sato K, Takamatsu M, et al. Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci Lett. 1995;202:81–84. doi: 10.1016/0304-3940(95)12206-0. [DOI] [PubMed] [Google Scholar]
- 81.Lund ET, McKenna R, Evans DB, et al. Characterization of the in vitro phosphorylation of human tau by tau protein kinase II (cdk5/p20) using mass spectrometry. J Neurochem. 2001;76:1221–1232. doi: 10.1046/j.1471-4159.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi H, Ishiguro K, Uchida T, et al. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 83.Liu WK, Williams RT, Hall FL, et al. Detection of a Cdc2-related kinase associated with Alzheimer paired helical filaments. Am J Pathol. 1995;146:228–238. [PMC free article] [PubMed] [Google Scholar]
- 84.Pei JJ, Grundke-Iqbal I, Iqbal K, et al. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res. 1998;797:267–277. doi: 10.1016/s0006-8993(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 85.Augustinack JC, Sanders JL, Tsai LH, et al. Colocalization and fluorescence resonance energy transfer between cdk5 and AT8 suggests a close association in pre-neurofibrillary tangles and neurofibrillary tangles. J Neuropathol Exp Neurol. 2002;61:557–564. doi: 10.1093/jnen/61.6.557. [DOI] [PubMed] [Google Scholar]
- 86.Hashiguchi M, Saito T, Hisanaga S, et al. Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem. 2002;277:44525–44530. doi: 10.1074/jbc.M207426200. [DOI] [PubMed] [Google Scholar]
- 87.Rudrabhatla P, Grant P, Jaffe H, et al. Quantitative phosphoproteomic analysis of neuronal intermediate filament proteins (NF-M/H) in Alzheimer’s disease by iTRAQ. FASEB J. 2010;24:4396–4407. doi: 10.1096/fj.10-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer’s NFTs. FASEB J. 2011;25:3896–3905. doi: 10.1096/fj.11-181297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou J, Wang H, Feng Y, Chen J. Increased expression of cdk5/p25 in N2a cells leads to hyperphosphorylation and impaired axonal transport of neurofilament proteins. Life Sci. 2010;86:532–537. doi: 10.1016/j.lfs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 90.Shah RS, Lee HG, Xiongwei Z, et al. Current approaches in the treatment of Alzheimer’s disease. Biomed Pharmacother. 2008;62:199–207. doi: 10.1016/j.biopha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 92.Maier M, Seabrook TJ, Lazo ND, et al. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piedrahita D, Hernandez I, Lopez-Tobon A, et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer’s mice. J Neurosci. 2010;30:13966–13976. doi: 10.1523/JNEUROSCI.3637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Helal CJ, Sanner MA, Cooper CB, et al. Discovery and SAR of 2-aminothiazole inhibitors of cyclin-dependent kinase 5/p25 as a potential treatment for Alzheimer’s disease. Bioorg Med Chem Lett. 2004;14:5521–5525. doi: 10.1016/j.bmcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 95.Helal CJ, Kang Z, Lucas JC, et al. Potent and cellularly active 4-aminoimidazole inhibitors of cyclin-dependent kinase 5/p25 for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett. 2009;19:5703–5707. doi: 10.1016/j.bmcl.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 96.Knockaert M, Wieking K, Schmitt S, et al. Intracellular targets of paullones. Identification following affinity purification on immobilized inhibitor. J Biol Chem. 2002;277:25493–25501. doi: 10.1074/jbc.M202651200. [DOI] [PubMed] [Google Scholar]
- 97.Zheng YL, Amin ND, Hu YF, et al. A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem. 2010;285:34202–34212. doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]