Abstract
Occlusion of the renal arteries can threaten the viability of the kidney when severe, in addition to accelerating hypertension and circulatory congestion. Renal artery stenting procedures have evolved from a treatment mainly for renovascular hypertension to a maneuver capable of recovering threatened renal function in patients with “ischemic nephropathy” and improving management of congestive heart failure. Improved catheter design and techniques have reduced, but not eliminated hazards associated with renovascular stenting. Expanded use of endovascular stent grafts to treat abdominal aortic aneurysms has introduced a new indication for renal artery stenting to protect the renal circulation when grafts cross the origins of the renal arteries. Although controversial, prospective randomized trials to evaluate the added benefit of revascularization to current medical therapy for atherosclerotic renal artery stenosis until now have failed to identify major benefits regarding either renal function or blood pressure control. These studies have been limited by selection bias and have been harshly criticized. While studies of tissue oxygenation using blood oxygen level dependent (BOLD) MR establish that kidneys can adapt to reduced blood flow to some degree, more severe occlusive disease leads to cortical hypoxia associated with microvascular rarefication, inflammatory injury and fibrosis. Current research is directed toward identifying pathways of irreversible kidney injury due to vascular occlusion and to increase the potential for renal repair after restoring renal artery patency. The role of nephrologists likely will focus upon recognizing the limits of renal adaptation to vascular disease and identifying kidneys truly at risk for ischemic injury at a time point when renal revascularization can still be of benefit to recovering kidney function.
“It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness, it was the epoch of belief, it was the epoch of incredulity…”
Charles Dickens: A Tale of Two Cities
Introduction
Rarely have competing technical advances in medicine, as in the case of managing renal artery stenosis by endovascular stenting or antihypertensive medical therapy, become so successful over precisely the same time interval. On the one hand, restoring blood flow to an ischemic kidney beyond vascular occlusion seems to provide an obvious means to restore kidney function and improve blood pressure, sometimes dramatically. One the other hand, prospective trials seeking to define the role for renal revascularization up to now have failed to establish a compelling added benefit for endovascular stenting when added to effective medical regimens [1][2]. Clinicians caring for patients with renovascular disease understandably find themselves confused by ambiguous clinical observations and disappointing results from prospective randomized trials. Many argue that the trials have been flawed and potentially misleading [3][4]. Issues of patient selection, statistical quirks, professional bias and flawed study designs continue to leave the role of stenting a matter of active debate and sometimes obscure basic truths that interfere with optimal patient care. To complicate matters further, newer aortic procedures for treatment of abdominal aneurysms introduce a new potential source of renal artery occlusion that can threaten kidney viability. The purpose of this review is to place the role of renal revascularization into context as a tool for management of atherosclerotic renal disease threatening renal function and blood pressure control.
PAST: Renal artery occlusion as a cause of Hypertension
It has been nearly 80 years since landmark studies of Goldblatt and Loesch established that sustained reduction of renal blood flow can raise systemic arterial pressure [5;6]. Since then, renovascular occlusion has been among the most extensively studied forms of experimental hypertension. Indeed, the premise that signals originating from the kidney could not only affect urine formation and solute excretion, but could also modify systemic hemodynamics, endocrine systems, central and peripheral nervous system pathways, vascular structure, cardiac function and systemic resistance provide the foundation for whole systems of understanding of animal and human physiology [7] [8].
From a clinical perspective, recognition that reduced renal blood flow sometimes triggers renovascular hypertension and can impair glomerular filtration provides a prototype for “secondary hypertension” that can sometimes be “cured” or “improved” after restoring kidney perfusion by revascularization. It should be emphasized that before surgical revascularization was technically possible in the 1960’s, clinicians had few effective treatments to reverse malignant phase hypertension. These sometimes included surgical thoraco-abdominal sympathectomy and/or nephrectomy to remove a “pressor” kidney [9].
Since that time, progress regarding imaging of the vascular anatomy, identification of pressor hormones from the kidney, and restoration of blood supply through endovascular methods has been stunning [10]. The ability to safely reach the vascular bed of major renal arteries and to restore blood flow allows endovascular intervention for many patients that would never be considered for open surgical procedures.
Initial renovascular imaging and surgical reconstruction
Vascular surgical techniques in the 1960’s developed sufficiently to allow control and successful operative intervention on the abdominal aorta for acute renal artery occlusion [11]. Partly because of these developments, intravascular contrast agents and vascular imaging became important to establish the diagnosis and anatomy of vascular disease. Early imaging of the abdominal aorta was undertaken through translumbar placement of a needle for contrast injection. Success at developing flexible catheters that could be introduced via a guidewire placed into the femoral artery came later and has been attributed to successful use of the “Seldinger technique” [12]. This was followed by a remarkable series of technical innovations in catheter design to allow selective imaging of vascular structures and selective venous sampling, including measurement of renal vein renin levels [13]
Cooperative Study of Renovascular Hypertension
Initial success at surgically restoring renal function after acute occlusion was followed by attempts to treat renal artery stenosis to improve blood pressure control. Detailed studies of individual kidney function based on measuring inulin clearance and para-amino-hippurate (PAH) testing could identify when blood flow to the kidney had been reduced sufficiently to reduce urine flow, increase sodium reabsorption, but continued have glomerular filtration [14]. The potential morbidity—and mortality—associated with renal artery surgery also led to concerns about the risks of surgical reconstruction and emphasized the need to select patients carefully that might benefit. In this context, early studies supported by the National Institutes of Health were undertaken to define clinical features, optimal imaging, diagnostic patterns and outcomes of surgery for renovascular disease [15–17]. This landmark study provided a registry of more than 500 patients, but was limited by lack of sampling for pressor hormones of the renin-angiotensin system. These studies provided a series of seminal papers regarding clinical features and comorbidity with atherosclerotic renal arterial disease [17]. These studies also identified a mortality risk above 6% associated with aortic surgery that limited the range of candidates most centers would consider for surgery. Renovascular surgery became a highly specialized clinical skill limited to high volume centers with focused interest, as Novick reviewed [18]. It remains so today.
Recent History: Establishing the role of vascular occlusion as a cause of renal insufficiency
In view of the complexity and potential risks, the decision to undertake surgical revascularization routinely included multiple clinicians, including hypertension specialists, nephrologists, cardiovascular specialists, radiologists and surgeons. Determining the likelihood of clinical improvement in blood pressure control was an overriding concern, moreso than any potential recovery of kidney function. In the 1970’s, measurement of the putative pressor signal for renovascular hypertension, plasma renin activity, became widely available and led to a series of seminal papers regarding lateralization of renin secretion from the affected kidneys [19], contralateral suppression of renin release, and additional maneuvers to reveal the role of the renin-angiotensin system for individual patients [20].
Early experience with surgical revascularization cast doubt that patients with reduced GFR would gain much benefit regarding blood pressure control from restoring blood supply. Such patients routinely were excluded from consideration for renovascular surgery, based both on experimental and clinical data suggesting that parenchymal damage to the “contralateral” kidney opposite the stenotic kidney would obviate a benefit regarding blood pressure control [21]. Remarkably, the concept that chronic vascular occlusion might be a reversible cause of renal insufficiency surfaced only in the 1980’s, and was greeted with skepticism [22] [23]. Recognition that atherosclerotic disease poses a risk of progressive vascular occlusion that could be stabilized or reversed regarding renal failure gradually led some centers to shift from “cure of hypertension” to “preservation of renal function” as a primary indication for revascularization [24] [25]. Identification of the concept of “critical perfusion pressure” for continued renal function that could be reversed by restoring renal blood flow supported this premise [26]. Several authors raised the possibility that unsuspected atherosclerotic renal arterial occlusion may contribute to advancing renal insufficiency more commonly than previously thought and coined the term “ischemic nephropathy” [27][28][29][30] [31].
PRESENT: Endovascular restoration of renal blood flow
In 1978, Andreas Gruntzig extended the practice of peripheral vascular dilation to the kidney in a seminal paper on renal artery balloon angioplasty [32;33], followed a year later by a description of initial experience with renal artery angioplasty in the United States [34]. Subsequent reports confirmed the potential for renal artery angioplasty to improve management of uncontrolled hypertension [35;36]. Technical success for angioplasty was generally good [37]. An obvious major benefit from these endovascular techniques was the potential for restoring kidney blood flow for many patients for whom the risks of surgical revascularization would be otherwise prohibitive.
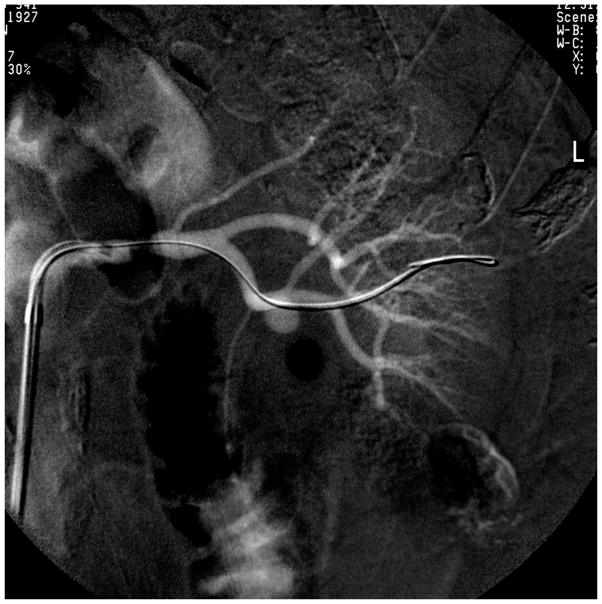
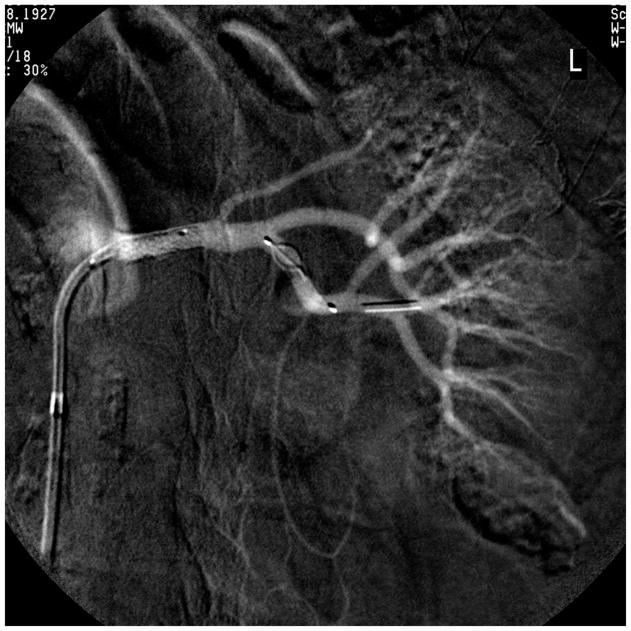
One shortcoming of renal artery angioplasty was the tendency for elastic recoil to occur which narrowed the lumen again immediately after balloon dilatation. This posed a major problem for ostial lesions which usually develop as an extension of aortic plaque into the renal artery. In order to prevent elastic recoil after angioplasty, Palmaz and colleagues in 1987 developed expandable metallic stents for renal arteries in a porcine model [38]. Initial reports of placing stents being placed in the renal artery in patients were positive [39;40]. These were followed by evaluation of the Palmaz stent in a clinical study [41], followed by a randomized trial comparing angioplasty to stenting that established significantly higher primary patency rates with the use of stents [42]. Today, primary stent placement for ostial renal artery stenosis has become the standard. FIGURE 1A and 1B.
Figure 1.
(A) Guidewire placement beyond a high-grade proximal stenosis
(B) Stent deployed with filter-wire embolic protection device, shown after removal in (C). Embolic debris is visible in the distal section of the EPD.
Since the original description of the procedure, additional improvements in the technology have occurred, including development of low profile catheters and semi-compliant balloon technology [43]. The original balloon catheters were constructed on a larger platform- 0.038”, but now are currently being delivered using smaller (0.014”) guide wire technology. These lower profile catheters help reduce peri-procedural complications including embolization and vessel dissection that can occur during the introduction of the stent across the stenosis.
Restenosis of treated vessels has been a practical limitation. Typically, one renal artery supplies each kidney and most renal arteries are 5-mm diameter or larger. A recent study using CT angiography of the renal arteries demonstrated that 30% of patients have more than one renal artery supplying each kidney and that the average renal artery diameter is 5.6 to 6.0-mm[44]. Restenosis rates for stents placed in vessels larger than 5-mm diameter are 15–20% at 9-months when measured by duplex ultrasound [45] [46]. Target vessel restenosis warranting re-intervention for clinical indications, such as recurrent hypertension, deteriorating kidney function (rise in creatinine by more than 20%) or recurrent pulmonary edema developed in 88 of 877 arteries (10.0%) from 748 patients followed longitudinally for a mean of 46 months [47]. Vessels less than 5 mm were twice as likely to restenose as compared to larger vessels [48]. Other predictors for restenosis include advanced age, a solitary functioning kidney and extensive peripheral vascular disease. Restenosis for symptomatic patients after stent placement can be treated by re-stenting or with angioplasty. Newer technologies continue to be developed, such as drug-eluting stents, drug coated balloons, and cutting balloons [49–51].
Drug eluting stents (DES) have been available to treat coronary arteries since early 2000 [52]. The major advantage of DES is reduced risk for restenosis when compared to bare metal stents, particularly in smaller vessels. Several groups have used DES in small diameter vessels such as accessory renal arteries, and some have observed patency rates of 70% in average vessel diameter of 3.5-mm as compared to 47% with bare metal stents [53]. Some trials have not observed major benefits from DES [54;55].
Complications of renal stenting
Despite major technical improvements, peri-procedural complications during renal artery stent placement can be problematic, especially in the presence of a diseased aorta. These include atheroembolism, renal artery or aortic dissection, renal artery rupture, and contrast-induced nephropathy.
Atheroembolism typically occurs during the manipulation of the catheter while selecting and engaging the renal artery or while advancing the stent across the stenosis [56]. As a preventive measure, embolic protection devices (EPD) created for saphenous bypass grafts for coronary artery disease or carotid artery stenosis [57–60] also have been applied in conjunction with renal artery stent placement [61] [62–68]. Currently, there are many different embolic protection devices that can be used to prevent embolization during the placement of stents in the renal artery.
To improve selection of a specific EPD, CT angiographic studies were applied to examine design criteria based on the diameter and length of the renal arteries. Some devices (including SpideRx, Angioguard, and Accunet) had more favorable characteristics for arteries with a diameter of 4–8 mm and a stent length of 12 mm in order to meet needed diameters for introduction and size with an adequate “landing zone” before renal artery bifurcation. [44] Single arm observational studies using EPD report recovery of embolic debris and kidney function stabilization after revascularization [66] [62–68;68]. Most of these reports indicate stabilization or improvement of kidney function in 80–93% of the patients after the procedure with variable capture rates of atheroembolic debris (Fig 1C) [61]. It should be emphasized that the only prospective, randomized trial of embolic protection device with renal stent placement demonstrated no difference between renal functional outcomes with or without use of the Angiogard [69]. This study utilized a device with a large profile which could itself cause thrombus formation during the procedure (Angioguard). This device was eventually removed from use in the CORAL trial due to its tendency to injure renal vessels. Remarkably, there appeared to be an interaction between platelet inhibition using abciximab and the EPD, favoring the combination above either specific measure alone. Clinical observations indicate that atheroemboli sometimes develop in the days and weeks after the procedure [70], limiting the clinical value of EPD’s deployed only during the stenting procedure itself.
Additional complications during renal artery stent placement include dissection, renal artery rupture, and contrast medium induced nephropathy. The reported cumulative risk of arterial dissection or rupture is less than 4% often treated with prolonged angioplasty or covered stent respectively [71]. Contrast medium induced nephropathy occurs in less than 5%. N-acetyl cysteine (Mucomyst1; Bristol Myers Squibb, Anagni, Italy) in a dose of 600 mg twice daily for 2 days can be given safely to reduce CIN [72]. Sodium bicarbonate has been advocated to the risk of CIN also. Alternative contrast agents such as CO2 are available, but image quality is not as good as conventional contrast agents. Patients commonly receive 325 mg of aspirin the day prior to the procedure. In patients with atherosclerotic RAS, clopidogrel (Plavix, Bristol-Myers Squibb, New York, NY) 300 mg is initiated the day before and 75 mg on the day of procedure. Patients who receive stents commonly are maintained on 325 mg aspirin for life and 75 mg Plavix for 1–3 months.
Renal functional outcome after renal artery stenting
Reports from several small studies indicate that renal artery stent placement in some patients undergoing hemodialysis sometimes allows recovery of sufficient kidney function to no longer require dialysis [73][74;75][76] [77]. These observations indicate that some individuals certainly obtain a major clinical benefit from successful stenting as illustrated in Figure 2A and B. Selection of patients for intervention remains highly controversial. How many patients reaching advanced renal insufficiency, even in the presence of large vessel occlusive disease, do so on the basis of impaired vascular supply is unknown. Many series of patients with various levels of renal dysfunction report little change on average after technically successful revascularization [78]. Although some reports present predictors of benefit, many of these suffer from relatively imprecise outcome definitions [79] [80][81][82].
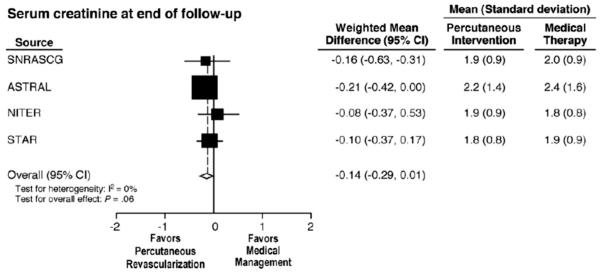
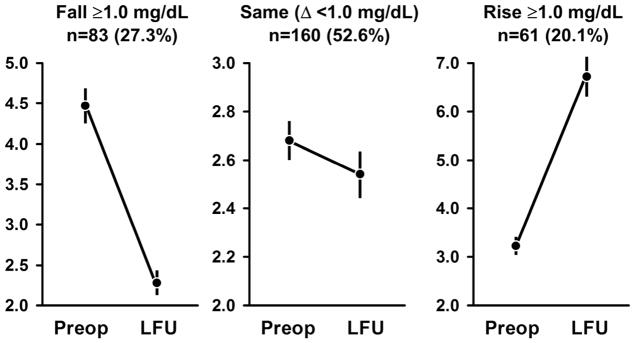
Figure 2.
(A) Meta-analysis of 4 randomized, controlled trials evaluating serial measurements of creatinine in patients treated either with angioplasty (SNRASG) or angioplasty and stenting. Mean values did not change during the follow-up periods reported. A subgroup of 20–28% of subjects were not revascularized in ASTRAL or STAR as assigned, mainly because finding only trivial stenosis at the time of angiography (see Text). (from [105] with permission.
(B) Serum creatinine levels before and after renal revascularization (mean f/u 28 mos) in 304 subjects with initial serum creatinine level above 2.0 mg/dL. These data underscore the differences with subgroups that are masked by group averages, which did not change overall. It should be emphasized that all of these underwent revascularization based upon clinician judgement as to likely benefit and were not randomized. A fall of more than 1.0 mg/dL was considered to be a meaningful decline, which occurred in 27.3% of this group (left panel). Most had little change (middle) panel, but the slight more than 20% experienced a rise of more than 1.0 mg/dL. of patients whose creatinine rose with significant deterioration of kidney function. Nearly all series of results from endovascular or surgical revascularization contain some variation these patterns, dependent in part on starting level of GFR and the degree of change in kidney function deemed significant. Some of the loss of GFR is likely related to atheroembolic complications which may be less common than before, partly related to improved technical expertise (see text). Reproduced from [151] with permission.
Observational reports most commonly include average levels of GFR (based on creatinine equation estimates, such as MDRD eGFR). Most often average GFR for the entire group changes little during follow-up intervals as long as several years. Within these reports, series with renal insufficiency and solitary kidneys often identify some individuals that reverse the trend to progressive renal failure [83][84]. In some reports this is as many as 67% of subjects with creatinine values above 1.5 mg/dL and stenosis affecting the entire renal mass [78]. Recovery of renal function in such patients offers a major benefit both in terms of blood pressure control and survival (Figure 2B). When one expresses clinical results as “improved” or “stabilized”, nearly all report at least 60% in these categories [73][85][86][87]. Most reports agree that a recent deterioration in kidney function is among the strongest predictors of likely recovery after endovascular repair [88][89]. Patients with continued progression despite technically successful revascularization commonly reach end-stage renal disease (ESRD). In nearly all reported series, a subset of patients progresses to advanced renal failure despite technically successful stenting [90].
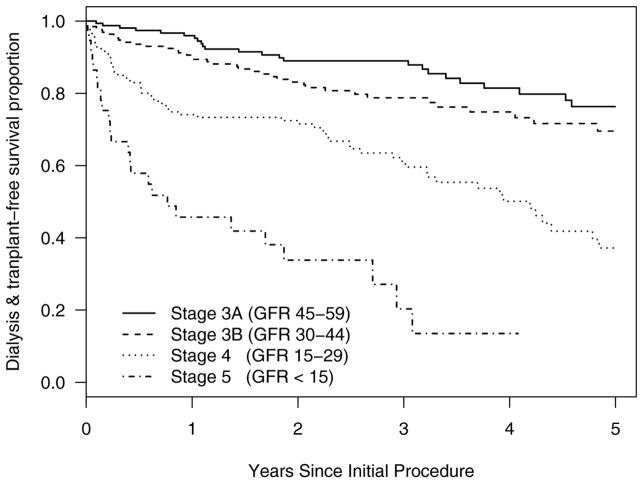
Renal artery revascularization has limited benefits for patients with established long-term loss of GFR [91] [1]. Results from studies of late outcome data in 550 patients treated at Mayo Clinic with renal artery stenting for either uncontrolled hypertension or worsening renal function indicate that established low-levels of eGFR indicate progressively worse renal outcomes for Stage 4 and 5 CKD. Renal outcomes including subsequent freedom from dialysis and/or transplantation were established by querying the United States Renal data Systems Database. The mean pre-treatment eGFR in this group overall was 34± 13 ml/min/1.73m2 (MDRD). (FIGURE 3) Not surprisingly, these data confirm that pre-treatment levels of kidney function portend major differences in long-term clinical outcomes and argue for careful identification and selection of patients for revascularization before far advanced loss of GFR. Even with technically successful revascularization, advanced CKD tended to progress for many patients in this cohort.
Figure 3.
Kaplan-Meier plots of freedom from requiring renal replacement therapy for 550 patients with variable pre-treatment levels of estimated GFR after technically successful renal artery angioplasty and stenting. Follow-up data were verified through the United States Renal Data System (USRDS). These data argue that advanced loss of GFR portends eventual progression to end-stage regardless of restoring renal artery patency.
Present Day Dilemmas: Evidence for and against revascularization in the era of effective medical therapy and highly prevalent atherosclerotic renal artery stenosis
Recent series for which imaging of the renal arteries is included as part of the evaluation of other vascular beds confirm that atherosclerosis is often widespread, with prevalence of visible occlusion (often taken as 50% lumen occlusion) rising from 10–14% for individuals with “suspected” renovascular hypertension to more than 35% of individuals with abdominal aortic disease [92]. These figures are far higher than estimates in the hypertensive population at large, for which most authors argue that no more than 1–2% of hypertensive individuals have “true renovascular hypertension” (defined in retrospect as a “cure” after successful revascularization)[93]. Advances in antihypertensive drug therapy now allow more patients to achieve acceptable blood pressure levels than before. A working classification of renal arterial disease based upon the functional role related to blood pressure and renal function is summarized in TABLE 1A and B. Some authors suspect that a sizable fraction of unexplained renal dysfunction, circulatory congestion (e.g. refractory heart failure) and other cardiovascular morbidity may be related to this disorder [94;95]. Observational series conclude that many patients have improved clinical outcomes regarding cardiovascular events, even mortality, after successful renal revascularization [96;97]. Position papers from some professional organizations support these positions [98], although they have been questioned by others [99]. These trends led to expansion of renal artery revascularization [100]. Within the United States, application of endovascular procedures for renal arterial disease in the Medicare population alone rose more than 4-fold in the decade between 1996–2005 [101]. Endovascular stenting of the renal arteries has become the primary procedure for revascularization for atherosclerotic disease. Surgical bypass procedures have declined substantially over the same interval [102].
Table 1.
| (A). Functional Classification for Atherosclerotic Renal Artery Stenosis |
|
| Table (B) |
Factors favoring medical therapy and revascularization for renal artery stenosis
|
Factors favoring medical therapy and surveillance of renal artery disease
|
From the writing group for Atherosclerotic Peripheral Vascular Disease Symposium [152]
A review of the expansion of endovascular stenting commissioned by the Centers for Medicare and Medicaid Services (CMS) in 2007 questioned the strength of evidence for this practice [103]. During roughly the same period, more widespread application of modern antihypertensive drug therapy—including agents that block the renin-angiotensin system and calcium channel blocking agents--- management of atherosclerotic disease with statins, aspirin, and withholding tobacco use was taking hold. Several small, prospective trials comparing medical therapy with or without either surgical or endovascular procedures to restore renal blood flow fail to demonstrate major advantages to renal revascularization up to now, as we and others have reviewed [104;105]. These trials have been small, conducted over short intervals, and have been ferociously criticized on methodologic grounds [3;106]. Most of these trials had profound challenges in recruiting, in part because of the established patterns, experience and beliefs summarized above. Nonetheless, widespread application of renal artery stenting has been declining in recent years, mainly because of the negative results from these trials.
Appearance of a new indication for renal artery intervention: Endovascular aortic repair (EVAR) and renal functional outcomes
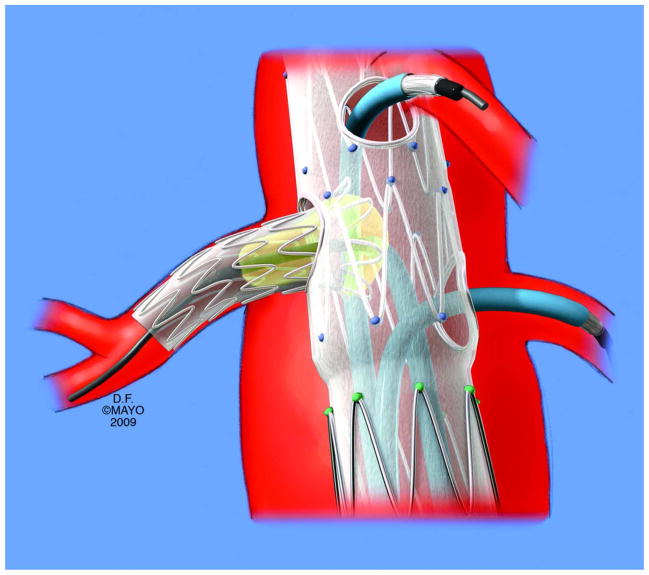
Current estimates indicate that up to 15,000 abdominal aortic aneurysms may rupture annually [107]. Traditionally, these patients have been treated by open surgical repair, with an average operative mortality of 4% in the largest reports [108]. Endovascular aortic aneurysm repair (EVAR) has gained widespread acceptance for treatment of infrarenal aortic aneurysms but offers potential hazards regarding the development of renal arterial obstruction. Results from prospective randomized studies confirm that EVAR reduces operative time, blood loss, transfusion requirements, hospital stay, morbidity and mortality compared to open surgical repair [109][110]. A critical requirement for successful EVAR is the presence of sufficient length of normal aorta (e.g. aortic neck) to provide adequate proximal and distal fixation to the endograft and to seal the aneurysm sac. Many stent grafts are placed in close proximity to the origins of the renal arteries. In some patients with short infrarenal necks or more extensive aneurysms, the renal arteries may be deliberately included in the repair with provision for placement of a protective renal artery stent through fenestrations or side cuffs, which are customized to the patient’s anatomy. FIGURE 4(A and B).
Figure 4.
(A and B)CT angiogram of endovascular stent graft placed above the renal arteries. Renal artery stents are placed through the graft struts to maintain renal artery patency, although some deterioration of kidney function and/or embolic injury can occur.
Preservation of renal artery patency during EVAR is critical for several reasons. A post-procedural decline in renal function is associated with increased mortality [111][112]. Although a modest decline in renal function commonly occurs after EVAR approaching 1 ml/min/1.73 m2 per year (typical of an aging population), more significant deterioration in renal function can develop associated with inadvertent renal artery occlusion, renal artery complications (stenosis, plaque dislodgement or dissection) and use of fenestrated endografts [113]. Haddad and associates reported that 23 of 72 patients (32%) treated with fenestrated endografts for juxtarenal aneurysms had greater than 30% decline in GFR, although the majority recovered by 6 months [114]. Results from another prospective non-randomized study of 287 patients treated by fenestrated endografts indicate that renal function deterioration occurred in 20% and was associated with presence of renal artery stenosis or kidney infarcts due to intra-procedural embolization or coverage of early branch bifurcations or accessory renal arteries by the stent graft [115].
Several factors likely contribute to renal injury observed in some patients undergoing EVAR, including exposure to repeated doses of contrast agents, implantation of stent grafts, and follow up surveillance imaging. Endovascular aortic instrumentation carries particular risk of atheroembolization, particularly for patients with complex aneurysms and thrombus involving the renal arteries or who need adjunctive renal artery stenting. Implantation of fenestrated endografts that cover the renal arteries and require placement of adjunctive renal artery stents can be technically challenging. These grafts are associated with higher rates of potential renal artery dissection and/or perforations compared to renal artery stent placement performed alone for occlusive disease [115][114][116].
Adjunctive renal artery stents during EVAR
Inadvertent coverage of the renal arteries is described in 0% to 6% of patients undergoing EVAR [109;110;113;117–119]. When identified and treated immediately by placement of a renal stent, this results in minimal adverse sequelae. However, introduction of renal stents in patients with suprarenal fixation can be difficult and may require use of stiff guide-wire system and large platform to provide enough support [118]. If renal artery impingement is not promptly recognized and treated, renal artery occlusion is a frequent complication [117].
Some aortic endografts use deliberate suprarenal fixation with an uncovered stent that extends above the renal arteries designed to improve fixation into the normal aorta and prevent distal migration. The presence of a suprarenal fixation structure may complicate subsequent placement of renal artery stents due to stent struts crossing the renal artery ostia, thereby encroaching on the renal artery lumen. These may limit the effective achievable renal blood flow or the cross-sectional luminal area [120][121][117][122]. Despite theoretical concerns that suprarenal fixation may impair renal function, results from experimental studies and clinical reports that compare infrarenal versus suprarenal fixation stents during EVAR identify only minor differences between these stent designs. Nevertheless, suprarenal fixation inevitably makes placement of renal artery stents more challenging in some patients, mainly as a result of adding difficulty in selective catheterization of the renal arteries, advancement of the sheath or guide catheter, and expansion of the stent which can be partially compressed by the suprarenal stent [118][119].
Prior placement of renal artery stents can limit suitability for treatment of the aneurysm using endografts. Because a renal artery stent is typically placed a few millimeters into the aorta, aortic grafts with suprarenal fixation or fenestrated stent grafts that extend above the renal arteries may no longer achieve adequate implantation. These are important considerations when deciding the timing and sequence of repair in patients who have aortic aneurysms and renal artery disease that warrants revascularization.
Recent developments include wider application of “fenestrated” stent grafts with construction of grafts to protect renal and mesenteric vessels. FIGURE 4(B) Migration is a rare occurrence given that these customized endografts have excellent fixation and are the designed to be implanted in normal aorta. Renal artery stent patency for patients with fenestrated aortic stent grafts averages 95% at 5-years in patients, higher than that reported for renal artery stents performed for occlusive disease [123][124][125][126][127][128]. In-stent stenosis affected the proximal portion of uncovered stents, while stenosis observed in covered stents affected the distal edge of the stent. Renal artery stent occlusion rate was 4.5% for uncovered and 2.2% for covered stents, and cumulative incidence of new permanent dialysis was 2% for the entire cohort.
The Future for Renal artery stenting: Patient Selection : Ambiguity and “Evidence Based Medicine”
Despite major successes in selected individual cases, persuasive evidence of benefit for many patients from renal revascularization has been lacking [101]. Several limited, prospective randomized controlled trials (RCT) for patients with atherosclerotic renal artery stenosis have been undertaken within the last seven years to examine the benefit of renal artery stenting when added to current medical therapy. Major features of these trials are summarized in TABLE 2. Numbers of subjects, age, levels of kidney function and selection criteria have been highly variable. None of these trials provided strong results supporting recovery or preservation of renal function. Remarkably different statistical power estimates were employed to define the number of subjects needed to identify clinical events, ranging from less than 100 to nearly 1000 participants [104][103]. Clearly, the investigators anticipated widely different rates of outcome events and disease progression, reflecting the inaccuracies of using historical data and selected patient series [129]. Many authors emphasize the heterogeneity between patients with widespread atherosclerosis and the comorbid disease risks that affect outcomes in this population independent of renovascular disease. Some have argued that the complexity of these confounders render patients so heterogeneous that meaningful prospective trials are practically impossible [130]. It is important for clinicians evaluating these trials to recognize that entry criteria for many of these trials were based on marginally significant vascular occlusion for many participants. In the STAR trial for example, fully 28% of patients assigned to stenting were not treated, mainly because of finding only trivial stenosis at the time of angiography [131]. Recent studies further challenge the premise that estimated GFR methods can identify changes in kidney function accurately in patients with renal artery stenosis in the 20% range applied to recent trials [132]. Some of the major limitations of these trials are summarized in TABLE 3.
Table 2.
Baseline characteristics of prospective, randomized treatment trials comparing medical therapy with or without renal artery intervention (PTRA with or without stents). None of these trials identified a meaningful benefit regarding changes in kidney function (see text and FIGURE 2). These were combined into a meta-analysis including approximately 1200 patients. Baseline assumptions regarding the risk of progression, diagnostic features, medications, and follow-up differed substantially (see text). Two trials of renal function (ASTRAL and STAR) failed to revascularize 20–28% of subjects mainly due to minimal lesions identified at angiography.
| EMMA | SNRASCG | DRASTIC | ASTRAL | STAR | NITER | |
|---|---|---|---|---|---|---|
| Location | France | UK | Netherlands | UK/Aust/NZ | Netherlands | Italy |
| Year | 1998 | 1998 | 2000 | 2009 | 2009 | 2009 |
| No. Pts. (Intervent./Med Rx) | 23/26 | 25/30 | 56/60 | 403/403 | 64/74 | 28/24 |
| Mean Age (yrs) | 59.4 | 61.1 | 59.9 | 70.5 | 66.5 | 72.0 |
| CAD (%) | NA | NA | NA | 48.5 | 39.3 | 63.5 |
| ASPVD (%) | NA | NA | NA | 40.5 | 40.0 | 46.2 |
| Creatinine Baseline mg/dl | 1.2 | 1.8 | 1.3 | 2.0 | 1.7 | 1.7 |
| Bilat (%) | 0 | 50.9 | 22.6 | 53.5 | 47.9 | 51.5 |
| ACE/ARB at F/U (%) | NA | 0 | NA | 45 | 57 | 61 |
| Crossover (%) | 26.9 | 6.2 | 44 | 3.2 | 1.3 | 0 |
| F/U Mean (mos) | 6 | 12 | 12 | 33.6 | 24 | 43 |
| Comment: Entry criteria | Unilateral only | “Resistant HTN” | “Resistant HTN” | “Uncertainty of benefit” | “Renal insufficiency” | Resistant HTN/CRI” |
Modified from [105].
Table 3.
Limitations of Clinical Trials in Atherosclerotic Renal Artery Stenosis
|
Modified from [104]
The most carefully performed of these trials to date is the U.S. based Cardiovascular Outcomes for Renal Arterial Lesions (CORAL) supported by the NHLBI. This trial has enrolled 947 subjects and randomized them to protocol-based antihypertensive and atherosclerotic medical therapy with or without renal artery stenting. Although investigators participating in this trial have taken care to assure that subjects have hemodynamically significant renovascular disease, how these enrollees compare to the many thousands of patients not enrolled will be difficult to ascertain.
Whether RCTs truly are appropriate for this condition and/or will provide meaningful guidance is not clear. In some respects, relying upon medical therapy alone to lower systemic arterial pressures in the face of deteriorating kidney function beyond a critical stenosis has flawed “face validity”. Further reduction of renal perfusion beyond a “critical” level obviously threatens viability of the kidney. Some authors argue that depending upon RCT data in this disorder is akin to arguing for a controlled trial of parachutes [133] or bullet proof vests [4]. The parachute analogy seems particularly apt, as some argue that the spectrum of renovascular disease truly includes a full range comparable to testing efficacy of parachutes for jumping from a footstool (no benefit) to jumping from a second story window (unknown, ?equipoise) to jumping from an airplane at 10,000 feet (near certain benefit, prospective trial not ethical) [4].
What to do? Limits to adaptation of reduced blood flow in the kidneys
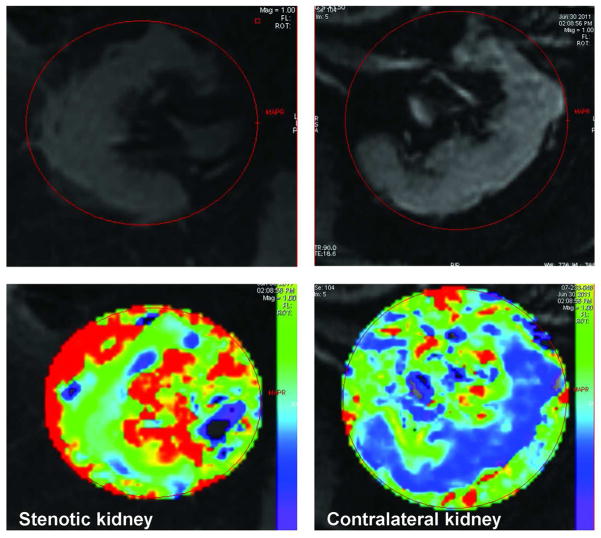
An important recent observation in renovascular disease is that gradually developing vascular occlusion leads to “adaptation” that protects intrarenal oxygenation for many subjects. Studies of tissue deoxyhemoglobin within the kidney using Blood Oxygen Level Dependent (BOLD) MR demonstrate complex adaptive processes that both prevent hyperoxia in the renal cortex –and thereby minimize generation of toxic reactive oxygen species—and at the same time preserve oxygenation in both between cortex and medulla despite substantial reductions in blood flow and GFR [134] FIGURE 5. Sudden loss of renal perfusion regularly produces acute hypoxia and kidney injury [135;136]. Gradual reduction of blood flow over years, however, more often leads to reduction in blood flow with preservation of oxygen levels and metabolic activity. Experimental studies confirm that medullary blood flow can be regulated independently of cortical hemodynamics, in part by modifications of changes in energy-dependent oxygen consumption, reduced filtration and changes in arterio-venous shunting [137]. These observations partly explain the relative stability of kidney function and lack of progressive renal injury in many patients treated medically, as observed in the ASTRAL trial [138].
Figure 5.
Blood Oxygen Level Dependent (BOLD) MR images with parametric maps depicting R2* levels that correspond to tissue levels of deoxyhemoglobin in axial images of the kidneys. Both of these kidneys had high grade renal arterial stenosis with velocities above 400 cm/sec. Serum creatinine was above 3.6 mg/dL, although the patient was treated with angiotensin receptor blockers and diuretics. The larger kidney (right panel, left kidney) has well-preserved cortical oxygenation (blue zone) and a normal cortico-medullary oxygen gradient. The smaller kidney (left panels) is developing overt cortical hypoxia with rising R2* levels and expanding zone of medullary hypoxia (inner red zone). These functional imaging tools may assist in defining kidneys that are “at risk” from critical vascular occlusion yet remain “salvageable” from the point of view of restoring renal blood flow. (See text).
It is equally clear, however, that renal adaptation has limits. When occlusive vascular disease reaches some “critical” level—perhaps based upon severity or duration—adaptive processes are overwhelmed and cortical hypoxia is apparent (FIGURE 5). Progressive renal damage ensues with microvascular “rarefication” and activation of inflammatory and fibrogenic processes [139;140]. At some point, the viability of the post-stenotic kidney is lost entirely.
Improving renal recovery
Is it possible that “adjunctive” measures, such as administration of specialized cells or cytokines and/or denervating the kidneys, beyond simply restoring blood flow can boost recovery of kidney function? In some cases, injury to kidney tissue may be magnified by “ischemia/reperfusion” injury with release of reactive oxygen species or mitochondrial injury related to flooding ischemic tissue with oxygenated blood [141;142]. Atheroemboli continue to be a major concern, and may develop in nearly all vessels subjected to instrumentation [56]. A worrisome observation from both observational and RCT’s is that restoring blood flow alone infrequently leads to full recovery of kidney function. Experimental data in a swine model indicate that endothelial progenitor cell infusion can boost restoration of the renal microvessels and facilitate repair of renal parenchymal structures [143]. Studies using mesenchymal stem cells suggest improved recovering of microvascular function can result from application of these methods at some point [144]. Recent studies using radiofrequency ablation of vascular nerve bundles alongside the renal arteries raise the possibility that both afferent and efferent adrenergic signals may affect both kidney function and blood pressure regulation [145]. A broad literature supports the notion that renal nerve traffic modulates renin release, blood flow, tubular function and can modify central nervous system signals affecting the circulation [146][147][148]. Some of these signals are changed after endovascular revascularization alone [149]. Up to now, studies of endovascular renal denervation have excluded subjects with primary renovascular lesions, specifically to avoid the question of whether such procedures induce renovascular injury. Some might argue that combining restoration of blood flow with interruption of renal nerve traffic may magnify potential clinical benefits of either treatment. Whether these measures will extend to human renal revascularization procedures and improve the potential for restoring lost renal functional capacity is not known.
Nephrologists and renal revascularization in the future
Clinicians caring for patients with renovascular disease should recognize that restoring blood flow is critical for some individuals. As a practical matter, clinicians must evaluate individual patients carefully –and repeatedly—to decide about the relative merits of relying on medical therapy or adding renal revascularization. We favor using the general guidelines summarized in TABLE 1(B) as they apply to a specific patient over time. The challenge for nephrologists is to identify patients whose kidneys are truly at risk from vascular occlusion at a point when restoring blood flow still may improve the outcome. Defining such patients remains difficult. Whether advances in imaging, evaluating oxygenation and/or identifying injury pathways will allow for more precise diagnosis and the role for stenting remains to be seen.
The limitations of trial data notwithstanding, current published studies in the general medical literature identify “substantial risks, but no evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renal artery stenosis” [138]. Hence, the pendulum that led initially to excessive enthusiasm for renal revascularization appears to have swung to the opposite extreme. Clinicians are aware of the fact that “average” values may obscure important individual differences in clinical trials [150]. We anticipate—and have already encountered—some patients managed solely with medical therapy until advanced loss of renal function occurs. Our contention is that clinicians—including nephrologists and cardiovascular specialists—will need to more vigilant to recognize the potential for recovery and/or stabilization of renal viability and blood pressure control than before. It likely will fall more to subspecialists in vascular medicine and nephrology to advocate for restoring blood flow and preventing kidney injury when possible. Developing more effective tools to identify viable kidneys and/or those at risk from irreversible vascular injury remains a top priority in this field.
Acknowledgments
The project described was supported by Award number P01HL085307 from National Heart, Lung and Blood Institute and NIH/NCRR CTSA Grant Number UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Contributor Information
Stephen C. Textor, Nephrology and Hypertension, Mayo Clinic
Sanjay Misra, Vascular and Interventional Radiology, Mayo Clinic
Gustavo Oderich, Vascular Surgery, Mayo Clinic
Reference List
- 1.Steichen O, Amar L, Plouin PF. Primary stenting for atherosclerotic renal artery stenosis. J Vasc surg. 2010;51:1574–1580. doi: 10.1016/j.jvs.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Textor SC, Lerman L, McKusick M. The uncertain value of Renal Artery Interventions: Where are we now? J Am Coll Cardiol Cardiovasc Interv. 2009;2:175–182. doi: 10.1016/j.jcin.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann SJ, Sos TA. Misleading results of randomized trials: the example of renal artery stenting. J Clin Hypertens. 2010;12:1–2. doi: 10.1111/j.1751-7176.2009.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White CJ. The need for randomized trials to prove the safety and efficacy of parachutes, bulletproof vests, and percutaneous renal intervention. Mayo Clinic Proc. 2011;86:603–605. doi: 10.4065/mcp.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldblatt H, Lynch J, Hanzal RE, Summerville WW. Studies on experimental hypertension I: the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loesch J. Ein Beitrag zur experimentellen Nephrtis und zum arteriellen Hochdruck III. Die Veranderungen in den Geweben. Zentralblatt fur Innere Medizin. 1933;7:177–185. [Google Scholar]
- 7.Guyton AC. Dominant role of the kidneys in long-term regulation of arterial pressure and hypertension: the integrated system for pressure control, chap. 19. In: Guyton AC, editor. Textbook of Medical Physiology. 8. Philadelphia: W.B. Saunders Company; 1991. pp. 205–220. [Google Scholar]
- 8.Basso N, Terragno NA. History about the discovery of the renin-angiotensin system. Hypertension. 2001;38:1246–1249. doi: 10.1161/hy1201.101214. [DOI] [PubMed] [Google Scholar]
- 9.Stanley JC. Surgical Treatment of Renovascular Hypertension. Am J Surg. 1997;174:102–110. doi: 10.1016/s0002-9610(97)00094-9. [DOI] [PubMed] [Google Scholar]
- 10.Textor SC, Lerman LO. Renovascular Hypertension and Ischemic Nephropathy: State of the Art. Am J Hypertens. 2010;23:1159–1169. doi: 10.1038/ajh.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris GC, DeBakey NE, Cooley DA. Surgical Treatment of renal failure of renovascular origin. JAMA. 1962;182:609–612. [Google Scholar]
- 12.King BF. Diagnostic imaging evaluation of renovascular hypertension. Abdom Imag. 1995;20:395–405. doi: 10.1007/BF01213259. [DOI] [PubMed] [Google Scholar]
- 13.Rossi GP, Cesari M, Chiesura-Corona M, Miotto D, Semplicini A, Pessina AC. Renal vein renin measurements accurately identify renovascular hypertension caused by total occlusion of the renal artery. J Hypertens. 2002;20:975–984. doi: 10.1097/00004872-200205000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Stamey TA, Nudelman JJ, Good PH, et al. Functional characteristics of renovascular hypertension. Medicine. 1961;40:347–394. doi: 10.1097/00005792-196112000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Simon N, Franklin SS, Bleifer KH, Maxwell MH. Clinical characteristics of renovascular hypertension. JAMA. 1972;220:1209–1218. [PubMed] [Google Scholar]
- 16.Bookstein JJ, Maxwell MH, Abrams HL, Buenger RE, Lecky J, Franklin SS. Cooperative Study of Radiologic Aspects of renovascular hypertension: Bilateral Renovascular disease. JAMA. 1977:1706–1709. [PubMed] [Google Scholar]
- 17.Franklin SS, Young JD, Maxwell MH, Foster JH, Palmer JM, Cerny J, Varady PD. Operative morbidity and mortality in renovascular disease. J Am Med Assoc. 1975;231:1148–1153. [PubMed] [Google Scholar]
- 18.Novick AC. Management of renovascular disease: a surgical perspective. Circulation. 1991;83(suppl I):I-167–I-171. [PubMed] [Google Scholar]
- 19.Maxwell MH, Marks LS, Lupu AN, Cahill PJ, Franklin SS, Kaufman JJ. Predictive value of renin determinations in renal artery stenosis. JAMA. 1977;238:2617–2620. [PubMed] [Google Scholar]
- 20.Vaughan ED, Jr, Buhler FR, Laragh JH, Sealey JE, Baer L, Bard RH. Renovascular hypertension: renin measurements to indicate hypersecretion and contralateral suppression, estimate renal plasma flow and score for surgery. Am J Med. 1973;55:402–414. doi: 10.1016/0002-9343(73)90139-3. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JM. Prognostic value of contralateral renal plasma flow in renovascular hypertension. JAMA. 1971;217:794–802. [PubMed] [Google Scholar]
- 22.Novick AC, Pohl MA, Schrieber M, Gifford RW, Jr, Vidt DG. Revascularization for preservation of renal function in patients with atherosclerotic renovascular disease. J Urol. 1983;129:907–912. doi: 10.1016/s0022-5347(17)52453-2. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber MJ, Pohl MA, Novick AC. Preserving renal function by revascularization. Annu Rev Med. 1990;41:423–429. doi: 10.1146/annurev.me.41.020190.002231. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber MJ, Pohl MA, Novick AC. The natural history of atherosclerotic and fibrous renal artery disease. Urologic Clinics of North America. 1984;11:383–392. [PubMed] [Google Scholar]
- 25.Novick AC. Atherosclerotic ischemic nephropathy. Urol Clin N Am. 1994;21:195–200. [PubMed] [Google Scholar]
- 26.Textor SC, Novick A, Tarazi RC, Klimas V, Vidt DG, Pohl M. Critical perfusion pressure for renal function in patients with bilateral atherosclerotic renal vascular disease. Ann Int Med. 1985;102:309–314. doi: 10.7326/0003-4819-102-3-308. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson HR. Ischemic renal disease: an overlooked clinical entity. Kidney International. 1988;34:729–743. doi: 10.1038/ki.1988.240. [DOI] [PubMed] [Google Scholar]
- 28.Breyer JA, Jacobson HR. Ischemic Nephropathy. Curr Opin Nephrol Hyper. 1993;2:216–224. doi: 10.1097/00041552-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Scoble JE, Maher ER, Hamilton G, Dick R, Sweny P, Moorhead JF. Atherosclerotic renovascular disease causing renal impairment- a case for treatment. Clinical Nephrology. 1989;31:119–122. [PubMed] [Google Scholar]
- 30.Scoble JE, Hamilton G. Atherosclerotic renovascular disease: remediable cause of renal failure in the elderly. Br Med J. 1990;300:1670–1671. doi: 10.1136/bmj.300.6741.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’neil EA, Hansen KJ, Canzanello VJ, Pennell TC, Dean RH. Prevalence of ischemic nephropathy in patients with renal insufficiency. Am Surg. 1992;58:485–490. [PubMed] [Google Scholar]
- 32.Gruentzig A, Kuhlmann U, Vetter W, Luetolf U, Meier B, Siegenthaler W. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal artery stenosis. Lancet. 1978;1:801–802. doi: 10.1016/s0140-6736(78)93000-3. [DOI] [PubMed] [Google Scholar]
- 33.Kuhlmann U, Gruentzig A, Vetter W, Lutolf U, Meier B, Siegenthaler W. Percutaneous translumial dilatation: a new treatment of renovascular hypertension? Klin. Wochenschr. 1978;56:703–707. doi: 10.1007/BF02429105. [DOI] [PubMed] [Google Scholar]
- 34.Katzen BT, Chang J, Knox WG. Percutaneous transluminal angioplasty with the Gruntzig balloon catheter A review of 70 cases. Arch Surg. 1979;114:1389–1399. doi: 10.1001/archsurg.1979.01370360043005. [DOI] [PubMed] [Google Scholar]
- 35.Sos TA, Saddekni S, Sniderman KW, Weiner M, Beinart C, Pickering TG, et al. Renal artey angioplasty: techniques and early results. Urol Radiol. 1981;3:223–231. doi: 10.1007/BF02938807. [DOI] [PubMed] [Google Scholar]
- 36.Tegtmeyer CJ, Ayers CA, Wellons HA. The axillary approach to percutaneous renal artery dilatation. Radiology. 1980;135:775–776. doi: 10.1148/radiology.135.3.7384473. [DOI] [PubMed] [Google Scholar]
- 37.Canzanello VJ, Millan VG, Spiegel JE, Ponce SP, Kopelman RI, Madias NE. Percutaneous transluminal renal angioplasty in management of atherosclerotic renovascular hypertension: results in 100 patients. Hypertension. 1989;13:163–172. doi: 10.1161/01.hyp.13.2.163. [DOI] [PubMed] [Google Scholar]
- 38.Palmaz JC. The Current Status of Vascular Intervention in Ischemic Nephropathy. J Vasc Intervent Radiol. 1998;9:539–543. doi: 10.1016/s1051-0443(98)70318-5. [DOI] [PubMed] [Google Scholar]
- 39.Mali WP. Follow-up of endovascular stented renal artery. Am J Roentgenol. 1990;154:902. doi: 10.2214/ajr.154.4.2107700. [DOI] [PubMed] [Google Scholar]
- 40.Joffre F, Bernadet P, Rousseau H, Nomblot C, Durand D, Chamontin B, et al. Usefulness of a percutaneous endoprosthesis in the treatment of renal artery stenoses. Arch Mal Coeur et Vaisseaux. 1989;82:11991–1204. [PubMed] [Google Scholar]
- 41.Rees CR, Palmaz JC, Becker GJ, Ehrman KO, Richter GM, Noeldge G, et al. Palmaz stent in atherosclerotic stenoses involving the ostia of the renal arteries: preliminary report of a multicenter study. Radiology. 1991;181:507–514. doi: 10.1148/radiology.181.2.1924796. [DOI] [PubMed] [Google Scholar]
- 42.van de Ven PJ, Kaatee R, Beutler JJ, Beek FJ, Woittiez AJ, Buskens E, Koomans HA, Mali WP. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999;353:282–286. doi: 10.1016/S0140-6736(98)04432-8. [DOI] [PubMed] [Google Scholar]
- 43.Nolan BW, Schermerhorn ML, Rowell E, Powell RJ, Fillinger MF, Rzucidlo EM, Wyers MC, Zwolak RM, Walsh DB, Cronenwett JL. Outcomes of renal artery angioplasty and stenting using low-profile systems. J Vasc surg. 2005;41:46–52. doi: 10.1016/j.jvs.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 44.Thatipelli MR, Sabater EA, Bjarnason H, McKusick MA, Mistra S. CT angiography of renal artery anatomy for evaluating embolic protection devices. J Vasc Interv Radiol. 2007;18:842–846. doi: 10.1016/j.jvir.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 45.Bakker J, Beutler JJ, Elgersma OE, de Lange EE, de Kort GA, Beek FJ. Duplex ultrasonography in assessing restenosis of renal artery stents. Cardiovasc and Intervent Radiol. 1999;22:468–474. doi: 10.1007/s002709900435. [DOI] [PubMed] [Google Scholar]
- 46.Girndt M, Kaul H, Maute C, Kramann B, Kohler H, Uder M. Enhanced flow velocity after stenting of renal arteries is associated with decreased renal function. Nephron. 2007;105:c84–c89. doi: 10.1159/000097866. [DOI] [PubMed] [Google Scholar]
- 47.Bates MC, Rashid M, Campbell JE, Stone PA, Broce M, Lavigne PS. Factors influencing the need for target vessel revascularization after renal artery stenting. Journal of Endovascular Therapy. 2007;13:569–577. doi: 10.1583/06-1861.1. [DOI] [PubMed] [Google Scholar]
- 48.Sapoval M, Zharinger M, Pattynama P, Rabbia C, Vignali C, Maleux G. Low-profile stent system for treatment of atherosclerotic renal artery stenosis: the GREAT trial. J Vasc Interv Radiol. 2005;16:1195–2002. doi: 10.1097/01.RVI.0000171765.67665.D3. [DOI] [PubMed] [Google Scholar]
- 49.Misra S, Sturludottir M, Matthews V, Bjarnason H, Ma M, Iyer V. Treatment of complex stenoses involving renal artery bifurcations using drug-eluting stents. J Vasc Interv Radiol. 2008;19:272–278. doi: 10.1016/j.jvir.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 50.Misra S, Thatipelli MR, Hunt C, Mathew V, Barsness GW, Pflueger A, Textor SC, Bjarnason H, McKusick MA. Preliminary study of the use of drug-eluting stents in atherosclerotic renal artery stenoses 4 mm in diameter or smaller. J Vasc Interv Radiol. 2008;19:833–839. doi: 10.1016/j.jvir.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Zeller T, Sixt S, Rastan A, Schwarzwalder U, Muller C, Frank U. Treatment of reoccurring instent restenosis following reintervention after stent-supported renal artery angioplasty. Catheterization & Cardiovascular Interventions. 2007;70:296–300. doi: 10.1002/ccd.21170. [DOI] [PubMed] [Google Scholar]
- 52.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R for RAVEL Study Group. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 53.Misra S, Thatipelli MR, Howe PW, Hunt C, Mathew V, Barsness GW, Pflueger A, Textor SC, Bjarnason H, McKusick MA. Preliminary study of the use of drug-eluting stents in atherosclerotic renal artery stenoses 4 mm in diameter or smaller. J Interv Radiol. 2008;19:833–839. doi: 10.1016/j.jvir.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Zahringer M, Sapoval M, Pattynama PM, Rabbia C, Vignali C, Maleux G, Boyer L, Szczerbo-Trojanowska M, Jaschke W, Hafsahl G, Downes M, Beregi JP, Veeger NJ, Stoll HP, Talen A. Sirolimus-eluting versus bare-metal low profile stent for renal artery treatment (GREAT Trial): angiographic follow-up after 6 months and clinical outcome up to 2 years. J Endovasc Therapy. 2007;14:460–468. doi: 10.1177/152660280701400405. [DOI] [PubMed] [Google Scholar]
- 55.Kiernan TJ, Yan BP, Eisenberg JD, Ruggiero NJ, Gupta V, Drachman D, Schainfeld RM, Jaff MR, Rosenfield K, Garasic J. Treatment of renal artery in-stent restenosis with sirolimus-eluting stents. Vascular Medicine. 2010;15:3–7. doi: 10.1177/1358863X09106897. [DOI] [PubMed] [Google Scholar]
- 56.Hiramoto J, Hansen KJ, Pan XM, Edwards MS, Sawhney R, Rapp JH. Atheroemboli during renal artery angioplasty: an ex-vivo study. J Vasc surg. 2005;41:1026–1030. doi: 10.1016/j.jvs.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 57.Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty with stenting with and without cerebral protection devices: a systematic review of the literature. Stroke. 2003;34:813–819. doi: 10.1161/01.STR.0000058160.53040.5F. [DOI] [PubMed] [Google Scholar]
- 58.Stone GW, Rogers C, Hermiller J, Feldman R, Hall P, Haber R, et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation. 2003;108:548–553. doi: 10.1161/01.CIR.0000080894.51311.0A. [DOI] [PubMed] [Google Scholar]
- 59.Baim DS, Wahr D, George B, Leon MB, Greenberg J, Cutlip DE. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation. 2002;105:1285–1290. [PubMed] [Google Scholar]
- 60.Mauri L, Rogers C, Baim DS. Devices for distal protection during percutaneous coronary revascularization. Circulation. 2006;113:2651–2656. doi: 10.1161/CIRCULATIONAHA.105.551770. [DOI] [PubMed] [Google Scholar]
- 61.Henry M, Henry I, Klonaris C, et al. Clinical Review: The role of embolic-protection devices in renal angioplasty and stenting. Vascular Disease Management. 2007;4:99–111. [Google Scholar]
- 62.Misra S, Gomes MT, Mathew V, Barsness GW, Textor SC, Bjarnason H, McKusick MA. Embolic protection devices in patients with renal artery stenosis with chronic renal insufficiency: a clinical study. J Vasc Interv Radiol. 2008;19:1639–1645. doi: 10.1016/j.jvir.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Thatipelli MR, Misra S, Sanikommu SR, Schainfeld RM, Sharma SK, Soukas PA. Embolic protection device use in renal artery stent placement. J Vasc Interv Radiol. 2009;20:580–586. doi: 10.1016/j.jvir.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: early experience. J Vasc Surgery. 2003;38:962–968. doi: 10.1016/s0741-5214(03)00606-2. [DOI] [PubMed] [Google Scholar]
- 65.Holden A, Hill A, Jaff MR, Pilmore H. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney International. 2007;70:948–955. doi: 10.1038/sj.ki.5001671. [DOI] [PubMed] [Google Scholar]
- 66.Hagspiel KD, Stone JR, Leung DA. Renal angioplasty and stent placement with distal protection: preliminary experience with the FilterWire EX. J Vasc Intervent Radiol. 2005;16:125–131. doi: 10.1097/01.RVI.0000147070.43605.76. [DOI] [PubMed] [Google Scholar]
- 67.Henry M, Henry I, Klonaris C, Polydorou A, Rath P, Kakshmi G, Rajacopal S, Hugel M. Renal angioplasty and stenting under protection: the way for the future? Catheterization and Cardiovascular Intervention. 2004;60:299–312. doi: 10.1002/ccd.10669. [DOI] [PubMed] [Google Scholar]
- 68.Henry M, Klonaris C, Henry I, Tzetanov K, Le Borgne E, Foliguet B, Hugel M. Protected renal stenting with the PercuSurge Guardwire device: a pilot study. Journal of Endovascular Therapy. 2001;8:227–237. doi: 10.1177/152660280100800301. [DOI] [PubMed] [Google Scholar]
- 69.Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Vimnani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D’Agostin RD, Pencina MJ, Khuder S. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752–2760. doi: 10.1161/CIRCULATIONAHA.107.730259. [DOI] [PubMed] [Google Scholar]
- 70.Scolari F, Tardanico R, Zani R, Pola A, Viola BF, Movilli E, Maiorca R. Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089–1109. doi: 10.1053/ajkd.2000.19809. [DOI] [PubMed] [Google Scholar]
- 71.Ivanovic V, McKusick MA, Johnson CM, Sabater EA, Andrews JC, Breen JF, Bjarnason H, Misra S, Stanson AW. Renal artery stent placement: complications at a single tertiary care center. J Vasc Intervent Radiol. 2003;14:217–225. doi: 10.1097/01.rvi.0000058324.82956.2a. [DOI] [PubMed] [Google Scholar]
- 72.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL. N-Acetylcysteine and Contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 73.Tuttle KF, Chouinard RF, Webber JT, Dahlstrom LR, Short RA, Henneberry KJ, Dunham LA, Raabe RD. Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis. 1998;32:611–622. doi: 10.1016/s0272-6386(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 74.Kaylor WM, Novick AC, Ziegelbaum M, Vidt DG. Reversal of end stage renal failure with surgical revascularization in patients with atherosclerotic renal artery occlusion. J Urol. 1989;141:486–488. doi: 10.1016/s0022-5347(17)40867-6. [DOI] [PubMed] [Google Scholar]
- 75.Dwyer JP, Greco BA, Lewis JB. Evaluation of renal artery stenosis in dialysis patients. Seminars in Dialysis. 2009;22:519–523. doi: 10.1111/j.1525-139X.2009.00618.x. [DOI] [PubMed] [Google Scholar]
- 76.Korsakas S, Mohaupt MG, Dinkel HP, Mahler F, Do DD, Voegele J, Baumgartner I. Delay of dialysis in end-stage renal failure: prospective study on percutaneous renal artery interventions. Kidney Int. 2004;65:251–258. doi: 10.1111/j.1523-1755.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 77.Thatipelli MR, Misra S, Johnson CM, Andrews JC, Stanson AW, Bjarnason H, et al. Renal artery stent placement for restoration of renal function in hemodialysis recipients with renal artery stenosis. J Vasc Interv Radiol. 2008;19:1563–1568. doi: 10.1016/j.jvir.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Kashyap VS, Sepulveda RN, Bena JF, Nally JV, Poggio ED, Greenberg RK, Yadav JS, Ouriel K. The management of renal artery atherosclerosis for renal salvage: does stenting help? J Vasc surg. 2007;45:101–108. doi: 10.1016/j.jvs.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 79.Bommart S, Cliche A, Therasse E, Giroux MF, Vidal V, Oliva VL, Soulez G. Renal artery revascularization: predictive value of kidney length and volume weighted by resistive index. Am J Roentgenol. 2010;194:1365–1372. doi: 10.2214/AJR.09.3558. [DOI] [PubMed] [Google Scholar]
- 80.Burket MW, Cooper CJ, Kennedy DJ, Brewster PS, Ansel GM, Moore JA, Venkatesan J, Henrich WL. Renal artery angioplasty and stent palcement: predictors of a favorable outcome. Am Heart J. 2000;139:64–71. doi: 10.1016/s0002-8703(00)90310-7. [DOI] [PubMed] [Google Scholar]
- 81.Dorros G, Jaff M, Mathiak L, Dorros I, Lowe A, Murphy K, He T. Four-Year follow-up of Palmaz-Schatz Stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98:642–647. doi: 10.1161/01.cir.98.7.642. [DOI] [PubMed] [Google Scholar]
- 82.Rundback JH, Sacks D, Kent KC, Cooper C, Jones D, Murphy T, Rosenfield K, White CJ, Bettman MA, Cortell S, Puschett JB, Clair DB, Cole P. Guidelines for reporting of renal artery revascularization in clinical trials. Circulation. 2002;106:1572–1585. doi: 10.1161/01.cir.0000029805.87199.45. [DOI] [PubMed] [Google Scholar]
- 83.Chatziioannou A, Mourikis D, Agroyannis B, Katsenis K, Pneumaticos S, Antoniou A, Dimakakos P, Vlachos L. Renal artery stenting for renal insufficiency in solitary kidney in 26 patients. Eur J Vasc& Endovasc Surg. 2002;23:49–54. doi: 10.1053/ejvs.2001.1535. [DOI] [PubMed] [Google Scholar]
- 84.Cioni R, Vignali C, Petruzzi P, Neri E, Caramella D, Vagli P, Bargellini I, Napoli V, Pinto S, Bartolozzi C. Renal artery stenting in patients with a solitary functioning kidney. Cardiovasc Intervent Radiol. 2001;24:372–377. doi: 10.1007/s00270-001-0045-3. [DOI] [PubMed] [Google Scholar]
- 85.Eklof H, Bergqvist D, Hagg A, Nyman R. Outcome after endovascular revascularization of atherosclerotic renal artery stenosis. Acta Radiologica. 2009;50:256–264. doi: 10.1080/02841850802668563. [DOI] [PubMed] [Google Scholar]
- 86.Gill KS, Fowler RC. Atherosclerotic renal arterial stenosis: clinical outcomes of stent placement for hypertension and renal failure. Radiology. 2003;226:821–826. doi: 10.1148/radiol.2263011244. [DOI] [PubMed] [Google Scholar]
- 87.Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. Q J Med. 1999;92:159–167. doi: 10.1093/qjmed/92.3.159. [DOI] [PubMed] [Google Scholar]
- 88.Muray S, Martin M, Amoedo ML, Garcia C, Jomet AR, Vera M, Oliveras A, Gomez X, Craver L, Real MI, Garcia L, Botey A, Montanya X, Fernandez E. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis. 2002;39:60–66. doi: 10.1053/ajkd.2002.29881. [DOI] [PubMed] [Google Scholar]
- 89.Cognet F, Garcier JM, Dranssart M, Defraissinette B, Cercueil JP, Ravel A, Boyer L. Percutaneous transluminal angioplasty in atheroma with renal failure: long-term outcomes in 99 patients. Europ Radiol. 2001;11:2524–2530. doi: 10.1007/s003300100862. [DOI] [PubMed] [Google Scholar]
- 90.Sahin S, Cimit C, Andac N, Baltaciolu F, Tulular S, Akolu E. Renal artery stenting in solitary functioning kidneys: technical and clinical results. European Journal of Radiology. 2006;57:131–137. doi: 10.1016/j.ejrad.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Singer GM, Remetz MS, Curtis JP, Setaro JF. Impact of baseline renal function on outcomes of renal artery stenting in hypertensive patients. J Clin Hypertens. 2009;11:615–620. doi: 10.1111/j.1751-7176.2009.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009;27:1333–1340. doi: 10.1097/HJH.0b013e328329bbf4. [DOI] [PubMed] [Google Scholar]
- 93.Calhoun DA, Jones D, Textor SC, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant Hypertension: Diagnosis, Evaluation and Treatment: A Scientific Statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 94.Kane GC, Xu N, Mistrik E, Roubicek T, Stanson AW, Garovic VD. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:813–820. doi: 10.1093/ndt/gfp393. [DOI] [PubMed] [Google Scholar]
- 95.Messerli FH, Bangalore S, Makani H, Rimoldi SF, Alleman Y, White CJ, Textor SC, Sleight P. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering Syndrome. Eur Heart J. 2011;32:2231–2237. doi: 10.1093/eurheartj/ehr056. [DOI] [PubMed] [Google Scholar]
- 96.Kalra PA, Chrysochou C, Green D, Cheung CM, Khavandi K, Sixt S, Rastan A, Zeller T. The benefit of renal artery stenting in patients with atheromatous renovascular disease and advanced chronic kidney disease. Catheterization & Cardiovascular Interventions. 2010;75:1–10. doi: 10.1002/ccd.22290. [DOI] [PubMed] [Google Scholar]
- 97.Dziemaianko I, Kuzniar J, Dorobisz A, Zynek-Litwin M, Garcarek J, Klinger M. Critical bilateral renal arterial stenosis presenting as cardio-renal syndrome: isolated ultrafiltration preceding percutaneous transluminal revascularization. Congestive Heart Failure. 2009;15:96–98. doi: 10.1111/j.1751-7133.2009.00052.x. [DOI] [PubMed] [Google Scholar]
- 98.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, White CJ, White J, White RA, Antman EM, Smith SC, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/ AHA Task force on Practice Guidelines. J Vasc Interv Radiol. 2006;17:1383–1397. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 99.Levin a, Linas SL, Luft FC, Chapman AB, Textor SC. Controversies in Renal Artery Stenosis: A review by the American Society of Nephrology Advisory Group on Hypertension. Am J Nephrol. 2007;27:212–220. doi: 10.1159/000101000. [DOI] [PubMed] [Google Scholar]
- 100.Bergqvist D, Bjock M, Lundgren F, Troeng T. Invasive treatment for renovascular disease: a twenty year experience from a population based registry. J Cardiovasc Surg. 2008;49:559–563. [PubMed] [Google Scholar]
- 101.Textor SC. Atherosclerotic renal artery stenosis: overtreated, but underrated? J Am Soc Nephrol. 2008;19:656–659. doi: 10.1681/ASN.2007111204. [DOI] [PubMed] [Google Scholar]
- 102.Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery inteventions by Medicare beneficiaries 1996–2000. Am J Roentgenol. 2004;183:561–568. doi: 10.2214/ajr.183.3.1830561. [DOI] [PubMed] [Google Scholar]
- 103.Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, Chew P, Gilbert SJ, Lau J. Effectiveness of management strategies for renal artery stenosis: a systematic review. Annals of Internal Medicine. 2006;145:901–912. doi: 10.7326/0003-4819-145-12-200612190-00143. [DOI] [PubMed] [Google Scholar]
- 104.Textor SC, McKusick MA, Misra S, Glockner J. Timing and selection for renal revascularization in an era of negative trials: what to do? Prog. Cardiovasc Dis. 2009;52:220–228. doi: 10.1016/j.pcad.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumbhani DJ, Bavry AA, Harvey JE, de Souza R, Scarpioni R, Bhatt DL, Kapadia SR. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am Heart J. 2011;161:622–630. doi: 10.1016/j.ahj.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 106.White CJ. Kiss my ASTRAL: one seriously flawed study of renal stenting after another. Catheterization & Cardiovascular Interventions. 2010;75:305–307. doi: 10.1002/ccd.22416. [DOI] [PubMed] [Google Scholar]
- 107.Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Blebea J, Littooy FN, Freischlag JA, Bandyk D, Rapp JH, Salam AA. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287:2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 108.Tallarita T, Sobreira ML, Oderich GS. Results of open repair of pararenal aortic aneurysm: a tabular review of the literature. Ann Vasc Surg. 2010;25:143–149. doi: 10.1016/j.avsg.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 109.EVAR Trial Participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysms (EVAR Trial 1): a randomized controlled trial. Lancet. 2005;365:2179–2186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 110.Blankensteijn JD, de Jong SECA, Prinssen M, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2011;352:2398–2405. doi: 10.1056/NEJMoa051255. [DOI] [PubMed] [Google Scholar]
- 111.Steyerberg EW, Kievit J, de Mol Van Otterloo JC, et al. Perioperative mortality of elective abdominal aortic aneurysm surgery: a clinical prediction rule based on literature and individual patient data. Arch Int Med. 2004;155:1998–2004. [PubMed] [Google Scholar]
- 112.Biancari F, Hobo R, Juvonen T. Glasgow aneurysm scocre predicts survival after endovascular stenting of abdominal aortic aneurysms in patients from the EUROSTAR registry. Br J Surg. 2006;93:191–194. doi: 10.1002/bjs.5262. [DOI] [PubMed] [Google Scholar]
- 113.Brown LC, Brown EA, Greenhalgh RM, Powell JT, Thompson SG, et al. Renal function and abdominal aortic aneurysm The impact of different management strategies on long-term renal function in the UK endovascular aneurysm repair trials. Ann Surgery. 2010;251:966–975. doi: 10.1097/SLA.0b013e3181d9767c. [DOI] [PubMed] [Google Scholar]
- 114.Haddad F, Greenberg RK, Walker E, Nally J, O’Neil S, Kolin G, et al. Fenestrated endovascular grafting: the renal side of the story. J Vasc Surgery. 2005;41:181–190. doi: 10.1016/j.jvs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 115.Mohabbat W, Greenberg RK, Mastracci TM, Cury M, Morales JP, Hernandez AV. Revised duplex criteria and outcomes for renal stents and stent grafts following endovascular repair of juxtarenal and thoracoabdominal aneurysms. J Vasc surg. 2009;49:827–837. doi: 10.1016/j.jvs.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 116.Verhoeven ELG, Vourliotakis G, Bos W, Tielliu IF, Zeebregts CJ, Prins TR. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: an 8-year single-center experience. Eur J Endovasc Surg. 2010;39:529–536. doi: 10.1016/j.ejvs.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Greenberg RK, Chuter TA, Lawrence-Brown M, Haulon S, Nolte L. Analysis of renal function after aneurysm repair with device using suprarenal fixation (Zenith AAA endovascular graft) in contrast to open repair. J Vasc surg. 2004;39:1219–1228. doi: 10.1016/j.jvs.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 118.Hiramoto JS, Chang CK, Reilly LM, Schneider DB, Rapp JH, Chuter TAM. Outcome of renal stenting for renal artery coverage during endovascular aortic aneurysm repair. J Vasc surg. 2009;49:1100–1106. doi: 10.1016/j.jvs.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Protrack CD, Saad WA, Davies MG. Renal artery interventions during infrarenal endovascular aortic repair: a greater potential of subsequent failure? J Vasc Interv Radiol. 2010;21:459–464. doi: 10.1016/j.jvir.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 120.Sun Z, O’Donnell ME, Winder J, Ellis PK, Blair PH. Effect of suprarenal fixation of aortic stent grafts on the renal ostia. J Endovasc Ther. 2007;14:650–660. doi: 10.1177/152660280701400508. [DOI] [PubMed] [Google Scholar]
- 121.Walsh SR, Boyle JR, Lynch AG. Suprarenal endografts fixation and medium-term renal function: a systematic review and meta-analysis. J Vasc surg. 2008;47:1364–1370. doi: 10.1016/j.jvs.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 122.O’Donnell ME, Sun Z, Winder RJ, Ellis PK, Lau LL, Blair PH. Supraenal fixation of endovascular aortic stent grafts: assessment of medium-term to long-term renal function by analysis of juxtarenal stent morphology. J Vasc surg. 2007;45:694–700. doi: 10.1016/j.jvs.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 123.Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair--a systematic review. J Endovasc Surg. 2009;38:35–41. doi: 10.1016/j.ejvs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Verhoeven EL, Zeebregts CJ, Kapman MR, Tielliu IF, Prins TR, van den Dungen JJ. Fenestrated and branched endovascular techniques for thoracoabdominal aneurysm repair. J Cardiovasc Surg. 2005;46:131–140. [PubMed] [Google Scholar]
- 125.Amiot S, Haulon S, Becquemen JP, Magnan PE, Lermusiaux P, Goueffic Y, et al. Fenestrated endovascular grafting: the French multicenter experience. J Endovasc Surg. 2010;39:537–544. doi: 10.1016/j.ejvs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 126.Troisi N, Donas KP, Austermann M, Tessarek J, Umscheid T, Torsello G. Secondary procedures after aortic aneurysm repair with fenestrated and branched endografts. J Endovasc Ther. 2011;18:146–153. doi: 10.1583/10-3274.1. [DOI] [PubMed] [Google Scholar]
- 127.Greenberg RK, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 2010;140:S171–S178. doi: 10.1016/j.jtcvs.2010.07.061. [DOI] [PubMed] [Google Scholar]
- 128.Manning BJ, Agu O, Richards T, Ivancev K, Harris PL. Early outcome following endovascular repair of pararenal aortic aneurysms-triple versus double or single-fenestrated stent grafts. J Endovasc Ther. 2011;18:98–105. doi: 10.1583/10-3122.1. [DOI] [PubMed] [Google Scholar]
- 129.Iqbal S, Sharma A, Wicky ST. Arterial Interventions for renovascular hypertension. Seminars in Interventional Radiology. 2009;26:245–252. doi: 10.1055/s-0029-1225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Main J. The problem with ASTRAL. J Renovascular Dis. 2002;1:19–23. [Google Scholar]
- 131.Bax L, Woittiez AJ, Kouwenberg HJ, Mali PTM, Buskens E, Beek FJA, Braam B, Huysmans FTM, Kool LJS, Rutten MJCM, Doorenbos CJ, Aarts JCNM, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PMT, van de Ven PJG, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent Placement in Patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Int Med. 2009;150:840–848. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 132.Madder RD, Hickman L, Crimmins GM, Puri M, Marinescu V, McCullough PA, Safian RD. Validity of estimated glomerular filtration rates for assessment of baseline and serial renal function in patients with atherosclerotic renal artery stenosis: implications for clinical trials of renal revascularization. Circulation: Cardiovasc Interv. 2011;4:219–225. doi: 10.1161/CIRCINTERVENTIONS.110.960971. [DOI] [PubMed] [Google Scholar]
- 133.Claus T, Schmitt R, Stabroth C, Luft FC. Where do we stand with renovascular hypertension? Nephrol Dial Transplant. 2005;20:1495–1498. doi: 10.1093/ndt/gfh722. [DOI] [PubMed] [Google Scholar]
- 134.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, Romero JC. Regional decrease in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R67–R71. doi: 10.1152/ajpregu.90677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65:944–950. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 137.Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295:F1259–F1270. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]
- 138.The ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 139.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 140.Lerman LO, Chade AR. Angiogenesis in the kidney: a new therapeutic target? Curr Opin Nephrol Hyper. 2009;18:160–165. doi: 10.1097/MNH.0b013e32831ec1db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thornton MA, Zager RA. Brief intermittent reperfusion during renal ischemia: effects on adenine nucleotides, oxidant stress, and the severity of renal failure. J Lab Clin Med. 1990;115:564–999. [PubMed] [Google Scholar]
- 143.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30:1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multi-centre safety and proof-of-principle cohort sudy. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 146.DiBona GF, Esler M. Translational Medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 147.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinski P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszedwicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 148.Egan BM. Renal Sympathetic Denervation: a novel intervention for resistant hypertension, insulin resistance and sleep apnea. Hypertension. 2011;58:542–543. doi: 10.1161/HYPERTENSIONAHA.111.179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17:1057–1062. doi: 10.1161/01.hyp.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 150.Kent D, Hayward R. When averages hide individual differences in clinical trials. American Scientist. 2007;95:60–68. [Google Scholar]
- 151.Textor SC, Wilcox CS. Renal Artery Stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421–442. doi: 10.1146/annurev.med.52.1.421. [DOI] [PubMed] [Google Scholar]
- 152.Rocha-Singh KJ, Eisenhauer AC, Textor SC, Cooper CJ, Tan WA, Matsumoto AH, Rosenfield K. Atherosclerotic peripheral vascular disease symposium II: Intervention for renal artery disease. Circulation. 2008;118:2873–2878. doi: 10.1161/CIRCULATIONAHA.108.191178. [DOI] [PubMed] [Google Scholar]