Abstract
Fatty liver disease is epidemiologically associated with type 2 diabetes (T2D), leading to a speculation of a reciprocal cause-effect relationship and a vicious cycle of pathology. Here, we summarize recent literature reporting dissociation of hepatosteatosis from insulin resistance, in genetic mouse models and clinical studies. We highlight rhythmic flows of metabolic intermediates between hepatic lipid synthesis and glucose production in normal circadian physiology. Blocking triglyceride (TG) secretion, subcellular lipid sequestration, lipolysis deficiency, enhanced lipogenesis, gluconeogenesis defects, or inhibition of fatty acid oxidation, all result in hepatosteatosis without causing hyperglycemia or insulin resistance, suggesting that the cause-effect relationship between hepatosteatosis and diabetes does not exist in all situations.
Hepatosteatosis and diabetes
Nonalcoholic fatty liver disease (NAFLD) affects over 20% of the population in the United States and emerges as a major metabolic disorder with increasing incidence worldwide [1]. Hepatosteatosis, abnormal accumulation of TGs in liver, is the defining feature of NAFLD. NAFLD is associated with T2D in many epidemiology studies, promoting speculation of a mutual cause-and-effect relationship between hepatosteatosis and insulin resistance in a “vicious cycle” as a unified pathogenesis mechanism [2][3]. On one hand, insulin resistance results in hyperinsulinemia that may drive hepatic lipogenesis and cause steatosis [3]. On the other, accumulation of various lipid species in the liver may activate several protein kinase cascades, either directly or indirectly through inflammation [4], or endoplasmic reticulum stress [5], which ultimately lead to disruption of the insulin signaling, and thus exacerbate insulin resistance [6][7].
The association of hepatosteatosis with insulin resistance is challenged by several considerations. First, lipid-induced disruption of the insulin signaling is proposed to occur on several sites including insulin receptor (IR), insulin receptor substrate (IRS), and AKT (protein kinase B) [4–7], all of which lie upstream of the bifurcation point for “selective insulin resistance” [8,9]. Therefore, the abovementioned vicious cycle seems self-limiting instead of self-reinforcing. Second, upon a closer look at the epidemiological studies that established the association, one can easily appreciate the fact that a lot of subjects with similar degree of hepatosteatosis can have very high or very low insulin sensitivity, suggesting the existence of a strong dissociation [10]. Third, from a therapeutic perspective, successful reversal of insulin resistance does not necessarily reverse hepatosteatosis, and vice versa, in both animal models [11–15] and clinical studies [16]. Fourth, numerous genetic animal models, human inborn defects, and human polymorphism studies demonstrate dissociation of hepatosteatosis from insulin resistance, and in some cases, even co-occurrence of hepatosteatosis with insulin hypersensitivity and hypoglycemia (Table 1). In this review, we focus on recent findings that demonstrate such dissociation and try to reconcile these data with the association argument towards a unified model on the intriguing interrelationship between the pathological hepatosteatosis and insulin resistance, or in more physiological terms, hepatic lipid and carbohydrate metabolism.
Table 1.
Summary of recent studies demonstrating a dissociation of fatty liver and diabetes in genetic animal models or human subjects with genetic variations
| Primary changes | Gene | Genetic manipulation | Phenotype compared to WT control on the same condition | References |
|---|---|---|---|---|
| Insulin signaling | IR | Liver-specific KO mice (LIRKO) | Hypotriglyceridemia, low lipogenesis, hyperinsulinemia, enhanced HGP, no change in BW | [18][19] |
| PI3K | Liver-specific KO of p85 (L-p85DKO) or p110a | Hypotriglyceridemia, low lipogenesis, hyperinsulinemia, glucose intolerance, on change in BW | [20][21] | |
| AKT2 | Liver-specific KO on ob/ob background or DIO | Absence of overnutrition-induced hepatosteatosis, hyperglycemia, hyperinsulinemia, lower BW | [22] | |
| PTEN | Liver-specific KO mice | Hepatosteatosis, enhanced lipogenesis, insulin hypersensitivity, no change in BW | [23] | |
| TG secretion | APOB | Truncation mutation in mice or human FHBL | Hepatosteatosis, no change on insulin sensitivity or BW | [24][25][26] |
| MTTP | MTTP f/f, Mx1-Cre mice | Hepatosteatosis with higher DAG and ceramide, no change in insulin sensitivity or BW | [27] | |
| PEMT | KO mice fed HFD | Hepatosteatosis, increased insulin sensitivity, lower BW | [28] | |
| CTa | Liver-KO on HFD | Hepatosteatosis with higher DAG and ceramide, no change in insulin sensitivity or BW | [28][29] | |
| Lipid sequestration | ATGL | Whole body or liver-specific KO | Hepatosteatosis, increased or unchanged insulin sensitivity, increased or unchanged BW | [33][36] |
| CGI-58 | ASO knockdown in liver in mice fed HFD | Hepatosteatosis with higher DAG and ceramide, increased insulin sensitivity, lower BW | [38] | |
| PNPLA3 | Variations in human | Hepatosteatosis, no change in insulin sensitivity | [41][42] | |
| PLIN2 | Overexpression in mice liver by adenovirus | Hepatosteatosis, increased systemic insulin sensitivity, no change in BW | [49] | |
| CIDEC | Overexpression in mice liver by adenovirus | Hepatosteatosis, fasting hypoglycemia | [52] | |
| Lipogenesis | DGAT2 | Transgenic over-expression in mouse liver, human variants | Hepatosteatosis with higher DAG and ceramide, no change in insulin sensitivity or BW | [56][58] |
| SREBP-1c | Overexpression in mice liver by adenovirus | Hepatosteatosis, lower blood glucose in streptozotocin-treated mice, no change in BW | [62] | |
| ChREBP | Overexpression in mice liver by adenovirus | Hepatosteatosis with higher DAG, increased insulin sensitivity on HFD, no change in BW | [64] | |
| HDAC3 | Liver-specific KO mice | Hepatosteatosis with higher DAG, increased insulin sensitivity, no change in BW | [49] | |
| FASN | Liver-specific KO mice | Hepatosteatosis upon fasting or zero fat diet, hypoglycemia, no change in BW | [68] | |
| ELOVL6 | KO or liver-specific knockdown by adenovirus | Hepatosteatosis on HF-HS diet, increased insulin sensitivity, slightly lower BW | [69] | |
| FA oxidation | CPT-1a | Heterozygous +/− mice | Hepatosteatosis, increased glucose tolerance on high carbohydrate diet, slightly lower BW | [73] |
| PPARa | KO mice fed HFD or on fasting | Hepatosteatosis, fasting hypoglycemia, increased systemic insulin sensitivity, no change in BW | [77] | |
| PGC-1a | KO mice, or liver- specific KO or knockdown | Hepatosteatosis, fasting hypoglycemia, increased glucose tolerance, unaltered or higher BW | [80][81][82] | |
| PGC-1b | KO mice on HFD | Hepatosteatosis, no change in glucose tolerance or insulin resistance, higher or lower BW | [85][86] | |
| BNIP3 | KO mice on fasting | Hepatosteatosis, improved glucose tolerance and insulin resistance, | [87] | |
| TBL1/TBLR1 | Double knockdown in liver by adenovirus, | Hepatosteatosis, increased insulin sensitivity, reduced FAO, no change in BW | [90] | |
| Gluconeogenesis | PCK1 | Liver-specific KO or knockdown | Hepatosteatosis, increased glucose tolerance and insulin sensitivity, no change in BW | [91][92] |
| G6PC | Liver-specific KO | Hepatosteatosis, fasting hypoglycemia, no change in BW | [93,94] | |
| FOXO | Liver-specific KO of FOXO1/3/4 | Hepatosteatosis, hypoglycemia, improved glucose tolerance, no change in BW | [96][97] | |
| CREB/CRTC2 | KO or dominant negative expression | Hepatosteatosis, hypoglycemia, improved insulin sensitivity | [102][101] | |
| GCGR | KO mice on HFD | Hepatosteatosis, hypoglycemia, insulin hypersensitivity, unaltered or lower BW | [103][104] |
KO: knock-out; WT: wild-type; HGP: hepatic glucose production; HFD: high fat diet; BW: body weight; DIO: diet induced obesity; DAG: diacylglycerol; ASO: anti-sense oligonucleotides.
Intact insulin signaling contributes to hepatosteatosis
Insulin exerts its action by binding to the extracellular portion of the insulin receptor (IR) and triggers a cascade of intracellular signaling events involving three critical nodes which are sequential phosphorylation and activation of IRSs, phosphoinositide 3-kinases (PI3Ks) and AKT [17]. In liver, intact insulin signaling is required for lipogenesis and subsequent hepatosteatosis and hypertriglyceridemia under hyperinsulinemia conditions, which constitutes one arm of the vicious cycle [2,3]. In the meantime, hepatic insulin signaling is also required for suppression of gluconeogenesis and subsequent glucose output. Therefore, defects in the upstream hepatic insulin signaling cause co-occurrence of glucose intolerance along with reduced TG levels in liver and blood, in other words, dissociation of hepatosteatosis from insulin resistance.
This was made evident by numerous genetic mouse models. Liver-specific insulin receptor knockout (LIRKO) mice display drastic hypotriglyceridemia, associated with reduced expression of hepatic lipogenic genes, despite hyperglycemia, hyperinsulinemia, glucose intolerance and insulin resistance [18][19]. In the same manner, liver-specific knockout of two p85 subunits of PI3Ks (L-p85DKO) impairs glucose tolerance, but causes hypotriglyceridemia associated with reduced hepatic lipogenic genes [20]. Deletion of p110alpha, another subunit of PI3K in liver also increases gluconeogenesis and results in reduced insulin sensitivity, but reduces TG levels in blood [21]. Liver-specific knockout of AKT2 abolishes hepatosteatosis and the abnormally elevated hepatic lipogenesis in the leptin-deficient hyperphagic model, despite that these mice have higher blood glucose level than wild-type controls in the same leptin-deficiency background [22]. These observations demonstrate the necessity of intact insulin signaling upstream of AKT for hepatic lipogenesis, hepatosteatosis, and hypertriglyceridemia under overnutrition conditions.
Another study demonstrates the sufficiency. Liver-specific deletion of phosphatase and tensin homolog (PTEN), a negative regulator of the insulin signaling that acts upstream of AKT, improves systemic glucose tolerance and causes massive fatty liver that is attributed mainly to enhanced hepatic lipogenesis [23]. Thus, hepatic insulin hypersensitivity is sufficient to drive hepatosteatosis.
If insulin sensitivity contributes to hepatosteatosis, how does insulin resistance contribute to a vicious cycle that exacerbates this condition? To resolve this paradox, Brown and Goldstein raised the concept of “selective insulin resistance” [8]. It is proposed that, although insulin-mediated suppression of hepatic gluconeogenesis is blunted due to disruption of some aspects of insulin signaling in liver, other insulin signaling mechanisms remain intact and continue to drive hepatic lipogenesis. Then, where is the bifurcation point of the insulin signaling? A recent study on rat suggests that mammalian target of rapamycin complex 1 (mTORC1) is such a bifurcation point that lies downstream of AKT [9]. Inhibition of mTORC1 blocks insulin-induced upregulation of lipogenic genes expression, but does not affect insulin-mediated suppression of gluconeogenic genes expression [9].
Herein lies another paradox related to the vicious cycle of insulin resistance and hepatosteatosis. The current prevailing view on how hepatosteatosis causes insulin resistance holds that it is mainly through modification and disruption of the insulin signaling at the sites of IR, IRS or AKT, that are all upstream of the bifurcation point mTORC1 [4–7]. However, if this were the complete story, hepatosteatosis would be self-limiting rather than self-reinforcing in a vicious cycle. It is no easy task to solve this paradox. Maybe the major site of lipid-mediated impairment of insulin signaling occurs further downstream than it is currently believed. Perhaps a significant portion of hepatic lipogenesis is independent of insulin signaling, or maybe hepatosteatosis and insulin resistance do not share a cause-and-effect relationship after all. Above all, we may need to integrate a more flux-centric view in our signaling-centric approaches towards a better understanding of metabolism. In the rest of this overview, we examine the interrelationships between hepatic lipid accumulation and glucose production in several conditions categorized by different metabolic flux pathways.
Blocking hepatic TG secretion causes hepatosteatosis without insulin resistance
During feeding, the liver converts surplus nutrients into TG, which are secreted into the blood in the form of very low-density lipoprotein (VLDL) for delivery to adipose tissues. Apolipoprotein B (APOB) is an essential component of VLDL particles. Patients with familial hypobetalipoproteinemia (FHBL), a disease caused by mutations in the APOB gene, have impaired hepatic TG secretion and therefore develop hepatosteatosis and hypotriglyceridemia. Recently, independent clinical studies using hyperinsulinemic-euglycemic clamp analysis showed that hepatosteatosis in FHBL is not associated with insulin resistance or diabetes [24][25]. Likewise, mice deficient in the APOB gene on several strain backgrounds develop hepatosteatosis, but do not display glucose intolerance or insulin resistance [26].
Microsomal triglyceride transfer protein (MTTP) is another key player in hepatic lipoprotein assembly. Mice with MTTP deleted in the liver have defective secretion of VLDL and develop hepatosteatosis, yet have normal glucose tolerance and insulin sensitivity, as assessed by hyperinsulinemic-euglycemic clamp studies [27]. Interestingly, in addition to increased TGs, these livers also contain elevated levels of ceramides and diacylglycerols (DAG), two lipid species alleged responsible for lipid-induced insulin resistance [27].
Phosphatidylcholine is the major component of the hydrophilic surface of VLDL and is required for VLDL generation and hepatic TG secretion. Phosphatidylcholine is synthesized either from phosphatidylethanolamine by phosphatidylethanolamine N-methyltransferase (PEMT) or from choline by CTP: phosphocholine cytidylyltransferase alpha (CTα) along with other enzymes [28]. PEMT knockout mice fed a high fat diet (HFD) develop more severe hepatosteatosis than wild-type controls, but gain less body weight and are protected from insulin resistance [28]. Liver-specific deletion of CTα in mice reduces VLDL secretion and increases TGs, ceramides and DAG levels in liver, but does not result in abnormal glucose tolerance on HFD [29].
Choline is an important precursor for phosphatidylcholine. Choline deficient diet causes fatty liver, presumably by blocking hepatic TG secretion, although the mechanism may also involve enhanced hepatic lipogenesis [30]. Nonetheless, hepatosteatosis caused by choline deficiency is not accompanied by insulin resistance. On the contrary, choline deficiency protects mice from HFD-induced insulin resistance without affecting body weight gain [31]. Taken together, the dissociation of insulin resistance from hepatosteatosis caused by blocking VLDL secretion indicates that elevated total hepatic TG, ceramides or DAG levels are not sufficient to cause insulin resistance.
Subcellular lipid sequestration prevents lipotoxicity
Before TGs are secreted as VLDL, they are stored in lipid droplets (LDs). LDs are also the place in the cell where lipolysis occurs [32]. Adipose triglyceride lipase (ATGL), officially annotated as patatin-like phospholipase domain-containing protein (PNPLA2), is the major lipase responsible for hydrolysis of TG to DAG [32]. Knockout of ATGL in mice blocks TG catabolism and causes massive steatosis in both liver and heart [33][34]. Despite slightly increased body weight, ATGL knockout mice display enhanced glucose tolerance and insulin sensitivity, mainly because skeletal muscles switch to use glucose as a major fuel source [33][35]. In line with this observation, liver-specific knockout of ATGL results in marked hepatosteatosis without causing changes in inflammation indices or insulin sensitivity [36]. In addition, overexpression of ATGL in liver using adenovirus reduces hepatic lipid accumulation but does not affect insulin sensitivity [37]. Comparative gene identification-58 (CGI-58) binds ATGL and activates the catalytic activity of ATGL on LD surfaces [32]. Mice treated with CGI-58 antisense oligonucleotides accumulate TG, DAG and ceramides in liver, but are protected from HFD-induced glucose intolerance and insulin resistance [38].
Recently, several independent genome-wide epidemiological studies have identified that variations in PNPLA3, a gene related to ATGL, are associated with NALFD [39][40]. Interestingly, PNPLA3-associated hepatosteatosis is not accompanied by insulin resistance, as revealed by oral glucose tolerance tests and hyperinsulinemic-euglycemic clamp analysis in several clinical studies [41][42]. PNPLA3 deficiency in mouse does not cause hepatosteatosis [43][44], a clear reminder that interspecies differences should always be kept under consideration when working with animal models. Meanwhile, overexpressing the NAFLD-associated PNPLA3 mutant I148M, but not the wide-type PNPLA3, causes hepatosteatosis in mice [45]. Whether I148M represents a gain-of-function mutation and whether PNPLA3 has lipase-independent activity is being investigated [46].
LDs are coated with numerous proteins that serve as dynamic scaffolds in regulating lipolysis [47]. Perilipin 2 (Plin2) is one of the first few proteins known to coat LDs and inhibit ATGL-mediated lipolysis in liver [48]. The protein levels of Plin2 correlates with liver TG levels and generally indicates the approximate quantity of LDs. Overexpression of Plin2 with adenovirus in mouse liver precipitates hepatosteatosis without changing body weight, and enhances whole-body insulin sensitivity as revealed by hyperinsulinemic-euglycemic clamp [49]. In like manner, muscle-specific Plin2 overexpression in rats increases intramyocellular TG and ceramide levels, but blunts the high-fat diet-induced insulin resistance [50]. Cell death-inducing DFFA-like effector c (Cidec), also known as fat-specific protein 27 (Fsp27), is another protein that associates with LDs and negatively regulates lipolysis [51]. Adenovirus-mediated overexpression of Cidec in liver causes hepatosteatosis associated with increased expression of hepatic lipogenic genes and decreased fasting blood glucose levels [52]. The interesting phenotypes of mice overexpressing LDs-coating proteins are consonant with those of lipolysis deficient mice. Together, these observations suggest that sequestering various lipid species within LDs and shielding them from lipolysis represents a protective mechanism against lipotoxicity-associated insulin resistance.
Excessively enhanced lipogenesis undermines gluconeogenesis
If the LD is a safe place for temporal storage of lipids before they are secreted or hydrolyzed, then channeling metabolic intermediates towards TGs synthesis would bring additional layer of protection against lipotoxicity, provided that the amount of synthesized lipids do not overwhelm the sequestration capacity of LDs. This putative protective role of lipid synthesis is demonstrated in several genetic mouse models and cell culture models. Mouse embryonic fibroblasts (MEFs) become more susceptible to fatty acid (FA)-induced cell death after depletion of diacylglycerol acyltransferase 1 (DGAT1), an enzyme catalyzing the final step of TG synthesis [53]. Transgenic mice overexpressing DGAT1 in muscles display increased intramyocellular TG levels, but are protected from high-fat diet-induced insulin resistance, recapitulating the “athlete’s paradox” [54]. Knockdown of DGAT2, another DGAT isoform catalyzing the same reaction, in mouse liver aggravates liver damage and fibrosis that is induced by methionine and choline-deficient diet (MCD) [55]. On the other hand, overexpression of DGAT2 in mouse liver increases intrahepatic TG, DAG and ceramides levels, but does not cause insulin resistance or glucose intolerance [56]. Although this result is not beyond dispute [57], a recent clinical study shows that a genetic variation in human DGAT2 gene is associated with fatty liver but not with insulin resistance, as analyzed by oral glucose tolerance tests and euglycemic-hyperinsulinemic clamp studies, suggesting that mutations in DGAT2 dissociate hepatosteatosis from diabetes in human [58].
Sterol regulatory element binding proteins (SREBPs) are generally recognized as master activators of lipid synthesis [59]. There are two SREBP genes in mammals, SREBP-1 and SREBP-2, with SREBP-1 mainly involved in FA synthesis and SREBP-2 in cholesterol synthesis. SREBP-1 proteins exist in two isoforms, SREBP-1a and SREBP-1c, with SREBP-1c as the predominant isoform in adult liver. Insulin drastically increases SREBP-1c mRNA levels in liver [60], and overexpression of either SREBP-1c or SREBP-1a in mouse liver markedly enhances expression of lipogenic genes and causes massive hepatosteatosis [61]. These findings suggest that SREBP-1 contributes to insulin-induced hepatic lipogenesis, and thus the first arm of the vicious cycle. Considering the second arm of the vicious cycle, one would readily expect that SREBP-1 activation could exacerbate hyperglycemia. However, on the contrary, adenovirus-mediated overexpression of SREBP-1c in liver decreases blood glucose levels in streptozotocin-induced diabetic mice, despite causing hepatosteatosis [62].
Carbohydrate responsive element binding protein (ChREBP) is another master transcriptional activator of hepatic lipogenesis [63]. ChREBP is activated by increased glucose influx in liver, and together with SREBP-1, drives expression of genes involved in FA synthesis and esterification. Overexpression of ChREBP in mouse liver using adenovirus increases hepatic TG and DAG levels, but blunts HFD-induced insulin resistance without changing body weight gain [64]. ChREBP overexpression also decreases expression of gluconeogenic genes and reduces hepatic glycogen storage in mice. In human, gene expression level of ChREBP is positively correlated with hepatosteatosis and negatively related to insulin resistance [64]. Conversely, ChREBP knockout mice fed high starch diet display mitigated hepatosteatosis, but develop severe insulin resistance along with hyperglycemia, hyperinsulinemia, and increased hepatic glycogen store, without changes in body weight [65].
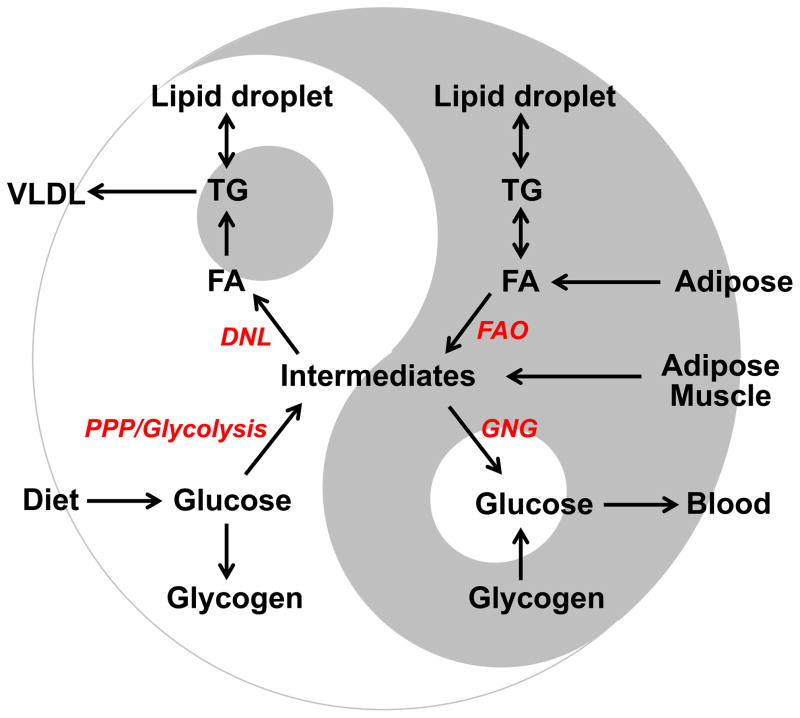
What does this noncanonical interplay between hepatic lipogenesis and gluconeogenesis actually mean from an evolutionary and teleological perspective? Recent studies on histone deacetylase 3 (HDAC3) provide some novel insights. Genome-wide occupancy of HDAC3 is highly enriched on lipogenic genes and displays robust circadian rhythm in mouse liver, with the maximum occupancy during the day and minimum occupancy at night, corresponding to fasting and feeding behavior, respectively, for nocturnal mice [66]. Such circadian rhythm is directly orchestrated by nuclear receptors Rev-erbα/β, key components of the internal circadian clock [66,67]. Liver-specific knockout of HDAC3 in mice drastically enhances lipogenesis flux and decreases gluconeogenesis flux, resulting in increased hepatic TG and DAG accumulation, reduced hepatic glycogen storage, hypoglycemia, and insulin hypersensitivity [49]. The decreased gluconeogenesis flux in HDAC3-deficient liver is a result of the metabolic re-routing towards lipid synthesis and subsequent lipid sequestration, rather than primary defects in gluconeogenic abilities [49]. Hence, hepatic lipogenesis and gluconeogenesis are mutually exclusive and are anti-phase to one another, in normal circadian physiology (Figure 1). While SREBPs and ChREBP respond to feeding behavior, HDAC3 anticipates it. Together, these mechanisms channel metabolic intermediates, including biosynthetic precursors, between lipid synthesis and glucose production in a rhythmic pattern. Breaking this dynamic balance by excessively enhancing lipogenesis undermines gluconeogenesis, leading to reduced glycogen storage and hypoglycemia (Figure 1).
Figure 1. Yin and Yang: counteraction and interdependence between hepatic lipid and carbohydrate metabolism harmonized in circadian rhythms.
In normal physiology, there is a well-balanced rhythmic flow of metabolic intermediates in between lipid synthesis and glucose production in liver. For diurnal organisms, intermediates supplied by carbohydrates catabolism are assimilated into lipids on the light cycle. While on the dark cycle, intermediates are re-routed towards glucose production, a process fueled by lipid oxidation. Excessively enhancing lipogenesis undermines gluconeogenesis because precursors are diverted away, leading to reduced glycogen storage and hypoglycemia. Blocking gluconeogenesis, on the other hand, shunts intermediates into lipogenesis and promotes steatosis. Inhibiting lipid oxidation accumulates triglycerides, but also impedes gluconeogenesis due to undersupply of energy and cofactors. Lipids, either generated intrahepatically or received externally, are handled by lipid droplets (LDs) before secretion or oxidation. When the amount of lipids exceeds the sequestration capacity of LDs, lipotoxicity ensues. The intricate relationship between hepatic lipid and carbohydrate metabolism exemplifies the ancient philosophy of Yin and Yang, which describes the world as two opposing existences that flow in a natural cycle seeking balance and interconnection to each other.
DNL: de novo lipogenesis; FAO: fatty acid oxidation; PPP: pentose phosphate pathway; GNG: gluconeogenesis; FA: fatty acids; TG: triglycerides. Intermediates include Krebs cycle intermediates, precursors and cofactors for biosynthesis, and energy.
In addition to direct effects on the metabolic re-routing, manipulation of lipogenesis may affect the availability of metabolites that serve as signaling molecules and hence causes unexpected metabolic changes. Mice with liver-specific knockout of fatty-acid synthase (FASN) display hypoglycemia, hepatosteatosis, and reduced hepatic glycogen storage when fasting or fed a zero-fat diet, without changes in body weight [68]. All of these three metabolic changes are reversed when mice are treated with a synthetic ligand for peroxisome proliferator-activated receptor alpha (PPARα), suggesting that FASN is involved in generating endogenous ligands for PPARα [68]. Similarly, mice deficient for fatty acid elongase 6 (ELOVL6), an enzyme catalyzing the elongation of palmitate to stearate, display slightly increased hepatic TG levels, but ameliorated insulin resistance on a high-fat high-sucrose diet [69]. Expression of several PPARα target genes is drastically downregulated in ELOVL6-deficient liver, suggesting that ELVOL6 may as well contribute to generating endogenous PPARα ligands. PPARα is a master transcriptional activator for FA oxidation (FAO). Both of the hepatosteatosis and hypoglycemia phenotypes in FASN or ELVOL6 deficient mice seem to be explained by a FAO defect, which is discussed below.
Blocking FAO impedes gluconeogenesis
In normal physiology, hepatic gluconeogenesis is activated upon fasting and is fueled by FAO. A defective FAO pathway fails to provide sufficient energy and cofactors that are needed for gluconeogenesis activation, resulting in fasting hypoglycemia and enhanced glucose tolerance. Meanwhile, lipids accumulate because they are not oxidized, leading to hepatosteatosis. Such dissociation of hepatosteatosis and insulin resistance is found in many situations. Human with inborn FAO errors develop Reyes-like syndrome characterized by hypoglycemia, fatty liver, fasting intolerance and other signs, which are recapitulated in genetic mouse models with deficiency in FAO enzymes such as long-chain acyl-CoA deydrogenase (LCAD), medium-chain acyl-CoA deydrogenase (MCAD), medium/short-chain 3-dydroxyacyl-CoA deydrogenase (M/SCHAD), short-chain acyl-CoA deydrogenase (SCAD), mitochondrial trifunctional protein α-subunit (TFPα) [70–72]. Carnitine palmitoyl transferase-1α (CPT-1α) contributes to transporting FAs into mitochondria, a rate-limiting step in FAO. Mice heterozygous for CPT-1α develop microvesicular hepatosteatosis and improved glucose tolerance on high carbohydrate diet [73]. Likewise, inhibition of CPT-1 by small molecule chemicals causes microvesicular hepatosteatosis with either unaltered or enhanced hepatic insulin sensitivity in mice [74–76].
PPARα is a master transcriptional activator that drives expression of many FAO genes. PPARα null mice display hepatosteatosis on HFD or after prolonged fasting, hypoglycemia, increased insulin sensitivity in insulin tolerance test, and reduced hepatic glycogen storage, without changes in body weight [77][78]. Hepatic glucose production from lactate is reduced in PPARα deficient liver, suggesting decreased gluconeogenesis flux, although the exact mechanism could be more complex [79]. In addition, the hypoglycemia phenotype in the whole-body PPARα knockout mice could be partially contributed to by muscles that may switch to oxidize more glucose. PPARγ coactivator 1α/β (PGC-1) are transcription coactivators for PPARα. Whole-body or liver specific knockout or knockdown of PGC-1α in mice causes hepatosteatosis, hypertriglycidemia, fasting hypoglycemia and improved glucose tolerance, although it is controversial whether or not the molecular insulin signaling is enhanced [80–82]. Detailed metabolic flux analysis and gene expression analysis revealed that the decreased gluconeogenesis in PGC-1α deficient liver is caused by the defective FAO and Krebs cycle flux, rather than an intrinsically impaired gluconeogenic capacity [83]. Conversely, overexpression of PGC-1α in mice enhances hepatic gluconeogenesis and glucose output, and thus results in hepatic insulin resistance [84]. PGC-1β knockout mice develop hepatosteatosis on HFD, but unaltered glucose tolerance or systemic insulin sensitivity [85][86]. Knockout of BNIP3, a PGC-1β target gene encoding a mitochondrial protein, reduces FAO and causes hepatosteatosis with improved glucose tolerance or systemic insulin sensitivity [87].
The transcriptional cofactors transducin beta-like 1 (TBL1) and TBL-related 1 (TBLR1) exist in multi-protein transcription co-repressor complexes containing HDAC3 [88,89]. Mice with both TBL1 and TBLR1 knocked-down in liver display hepatosteatosis, reduced blood ketone levels, and improved glucose tolerance and insulin sensitivity without changes in body weight [90]. It is suggested that TBL1 and TBLR1 facilitate PPARα-mediated activation of FAO and that TBL1/TBLR1 deficiency phenocopies PPARα ablation [90], although it is also possible that TBL1/TBLR1 may play a role in the HDAC3-containing co-repressor complexes and thus directly affect lipogenesis [49]. In summary, while hepatic gluconeogenesis competes against lipid synthesis for metabolic intermediates, it depends on lipid oxidation for energy and cofactors. Such counteraction and interdependence between carbohydrates and lipid metabolism is harmonized by circadian re-routing of metabolic intermediates. This intriguing interrelationship reflects the ancient philosophy of Yin and Yang, which describes the world as two opposing existences that flow in a natural cycle seeking balance and interconnection to each other (Figure 1).
Defects in gluconeogenesis promote hepatosteatosis
If hepatic gluconeogenesis and lipogenesis are indeed mutually exclusive, blocking gluconeogenesis would shunt metabolic intermediates into lipogenesis pathway and promote hepatosteatosis, resulting in dissociation of hepatosteatosis from hyperglycemia and diabetes. It is exactly what happens. Human genetic defects in gluconeogenic enzymes, such as phosphoenolpyruvate carboxykinase 1 (PCK1), PCK2, glucose-6-phosphatase, catalytic (G6PC), fructose-1,6-bisphosphatase 1 (FBP1), pyruvate carboxylase (PC), manifest hypoglycemia, hepatomegaly and fatty infiltration in liver (OMIM database). The central role of liver in developing these phenotypes is demonstrated in mouse models. Liver-specific knockout or knockdown of PCK1 reduces gluconeogenesis flux, improves glucose tolerance and insulin sensitivity, and even improves insulin signaling in liver, despite causing hepatosteatosis [91][92]. Liver-specific knockout of G6PC in mice causes massive hepatosteatosis and fasting hypoglycemia without changes in body weight [93][94].
Forkhead box O proteins (FOXOs) are master transcriptional activators of gluconeogenic genes. There are four mammalian FOXO isoforms, FOXO1/3/4/6 that are activated by sirtuin-mediated deacetylation and repressed by AKT-mediated phosphorylation in response to fasting and feeding, respectively [95]. Combined liver-specific deletion of FOXO1/3 or FOXO1/3/4 in mice fed normal chow, causes hypoglycemia and improves glucose tolerance, and at the same time increases lipogenesis flux leading to hepatosteatosis and hypertriglyceridemia [96,97]. Conversely, expression of a constitutively active FOXO1 that is either dephosphorylated [98] or deacetylated [99], increases glucose production and induces insulin resistance, but reduces lipogenesis resulting in hypotriglyceridemia. Cyclic AMP-responsive element-binding protein (CREB) is another transcriptional activator of gluconeogenesis and is activated by glucagon through protein kinase A (PKA)-mediated phosphorylation [100]. Deficiency of CREB or its coacitvator CRTC2 causes fasting hypoglycemia and increases insulin sensitivity [101]. And CREB deficient mice develop hepatosteatosis accompanied with enhanced lipogenesis [102]. Likewise, glucogan receptor (GCGR) knockout mice display increased lipogenesis flux and develop hepatosteatosis on HFD, despite improved insulin sensitivity [103,104]. Taken together, master transcriptional regulators, such as FOXOs, CREB, SREBPs or ChREBP, specifically channel metabolic intermediates to gluconeogenesis or lipogenesis in response to fasting or feeding, respectively. This feedback mechanism is coupled with the anticipatory mechanism mediated by HDAC3 that receives signals directly from the circadian clock. Together, they orchestrate the dynamic metabolic fluxes precisely and reliably, which is ensured by natural selection during evolution (Figure 1).
Concluding remarks
The observations that hepatosteatosis is dissociated from diabetes in many mechanistic studies despite their positive correlation in epidemiology studies (Table 1) strongly argue that fatty liver disease is a complex spectrum of disorders with many underlying causes. Current classification and definition of “nonalcoholic” and “alcoholic” fatty liver disease is vague, arbitrary, oversimplified, and even unnecessary [105]. Fatty liver disease needs to be classified according to more detailed etiologies in order to develop efficient therapy and prevention strategies [106]. The same is true for diabetes that is currently classified into only two categories: type 1 and type 2. Technological advances in mass spectrometry (MS)-based metabolomics, stable isotope nuclear magnetic resonance spectroscopy (NMRS)-based noninvasive fluxomics, and next-generation DNA sequencing will allow identification of biomarkers, characterization of metabolic flux derangements, and profiling of genetic variations towards a more detailed classification of fatty liver disease and diabetes, which will in turn guide mechanistic studies and personalized treatment.
The dissociation of hepatosteatosis and insulin resistance is summarized and explained from a flux-centric cycle that reflects normal physiology (Figure 1). However, when normal physiology is disturbed, disease arises. A key balance-tipping factor seems to be the ability of the liver to handle lipids. This includes the ability to accommodate lipids in lipid droplets, to shield them from lipolysis, and to partition them into secretion or oxidation pathways. Evolution ensures that liver has sufficient lipid-handling capacity during circadian shifts in metabolic flux, but pathological overnutrition provides so many intermediates that drive up gluconeogenesis and lipogenesis simultaneously, producing way too much lipid than liver can handle. As a result, lipotoxicity is concurrent with hyperglycemia, the so-called association of fatty liver and diabetes. From a therapeutic perspective, efforts to reverse hepatosteatosis and insulin resistance have generated mixed results and produced conflicting data. In some case, successful treatment of hepatosteatosis does not reverse insulin resistance [11–14] and vice versa [16,15], again suggesting that the cause-effect relationship between the two does not exist in all situations.
Acknowledgments
Work in the authors’ laboratory is supported by NIH grants DK43806 and DK45586, the Cox Institute for Medical Research, and the JPB Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen JC, et al. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 3.Moore DD. Nuclear receptors reverse McGarry’s vicious cycle to insulin resistance. Cell Metab. 2012;15:615–622. doi: 10.1016/j.cmet.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu S, et al. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Li S, et al. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefan N, et al. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 11.Moon YA, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu XX, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 13.Mao J, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendel AA, et al. Glycerol-3-phosphate acyltransferase 1 deficiency in ob/ob mice diminishes hepatic steatosis but does not protect against insulin resistance or obesity. Diabetes. 2010;59:1321–1329. doi: 10.2337/db09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wunderlich FT, et al. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc Natl Acad Sci USA. 2008;105:1297–1302. doi: 10.1073/pnas.0707849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonardo A, et al. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient - death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279–289. doi: 10.1586/egh.11.19. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi CM, et al. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 18.Michael MD, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 19.Biddinger SB, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi CM, et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Sopasakis VR, et al. Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11:220–230. doi: 10.1016/j.cmet.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leavens KF, et al. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiles B, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc Natl Acad Sci USA. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaro A, et al. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visser ME, et al. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54:2113–2121. doi: 10.1007/s00125-011-2157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonfeld G, et al. Fatty liver and insulin resistance: not always linked. Trans Am Clin Climatol Assoc. 2008;119:217–223. discussion 223–224. [PMC free article] [PubMed] [Google Scholar]
- 27.Minehira K, et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res. 2008;49:2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs RL, et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285:22403–22413. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niebergall LJ, et al. Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim Biophys Acta. 2011;1811:1177–1185. doi: 10.1016/j.bbalip.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Walker AK, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raubenheimer PJ, et al. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–2020. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- 32.Lass A, et al. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 34.Haemmerle G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kienesberger PC, et al. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem. 2009;284:30218–30229. doi: 10.1074/jbc.M109.047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JW, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 37.Turpin SM, et al. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54:146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- 38.Brown JM, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romeo S, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speliotes EK, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantartzis K, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krarup NT, et al. The PNPLA3 rs738409 G-Allele Associates with Reduced Fasting Serum Triglyceride and Serum Cholesterol in Danes with Impaired Glucose Regulation. PLoS ONE. 2012;7:e40376. doi: 10.1371/journal.pone.0040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basantani MK, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, et al. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He S, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumari M, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenberg AS, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Listenberger LL, et al. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007;48:2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Sun Z, et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nature medicine. 2012 doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bosma M, et al. Perilipin 2 Improves Insulin Sensitivity in Skeletal Muscle Despite Elevated Intramuscular Lipid Levels. Diabetes. 2012 doi: 10.2337/db11-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong J, et al. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20:121–126. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 52.Uno K, et al. Hepatic peroxisome proliferator-activated receptor-γ-fat-specific protein 27 pathway contributes to obesity-related hypertension via afferent vagal signals. Eur Heart J. 2012;33:1279–1289. doi: 10.1093/eurheartj/ehr265. [DOI] [PubMed] [Google Scholar]
- 53.Listenberger LL, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi K, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 56.Monetti M, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Jornayvaz FR, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci USA. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kantartzis K, et al. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin Sci. 2009;116:531–537. doi: 10.1042/CS20080306. [DOI] [PubMed] [Google Scholar]
- 59.Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimomura I, et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimano H, et al. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bécard D, et al. Adenovirus-mediated overexpression of sterol regulatory element binding protein-1c mimics insulin effects on hepatic gene expression and glucose homeostasis in diabetic mice. Diabetes. 2001;50:2425–2430. doi: 10.2337/diabetes.50.11.2425. [DOI] [PubMed] [Google Scholar]
- 63.Poupeau A, Postic C. Cross-regulation of hepatic glucose metabolism via ChREBP and nuclear receptors. Biochim Biophys Acta. 2011;1812:995–1006. doi: 10.1016/j.bbadis.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Benhamed F, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iizuka K, et al. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bugge A, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakravarthy MV, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Matsuzaka T, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 70.Bennett MJ. Pathophysiology of fatty acid oxidation disorders. J Inherit Metab Dis. 2010;33:533–537. doi: 10.1007/s10545-010-9170-y. [DOI] [PubMed] [Google Scholar]
- 71.Spiekerkoetter U, Wood PA. Mitochondrial fatty acid oxidation disorders: pathophysiological studies in mouse models. J Inherit Metab Dis. 2010;33:539–546. doi: 10.1007/s10545-010-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagle CA, et al. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(Suppl):S74–79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyman LR, et al. Long term effects of high fat or high carbohydrate diets on glucose tolerance in mice with heterozygous carnitine palmitoyltransferase-1a (CPT-1a) deficiency: Diet influences on CPT1a deficient mice. Nutrition & diabetes. 2011;1:e14. doi: 10.1038/nutd.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grefhorst A, et al. Acute hepatic steatosis in mice by blocking beta-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am J Physiol Gastrointest Liver Physiol. 2005;289:G592–598. doi: 10.1152/ajpgi.00063.2005. [DOI] [PubMed] [Google Scholar]
- 75.Duivenvoorden I, et al. Acute inhibition of hepatic beta-oxidation in APOE*3Leiden mice does not affect hepatic VLDL secretion or insulin sensitivity. J Lipid Res. 2005;46:988–993. doi: 10.1194/jlr.M400505-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Conti R, et al. Selective reversible inhibition of liver carnitine palmitoyl-transferase 1 by teglicar reduces gluconeogenesis and improves glucose homeostasis. Diabetes. 2011;60:644–651. doi: 10.2337/db10-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kersten S, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peeters A, Baes M. Role of PPARα in Hepatic Carbohydrate Metabolism. PPAR Res. 2010;2010 doi: 10.1155/2010/572405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu J, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) influences substrate utilization for hepatic glucose production. J Biol Chem. 2002;277:50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- 80.Estall JL, et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes. 2009;58:1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 82.Leone TC, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burgess SC, et al. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J Biol Chem. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang H, et al. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab. 2009;296:E945–954. doi: 10.1152/ajpendo.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonoda J, et al. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lelliott CJ, et al. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glick D, et al. BNip3 Regulates Mitochondrial Function and Lipid Metabolism in the Liver. Mol Cell Biol. 2012;32:2570–2584. doi: 10.1128/MCB.00167-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guenther MG, et al. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- 89.Li J, et al. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 2000;19:4342–4350. doi: 10.1093/emboj/19.16.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kulozik P, et al. Hepatic deficiency in transcriptional cofactor TBL1 promotes liver steatosis and hypertriglyceridemia. Cell Metab. 2011;13:389–400. doi: 10.1016/j.cmet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Gómez-Valadés AG, et al. Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57:2199–2210. doi: 10.2337/db07-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burgess SC, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mutel E, et al. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J Hepatol. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Mutel E, et al. Control of Blood Glucose in the Absence of Hepatic Glucose Production During Prolonged Fasting in Mice: Induction of Renal and Intestinal Gluconeogenesis by Glucagon. Diabetes. 2011 doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vogt PK, et al. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 96.Tao R, et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem. 2011;286:14681–14690. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang K, et al. Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. doi: 10.1210/en.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang W, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 99.Banks AS, et al. Dissociation of the Glucose and Lipid Regulatory Functions of FoxO1 by Targeted Knockin of Acetylation-Defective Alleles in Mice. Cell Metab. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herzig S, et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 103.Conarello SL, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- 104.Longuet C, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8:359–371. doi: 10.1016/j.cmet.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Völzke H. Multicausality in fatty liver disease: Is there a rationale to distinguish between alcoholic and non-alcoholic origin? World J Gastroenterol. 2012;18:3492–3501. doi: 10.3748/wjg.v18.i27.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanal MG. The blind men “see” the elephant-the many faces of fatty liver disease. World J Gastroenterol. 2008;14:831–844. doi: 10.3748/wjg.14.831. [DOI] [PMC free article] [PubMed] [Google Scholar]