Abstract
Systemic antagonists of the histamine type 1 and 2 receptors (H1/2r) are widely used as anti-pruritics and central sedatives, but demonstrate only modest anti-inflammatory activity. Because many inflammatory dermatoses result from defects in cutaneous barrier function, and because keratinocytes express both Hr1 and Hr2, we hypothesized that H1/2r antagonists might be more effective, if they were used topically to treat inflammatory dermatoses. Topical H1/2r antagonists additively enhanced permeability barrier homeostasis in normal mouse skin by: i) stimulation of epidermal differentiation, leading to thickened cornified envelopes; and ii) enhanced epidermal lipid synthesis and secretion. Since barrier homeostasis was enhanced to a comparable extent in mast cell-deficient mice, with no further improvement following application of topical H1/2r antagonists, H1/2r antagonists likely oppose mast cell-derived histamine. In four immunologically-diverse, murine disease models, characterized by either inflammation alone (acute irritant contact dermatitis, acute allergic contact dermatitis), or by prominent barrier abnormalities (subacute allergic contact dermatitis, atopic dermatitis), topical H1/2r agonists aggravated, while H1/2r antagonists improved inflammation and/or barrier function. The apparent ability of topical H1r/2r antagonists to target epidermal H1/2r could translate into increased efficacy in the treatment of inflammatory dermatoses, likely due to decreased inflammation and enhanced barrier function. These results could shift current paradigms of antihistamine utilization from a predominantly-systemic to a topical approach.
Keywords: Antihistamines, epidermal differentiation, epidermal barrier function, epidermal lipid synthesis, H1 receptor, H2 receptor, treatment (therapy)
INTRODUCTION
Because of their common embryologic origin, epidermis not surprisingly expresses multiple neuroreceptors, neurotransmitters and neurohormones that mediate important functions in the central nervous system (Denda, 2002, Denda, et al., 2007). One of these mediators is histamine (Travis, et al., 2000), an aminergic neurotransmitter that is produced not only by neurons, but also by mast cells, eosinophils and basophils (Endo, et al., 1992, Endo, et al., 1995, Yamaguchi, et al., 2000). In contrast to the limited number of cell types that synthesize histamine, one or more of four histamine receptors (H1r-4r), belonging to the superfamily of G-protein-coupled receptors, are ubiquitous and modulate a variety of pathophysiological responses, including pruritus and inflammation in the skin (Hill, et al., 1997, Oda, et al., 2000). Yet, though systemic antihistamines are widely deployed in clinical settings, they demonstrate only modest anti-inflammatory activity in diseases such as atopic dermatitis (Klein and Clark, 1999, Diepgen, 2002, Kawashima, et al., 2003, Buddenkotte, et al., 2010, Eschler and Klein, 2010). Moreover, their utilization can be limited by important side effects, including sedation and cardiotoxicity, particularly in the elderly [rev. in (Greaves, 2005)]. The reason for the limited anti-inflammatory activity of systemic antihistamines is not known, but it seems plausible that either their bioavailability to peripheral tissues could be limited at current dosage levels, or that they could be substantially metabolized prior to their arrival in the skin. Because the H1r and H2r are strongly expressed in epidermis [(Gschwandtner, et al., 2011) and these studies], and because one prior study showed that topical H1/2r antagonists improve barrier function in normal skin (Ashida, et al., 2001), we hypothesized here that the bioavailability and efficacy of antihistamines could be enhanced, were they deployed as topical, rather than as systemic agents to treat inflammatory dermatoses, with or without associated barrier abnormalities.
Epidermis mediates a set of protective functions, including maintenance of permeability barrier homeostasis. This critical function, which allows survival in a terrestrial environment, is mediated by the unique two-compartment organization of the stratum corneum (SC) into anucleate corneocytes embedded in an expanded, lipid-enriched extracellular matrix [rev. in (Elias and Menon, 1991)]. Inflammatory dermatoses are now increasingly recognized to result from inherited abnormalities that compromise epidermal barrier function (Sandilands, et al., 2009, Irvine, et al., 2011). A likely pathogenic sequence that leads to inflammation invokes both increased allergen penetration, through a genetically-impaired barrier, leading to Th2 inflammation (Irvine, et al., 2011) and an epidermis-initiated ‘cytokine cascade’ that recruits downstream inflammation (Wood, et al., 1992, Nickoloff and Naidu, 1994). Accordingly, immune abnormalities, once seen as the primary disease instigator, are now increasingly considered as downstream or disease-modifying participants (Elias, et al., 2008a, Elias and Steinhoff, 2008). Then, inflammation once established, can further aggravate the barrier abnormality by multiple mechanisms, establishing an ‘outside-to-inside, back-to-outside’ pathogenic circle (Elias, et al., 2008a, Elias and Steinhoff, 2008, Elias and Schmuth, 2009). Accordingly, disorders such as atopic dermatitis, psoriasis, and the inherited ichthyoses are increasingly being treated with various forms of topical ‘barrier repair therapy,’ strategies that can themselves be anti-inflammatory by multiple mechanisms [rev. in (Elias and Wakefield, 2011)]. We show here that topical antihistamines could comprise effective therapy not only through enhanced anti-inflammatory activity, but also in part through their ability to improve epidermal structure and function. Since mast cell deficient mice (MCDM) displayed a comparable enhancement in barrier function, and since topical H1/2r antagonists provided no further benefits, the H1/2r antagonists likely oppose mast cell-derived histamine. Finally, the H1r and H2r antagonists markedly improved inflammation in four different inflammatory dermatoses models, characterized by either inflammation alone and/or a prominent barrier abnormality.
RESULTS
Topical Antihistamines Enhance Cutaneous Permeability Barrier Homeostasis By Opposing Mast Cell-Derived Histamines
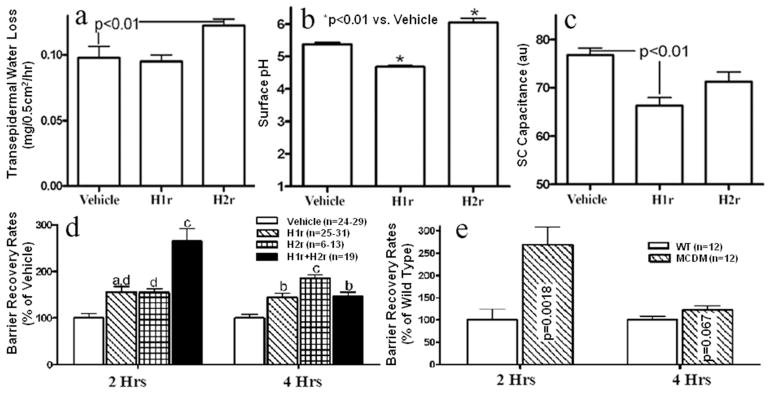
As recently reported by Gschwandtner, et al., (2011), we initially found that only the H1r and H2r are expressed in abundance in normal mouse epidermis (suppl. Fig. 1). We utilized the H1r and H2r antagonists, diphenhydramine and cimetidine here, because they most-potently improved barrier function among several agents tested in preliminary studies. Twice-daily topical applications of these H1/2r antagonists to intact skin produced only modest changes in basal barrier function, SC hydration, and surface pH, which all fell within the normal range (Figs. 1a-c). In contrast, when skin sites, previously-treated with H1/2r antagonists, were disrupted by sequential tape stripping, permeability barrier restoration accelerated (by ≈100%) in comparison to vehicle-treated sites (Fig. 1d). Moreover, co-applications of the H1r and H2r antagonists additively improved barrier function at two hours post-barrier disruption, but no additive or synergic effects were observed at four hours after barrier abrogation (Fig. 1d). Finally, when the H1r and H2r antagonists were applied unilaterally (vehicle alone applied to the opposing, similarly tape-stripped flank), barrier homeostasis improved only on the antagonist-treated side (not shown), indicating that the H1/2r antagonists enhance epidermal function locally, rather than after prior systemic absorption.
Figure 1. Topical H1/2r Antagonists Enhance Permeability Barrier Homeostasis in Normal Skin.
The flanks of hairless mice (n=4–5 each) were treated with topical applications of either diphenhydramine chlorhydrate (H1r), cimetidine (H2r), or ethanol (vehicle) twice-daily for four days (see Methods for further details). At the end of treatments, changes in basal barrier function, assessed as transepidermal water loss (TEWL, a), surface pH (b), and electrical conductance (SC hydration, c) were measured. (d) Barrier disruption was induced by sequential cellophane tape strippings until TEWL levels ≥ 10-fold increase over baseline, and barrier recovery rates were assessed two and four hrs later (a=p<0.05 vs. vehicle, b=p<0.01 vs. vehicle, c=p<0.001 vs. vehicle, and d=p<0.001 vs. H1r+H2r)66. e: Percent barrier recovery was compared in mast cell-deficient KitW/KitW-v double heterozygote mice (MCDM) vs. wild-type (WBB6F1) mice two and four hrs after tape stripping, as above.
The putative source for endogenous histamine in the skin are cutaneous mast cells, which are present in substantial numbers in normal skin (Janssens, et al., 2005). To assess whether the H1/2r antagonists improve barrier function by opposing mast cell-derived histamine, we next compared barrier homeostasis in mast cell-deficient mice (MCDM, kitw/kitw-v) vs. age-matched, same-strain (WBB6F1), mast cell-replete mice. Although basal barrier function, hydration and surface pH did not differ in MCDM vs. wild-type mice, barrier recovery accelerated significantly (≈160%) in MCDM at 2 hrs vs. control mouse skin (Fig. 1e; p<0.002), an enhancement of barrier homeostasis that was comparable to that produced by the topical antihistamines. But, no further improvements in permeability barrier homeostasis occurred when MCDM were treated topically with either the H1r or H2r antagonist (Suppl. Fig. 2). Interestingly, barrier disruption provoked a modest, though significant increase in the density of mast cells (Suppl. Fig. 3). Together, these results show that: 1) topical H1r and H2r antagonists improve permeability barrier homeostasis in acutely-perturbed, but otherwise normal mouse skin [see also (Ashida, et al., 2001)]; 2) improvement is due to local effects, ruling out efficacy due to prior systemic absorption; 3) mast cell-derived histamine is likely the primary source of ligand opposed by the H1/2r antagonists; 4) barrier disruption stimulates proliferation of mast cells in the dermis; and 5) H1/2r antagonists improve barrier function specifically by opposing mast cell-derived histamine.
Mechanisms Whereby Topical Antihistamines Enhance Barrier Function
Corneocytes and extracellular lipids together mediate epidermal permeability barrier homeostasis (Elias, 2006, Feingold, 2007). To explore the mechanistic basis for enhanced permeability barrier function, we first assessed whether these agents alter epidermal proliferation after barrier disruption. In H+E stained sections, both H1r and H2r antagonists modestly stimulated epidermal hyperplasia (suppl. Fig. 4a–c), but the increase in thickness achieved statistical significance only in H2r antagonist–treated skin (suppl. Fig. 4d–g). Likewise, epidermal proliferation, assessed as the density of PCNA-positive cells in the basal layer, increased more in H2r- than in H1r-antagonist treated skin (suppl. Fig. 4h).
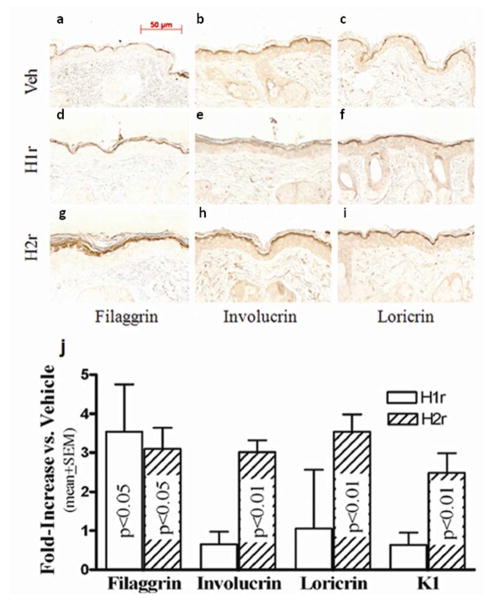
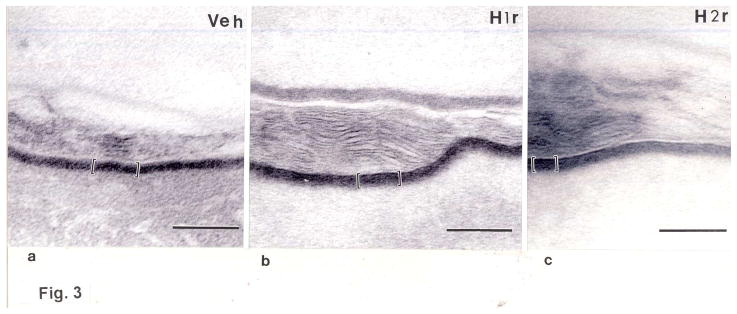
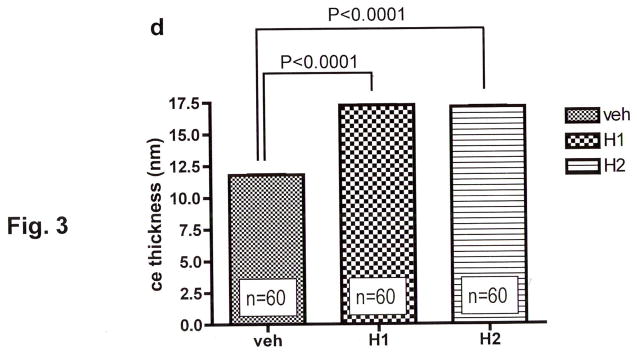
We next assessed whether one or both of these agents enhance expression of epidermal differentiation-related proteins. Applications of the H1/2r antagonists to intact skin enhanced expression of involucrin, loricrin, and particularly filaggrin in immunohistochemical preparations (Figs. 2a–i). Accordingly, epidermal mRNA levels, assessed by real-time quantitative PCR [rt(Q)-PCR] (see suppl. Table 1 for list of all primers used in the studies), increased after H1/2r antagonist applications (Fig. 2j). Finally, we assessed whether increased differentiation-linked protein expression translated into altered corneocyte structure. Electron microscopy demonstrated an apparent increase in the thickness of cornified envelopes in both H1r and H2r antagonist-treated epidermis (Fig. 3a-c), validated further in quantitative studies, utilizing randomized, coded micrographs (≈ 40% increase; p < 0.0001 for both H1/2r antagonists) (Fig. 3d). Together, these studies show that the H1r/H2r antagonist-induced improvements in barrier function can be attributed in part to enhanced epidermal differentiation, leading to more-robust corneocytes.
Figure 2. Topical H1/H2r Antagonists Stimulate Epidermal Differentiation.
Hairless mice were treated as above, and paraffin-embedded sections (6 μm) then were immunostained to detect changes in filaggrin, involucrin, and loricrin content and localization (a-i). j: In parallel, mRNA was isolated from freshly-obtained epidermal sheets after treatments as in Fig. 1, above (n=4), and changes in mRNA levels for filaggrin, loricrin, and involucrin were assessed by rt(Q)-PCR (see Methods and suppl. Table 2 for further details). Scale bar = 50 μm.
Figure 3. Topical H1r/H2r Antagonists Enhance Corneocyte Envelope Thickness.
Electron micrographs of biopsies of H1/2r antagonist-treated skin were processed for electron microscopy (a-c), as in Methods. d: 10 micrographs each of perpendicular sections taken at random from 6 different biopsy samples. CE dimensions were measured directly on the electron microscope, as in Methods. a-c, Osmium tetroxide post-fixation; Scale bar = 100μm.
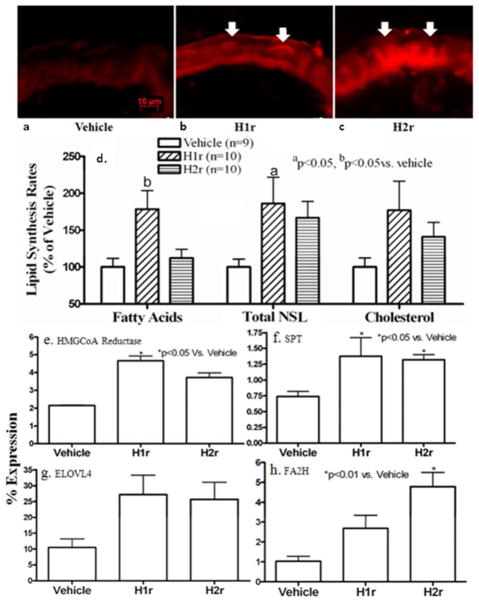
Epidermal permeability barrier function requires both the synthesis and ultimately the secretion of hydrophobic lipids from epidermal keratinocytes into the SC extracellular matrix (Feingold, 2009). Therefore, we next asked whether topical H1r and H2r antagonists enhance lipid production and/or secretion in normal epidermis. To assess the global impact of the antihistamines on lipid production, we initially compared fluorescence intensity after applications of nile red, a fluorophore that selectively depicts lipids, to frozen sections of topical H1/2r antagonist-treated normal skin. Both H1r and H2r antagonists markedly enhanced the overall lipid content of epidermis (Figs. 4a-c, arrows; H2r>H1r).
Figure 4. Global Stimulation of Epidermal Lipid Synthesis by Topical H1/2r Antagonists.
Hairless mice were treated as above (Fig. 1), and biopsies were snap-frozen in liquid nitrogen. Frozen sections (5 μm) were incubated with either the fluorophore, nile red, or vehicle, and viewed in a fluorescence microscope, as in Methods (a-c; arrows depict sites of enhanced staining for lipids in epidermis). d: Freshly-obtained, full-thickness skin biopsies were incubated with 14C-acetate, followed by epidermal isolation, lipid extraction, saponification, fractionation by thin layer chromatography, and quantitation of changes in non-saponifiable (NSL) and saponifiable (total fatty acid) lipids, as in Methods. e-j: mRNA was isolated from epidermal sheets as above (n=4 mice each), and changes in mRNA levels for lipid synthetic enzymes (HMGCoA reductases, serine palmitoyl transferase [SPT], fatty acid modifying enzyme, fatty acid 2-hydroxylase [FA2H], and the acylceramide-generating elongation of very-long chain fatty acid-4 [ELOVL4]) were assessed by rt(Q)-PCR, as in Methods and suppl. Table 2. Scale bar = 10μm.
We next assessed whether topical H1/2r antagonist treatments enhance epidermal lipid synthesis. After four days of topical treatment, both the H1r and H2r antagonists significantly stimulated both epidermal non-saponifiable lipid and cholesterol synthesis, but only the H1r antagonist upregulated saponifiable lipid (i.e., fatty acid) synthesis (Fig. 4d). To assess the basis for enhanced lipid synthesis, we next compared changes in expression of several key lipid synthetic and lipid-modifying enzymes in epidermis two hrs after topical H1/2r antagonist applications. By rt(Q)-PCR, mRNA levels of HMGCoA reductase and serine palmitoyl transferase, the rate-linking enzymes of cholesterol and sphingoid base (ceramide) synthesis, respectively, increased significantly after topical H1/2r antagonist applications (Figs. 4e&f). Moreover, mRNA levels for two key enzymes that modify fatty acid structure, the α-hydroxylating enzyme, fatty acid 2-hydroylase (FA2H), and the elongation enzyme (elongation of very long-chain fatty acid-4, ELOVL4), which is required for acylceramide production, increased significantly after H1/2r antagonist applications (Figs. 4g&h). Together, these results demonstrate that both H1r and H2r antagonists stimulate epidermal lipid production by multiple pathways.
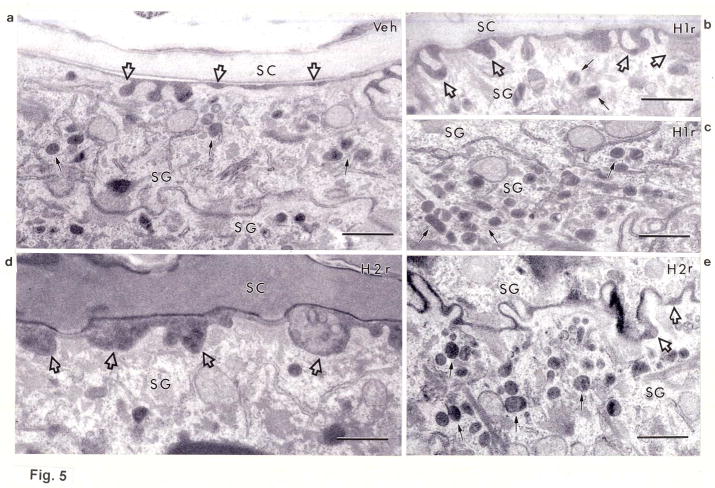
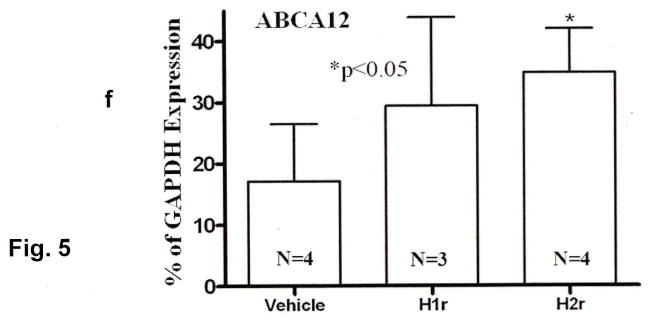
Newly-synthesized lipids are packaged into epidermal lamellar bodies, which then deposit their cargo at the stratum granulosum-SC interface. To determine whether the antihistamines stimulate lipid secretion, we next assessed changes in lamellar body formation after topical H1/2r antagonist applications to intact skin (Figs. 5a–f). While the density of lamellar bodies in the granular cell cytosol appeared to increase after treatment with the H2r antagonist, the H1r antagonist instead appeared to stimulate premature secretion of lamellar body contents between cells deep within the granular layer, a feature that was not evident following H2r antagonist applications (Figs. 5e vs. c). To determine the basis for enhanced lamellar body production, we next assessed mRNA levels of the epidermal-specific, transmembrane transporter, ABCA12, which delivers glucosylceramides into nascent lamellar bodies. Transporter mRNA levels increased significantly after H2r antagonist applications, a finding that correlated with increased lamellar body density in parallel samples (cf., Fig. 5e; increase after H1r antagonist applications did not achieve statistical significance, Fig. 5f). Finally, accelerated production, with or without premature secretion, correlated with enhanced deposition of lamellar body contents at the stratum granulosum-SC interface, a change more evident in H2r than in H1r antagonist-treated epidermis (Figs. 5b&d, open arrows). Together, these results suggest that the H1/2r antagonists also improve barrier function by stimulating lipid secretion.
Figure 5. Topical H1/2r Antagonists Stimulate Lamellar Production and/or Secretion.
Biopsies of H1/2r antagonist- and vehicle-treated skin samples (as in Fig. 1) were processed for electron microscopy, as in Methods. Solid arrows point to individual or aggregated lamellar bodies, and open arrows depict changes in secreted contents at stratum granulosum (SG)-SC interface. Representative samples of outer epidermis of vehicle-treated (Veh)- (a), H1r antagonist (diphenhydramine chlorhydrate)-treated- (b+c), and H2r (cimetidine)-treated- (d) skin. Note increased organelle density in the SG of H2r-treated epidermis, and premature secretion of lamellar bodies in H1r antagonist-treated skin, (b-e), as well as enhanced secretion of lamellar body contents at the stratum granulosum (SG)-SC interface (H2r>H1r). f: Enhanced formation of lamellar bodies correlated with increased mRNA levels of ABCA12 in H2r antagonist-treated skin (n=4); the increase in ABCA12 mRNA following H1r antagonist treatments did not achieve statistical significance. a-e: Osmium tetroxide post-fixation. Scale bar = 0.5 μm.
Topical Antihistamines Improve Inflammation and Barrier Function in Diverse Murine Models
The studies above demonstrate that topical H1/2r antagonists substantially enhance epidermal structure and function in otherwise normal skin by multiple mechanisms. Many inflammatory skin diseases are characterized not only by inflammation, but also by permeability barrier abnormalities. A primary barrier abnormality can induce inflammation (Elias and Schmuth, 2009, Elias, 2010); or conversely, a primary immunologic abnormality, as in HIV (Gunathilake, et al., 2010), can lead to abnormalities in barrier function that further stimulate inflammation (Elias, et al., 1997, Elias, et al., 1999, Elias and Feingold, 2001). Hence, we next asked whether topical H1/2r antagonists could have favorable effects in four different mouse models of cutaneous disease (suppl. Table 2).
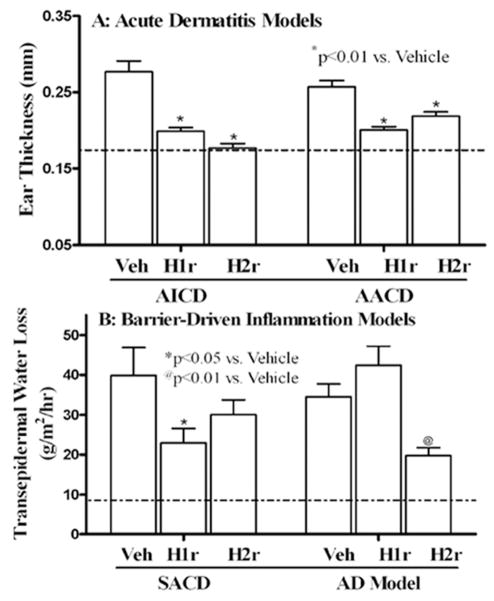
Acute irritant contact dermatitis (AICD), induced by topical phorbol ester [12-O-tetradecanoylphorbol-13-acetate (TPA)] treatment, is characterized by inflammation, but barrier function remains within the range of normal, even after inflammation appears (2 hrs) (8.1 +/− 2.4 vs. 6.4 +/− 1.9 [SD]; n = 29-31; normal ≤ 10) (suppl. Table 2). A single application of either the H1r or H2r antagonist, immediately after the phorbol ester application, significantly reduced inflammation in AICD, quantitated as reductions in ear thickness (Fig. 6a). In parallel, dermal inflammation and epidermal hyperplasia, assessed in H+E sections, declined markedly (suppl. Fig. 6). In contrast, pre-treatment with H1/2r antagonists prior to TPA application did not prevent inflammation (ear thickness, 0.32+0.01 for vehicle, 0.29+0.01 for H1r antagonist, and 0.34+0.01 for H2r antagonist).
Figure 6. Topical H1/2r Antagonists Improve Inflammatory Dermatosis, Independent of Benefits for Barrier Function.
a: Changes in ear thickness in acute irritant and acute allergic contact dermatitis (AICD and AACD, respectively) 16 hrs after prior topical application of the H1/2r antagonists. b: Changes in transepidermal water loss 16 hrs after a single topical application of the H1r or H2r antagonist or vehicle to opposing flanks of previously-sensitized and subsequently challenged mice (3× = subacute allergic contact dermatitis [SACD]); (atopic dermatitis [AD] =10 hapten challenges). Dotted line indicates upper level of water loss in normal mice.
Acute allergic contact dermatitis (AACD), produced by a single hapten (oxazolone, Ox) challenge, after prior sensitization, also induces inflammation, without provoking an immediate barrier abnormality (Sheu, et al., 2002, Fowler, et al., 2003) (3.9 +/− 1.7 vs. 3.4 +/− 1.5 [SD]; n = 26–31; p<0.5). Both H1r and H2r antagonists markedly reduced histological evidence of inflammation (suppl Fig. 6), further quantified as a reduction in ear thickness in AACD (Fig. 6a). Yet, pretreatment again did not prevent development of inflammation (not shown). Together with the studies in AICD, these results demonstrate that topical H1/2r antagonists exhibit potent anti-inflammatory activity in dermatoses that lack a primary barrier abnormality.
Subacute allergic contact dermatitis (SACD), induced by repeated hapten challenges (3×), is characterized by both a substantial barrier abnormality (Fig. 6b), as well as inflammation. Treatment with both the H/2r antagonists significantly improved barrier function and decreased inflammation in the SACD model (Fig. 6b; but pretreatment with the antagonists again did not prevent the development of inflammation).
Atopic dermatitis [AD]-like dermatosis
With further hapten challenges (10×), atopic dermatitis-like inflammation develops, in which a prominent barrier abnormality is currently thought to ‘drive’ downstream inflammation, characterized by a prominent th2-dominant immunophenotype (suppl. Table 2) (Elias, et al., 2008a, Elias and Steinhoff, 2008, Irvine, et al., 2011). When we applied specific H1/2r receptor agonists, both exacerbated inflammation in the AD model (suppl. Figs. 6&7). In contrast, H1r and H2r antagonists reduced inflammation (suppl. Figs. 6&7), but only the H2r antagonist significantly improved barrier function in this model (Fig. 6b). Since the H1/2r antagonists improved inflammation and barrier function only at sites of local application in both the SACD and AD models, systemic activity did not account for disease improvement. Yet again, neither antagonist exhibited preventive benefits in these models. These results show that H1/2r antagonists improve inflammation in the AD model, often with parallel improvements in barrier function.
DISCUSSION
Although histamine is a potent inflammatory mediator, whose levels increase markedly in inflammatory dermatoses [rev. in (Pavlinkova, et al., 2003, Greaves, 2005)], systemic antihistamines have proven ineffective as anti-inflammatory therapy for these disorders (Belsito, et al., 1990, Klein and Clark, 1999, Diepgen, 2002, Kawashima, et al., 2003, Buddenkotte, et al., 2010). Thus, these agents are largely deployed for their relatively-modest anti-pruritic or central sedating properties (Buddenkotte, et al., 2010, Eschler and Klein, 2010). Yet, their use for these purposes can be limited by important side effects, particularly in the elderly (Greaves, 2005). Systemic antihistamines could be minimally effective, either because of poor peripheral bioavailability, and/or metabolism to inactive compounds prior to their arrival in the skin (Levi-Schaffer and Eliashar, 2009). Although topical antihistamines are widely used as anti-pruritics (Eschler and Klein, 2010, Baumer, et al., 2011), whether the topical approach could provide further anti-inflammatory benefits has not yet been examined. After showing that H1r and H2r are highly-expressed in epidermis, we hypothesized that topical deployment of H1/H2r antagonists could provide a boost in anti-inflammatory activity, because of their greater bioavailability, and perhaps by improving barrier function. Pertinently, several inherited inflammatory dermatoses, including atopic dermatitis, inflammatory ichthyoses, and even psoriasis, are now seen as barrier-initiated (Sun, et al., 2006, Schmuth, et al., 2007, Tschachler, 2007, Elias, et al., 2008b, Chen, et al., 2009, Sandilands, et al., 2009, Elias, et al., 2010, Strange, et al., 2010). Hence, after initially determining whether and how these agents improve barrier function in normal epidermis, we then assessed their efficacy in several unrelated mouse models of inflammatory dermatoses.
We described here a marked improved barrier function following topical H1r and H2r antagonist applications to normal skin, confirming prior studies (Ashida, et al., 2001). We further demonstrated that H1r and H2r additively improved barrier function, at least at early time points. These results suggest that H1r and H2r could regulate epidermal function via different downstream mechanisms. Moreover, these agents appear to target the appropriate receptors, since H1/2r antagonist applications to mast cell deficient mice (MCDM) provided no further benefits for the barrier. Since the MCDM also demonstrated enhanced barrier function, and since topical H1/2r antagonists exert no further benefits, these results strongly suggest that mast cells must be the primary source of the ligand that is opposed by the H1/2r antagonists. Yet, these studies did not completely rule out other cell types as potential sources of histamine.
How these agents enhance epidermal structure and function is not yet known. We identified several mechanisms that account for enhanced permeability barrier in H1/2r antagonist-treated normal skin. First, acute barrier disruption increased the density of mast cells in the dermis, raising the question whether recruitment of mast cells to the skin contributes to the development of inflammation in dermatoses characterized by barrier abnormalities. But perhaps more importantly, the topical H1/2r antagonists directly impact epidermal structure and function. Topical applications of H1r and H2r antagonists enhanced epidermal differentiation, the latter at both the mRNA and protein levels, which could reflect the ability of these agents to mobilize intracellular calcium (Koizumi and Ohkawara, 1999). Enhanced differentiation translated further into a significant increase in the thickness of cornified envelopes, which should yield more robust corneocytes, also a Ca++-dependent process (Kim and Bae, 1998, Nemes and Steinert, 1999). The converse certainly proves this point -- effete cornified envelopes occur in several inherited disorders of cornification, including loss-of-function mutations in transglutaminase 1-deficient (lamellar) ichthyosis [rev. in (Schmuth, et al., 2007)], that display subnormal barrier function. Thus, the more robust corneocytes in topical H1/H2r-antagonist-treated skin likely contribute to enhanced barrier function.
We also show that topical antihistamines enhance barrier function by stimulating the synthesis and secretion of epidermal lipids. Multiple steps in the initial synthesis, later modification, and subsequent secretion of epidermal lipids were stimulated by topical applications of the H1r and/or H2r antagonists. Pertinently, hepatic lipid synthesis is similarly enhanced in both H1r and H2r knockout mice (Wang, et al., 2010). Yet, there were subtle differences in the effects of H1r and H2r antagonists on these metabolic pathways in normal epidermis. While both the H1r and H2r antagonists stimulate epidermal lipid synthesis, the H2r antagonists more-potently stimulate lamellar body production, which parallel enhancement of ABCA12 expression after H2r (but not H1r) antagonist applications. While the H1r antagonist displayed a lesser impact on organelle production (and ABCA12 expression), it instead appeared to accelerate lamellar body secretion. Thus, in addition to profound effects on epidermal differentiation, H1/2r antagonists strongly stimulate epidermal lipid synthesis, metabolism and secretion.
Based upon the putative link between abnormalities in barrier function and downstream inflammation, we reasoned that topical antihistamines could reduce inflammation in inflammatory dermatosis, at least in part by improving barrier function, as we showed previously with activators of the liposensor subclass of nuclear hormone receptors (i.e., PPARα, γ, β/δ and LXR). These agents not only improve barrier function in normal skin (Man, et al., 2006, Schmuth, et al., 2008), but also reduce inflammation in diverse inflammatory dermatosis models that may or may not be characterized by a barrier abnormality (Komuves, et al., 2000, Sheu, et al., 2002, Fowler, et al., 2003). Indeed, our results strongly suggest that the benefits of the topical H1/2r antagonists extend beyond their impact on barrier function, because they also reduced inflammation in two models, where barrier function remained normal (i.e., AICD and AACD). Thus, the topical H1/2r antagonists exhibit potent anti-inflammatory activity that could operate independently of, or in parallel to improved barrier function. In contrast, the SACD and AD models display progressively more-severe barrier abnormalities [(Man, et al., 2008, Hatano, et al., 2009, Hatano, et al., 2010) and these results]. Since both the H1r and H2r antagonists improve barrier function in both of these models, it is tempting to argue that this result reflects the impact of the antagonists on barrier function. The H1r antagonist, though highly anti-inflammatory, did not significantly improve barrier function in the AD model. Hence, it is not possible to discriminate which of these two mechanisms (anti-inflammatory vs. barrier enhancement) predominates in reducing inflammation. Yet, even the anti-inflammatory benefits could reflect direct effects on the epidermis, because both H1r and H2r antagonists oppose production of multiple keratinocyte-derived cytokines (Shimizu, et al., 2004, Matsubara, et al., 2005, Kobayashi, et al., 2009), independent of their well-known ability to stabilize histamine production by mast cells (Levi-Schaffer and Eliashar, 2009). Moreover, anti-inflammatory benefits can accrue with improved barrier function via a reduction in the barrier-initiated ‘cytokine cascade’ (Elias, et al., 2008a, Elias and Steinhoff, 2008). Pertinently, topical H1/2r agonists instead aggravated inflammation, perhaps by direct pro-inflammatory effects, or by further compromising barrier function, as they do after topical applications to normal skin [(Ashida, et al., 2001) and these studies].
Not only filaggrin-deficient atopic dermatitis (Sandilands, et al., 2009), but also all of the inherited ichthyoses studied to date (Schmuth, et al., 2007, Elias, et al., 2008b, Elias, et al., 2010), and most recently even psoriasis (Sun, et al., 2006, Tschachler, 2007, Chen, et al., 2009, Strange, et al., 2010), appear to be provoked by primary genetic alterations that compromise epidermal structure and function. Since these dermatoses are often driven or accompanied by prominent barrier abnormalities, not surprisingly, recent studies show that a variety of ‘barrier repair’ strategies comprise effective (and inherently safer) therapy for these disorders (Elias and Wakefield, 2011). The topical H1/2r antagonists, if they prove equally effective when deployed topically for their human disease counterparts, could be added to this list. Nonetheless, it now seems reasonable to propose that H1r and H2r antagonists could be deployed topically to treat a broad range of inflammatory dermatoses.
MATERIALS AND METHODS
(Please read supplemental information for further details of Materials and Methods)
Materials
Female albino hairless (Skh1) mice, aged six-eight weeks, were from Charles River Laboratories (Wilmington, MA). Mast cell deficient mice (MCDM, KitW/KitW-v double heterozygous mice) and age- and gender-matched wild-type littermates (WBB6F1) were from Jackson Labs (Bar Harbor, ME). Ethanol and propylene glycol were from Fisher Scientific (Fairlane, NJ); diphenhydramine chlorhydrate and cimetidine were from Sigma (St Louis, MO), and affinity-purified, rabbit anti-mouse filaggrin, involucrin, and loricrin antibodies were from BabCo (Richmond, CA). Secondary biotinylated, goat anti-rabbit IgG and ABC-peroxidase kit were from Vector laboratories (Burlingame, CA). Anti-proliferating cell nuclear antigen antibody (PCNA, Ki-67) was from CalTag Laboratories (Burlingame, CA).
Experimental protocols and functional studies
Animal procedures were approved and performed in accordance with guidelines of the Animal Studies Subcommittee (IACUC), San Francisco VA Medical Center. Mice were maintained in temperature- and humidity-controlled rooms, and given standard laboratory food and tap water ad libitum. Barrier disruption on hairless mice was achieved by repeated tape-stripping until 10 fold increase in transepidermal water loss. Mice were treated topically on one or both flanks with 5% diphenhydramine or 5% cimetidine or vehicle alone (propylene glycol:ethanol:water = 1:2:2, volume) twice-daily for four days. Changes in transepidermal water loss (TEWL), measured with an electrolytic water analyzer (Meeco, Warrington, PA), were measured 0, 2 and 4 hrs after sequential tape stripping, resulting in a 10-fold increase in TEWL, and percent barrier recovery rates was calculated (Man, et al., 1993, Man, et al., 2006, Man, et al., 2008). SC hydration was measured as changes in electrical capacitance, and surface pH with a flat surface electrode (Ibid.). For studies in MCDM, additional groups of WBB6F1 mice, treated with vehicle, served as controls.
Quantitation of Mast Cell Densities
Skin biopsies were taken from normal, 30 min, 3 hr and 6 hrs after barrier disruption. Mast cell infiltrates in the dermis were identified with 1% toluidine blue staining of 5 um paraffin sections. Pictures were taken at 20X with a Leica DM400B digital microscope, equipped with LAS v4.0 software. The density of mast cells was determined on every 25 cm2 area at regions between basement membrane and 5 cm below basement membrane in printed micrographs.
Statistical Analyses
Data are expressed as the means ± SEM. Unpaired two-tailed student t test with Welch’s correction was used to determine significant differences when two groups were compared, and a one-way ANOVA with a post-Tukey Test or Dunnett post-correction was used to determine significant differences when three of more groups were compared.
Supplementary Material
Acknowledgments
These studies were supported by the San Francisco Veterans Affairs Medical Center, an NIH grant, AR019098, a grant from ISDIN, Ltd. (Barcelona, Spain), and an Austrian Science Fund grant T545-B19. Ms. Joan Wakefield provided superb editorial assistance. These contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Abbreviations
- AACD
acute allergic contact dermatitis
- AD
atopic dermatitis
- AICD
acute irritant contact dermatitis
- CE
cornified envelope
- Hr
histamine receptor
- MCDM
mast cell deficient mice
- Ox
oxazolone
- rt(Q)-PCR
real time quantitative PCR
- SACD
subacute allergic contact dermatitis
- SC
stratum corneum
- TEWL
transepidermal water loss
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
The authors state no conflict of interest.
References
- Ashida Y, Denda M, Hirao T. Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J Invest Dermatol. 2001;116:261–5. doi: 10.1046/j.1523-1747.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- Baumer W, Stahl J, Sander K, et al. Lack of preventing effect of systemically and topically administered histamine H(1) or H(4) receptor antagonists in a dog model of acute atopic dermatitis. Exp Dermatol. 2011;20:577–81. doi: 10.1111/j.1600-0625.2011.01268.x. [DOI] [PubMed] [Google Scholar]
- Belsito DV, Kerdel FA, Potozkin J, et al. Cimetidine-induced augmentation of allergic contact hypersensitivity reactions in mice. J Invest Dermatol. 1990;94:441–5. doi: 10.1111/1523-1747.ep12874535. [DOI] [PubMed] [Google Scholar]
- Buddenkotte J, Maurer M, Steinhoff M. Histamine and antihistamines in atopic dermatitis. Adv Exp Med Biol. 2010;709:73–80. doi: 10.1007/978-1-4419-8056-4_8. [DOI] [PubMed] [Google Scholar]
- Chen H, Toh TK, Szeverenyi I, et al. Association of skin barrier genes within the PSORS4 locus is enriched in Singaporean Chinese with early-onset psoriasis. J Invest Dermatol. 2009;129:606–14. doi: 10.1038/jid.2008.273. [DOI] [PubMed] [Google Scholar]
- Denda M. New strategies to improve skin barrier homeostasis. Adv Drug Deliv Rev. 2002;54(Suppl 1):S123–30. doi: 10.1016/s0169-409x(02)00115-1. [DOI] [PubMed] [Google Scholar]
- Denda M, Nakatani M, Ikeyama K, et al. Epidermal keratinocytes as the forefront of the sensory system. Exp Dermatol. 2007;16:157–61. doi: 10.1111/j.1600-0625.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Diepgen TL. Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol. 2002;13:278–86. doi: 10.1034/j.1399-3038.2002.01047.x. [DOI] [PubMed] [Google Scholar]
- Elias P, Williams M, Crumrine D, et al. Ichthyoses - clinical, biochemical, pathogenic, and diagnostic assessment. Vol. 39. Basel: S. Kargar AG; 2010. p. 144. [Google Scholar]
- Elias P, Wood L, Feingold K. Relationship of the epidermal permeability barrier to irritant contact dermatitis. Immunol Allergy Clin North America. 1997;17:417–430. [Google Scholar]
- Elias PM. The epidermal permeability barrier: from Saran Wrap to biosensor. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 25–31. [Google Scholar]
- Elias PM. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Ann Dermatol. 2010;22:245–54. doi: 10.5021/ad.2010.22.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–81. [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008a;121:1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437–46. doi: 10.1097/ACI.0b013e32832e7d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–70. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–95. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Williams ML, Holleran WM, et al. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008b;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Endo Y, Kikuchi T, Takeda Y, et al. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992;33:9–13. doi: 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nakamura M, Nitta Y, et al. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin 1 and tumour necrosis factor. Br J Pharmacol. 1995;114:187–93. doi: 10.1111/j.1476-5381.1995.tb14924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschler DC, Klein PA. An evidence-based review of the efficacy of topical antihistamines in the relief of pruritus. J Drugs Dermatol. 2010;9:992–7. [PubMed] [Google Scholar]
- Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–46. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–22. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AJ, Sheu MY, Schmuth M, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–55. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- Greaves MW. Antihistamines in dermatology. Skin Pharmacol Physiol. 2005;18:220–9. doi: 10.1159/000086667. [DOI] [PubMed] [Google Scholar]
- Gschwandtner M, Mommert S, Kother B, et al. The histamine H4 receptor is highly expressed on plasmacytoid dendritic cells in psoriasis and histamine regulates their cytokine production and migration. J Invest Dermatol. 2011;131:1668–76. doi: 10.1038/jid.2011.72. [DOI] [PubMed] [Google Scholar]
- Gunathilake R, Schmuth M, Scharschmidt TC, et al. Epidermal barrier dysfunction in non-atopic HIV: evidence for an “inside-to-outside” pathogenesis. J Invest Dermatol. 2010;130:1185–8. doi: 10.1038/jid.2009.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano Y, Man MQ, Uchida Y, et al. Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators. J Allergy Clin Immunol. 2010;125:160–9. e1–5. doi: 10.1016/j.jaci.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano Y, Man MQ, Uchida Y, et al. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J Invest Dermatol. 2009;129:1824–35. doi: 10.1038/jid.2008.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ, Ganellin CR, Timmerman H, et al. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev. 1997;49:253–78. [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Janssens AS, Heide R, den Hollander JC, et al. Mast cell distribution in normal adult skin. J Clin Pathol. 2005;58:285–9. doi: 10.1136/jcp.2004.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Tango T, Noguchi T, et al. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol. 2003;148:1212–21. doi: 10.1046/j.1365-2133.2003.05293.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Bae CD. Calpain inhibitors reduce the cornified cell envelope formation by inhibiting proteolytic processing of transglutaminase 1. Exp Mol Med. 1998;30:257–62. doi: 10.1038/emm.1998.38. [DOI] [PubMed] [Google Scholar]
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–5. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kabashima K, Nakamura M, et al. Downmodulatory effects of the antihistaminic drug bepotastine on cytokine/chemokine production and CD54 expression in human keratinocytes. Skin Pharmacol Physiol. 2009;22:45–8. doi: 10.1159/000183924. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Ohkawara A. H2 histamine receptor-mediated increase in intracellular Ca2+ in cultured human keratinocytes. J Dermatol Sci. 1999;21:127–32. doi: 10.1016/s0923-1811(99)00027-4. [DOI] [PubMed] [Google Scholar]
- Komuves LG, Hanley K, Man MQ, et al. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPARalpha activators restores tissue homeostasis. J Invest Dermatol. 2000;115:361–7. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F, Eliashar R. Mast cell stabilizing properties of antihistamines. J Invest Dermatol. 2009;129:2549–51. doi: 10.1038/jid.2009.256. [DOI] [PubMed] [Google Scholar]
- Man MQ, Choi EH, Schmuth M, et al. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. J Invest Dermatol. 2006;126:386–92. doi: 10.1038/sj.jid.5700046. [DOI] [PubMed] [Google Scholar]
- Man MQ, Feingold KR, Elias PM. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Arch Dermatol. 1993;129:728–38. [PubMed] [Google Scholar]
- Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara M, Tamura T, Ohmori K, et al. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/I kappa B/NF-kappa B signal cascades. Biochem Pharmacol. 2005;69:433–49. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–46. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, et al. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–6. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- Pavlinkova G, Yanagawa Y, Kikuchi K, et al. Effects of histamine on functional maturation of dendritic cells. Immunobiology. 2003;207:315–25. doi: 10.1078/0171-2985-00247. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Sutherland C, Irvine AD, et al. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Gruber R, PME, et al. Ichthyosis update: towards a function-driven model of pathogenesis of the disorders of cornification and the role of corneocyte proteins in these disorders. Adv Dermatol. 2007;23:231–256. doi: 10.1016/j.yadr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Jiang YJ, Dubrac S, et al. Thematic Review Series: Skin Lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- Sheu MY, Fowler AJ, Kao J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nishihira J, Watanabe H, et al. Cetirizine, an H1-receptor antagonist, suppresses the expression of macrophage migration inhibitory factor: its potential anti-inflammatory action. Clin Exp Allergy. 2004;34:103–9. doi: 10.1111/j.1365-2222.2004.01836.x. [DOI] [PubMed] [Google Scholar]
- Strange A, Capon F, Spencer CC, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985–90. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mathur P, Dupuis J, et al. Peptidoglycan recognition proteins Pglyrp3 and Pglyrp4 are encoded from the epidermal differentiation complex and are candidate genes for the Psors4 locus on chromosome 1q21. Hum Genet. 2006;119:113–25. doi: 10.1007/s00439-005-0115-8. [DOI] [PubMed] [Google Scholar]
- Travis ER, Wang YM, Michael DJ, et al. Differential quantal release of histamine and 5-hydroxytryptamine from mast cells of vesicular monoamine transporter 2 knockout mice. Proc Natl Acad Sci U S A. 2000;97:162–7. doi: 10.1073/pnas.97.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E. Psoriasis: the epidermal component. Clin Dermatol. 2007;25:589–95. doi: 10.1016/j.clindermatol.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Wang KY, Tanimoto A, Yamada S, et al. Histamine regulation in glucose and lipid metabolism via histamine receptors: model for nonalcoholic steatohepatitis in mice. Am J Pathol. 2010;177:713–23. doi: 10.2353/ajpath.2010.091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, et al. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Motegi K, Kurimoto M, et al. Induction of the activity of the histamine-forming enzyme, histidine decarboxylase, in mice by IL-18 and by IL-18 plus IL-12. Inflamm Res. 2000;49:513–9. doi: 10.1007/s000110050624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.