Abstract
Background
There are no descriptions of stroke mechanisms from intracranial atherosclerotic disease (ICAD) in native South Asian Pakistanis.
Methods
Men and women aged >/= 18 years with acute stroke presenting to four tertiary care hospitals in Karachi, Pakistan were screened using Magnetic Resonance Angiography/Transcranial Doppler scans. TOAST criteria were applied to identify strokes from ICAD.
Results
245 patients with acute stroke due to ICAD were studied. 230 scans were reviewed. 206 /230(89.0%) showed acute ischemia.
The most frequent presentation was with cortically based strokes in 42.2% (87/206) followed by border zone infarcts (52/206, 25.2%).Increasing degrees of stenosis correlated with the development of both cortical and border zone strokes (p=.002). Important associated findings were frequent atrophy (166/230, 72.2%) silent brain infarcts (66/230, 28%) and a marked lack of severe leukoaraiosis identified in only 68 /230 (29.6 %).
A total of 1870 arteries were studied individually. MCA was the symptomatic stroke vessel in half, presenting with complete occlusion in 66%. Evidence of biological disease, symptomatic or asymptomatic was identified in 753 (40.2%) vessels of which 543 (72%) were significantly (>50%) stenosed at presentation.
Conclusion
ICAD is a diffuse process in Pakistani South Asians, with involvement of multiple vessels in addition to the symptomatic vessel. The MCA is the most frequent symptomatic vessel presenting with cortical embolic infarcts. There is a relative lack of leukoaraiosis. Concomitant atrophy, silent brain infarcts and recent ischemia in the symptomatic territory are all frequently associated findings.
INTRODUCTION
Pakistan is the sixth most populous country in the world with a population of 170 million 1. Over the last four decades, the incidence of stroke has increased by more than a 100% in middle-low income countries like Pakistan 2. The estimated lifetime prevalence of cerebrovascular disease in Pakistan has been reported to be 20% 3 which translates into 34 million individuals with either stroke or TIA. Intracranial atherosclerotic disease (ICAD) may be the most common cause of stroke in the world especially in Pakistani South Asians 4, 5. To inform better strategies for prevention and treatment of ICAD, a clearer recognition of its presentation and pathophysiologic mechanisms in susceptible populations will be helpful. In Pakistani South Asians however this has not been reported to date.
The Karachi Intracranial Stenosis Study (KISS) is a multicenter case-control study describing the patterns and determinants of intracranial disease in an urban Pakistani population- those most at risk for ICAD6. In this paper we describe the detailed radiologic presentations of ICAD stroke and their mechanistic implications.
METHODS
KISS is a multicenter, hospital based, case control study of patients with intracranial atherosclerosis and healthy stroke-free controls. Methods have been described in detail earlier 6.
Study Site
Subjects were recruited from 4 tertiary care hospitals in Karachi, a southern metropolis of Pakistan with a socioeconomically diverse multiethnic population. Of the contributing hospitals, two are academic referral centers following fee-for-service models and the rest are heavily-subsidized publicly funded hospitals. Data collection was carried out from November 2007 to March 2010.
Sample Size
Over the study inclusion period, 1604 patients with stroke were reviewed. Of these 385 (24%) patients were found to have large artery atherosclerosis (intra or extracranial disease) as the stroke mechanism. Within this group 314 (81.5%) were diagnosed ICAD, rest were extracranial atherosclerosis. We further excluded 69 patients because the certainty of diagnosis was weaker for these patients for definite ICAD as a stroke mechanism. This study describes the radiology and stroke mechanisms of the 245 participants who had intracranial atherosclerotic disease (ICAD), as per the modified TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria.7
Study Participants
All men and women aged 18 years or older presenting with stroke fulfilling WHO criteria were recruited by trained study physicians who actively reviewed all admissions to the medicine or neurology wards on a daily basis. Neuro-imaging was mandatory for inclusion. Patients receiving medical attention after 72 hours since they were last seen normal, those diagnosed with non-vascular or iatrogenic stroke, those presenting with concomitant acute coronary syndrome or those who refused to consent were excluded from this study. Surrogate consent was sought if the patient was unable to consent independently.
Study procedures
Appropriate ancillary vascular and cardiac investigations to assign the patient to a diagnosis of ICAD according to TOAST criteria were reviewed in each individual case. No patient was assigned ‘concurrent mechanism’. If a patient had atrial fibrillation, they were classified under cardioembolism and excluded for this review. For this analysis, those patients where the diagnosis of ICAD was most definite were selected. All scans were reviewed by a stroke neurologist allowing appropriate classification of each stroke patient; 10% of the scans were read by a second neurologist to assure reliability and consistency of the angiographic data with minimal inter-observer correlation..
A standardized Computerized Tomography (CT)/Magnetic Resonance Imaging (MRI) reading form adapted from the work of J Wardlaw8 was used to document imaging findings. Modifications made to the form included using angiographic data to record information on cerebral vasculature. Every artery was examined for the number, degree and distribution of stenosis. Biologically relevant disease ranging from atherosclerotic irregularity to measurable stenosis was noted and measured. Another modification was made to draw out the visualized strokes on “stroke maps”. These were derived from published papers9, 10. This was done to better visualize stroke topography in each individual patient. The following radiologic variables were reviewed in each patient: Stroke11, acute stroke lesions12, silent brain infarcts13, border zone infarcts14, cortical infarctions15, lacunar infarcts15, atrophy16, leukoaraiosis17, arterial review and assignment of symptomatic vs. asymptomatic status9, 10.
All radiologic data was directly archived into a centralized computer using specially designed software. The software allowed for measurements to be made for calculation of intracranial stenosis using computerized calipers, magnify areas of interest, and quantify stroke volumes. We used the WASID method to calculate stenosis18.
Ethical Considerations
The study protocol was approved by the relevant Ethical Review Committee. Written informed consent and verbal assent was given by all participants or their surrogate respondents prior to inclusion.
Statistical Analysis
All data was entered and rechecked by two different investigators. Analysis was carried out using Statistical Package for Social Sciences (SPSS) Version 17.0 (SPSS Inc., Chicago, IL). We calculated simple frequencies for stroke locations and subtypes, symptomatic vessels, old vascular lesions and brain atrophy and leukoaraiosis. Means and standard deviations were computed for percentage of stenosis in various symptomatic and asymptomatic vessels and proportions were used for the categorical variables. Chi-square test of independence was applied for correlations between symptomatic vessel stenosis and stroke mechanism, as well as association of cerebral atrophy and leukoaraiosis with the number of diseased vessels. Two sided p-value of ≤ 0.05 was taken significant.
RESULTS
Stroke due to ICAD was the predominant mechanism of stroke due to large artery atherosclerosis. Two hundred and forty five patients with stroke due to definite ICAD presented to the study centers between November 2007 and March 2010. Scans (CT/MRI) data were missing for 15 of these patients, who were, therefore, excluded. Out of the final 230 scans reviewed, a majority were judged to be of good quality (72.2%) with only 8.3% being of poor quality. Most of the scans, 161 (70%) were MRIs with Diffusion Weighted Imaging, 49 (21%) were non contrast CT head and 20 (9%) were plain MRIs. MR Angiography (MRA) was available for 170 of these 230 patients.
Acute Stroke Findings
Out of the 230 scans, 24 (10.4%) had no sign of acute ischemic change at presentation. Of these 24, 5 scans were non contrast head CT, these were not repeated. The rest (n=206, 89%) had evidence of acute ischemia with about 11 (5.4%) showing a concomitant recent ischemic lesion. Nine of these patients had acute lesions in two territories. Only 7 (3%) showed hemorrhagic transformation, while 56 (27.2%) had evidence of mass effect with large infarctions. Old vascular lesions were seen in 66 of the 230 scans (28%). Most of these were either old lacunar strokes (43.9%) or old cortical infarcts (33.3%). Only two of these old lesions were hemorrhagic. There were no non-stroke lesions identified in the 230 scans.
Stroke Distributions and Mechanisms
(Figure 1) Of the acute strokes 151/206 (73.3%) were in the anterior circulation and the rest (55/206, 26.7%) were in the posterior circulation. The majority were cortically based strokes (87/206, 42.2%), followed by border-zone infarcts (52/206, 25.2%). Lacunar infarcts were 23 /206 (16%) and were the least common presentation. Cortically based strokes were more frequent in the middle cerebral artery (MCA) territory (66/87; 75.8%). Of the borderzone infarctions, majority were in the internal borderzone (37/52; 71.2%).
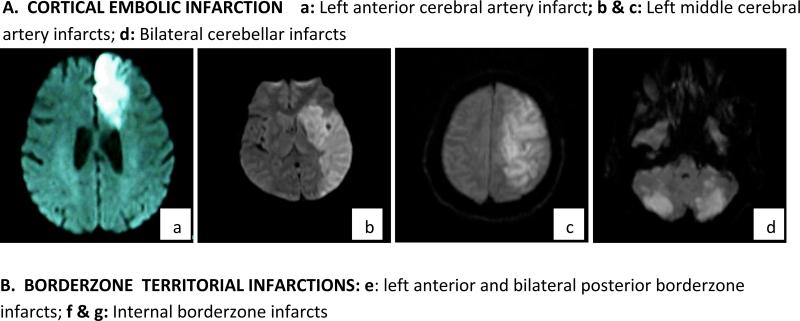
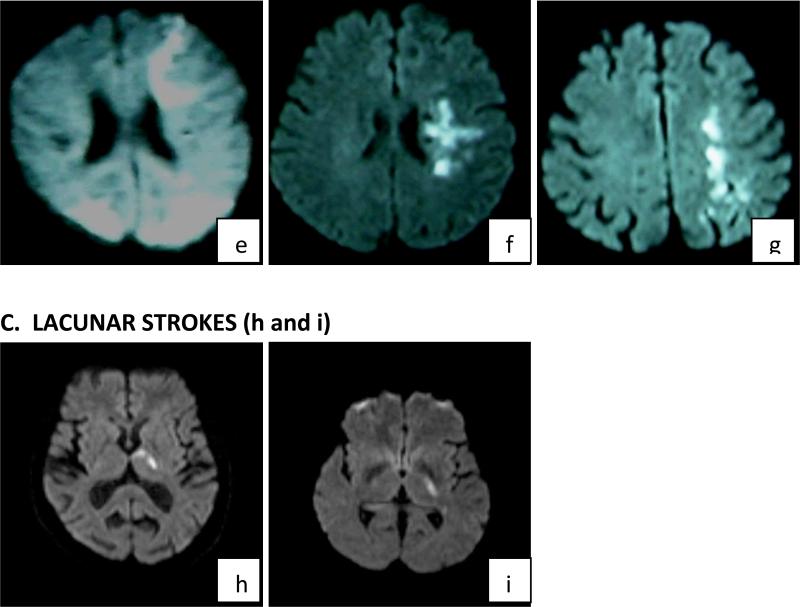
Figure 1. Diffusion weighted images showing the stroke location and mechanisms in order of frequency – most frequent to least.
A. CORTICAL EMBOLIC INFARCTION a: Left anterior cerebral artery infarct; b & c: Left middle cerebral artery infarcts; d: Bilateral cerebellar infarcts
B. BORDERZONE TERRITORIAL INFARCTIONS: e: left anterior and bilateral posterior borderzone infarcts; f & g: Internal borderzone infarcts
Correlation with stroke mechanism and vessel stenosis
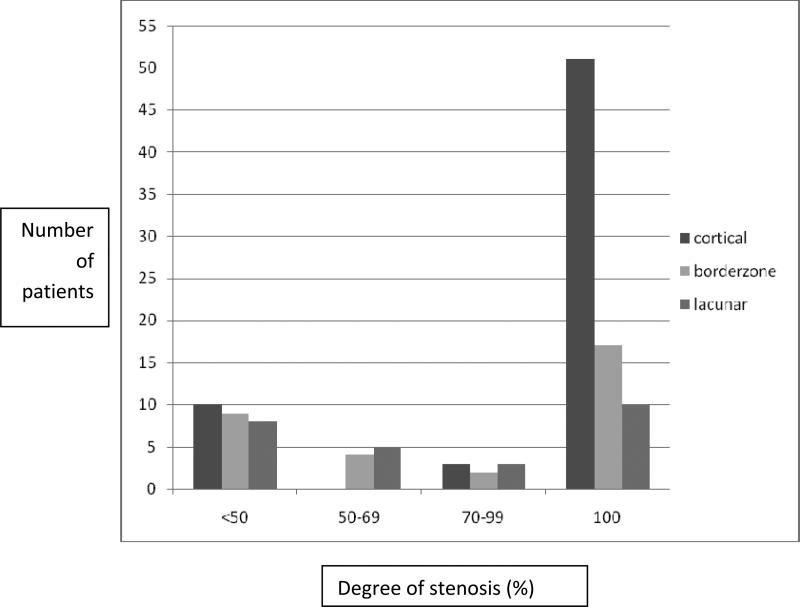
(Figure 2) As the degree of arterial stenosis increased from <50% to complete occlusion, there was a statistically significant increase observed in both cortical and borderzone infarcts (p=0.002). This difference was more marked for cortically based infarctions than for borderzone infarctions and was negligible for lacunar strokes.
Figure 2.
Correlation of stroke mechanisms with increasing degree of atherosclerotic stenosis (p=0.002). Advanced stenosis increases hemodynamic strokes and major cortical embolisms
Description of Angiographic findings
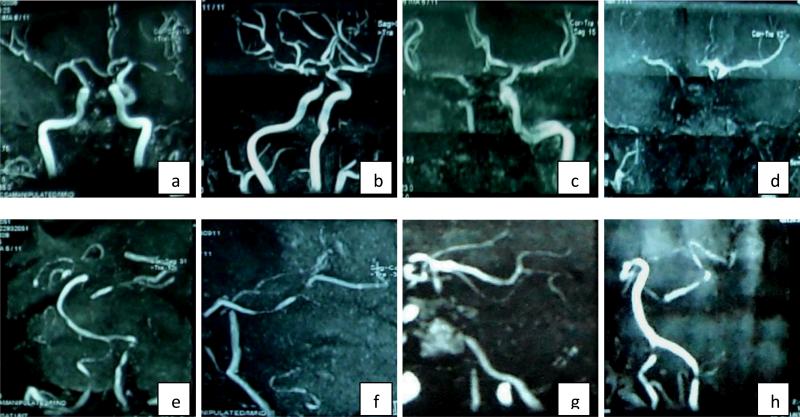
(Figure 3) A total of 1,870 intracranial arteries of 170 patients were studied. Of these 170 patients, 151 had acute symptomatic stroke. MCA was the symptomatic vessel in nearly half the patients (69/151, 45.7%) with complete occlusion observed in 66% of these MCAs. ICA and Basilar (BA) were the next common culprit vessels (25/151 each, 16.6%) (Table 1).
Figure 3. MRA images showing diffuse severe atherosclerotic disease in South Asian Patients with Intracranial Atherosclerotic Disease (ICAD).
a-d: anterior circulation; frequent bilateral MCA involvement in addition to the symptomatic vessel; e-h: posterior circulation; frequent PCA disease in addition to the symptomatic vessel
Table 1.
Symptomatic vessel and atherosclerotic stenosis in decreasing order of frequency (n=151)
| Vessel | Symptomatic vessel, n (%) | Mean stenosis (SD) | 100% stenosis, n (%) |
|---|---|---|---|
| MCA | 69 (45.7) | 77.4 (36.3) | 46 (66) |
| ICA | 25 (16.6) | 81.5 (27.6) | 16(64) |
| Basilar | 25 (16.6) | 44.6 (45.2) | 8 (32) |
| PCA | 23 (15.2) | 67.6 (39.7) | 11 (47.8) |
| ACA | 7 (4.6) | 85.7 (37.7) | 6 (85.7) |
| Vertebrals | 2(1.3) | 72.1 (39.3) | 1 (50) |
ICA= internal carotid artery; MCA= middle cerebral artery; ACA= anterior cerebral artery; PCA= posterior cerebral artery;
Evidence of biological disease (any degree of stenosis) was identified in 753 of the 1870 (40.2%) vessels studied. Significant stenosis (>50%) was found in 543 of the 753 (72%) arteries; 433 of these 543 (79.9%) vessels with significant stenosis were asymptomatic (i.e. not corresponding to the arterial territory of the presenting ischemic change).The greatest number of asymptomatic significant stenosis was observed in PCAs. A mean of 4.4 vessels were diseased per patient with the highest number of affected vessels being 10 of 11.
Associated Radiologic findings and correlations
Important associated radiologic findings were frequent atrophy 166 /230 (72.2%) and the marked lack of leukoaraiosis identified in only 68 of the 230 patients (29.6%). There were no significant associations between number of diseased vessels and leukoaraiosis (p =0.55), as well as central/ cortical atrophy (p = 0.87, 0.96 respectively).
DISCUSSION
We found that ICAD presents most frequently with cortical embolic stroke followed by internal borderzone ischemia in Pakistani South Asians. The stroke is most often due to advanced stenosis of the MCA, and the chances of getting a disabling cortically based stroke correlates with advancing degrees of stenosis. ICAD interestingly, not infrequently presents with lacunar stroke, however this presentation is not influenced by increasing stenosis in the parent artery. Noteworthy concomitants are, associated remote silent brain infarcts, of which one third were cortical strokes, atrophy, recent ischemia, and diffuse atherosclerosis. Unexpectedly infrequent is diffuse white matter disease—given the distribution of risk factors for both being similar – this observation is puzzling and unclear.
The mechanisms of stroke from ICAD in Pakistanis are heterogeneous and it is important for the clinician to recognize these mechanisms for their potential therapeutic implications. Studies from similar populations have shown that cortical embolic stroke in ICAD are often accompanied by platelet microembolism measurable by doppler ultrasound19. Clinical intervention by the use of double antiplatelet agents in this setting 20, 21 have shown both reductions in micro-embolic signals and a reduction of recurrent stroke. Internal border-zone stroke, the second commonest mechanism, when measured by Positron Emission Tomography (PET) shows increased Oxygen Extraction Fraction (OEF)22 and by Single Photon Emission CT (SPECT) shows decreased reserve on acetazolamide challenge23 and is often also prone to progressive infarction24, 25. In actuality there are very few ICAD based studies that tell us what intervention would be reasonable in the face of acute border-zone ischemia. However emerging data suggests that it may not be safe to acutely reduce pressure aggressively26 and perhaps increasing pressure may enhance flow through collaterals and stabilize the progression.
Important also are the lacunar presentations of ICAD, these have been described in other populations due to atheroma in the parent artery 27. Getting an expensive intracranial MRA, when the presentation is lacunar is not intuitional for most developing country neurologists, but recognizing this presentation for prognostic reasons is essential. ICAD carries the worst recurrent stroke rate (15% at one year)28 amongst all ischemic stroke presentations- and at this time evidence from Warfarin versus Aspirin in Symptomatic Intracranial Disease (WASID) study clearly informs us that a strategy of aggressive risk factor intervention is the only way forward 29.
Important to note is that although the chances of stroke increased with increasing degree of stenosis, strokes of varied mechanisms were seen at all degrees of stenosis30. Autopsy studies on fatal strokes31 and high strength magnets 32 are informing us that we need to appreciate ICAD as a mechanism of stroke at lesser degrees of stenosis as plaque hemorrhage and instability will ensue in both kinds of strokes regardless of the degree of initial stenosis33. The associated “preclinical silent brain infarcts” that we report are an additional observation supporting the emergent data on the effect of less than 50% stenosis on brains.
Evidence of atherosclerotic disease was present in nearly half the vessels studied, with more than 70% vessels having significant (≥ 50%) stenosis. Sixty five percent of the culprit vessels had >70% stenosis in our study as compared to WASID which reports 40% of its subjects having severe stenosis (>70% in single or >50% in multiple arteries) 34. A study from the Chinese population showed significant (≥50%) stenosis in 65% of their patients 35. 72.8% of our patients had ≥50% stenosis in their culprit vessels.
The ratio of asymptomatic stenosis was considerably high in our study (79% vs. 27% in MRAs reviewed for WASID) 36. There are currently limited studies on asymptomatic cerebrovascular disease. MRAs in healthy Japanese and Koreans had 3-3.5% ICAD 37, 38. Some studies suggest that asymptomatic intracranial disease has a low risk of recurrence 36, 39. At least in our cross sectional observation, the involvement of the middle cerebral artery was more likely to be symptomatic and the anterior and posterior cerebral asymptomatic, suggesting that location may determine asymptomatic status due to collaterals 40. This hypothesis will need further testing. At the very least, if there is diffuse involvement, intervention in high risk populations presents an opportunity to prevent symptomatic stroke. At this stage intervention may not necessarily be technically demanding and expensive like stents or bypass- both difficult interventions in most resource poor countries where ICAD is common.
Leukoaraiosis was surprisingly low. As the burden of hypertension is significantly high in our population, more was expected. In some Asian populations, significant association of leukoaraiosis has been reported with large artery atherosclerosis 27 and with multiple vessels 35. We are puzzled by this observation and unclear as to why there is small vessel protection.
Lastly the cortical atrophy may be the result of chronic ischemia of multiple vessels, as in a recent observation, revascularization reverses cortical thinning in these patients41 although our data set failed to find a statistically significant association.
Our study is unique because the disease burden in an individual patient of ICAD has never been quantified before in native South Asians. Our participating locations are national referral centers for stroke, so there is broader generalizability. We followed a systemized, centralized, standardized and supervised protocol. All our variables were predefined and measured exactly using international guidelines. The main limitation of this study is its cross sectional design and lack of longitudinal data. Our study population was urban and hospital based, and our investigation uses non invasive modalities, which might lead to detection bias of the true burden of ICAD. Also, not all scans were MRIs; CT may have missed out on some of the asymptomatic strokes. This may have decreased the detection rate of silent strokes –and the asymptomatic disease burden may be underreported.
Looking at the broader picture, studies aimed at alleviating stenosis in a single vessel may be ignoring the larger problem which perhaps may respond to global interventions aimed at halting the process of progressive pre-symptomatic ICAD42.
Acknowledgements
The authors would like to acknowledge the patience, kindness, time and cooperation of all patients and their families who contributed to this study.
Funding Disclosures:
The KISS project was funded by a grant awarded to Ayeesha Kamran Kamal (PI) and Philippe M. Frossard (Co PI) by the Higher Education Commission of the Government of Pakistan. Ayeesha Kamran Kamal was also the recipient of a seed money grant from the University Research Council. Dr Maria Khan is a neurovascular research fellow whose training is currently funded by Award Number D43TW008660 from the Fogarty International Center and the National Institute of Neurologic Disorders and Stroke. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center, National Institute of Neurologic Disorders and Stroke or the National Institute of Health.
REFERENCES
- 1. [03-11-2010]; Www.Census.Gov.Pk.
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.Kamal AK, Itrat A, Murtaza M, Khan M, Rasheed A, Ali A, Akber A, Akber Z, Iqbal N, Shoukat S, Majeed F, Saleheen D. The burden of stroke and transient ischemic attack in pakistan: A community-based prevalence study. BMC Neurol. 2009;9:58. doi: 10.1186/1471-2377-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: A review. Stroke. 1986;17:648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 5.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 6.Kamal AK, Taj F, Junaidi B, Rasheed A, Zaidi M, Murtaza M, Iqbal N, Hashmat F, Alam SV, Saleem U, Waheed S, Bansari L, Shah N, Samuel M, Yameen M, Naz S, Khan FS, Ahmed N, Mahmood K, Sheikh N, Makki KU, Ahmed MM, Memon AR, Wasay M, Syed NA, Khealani B, Frossard PM, Saleheen D. The karachi intracranial stenosis study (kiss) protocol: An urban multicenter case-control investigation reporting the clinical, radiologic and biochemical associations of intracranial stenosis in pakistan. BMC Neurol. 2009;9:31. doi: 10.1186/1471-2377-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams HPBB, Jr, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 8. [03-11-2010]; Sbirc website, www.Sbirc.Ed.Ac.Uk/imageanalysis.Html, j wardlaw.
- 9.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: Cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 10.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: Brainstem and cerebellum. Neurology. 1996;47:1125–1135. doi: 10.1212/wnl.47.5.1125. [DOI] [PubMed] [Google Scholar]
- 11.Asplund K, Tuomilehto J, Stegmayr B, Wester PO, Tunstall-Pedoe H. Diagnostic criteria and quality control of the registration of stroke events in the monica project. Acta Med Scand Suppl. 1988;728:26–39. doi: 10.1111/j.0954-6820.1988.tb05550.x. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Sellar R. A simple practical classification of cerebral infarcts on ct and its interobserver reliability. AJNR Am J Neuroradiol. 1994;15:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeer SE, Longstreth WT, Jr., Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 14.Damasio H. A computed tomographic guide to the identification of cerebral vascular territories. Arch Neurol. 1983;40:138–142. doi: 10.1001/archneur.1983.04050030032005. [DOI] [PubMed] [Google Scholar]
- 15.Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol. 2005;15:416–426. doi: 10.1007/s00330-004-2633-5. [DOI] [PubMed] [Google Scholar]
- 16.Farrell C, Chappell F, Armitage PA, Keston P, Maclullich A, Shenkin S, Wardlaw JM. Development and initial testing of normal reference mr images for the brain at ages 65-70 and 75-80 years. Eur Radiol. 2009;19:177–183. doi: 10.1007/s00330-008-1119-2. [DOI] [PubMed] [Google Scholar]
- 17.van Swieten JC, Hijdra A, Koudstaal PJ, van Gijn J. Grading white matter lesions on ct and mri: A simple scale. J Neurol Neurosurg Psychiatry. 1990;53:1080–1083. doi: 10.1136/jnnp.53.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 19.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, Kay R, Ringelstein EB. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: A diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 20.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P, Zhao Y, Koh A, Hao Q, Markus HS. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (clair study): A randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–497. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TN, Zaidat OO, Gupta R, Nogueira RG, Tariq N, Kalia JS, Norbash AM, Qureshi AI. Balloon angioplasty for intracranial atherosclerotic disease: Periprocedural risks and short-term outcomes in a multicenter study. Stroke. 2011;42:107–111. doi: 10.1161/STROKEAHA.110.583245. [DOI] [PubMed] [Google Scholar]
- 22.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 23.Ozgur HT, Kent Walsh T, Masaryk A, Seeger JF, Williams W, Krupinski E, Melgar M, Labadie E. Correlation of cerebrovascular reserve as measured by acetazolamide-challenged spect with angiographic flow patterns and intra- or extracranial arterial stenosis. AJNR Am J Neuroradiol. 2001;22:928–936. [PMC free article] [PubMed] [Google Scholar]
- 24.Del Sette M, Eliasziw M, Streifler JY, Hachinski VC, Fox AJ, Barnett HJ. Internal borderzone infarction: A marker for severe stenosis in patients with symptomatic internal carotid artery disease. For the north american symptomatic carotid endarterectomy (nascet) group. Stroke. 2000;31:631–636. doi: 10.1161/01.str.31.3.631. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Hemodynamic compromise as a cause of internal border-zone infarction and cortical neuronal damage in atherosclerotic middle cerebral artery disease. Stroke. 2009;40:3730–3735. doi: 10.1161/STROKEAHA.109.560011. [DOI] [PubMed] [Google Scholar]
- 26.Sandset EC, Bath PM, Boysen G, Jatuzis D, Korv J, Luders S, Murray GD, Richter PS, Roine RO, Terent A, Thijs V, Berge E. The angiotensin-receptor blocker candesartan for treatment of acute stroke (scast): A randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: Early diffusion-weighted imaging study. Stroke. 2005;36:2583–2588. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 28.Turan TN, Maidan L, Cotsonis G, Lynn MJ, Romano JG, Levine SR, Chimowitz MI. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–509. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turan TN, Derdeyn CP, Fiorella D, Chimowitz MI. Treatment of atherosclerotic intracranial arterial stenosis. Stroke. 2009;40:2257–2261. doi: 10.1161/STROKEAHA.108.537589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein IF, Labreuche J, Lavallee PC, Mazighi M, Duyckaerts C, Hauw JJ, Amarenco P. Is moderate atherosclerotic stenosis in the middle cerebral artery a cause of or a coincidental finding in ischemic stroke? Cerebrovasc Dis. 2010;29:140–145. doi: 10.1159/000262310. [DOI] [PubMed] [Google Scholar]
- 31.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–1147. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 32.Arenillas JF. Intracranial atherosclerosis: Current concepts. Stroke. 2011;42:S20–23. doi: 10.1161/STROKEAHA.110.597278. [DOI] [PubMed] [Google Scholar]
- 33.Ogata J, Masuda J, Yutani C, Yamaguchi T. Mechanisms of cerebral artery thrombosis: A histopathological analysis on eight necropsy cases. J Neurol Neurosurg Psychiatry. 1994;57:17–21. doi: 10.1136/jnnp.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turan TN, Makki AA, Tsappidi S, Cotsonis G, Lynn MJ, Cloft HJ, Chimowitz MI. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke. 2010;41:1636–1640. doi: 10.1161/STROKEAHA.110.584672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pu Y, Liu L, Zou X, Chen P, Wang Y, Zhou Y, Dong K, Zhao X, Wang C, Wang Y. Relationship between leukoaraiosis and cerebral large artery stenosis. Neurol Res. 2009;31:376–380. doi: 10.1179/174313209X444071. [DOI] [PubMed] [Google Scholar]
- 36.Nahab F, Cotsonis G, Lynn M, Feldmann E, Chaturvedi S, Hemphill JC, Zweifler R, Johnston K, Bonovich D, Kasner S, Chimowitz M. Prevalence and prognosis of coexistent asymptomatic intracranial stenosis. Stroke. 2008;39:1039–1041. doi: 10.1161/STROKEAHA.107.499475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park KY, Chung CS, Lee KH, Kim GM, Kim YB, Oh K. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic korean population. J Clin Neurol. 2006;2:29–33. doi: 10.3988/jcn.2006.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007;68:2035–2038. doi: 10.1212/01.wnl.0000264427.09191.89. [DOI] [PubMed] [Google Scholar]
- 39.Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2009;40:1999–2003. doi: 10.1161/STROKEAHA.108.546150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2010 doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fierstra J, Maclean DB, Fisher JA, Han JS, Mandell DM, Conklin J, Poublanc J, Crawley AP, Regli L, Mikulis DJ, Tymianski M. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke. 2011;42:1631–1637. doi: 10.1161/STROKEAHA.110.608521. [DOI] [PubMed] [Google Scholar]
- 42.Spence JD, Hackam DG. Treating arteries instead of risk factors: A paradigm change in management of atherosclerosis. Stroke. 2010;41:1193–1199. doi: 10.1161/STROKEAHA.110.577973. [DOI] [PubMed] [Google Scholar]