Abstract
Since the original characterization of the ventral visual pathway our knowledge of its neuroanatomy, functional properties, and extrinsic targets has grown considerably. Here we synthesize this recent evidence and propose that the ventral pathway is best understood as a recurrent occipitotemporal network containing neural representations of object quality both utilized and constrained by at least six distinct cortical and subcortical systems. Each system serves its own specialized behavioral, cognitive, or affective function, collectively providing the raison d’etre for the ventral visual pathway. This expanded framework contrasts with the depiction of the ventral visual pathway as a largely serial staged hierarchy that culminates in singular object representations for utilization mainly by ventrolateral prefrontal cortex and, more parsimoniously than this account, incorporates attentional, contextual, and feedback effects.
History and Overview
Cortical visual processing is commonly thought to proceed along two distinct pathways, a dorsal pathway projecting into parietal cortex, and a ventral pathway, projecting into temporal cortex. The dorsal and ventral visual pathways were identified in monkey as anatomically and functionally distinct systems of multisynaptic connections emerging from the striate cortex[1] (Fig. 1A). The dorsal pathway was described as coursing through the occipitoparietal cortex to the posterior part of the inferior parietal lobule (area PG)[1, 2], with a likely further extension to the dorsolateral prefrontal cortex (DLPFC/area FD^)(Fig. 1A)[3]. The ventral pathway was described as coursing through the occipitotemporal cortex to the anterior part of the inferior temporal gyrus (area TE)[1, 2], with a likely extension into the ventrolateral prefrontal cortex (VLPFC/area FDv)[3]. In monkey, lesions in the dorsal and ventral pathways yielded dissociable deficits in spatial and object vision, leading to their characterization as ‘Where’ and ‘What’ pathways, respectively[1–3]. While the general functional characterization of the dorsal stream as a ‘Where’ pathway has been extensively debated (e.g. [4, 5]), the characterization of the ventral stream as a ‘What’ pathway supporting the processing of object quality or identity (Text Box 1) has remained largely unchallenged (but see[6]). The aim of this review will not be to fundamentally challenge this characterization but rather to integrate the now better explored neuroanatomical and functional properties of the ventral pathway into an expanded and more fully specified framework.
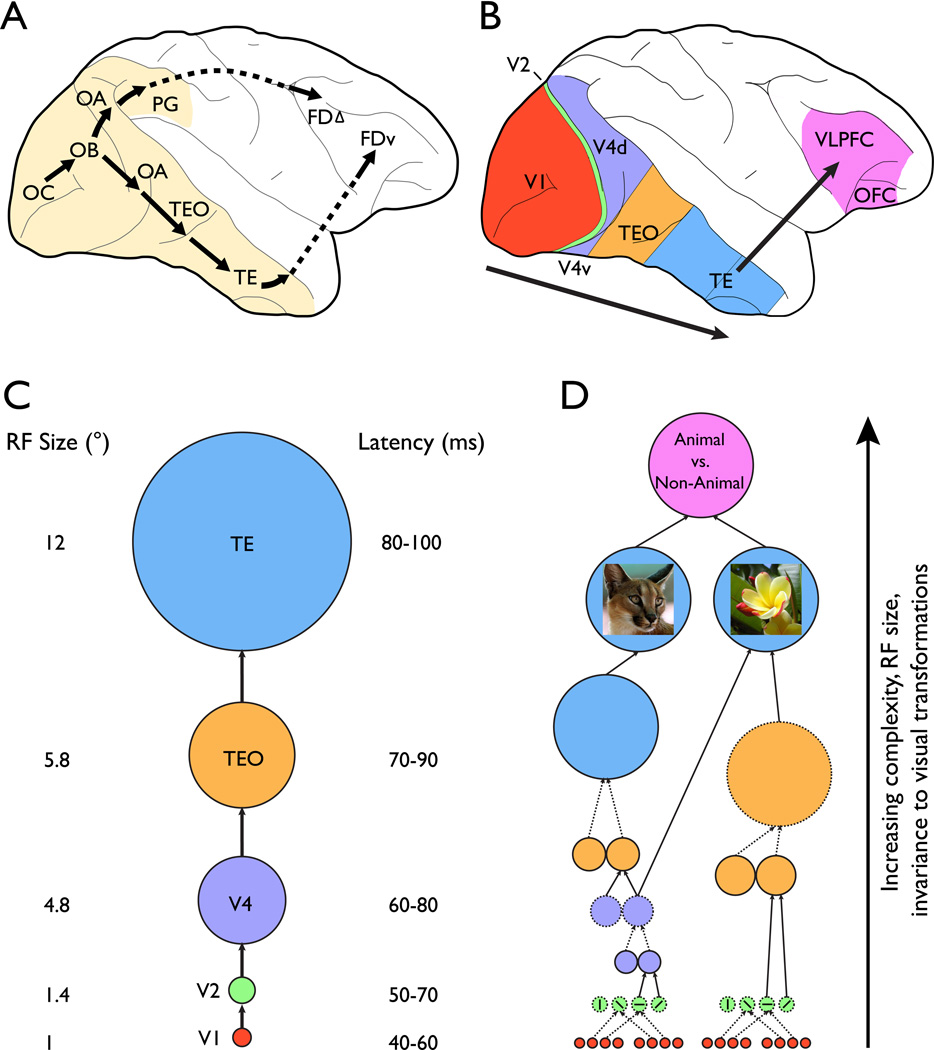
Figure 1. Frameworks of object quality processing.
A. Original formulation of the dorsal and ventral pathways in macaque monkey. The ventral pathway was described as a multisynaptic pathway projecting from striate cortex (cytoarchetectonic area OC) to area TE in the inferior temporal cortex, with a further projection from TE to ventral prefrontal region FDv. The dorsal pathway was described as a multisynaptic pathway projecting from striate cortex to area PG in the inferior parietal lobule, with a further projection from PG to dorsolateral prefrontal region FDΔ. On the basis of behavioral effects of lesions in the monkey, the ventral pathway was characterized as supporting object vision (‘what’), whereas the dorsal pathway was characterized as supporting spatial vision (‘where’). (Based on [1]; [2]; [3])
B. Schematic of the current understanding of the components of the ventral pathway that lie along the lateral surface of the macaque brain and their projection to the ventrolateral prefrontal cortex (VLPFC) and orbitofrontal cortex (OFC). Note the similarity between the original characterization and the current view. (Adapted from [12]).
C. Schematic of the commonly assumed model of serial information flow from V1 through aIT (central route). The size of each circle reflects the average receptive field (RF) size of neurons in that region from recent recordings (V1[9], V2/V4[41], TEO[312], TE[60, 61, 63]: Note that as few functional studies differentiate between TEpd (posterodorsal) and TEad (anterodorsal), the RF size for area TE is an approximation based on the three cited studies). The numbers to the right of each region give the approximate range of latencies of first response[9].Color scheme as in panel B. (Adapted from [9] and [12]).
D. Schematic of a recent version of the HMAX model[7] of object recognition. The model consists of a number of units each of which either sums (solid lines) or takes the max (dotted lines) over its inputs. The earliest units (red/green) loosely correspond to V1 and V2, have very small RFs, and are selective for simple features (e.g. oriented lines). The next layers (purple/V4) aggregate the responses of these simple feature detectors to support units with larger RFs and selectivity for more complex stimuli. This process is applied at each subsequent layer of the model through pools of units analogous to TEO (orange) and TE (cyan) until units are reached with large RFs and selectivity for whole objects. These units then project to decision units thought to be analogous to neurons in VLPFC and OFC. According to the model, the complexity of the representations, RF size, and, critically, invariance to visual transformations increase from the early to the late units through the iterative sum and max operations applied by each unit to their inputs. Note that in the model there are some bypass connections (units on the right) that constitute an “indirect” route for information transfer (see also Figure 3), which do not appear in the central route depicted in panel C. (Adapted from [7]).
Text Box 1: The general functions of the ventral and dorsal pathways.
One of the key principles that we highlight in both this review and our previous review of the dorsal pathway[5] is that the functional properties of a region are intimately related to its connectivity. This principle makes it very difficult to describe the general function of an entire pathway given its complex and diverse connectivity. Nonetheless, here we will try to provide a high-level intuition about the general functions of the ventral and dorsal pathways.
The dorsal pathway is an occipitoparietal network that lies between early visual cortex and specialized cortical structures involved in visually-guided action, somatosensation, spatial audition, navigation, and spatial working memory. The type of visual information required by these processes is very general. For example an observer’s hand and the target of a reaching movement can appear in any retinotopic positions and the occipitoparietal network must still create an accurate map of their relative positions to effectively guide the action. The need to represent these sorts of relationships naturally leads to the formation of coordinate systems and general reference frames. Thus, the dorsal pathway specializes in capturing arbitrary and dynamic spatiotemporal relationships between multiple items. Over time, however, the occipitoparietal cortex also contributes to the long-term representation of non-arbitrary spatiotemporal relationships as demonstrated by findings of apraxias[4] and tool-selective regions[283] localized within the posterior parietal cortex.. This framework has the advantage of parsimoniously incorporating a number of non-visual functions associated with the dorsal pathway (e.g. number[284], sequences[285], melody[286], prosody[287]).
In contrast to the dorsal pathway, the ventral pathway does not need to capture these sorts of arbitrary relationships to support the functions of its extensions, nor does it have a strong connection with motor output. Rather it is an occipitotemporal network that bridges the early visual cortex and specialized cortical and subcortical structures involved in various forms of memory and learning, specifically habit formation, emotion, long- and short-term memory, reward, and value. These structures are involved in forming specific representations or associations involving stable aspects of visual information, rather than capturing arbitrary dynamic relationships amongst multiple items. The term ‘processing of object quality’ (see also[1, 2]) is an inclusive term meant to capture the wide variety of stable visual information that can be processed as the basis of these associations. Specifically, it refers to the processing of the features or perceptual dimensions (e.g. shape, color, size, brightness) that are available in the information passed to striate cortex from the retina. These dimensions might be readily available in the input (e.g. retinotopic position, brightness) or they may be a conjunction of basic dimensions (e.g. shape, faceness). Any stimulus can be represented as a coordinate or configuration along all of the dimensions that the occipitotemporal network represents. The key aspect of ventral pathway representations is not that they are tied to particular physical objects, but that they capture a stable configuration of visual information (e.g. texture, scenes). Thus, the standard dichotomy of spatial and object vision being supported by the dorsal and ventral pathway, respectively, is too restrictive. Any number of spatial dimensions (e.g. retinotopic position, stimulus motion, etc), can contribute to ventral pathway representations and some aspects of object shape must be captured in the dorsal pathway to effectively guide action.
In particular, we argue that the ventral visual pathway is a recurrent and highly interactive occipitotemporal network linking early visual areas and the anterior inferior temporal cortex along multiple routes through which visual information is processed. Extrinsically, projections to at least six major cortical and subcortical regions arise from different parts of the pathway; supporting many different forms of object quality processing (Text Box 1). This framework synthesizes the neuroanatomical and functional findings in monkey and human and can more parsimoniously incorporate a number of effects clearly dependent on recurrent processing such as attention, masking, and context. In explicitly associating neuroanatomy and function, this framework also provides traction on a number of difficult theoretical issues, including why different areas in the network show such diverse and clustered categorical selectivity, why there is consistency across individuals in the location of these clusters, and how regions from different putative levels of the hierarchy communicate to enable recurrent processing.
For the purpose of stepping through this complex and detailed framework it is useful to contrast it with the common conception of the ventral pathway as a serial staged hierarchy (Fig. 1B). According to this view, visual information from the striate cortex (V1) passes through a sequence of processing stages in extrastriate cortex until complex object representations are formed in the anterior portion of the inferior temporal cortex (IT), area TE (Fig. 1B, C). It was proposed that each stage of this hierarchy created successively more complex representations by aggregating the output of the simpler feature detectors in the previous stage (Fig. 1D). This idea was based partly on the apparently hierarchical response properties of cells in V1 and V2 which gave rise to formal computational models of processing in the ventral pathway[7, 8]. The hierarchical view also drew support from the gradually increasing receptive field (RF) size, onset latencies, and complexity of stimulus selectivity of neurons (e.g[9–11]) as one proceeds rostrally along the ventral pathway (Fig. 1C). This view is rarely explicitly stated in its totality (but see[12, 13]) although components of it were present in the original characterization of the pathway[1] and continue to underlie a number of theories in the field.
We will highlight three aspects of our modified neuroanatomical and functional framework that distinguish it from the original conception. First, anatomical evidence indicates that the ventral pathway is actually a complex network of feedforward and feedback projections, some of which are unidirectional (i.e. non-reciprocal) feedback connections and others of which bypass intermediate areas, allowing for direct communication between putative early and late stages of the hierarchy (Fig. 2A). Second, there appears to be a strong link between retinotopic position and the intrinsic neuroanatomy, such that even high-level object representations are constrained by retinotopic position. Finally, various regions within the occipitotemporal network project differentially to at least six distinct cortical and subcortical structures. First, a unidirectional occipitotemporo-neostriatal pathway originates from nearly every area in the occipitotemporal network and projects to the neostriatum (Fig. 2B), supporting the formation of stimulus-response associations. Second, different areas within IT give rise to the occipitotemporo-amygdaloid pathway, supporting the processing of emotionally salient stimuli (Fig. 2B). A third cortico-subcortical projection is the unidirectional occipitotemporo-ventral striatum pathway, which also originates in aIT and supports the assignment of stimulus valence. Finally, there are three major cortico-cortical projections, all of which are reciprocal with their sources in aIT, including but not limited to area TE. These three projections are the occipitotemporo-medial temporal, occipitotemporo-orbitofrontal, and occipitotemporo-ventrolateral prefrontal pathways (Fig. 2B), which support, briefly, long-term memory, object-reward association, and object working memory, respectively.
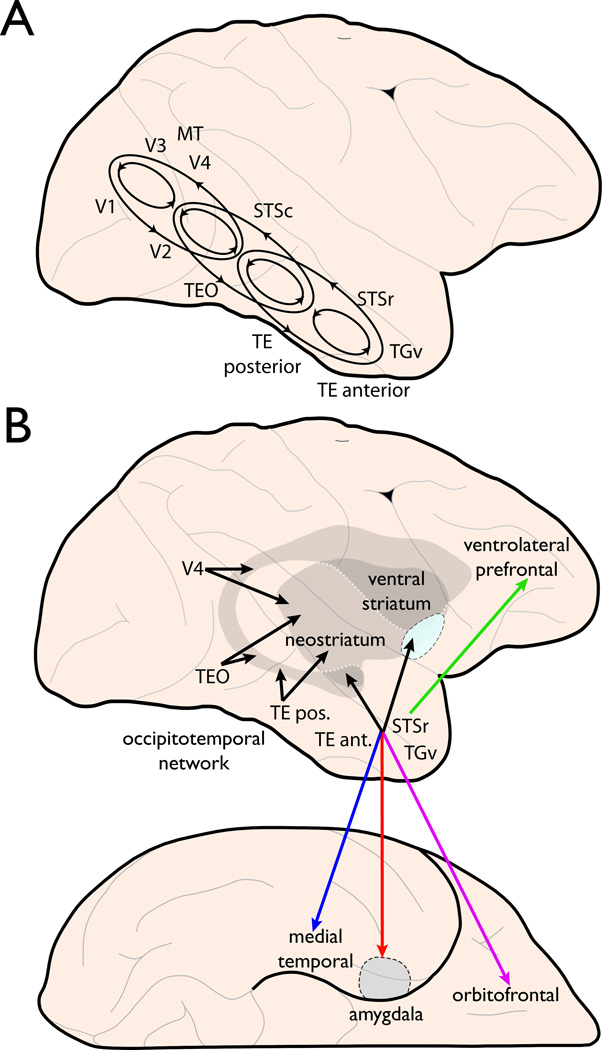
Figure 2. Schematic of proposed framework.
A. A schematic of the intrinsic connectivity of the ventral pathway on the lateral surface of the macaque brain. Note the inclusion of V3, the MT/MST complex (labeled MT), and the superior temporal sulcus (STS), which are typically not included in reference to the ventral pathway. Rather than a simple sequence of projections leading to aIT, the pathway is actually composed of a series of overlapping recurrent networks of various scales. At the most local level there are roughly four subnetworks (small black ellipses), each with strong bidirectional connections among its components. These include, (1) all of the early visual areas (V1, V2, V3, V4) and MT share strong bidirectional connections; (2) V2, V4, MT, caudal STS (STSc), and TEO; (3) TEO, STSc, rostral STS (STSr), and TEposterior; and finally (4) STSr, TEposterior, TEanterior, and TGv. Beyond their intrinsic components, these subnetworks are connected to each other via more extended, bidirectional and non-reciprocal feedback connections that bypass intermediate regions (large black ellipses).
B. A summary of the extrinsic connectivity of the ventral pathway. At least six distinct pathways emanate from the occipitotemporal network. The occipitotemporo-neostriatal pathway (black lines) orginates from every region in the network and supports visually-dependent habit formation and skill learning. All the other projections originate in the network’s rostral portion, which encompasses areas TEanterior, STSr, TGv, and the ventral portion of TEposterior (not shown) though not all these contribute equally to every pathway. One such projection targets the ventral striatum (or nucleus accumbens) and supports the assignment of stimulus valence. Another forms the occipitotemporo-amygdaloid pathway (red line) and supports the processing of emotional stimuli. The occipitotemporo-medial temporal pathway (blue line) targets the perirhinal and entorhinal cortices as well as the hippocampus and supports long-term object and object-context memory. Finally, the occipitotemporo-orbitofrontal pathway (purple line) and the the occipitotemporo-ventrolateral prefrontal pathway (green line; see also Fig. 1A,B) mediate reward processing and object working memory, respectively.
We begin with a review of anatomical and functional findings from within the occipitotemporal network, laying out the intrinsic reciprocal and nonreciprocal connections and their relationship to function. We then review the anatomical and functional organization of the six major output pathways (Fig. 2B), highlighting implications for our understanding of the occipitotemporal network. Throughout the review we will also highlight critical findings that are accounted for by viewing the ventral visual pathway as a recurrent occipitotemporal network.
Occipitotemporal Network: Intrinsic Connectivity
The intrinsic connectivity of this network in monkey consists of a set of bidirectional projections along a caudal-rostral axis from early visual cortical areas through the preoccipital gyrus, the inferior temporal gyrus (areas TEO and subdivisions of TE), and, most rostrally, to anterior TE (TEa), the ventral temporal pole (area TGv), and the most anterior parts of the ventral bank and fundus of the superior temporal sulcus (STSv/f) (collectively, the anterior inferior temporal cortex; aIT; Fig 2B). The ventral visual pathway was originally viewed as a series of sequential projections (the single “central route” in Fig. 1C) between V1, V2, V4, TEO, and TE (also TGv; Fig. 3A)[7, 12, 14–26]. The connections between the earliest visual areas (V1 and V2) established the rules for the lamina of origin and termination of feedforward and feedback connections[15, 18, 23, 27], namely that feedforward projections originate in layer 3 and terminate in layer 4, whereas feedback projections originate in layers 5/6 and terminate in layer 1. Also, the functional properties of single cells within V2 were well predicted by a simple aggregation of responses across several V1 cells, with a slight increase in both receptive field (RF) size and selectivity for more complex stimuli (angles) than the orientation selectivity observed in V1. These basic functional observations gave rise to the idea that the ventral pathway was a serial hierarchy, with each sequential stage having progressively more complex selectivity and invariance to simple visual transformations, like retinotopic position.
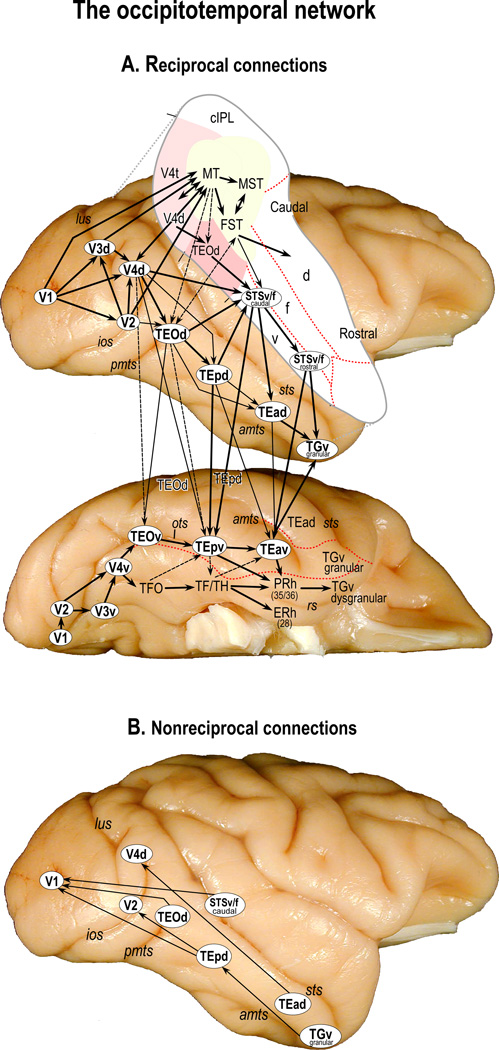
Figure 3. Intrinsic connectivity of the occipitotemporal network.
A. The connections which form the occipitotemporal network on the lateral and ventral views of the rhesus monkey brain. This network consists of a set of direct and indirect reciprocal projections (shown by single arrow-head; double arrow head: areas at the same hierarchical level) along a caudal to rostral axis from primary visual cortex (V1) through different subregions of preoccipital (V2/V3d/v), prelunate (V4d/v), posterior inferotemporal (TEOd/v) and posterior ventral bank and fundus of the superior temporal sulcus (STSv/f caudal), into the anterior inferotemporal lobe, including area TE (TEpd, TEpv, TEad, and TEav) and temporal pole (TGv), as well as rostral STSv/f. Also shown is a connection between V4v through the medial temporal lobe (parahippocampal (TF/TH/TFO), perirhinal (PRh), and entorhinal (ERh) cortices). The STS is opened to show the borders (red dashed lines), and connections of different visuo-spatial cortical areas (different shades of pink and yellow, and white) in the ventral bank (v), fundus (f), and dorsal bank (d) of the STS (see also[35]). Subdivisions within the inferotemporal cortex are based on the anatomical studies of Saleem and colleagues[34, 140, 201]. Note that the strength of the projection between different cortical areas is not uniform: some areas receive strong projections (Thick lines), whereas others receive moderate to sparse projections (thin lines) or very sparse projections (dashed lines). Based on this complex network of connections, there are at least four somewhat parallel routes (with cross-connections between them) through the occipitotemporal lobe: 1) within the STS, 2) in the dorsal or lateral portion of the inferior temporal lobe, 3) ventral or medial part of the inferior temporal lobe, and finally 4) within the medial temporal lobe. Abbreviations: 28, entorhinal cortex (or ERh); 35/36, areas 35 and 36 of the perirhinal cortex (or PRh); amts, anterior middle temporal sulcus; cIPL, caudal intraparietal lobule; ERh, entorhinal cortex; FST, floor of superior temporal sulcus; ios, inferior occipital sulcus; lus, lunate sulcus; MST, medial superior temporal area; MT, middle temporal area; ots, occipitotemporal sulcus; pmts, posterior middle temporal sulcus; PRh, perirhinal cortex; rs, rhinal sulcus; sts, superior temporal sulcus; STSf, fundus of the superior temporal sulcus; STSv, lower (ventral) bank of the superior temporal sulcus; TEad, dorsal subregion of anterior TE; TEav, ventral subregion of anterior TE; TEOd, area TEO, dorsal part; TEOv, area TEO, ventral part; TEpd, dorsal subregion of posterior TE; TEpv, ventral subregion of posterior TE; TF/TH, areas TF and TH of the parahippocampal cortex; TFO, area TFO of the parahippocampal cortex; TGv, ventral temporal pole; V1, visual area 1 (primary visual cortex); V2, visual area 2; V3d, visual area 3, dorsal part; V3v, visual area 3, ventral part; V4d, visual area 4, dorsal part; V4t, V4 transitional area; V4v, visual area 4, ventral part.
B. Non-reciprocal connections on the lateral view of the rhesus monkey brain. Note that unlike feedforward projections, the dorsal subregion of TEO (TEOd), posterior TE (TEpd), and ventral bank and fundus of the caudal STS (STSv/f) send direct feedback projections to area V1 (see Fig. 3A).
However, there is now a wealth of anatomical evidence suggesting that the ventral visual pathway is actually a complex recurrent network. V1 projects directly not just to V2, but also to V3[24], V4[25, 28], and MT[29–32]. Thus, visual information from V1 can reach TEO in two steps via V2, V4, or MT[16, 19, 25], violating a strict serial hierarchy at even the earliest stages of visual processing. There are also direct projections between V4d and TEpd[25] and between TEOd and TEad[16, 20, 33] that bypass the intermediate regions (Fig. 3A). In addition, there are unidirectional, nonreciprocal projections from putative late stages in the pathway to early stages (Fig. 3B). Finally, while the laminar structure of projections has been used to determine levels in the hierarchy, projections beyond V1 to V2 do not strictly follow those rules, with feedforward connections terminating instead across a broad range of lamina in the target areas. For example, connections from TEO to TEpd/TEad and from TEad/TEav to STSv/f terminate throughout all 6 cortical layers[20, 34].
The neuroanatomy of the occipitotemporal network and the putative central route (Fig. 1C) is further complicated by the connectivity of STSv/f (Fig. 3A). V1 and V3d project directly to MT which in turn projects to MST, FST, V4d, and TEOd[16, 30, 35]. Areas V4d, TEOd, and TEpd also project to STSv/f[20, 25], which then projects to aIT[34] (areas TEad, TEav, and the ventral temporal pole area TGv) (Fig. 3A). Thus, the STS provides another major route through which visual information from “early” stages of the central route can be transmitted to the most rostral temporal areas or “final” visual processing stages without passing through the intermediate areas of the central route. These indirect channels may underlie the persistence of complex selectivity in aIT even after posterior regions have been extensively damaged (see section on Intrinsic Connectivity function section below).
The details of the intrinsic connectivity also highlight the importance of retinotopic position. First, V1, and indeed all of the subcortical inputs[36] to the occipitotemporal network as well, represent only the contralateral side of space, and there is evidence that a contralateral bias persists even into aIT (see Propagation of Retinotopic Biases section below). Second, there is a neuroanatomical distinction between the input from the central and peripheral visual field into IT, resulting in a strong foveal bias throughout TEO and TE[12]. Conversely, the portions of V4v that most strongly represent the periphery project strongly to TFO, which in turn provides input to TF/TH in the parahippocampal gyrus (Fig. 3A)[25, 37]. This medial pathway has only weak connections with the ventral portions of either TEO or TE, making it a somewhat distinct channel for object quality information biased toward peripheral portions of the visual field (see[5] for a further review of the connectivity of these regions). Finally, there is a bias in the input to dorsal and ventral IT for the lower and upper field, respectively, with relatively weak connections between these regions, particularly posterior to area TE (Fig. 3A). Specifically, the ventral portion of V4 (V4v), which represents the upper visual field, provides the majority of direct cortical visual input to both TFO (in the posterior parahippocampal gyrus) and to the ventral portion of posterior IT (TEOv)[25], which, in turn, provide strong input to ventral TE (TEpv/TEav)[34]. In contrast, the dorsal portion of V4 (V4d), which represents the lower visual field, provides the majority of direct cortical visual inputs to dorsal posterior IT (TEOd)[25]. Area TEOd then projects anteriorly through dorsal TE (TEpd/TEad) to TGv[16, 20, 33, 34] (Fig. 3A). These biases for the upper and lower visual field are reflected in the object representations of IT in human (see Retinotopy function section below).
Overall, the neuroanatomy thus argues against a strict serial hierarchy and appears to propagate strong retinotopic biases along at least three dimensions of the visual field (contralateral vs. ipsilateral, lower vs. upper, and central vs. peripheral). In combination with the unique input of the MT/MST complex into STSv/f, this suggests that rather than having just a single central route, the occipitotemporal network has at least four somewhat independent parallel routes, which, arranged dorsoventrally, pass through either STSv/f, TEOd, TEOv, or TFO (Fig. 3A).
Occipitotemporal Network: Intrinsic Function
Our review of the functional properties of the occipitotemporal pathway begins with functional findings related to the complex intrinsic connectivity and its relationship to and propagation of retinotopic biases. We then review two domains in which the proposed framework offers new traction: clustering of different types of selectivity and attentional/contextual effects. The purpose of the following sections is not to fully review the very large functional literature on the ventral visual pathway, but to provide a functional framework, comprising key results, theories, and debates, that complements the neuroanatomical framework specified in the previous section (see Text Box 2 for a discussion of the functions of the anterior inferior temporal cortex).
Text Box 2: Functions of the anterior temporal lobe and the difficulties of establishing cross-species homologies.
A major stumbling block to advancing our understanding of the ventral visual pathway is the paucity of knowledge about what visual functions, if any, are subserved by the anterior temporal lobe (aIT) in humans. One persistent problem is the lack of good fMRI signal from this region[288], though some fMRI studies and those using other methods (PET, lesions) have implicated the region in semantic processing[289]. Importantly, compared to the pronounced visual impairments that are produced by aIT lesions in monkey, the visual effects of damage to this region in humans are far less striking. This has led to the proposal that the human homolog of aIT in the monkey is posterior IT cortex, where visual information and deficits are more typically localized. However, this proposal is difficult to reconcile with the primacy of aIT as the source of many output projections from the occipitotemporal network in monkey (Fig. 3A), and the likely conservation of those projections in human.
PET studies have revealed responses in anterior temporal cortex (e.g.[290]) during object vision and a recent fMRI study found that the ventral portions of the temporal pole (area TGv; Fig 2) are activated more by visual than by auditory stimuli[291], with different subdivisions showing differential activations for pictures of animals and tools[292]. Further, recent studies have reported a face-selective cluster in aIT[293], evidence that other areas in aIT contain information about facial expression, gaze direction, identity, and arbitrary facts associated with faces[294, 295], and a face-specific deficit following damage restricted to aIT[90, 296]. Finally, intracranial recording studies have demonstrated categorization for faces and animals in the anterior temporal lobe[110]. These studies clearly demonstrate that the human anterior temporal lobe does process visual stimuli and does contain stored visual information.
Beyond the domain of vision, the anterior temporal lobe has a number of characteristics consistent with its extensive connectivity with the cognitive memory system in the medial temporal lobe (Fig 7) (see also occipitotemporo-medial temporal section in the main text). Beyond the deficits in object naming[297, 298] observed after left hemisphere damage, visual representations in the anterior temporal lobe are stronger for familiar than for unfamiliar stimuli[297–299], and the region is strongly activated during the learning of associations between arbitrary facts about individuals (e.g. profession) and particular people[294]. Yet, much more work is needed to identify precisely where functional homologies in the visual functions of the human and monkey anterior temporal lobe begin and end.
Functional implications of the complex intrinsic connectivity
As discussed above, the gross neuroanatomy highlights a number of bypasses and other nonhierarchical patterns of connectivity (Fig. 3) that complicate the central route. Critically, there is also strong evidence that these connections have functional consequences, as lesions of areas along the central route do not cause the profound effects in later regions that would be expected in a single serial system. For example, even though extensive damage to V4d and TEO in macaques (Fig. 4A) reduces the ability of neurons in TE to filter distracters, their basic firing rate and selectivity remains largely unaffected[38, 39] (Fig. 4B). This result implies either that information is flowing through an indirect and seldom considered route (via MT/MST) or that some very small spared portion of V4/TEO is capable of driving neurons in TE. In either case, at a minimum, the majority of the central route (Fig. 1C) is not necessary for the functions being assumed and formally modeled (Fig. 1D) in area TE. Nor are these findings unique; a great many bilateral lesion and disconnection studies have failed to silence neurons in aIT (see[40] for a review, including the lack of effects following pulvinar lesions). Consistent with the multiple parallel routes suggested by the neuroanatomical framework (Fig. 3A), abolishing TE-dependent behavior requires the bilateral removal of the entire prestriate cortex (V2, V3, V4, and portions of the MT/MST complex) on both the dorsal and ventral surfaces[40] (Fig 4C). Taken together, these results suggest that great care must be taken in proposing theoretical and computational models of IT that presuppose a largely serial flow of information along a single central route through what is, in fact, an extremely complicated recurrent network.
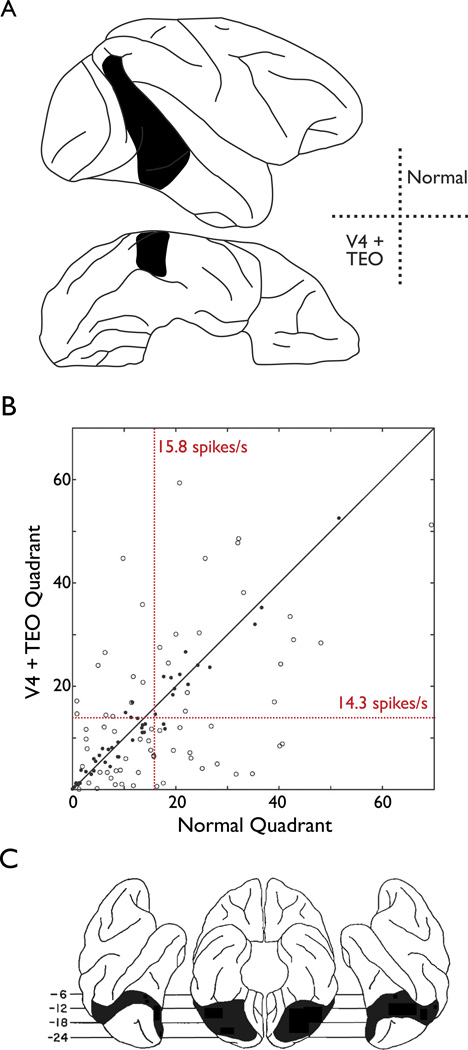
Figure 4. Test of the strict hierarchical model of object quality processing.
A. Schematic of prestriate lesions in two macaque monkeys. In the left hemisphere (upper panels) only V4d has been removed, whereas in the right hemisphere both V4d and all of TEO have been removed. Under the serial processing model in Fig. 1A, this should lead to profound reductions in the responsiveness and selectivity of TE neurons to stimuli in the lower right quadrant (Adapted from [38]).
B. Recordings from neurons in TE of the right hemisphere following the lesions depicted in panel A. The x-axis gives the average spikes per second for 57 unique stimuli presented in the normal upper right quadrant. The y-axis gives the average response for those same stimuli in the affected lower left quadrant. Filled circles denote stimuli for which there is no difference in response between the two quadrants. Open circles denote stimuli where there was a difference in response. The black diagonal line is the identity line and any shift in response magnitude due to the lesions will be reflected in the majority of points being either above or below the line. Note that there is no such bias in the plotted points. The average response in both quadrants (red dotted lines and numbers) do not differ significantly (Adapted from [38]).
C. Depiction of the lesions required to abolish area TE-dependent behavior. Note the extensive bilateral removal of the prestriate cortex, corresponding to areas V2,V3,V4, and portions of MT/MST (Adapted from [40]).
The neuroanatomy also highlights that, even among early visual areas, the complex pattern of connectivity (Fig. 3A,B) makes it difficult to assign a hierarchical level to any region within the pathway. This matches well with the relatively weak functional distinctions between adjacent stages of the putative central route that are observed anterior to V2 in monkey, with even V2 and V4 showing largely overlapping functional properties (e.g. RF size and stimulus selectivity)[41]. Further, in both monkey and human, the predominant distinctions identified have been different visual selectivity amongst areas that occupy the same putative level of the hierarchy (e.g. face selective regions: see Clustering section below). The best evidence for distinctions between levels of the putative hierarchy comes from a series of studies using the same artificial stimulus set, reporting a slowly (~50ms) evolving representation of complex curvature in V4 and 3D shape in IT[10, 11, 42]. These functional studies, along with many others, provide strong support for an increase in the complexity of representations from posterior to aIT but cannot address either the necessity or sufficiency of the central route for this increase.
Propagation of retinotopic biases
The intrinsic connectivity of the occipitotemporal network reveals a strong relationship between neuroanatomy and retinotopic position, with strong retinotopic biases in the primary inputs to the medial temporal lobe (via TFO), ventral surface (via TEOv), and dorsal surface (via TEOd). There is also some behavioral and functional evidence that even high-level visual object representations are at least somewhat position-dependent (e.g.[43–45]; see[46] for a full review). This contrasts with the general assumption that visual object representations become increasingly position invariant along the hierarchy, culminating in abstract representations of object identity in aIT. Below, we highlight the neural effects of retinotopic position along each of the three different divisions of the visual field: contra vs. ipsi, upper vs. lower, and central vs. peripheral.
First, it is important to consider the contralateral bias present throughout the occipitotemporal network. Visual input from the left and right visual fields, even within the fovea, initially projects only to the contralateral V1[47]. In both human (e.g.[48]) and monkey (e.g.[49, 50]), each of the early visual areas (V1,V2,V3,V4) contains a map of the contralateral visual field. These early visual areas seem to be largely comparable in the two species (Fig. 5A,B) [51, 52], with the possible exception of human V4d, the existence of which is a matter of current debate[53–55]. Human neuroimaging has revealed a strong contralateral retinotopic bias that extends far into posterior IT (e.g.[56–58]). Although RF sizes do increase[59–61], they still have a strong contralateral bias even in monkey aIT where they range in size between 2.8 and 26° with a reported mean size of 12°[62, 63] (Fig. 5C). There is also growing evidence that the functional consequences of these biases extend to high-level object and pattern representations. In monkey, unilateral lesions of TE/TEO cause only contralesional impairments in a broad array of visual discriminations, including shape and color[64] (Fig. 5D). In human, the identity of individual objects in a given hemifield can be decoded much more accurately from the fMRI response of the contralateral object-selective posterior fusiform sulcal cortex (pFs) than from its ipsilateral homolog, and more accurately within one quadrant of the contralateral visual field than across quadrants (Fig. 5E)[43] (see also[65] for other demonstrations of stronger decoding within than across quadrants). Further, the identity of body parts can be more accurately decoded in the body-selective extrastriate body area (EBA) when they are presented in their typical field (e.g. right body in the left field) than in an atypical field (e.g. right body in the right field), indicating that long-term retinotopic experience shapes high-level representations (Fig. 5F).
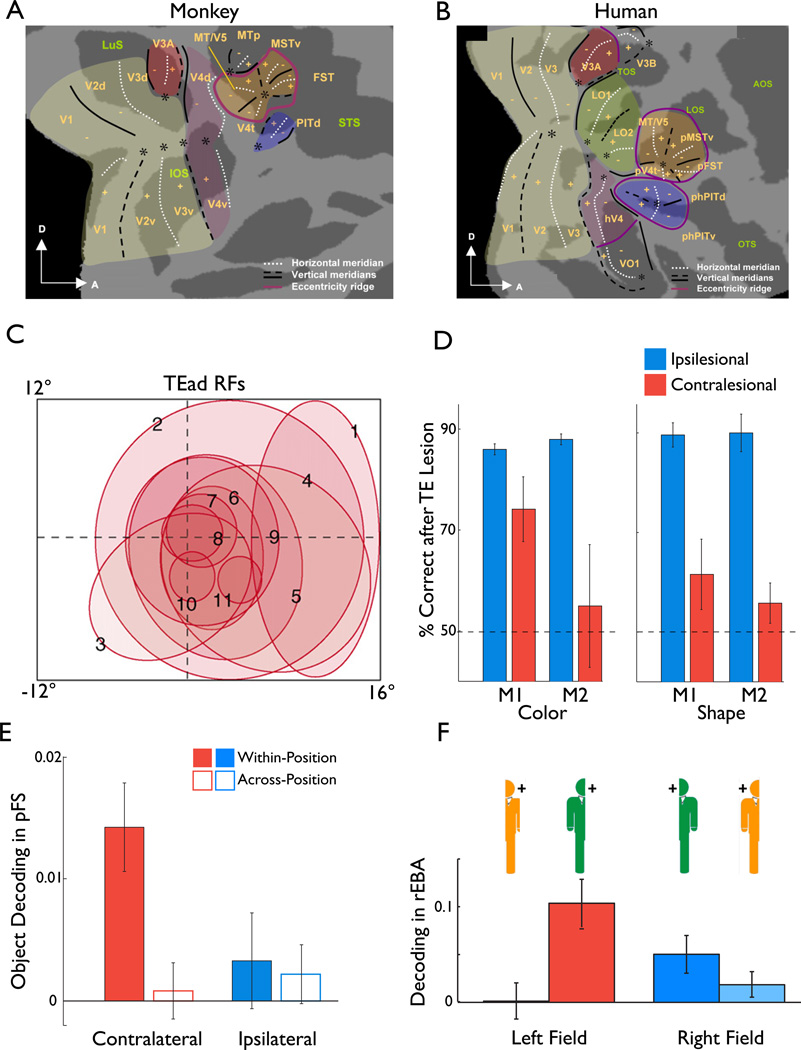
Figure 5. Retinotopy: Contralateral vs. ipsilateral.
A. Flat map representation of the known retinotopic maps in macaque. The map is created by inflating the cortical surface (see panel C) to bring the depths of sulci to the surface and cutting along the calcarine sulcus to flatten the map. Note the predominant lower field representations (−) in the dorsal early visual areas and the upper field representations in the ventral early visual areas (+). The various representations of the fovea are marked (*). (Adapted from [52])
B. As in panel A but for human. Note the large degree of correspondence between monkey and human in the early visual areas V1-V4. Note also the additional retinotopic areas anterior to V4 adjacent to both the dorsal and ventral portions of the early visual areas. Note that VO2 (Fig. 6C) lies just anterior to VO1 (not shown). (Adapted from [52])
C. Schematic of RF size and location of 11 example neurons in macaque area TEad. Note the bias towards the contralateral field and the correlation between RF size and the eccentricity of the RF centers and that most RFs overlap the fovea. (Adapted from[46] and [63]).
D. Results of color and shape matching tasks performed by two macaques (M1, M2) following unilateral lesions to either TE (M1) or both TE and TEO (M2). Note that performance is spared in the hemifield ipsilateral to the lesion but severely impaired in the contralesional field in both macaques for both tasks. Error bars denote standard errors across trials. Similar results were observed for a color matching task. (Adapted from[64]).
E. Plot of object identity decoding (individuation among 24 objects) from fMRI BOLD responses in human ventral object-selective cortex (area pFs) as a function of 1) stimulus presentations in the contralateral or ipsilateral field and 2) decoding within a single quadrant or across quadrants. Within-quadrant decoding was quantified as the correlation in multivariate response patterns to two independent presentations of the same object in the same quadrant minus the average correlation between that object and all the other objects in that same quadrant. Across-quadrant decoding was defined as the correlation in response patterns between an object and that same object in a different quadrant minus the average correlation between that object and all the other objects in the different quadrant. Note that significant decoding was observed only in the contralateral field and that this decoding was greater than that observed either across-positions or in the ipsilateral field. Complementary to the lesion results in panel E, these results indicate that 1) objects evoke reproducible patterns of activity only within the contralateral field and 2) that the same object produces distinct patterns of response in different positions even in high-level object-selective cortex. (Adapted from[43]).
F. Plot of body part decoding (e.g. arm, torso) from fMRI BOLD responses in human right EBA as a function of 1) stimulus presentations in the left or right visual field and 2) presentations of right or left body parts. Decoding was defined analogously to within-position decoding in panel E. The combination of visual field and side of body is given by the body and fixation cross icons above each bar. Note that decoding is stronger for body parts in presented in their typical combination of field and side of body (bars beneath green figures) than in atypical combinations (bars beneath orange figures). Note also the slightly stronger decoding of body part in the left (contralateral; red bars) than in the right (ipsilateral; blue bars) field.
Second, there are widespread functional eccentricity biases throughout the occipitotemporal network. Across early visual areas, there is a map of eccentricity with an expanded representation of the central visual field relative to the periphery (Fig. 6A). In human as in the monkey, many of the IT regions within the occipitotemporal network lie immediately anterior to foveally biased early visual cortex (e.g.[55, 66, 67]) (Fig. 6A), whereas the parahippocampal gyrus (TFO, TF/TH; Fig. 6A) lies immediately anterior to the peripheral representations in early visual areas[68, 69]. In monkey, neurons within IT generally show a strong foveal bias (e.g[63]), while neurons in TF/TH have larger RFs and a very weak foveal bias[70] and receive strong input from the parietal cortex and hippocampus[5]. In human, the parahippocampal gyrus contains a scene-selective region (parahippocampal place area; PPA) that primarily represents the peripheral spatial boundary of scenes[71, 72], whereas the portions of IT aligned with the foveally biased portions of early visual areas contain regions selective for faces[68, 69] and words[73], suggesting that the availability of information about certain parts of space constrains the location of high-level selectivity (see also Clustering section below). There is also recent evidence that object representations might be organized along a lateral-medial axis across IT according to real-world size regardless of retinal projection size in correspondence with the eccentricity gradient (i.e. small objects are represented laterally and large objects medially)[74], suggesting that eccentricity serves as a general organizing principle across the occipitotemporal network.
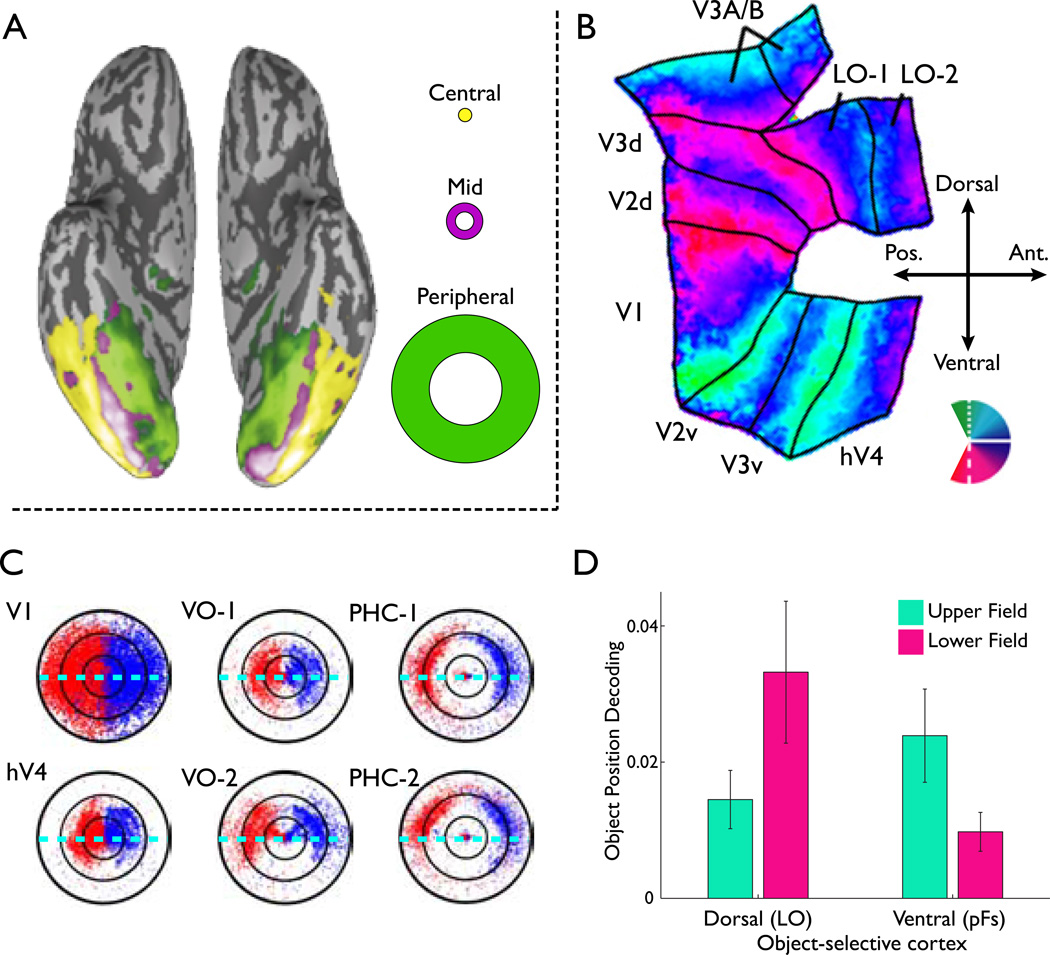
Figure 6. Retinotopy: Fovea vs. Periphery and Upper vs. Lower.
A. Plot of the effect of eccentricity across the inflated ventral surface of the posterior temporal lobe. Participants were presented with concentric rings of a variety of objects (see[69]) at one of three eccentricities (right inset shows rings). The colors on the inflated surface represent which of the three eccentricities generated the strongest response in that cortical region. Note that the more medial regions of the temporal lobe evidence a peripheral bias whereas the more lateral regions evidence a foveal bias. Scene- and face-selective regions occur within these peripherally and foveally biased regions, respectively. (Adapted from[69]).
B. Flattened plot of retinotopic maps from V1 through hV4 (human V4) on the ventral surface and through LO-2 on the lateral surface. The colors plotted represented the position of the rotating wedge that generated the strongest response (see partial pinwheel inset). Note the clear division between the upper and lower field representations for the dorsal and ventral portions of the early visual areas (V1-V4). Note also that the bias for the lower field on the dorsal surface extends into LO-1/2 as shown by the lack of a strong representation of the upper vertical meridian (green) in either region. (Adapted from[67]).
C. Plot of the aggregated location in the visual field of the peak response across nine participants from voxels within different visual areas. The VO regions lie ventrally adjacent to hV4 (Fig. 5B). The PHC regions lie medial to the VO regions, and closer to the peripheral representation (panel A). The horizontal dashed lines in blue represent the horizontal meridian. Note that in V1 there is an even distribution of peak responses across the entire visual field, with equal representation of the upper and lower visual field (see also panel D). In hV4, VO-1, and VO-2, all of which lie on the ventral surface (panel B) there is a clear bias towards the contralateral upper visual field (but see[53]). This same bias is also seen in the PHC regions, which also show a bias for peripheral space in keeping with their position in peripherally biased extrastriate cortex (panel A; adapted from[78]).
D. Plot of object position decoding as a function of 1) location of the object-selective region (dorsal or ventral surface) and 2) presentation of objects in the upper or lower contralateral visual field. Object position decoding in object-selective cortex was stronger dorsally in LO for the lower visual field and stronger ventrally in pFs for the upper visual field in ventral object-selective cortex, respectively. These biases are consistent with the position of the regions relative to the upper and lower field representations in early visual areas. (Adapted from[43]).
Finally, the upper and lower visual fields are represented in the ventral and dorsal portions of early visual areas, respectively (Fig. 7B,C). The ventral visual areas project primarily to the ventral surface of IT and the dorsal areas to the dorsal surface, with only weak projections between them caudal of area TE (Fig. 3A). In human, category-selective regions often come in pairs[75], with one region on each of the dorsal and ventral surfaces of IT (see also Clustering section below). For example, the lateral occipital complex (LOC)[76], which shows stronger fMRI responses to veridical than to scrambled objects, has ventral (pFs) and dorsal (lateral occipital, LO) components. LO has been reported to have a bias for the lower visual field[43, 66, 77], whereas pFS was recently shown to have a bias for the upper visual field[43] (Fig. 6D) (see also[77, 78] for upper field biases in other ventral regions). Further, many areas in the dorsal pathway, which receive most of their input from dorsal portions of the early visual areas, have a bias for the lower visual field (e.g.[79–81])(Fig. 6C). It therefore appears that the differential representation of the upper and lower visual fields may be an additional organizing principle across both the ventral and dorsal visual pathways.
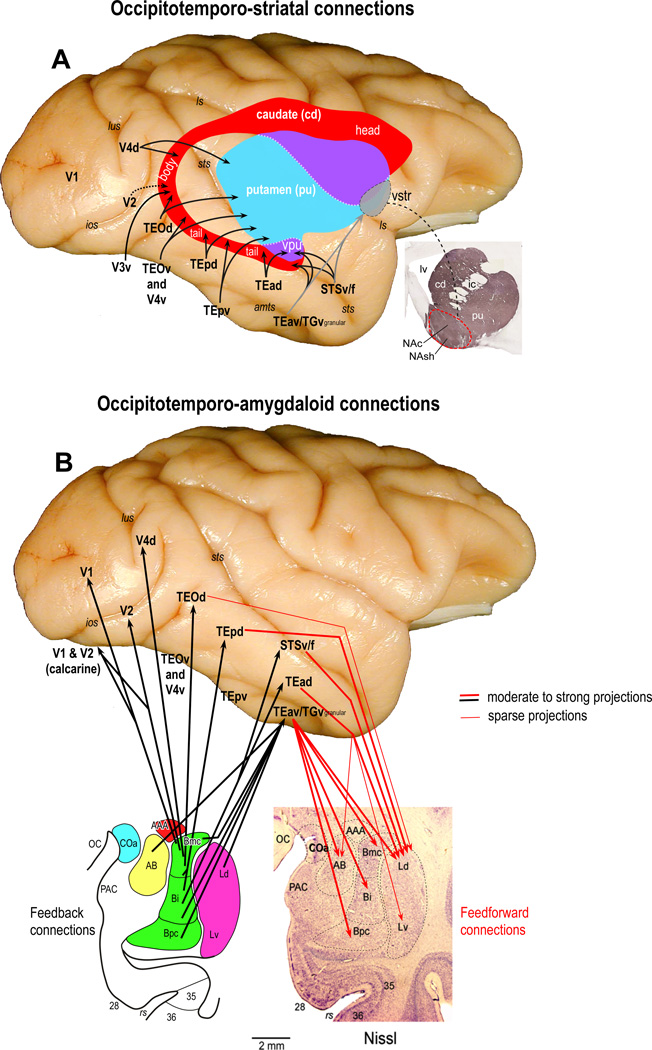
Figure 7. Subcortical pathway anatomy.
A. This illustration shows the topographic organization of projections from occipitotemporal network to the neostriatum (caudate nucleus and putamen; red and blue/purple shaded areas), and the ventral striatum (VStr; gray shaded area). Inset: the photomicrographic section stained for tyrosine hydroxylase shows the ventral striatum (red dashed region), which includes the ventral part of the head of the caudate nucleus (cd) and putamen (pu), and the shell and core regions of the nucleus accumbens (NAsh and NAc, respectively). Note that the caudal visual areas V2 and V4 project to the most caudal part of the body of the caudate nucleus (genu) and more rostral visual areas (subregions of TEO, TE, TGv granular, and STSv/f) project to overlapping but progressively more rostral portions of the tail of the caudate nucleus and the caudoventral parts of the putamen. The subregions within aIT (TEav, TGv granular, and rostral STSv/f) also give rise to projections that target the ventral striatum (see main text for other details). Abbreviations: cd, caudate nucleus; ic, internal capsule; ls, lateral sulcus; lv, lateral ventricle; NAc, nucleus accumbens, core; Nash, nucleus accumbens, shell; pu, putamen; vpu, ventral putamen; Vstr, ventral striatum. For other abbreviations see Figure 3.
B. This figure illustrates the organization of reciprocal (feedforward and feedback) connections between the occipitotemporal network and the amygdala. In contrast to neostriatum, which receives input from nearly every cortical area along the occipitotemporal pathway but projects directly back to none, the amygdala receives the vast majority of feedforward projections (red arrows) from regions within aIT (TEad, TEav, TGv granular and STSv/f), and sends feedback projections (black arrows) to almost every area in the network, including V1. Note that the projections from basal nucleus of amygdala to TEpv, TEOv, and V4v are not shown (no published data). Thick lines indicate the moderate to strong projections, and thin lines show the sparse projections. Abbreviations: AAA, anterior amygdaloid area; AB, accessory basal nucleus of amygdala; Bi, basal nucleus of amygdala, intermediate subdivision; Bmc, basal nucleus of amygdala, magnocellular subdivision; Bpc, basal nucleus of amygdala parvicellular subdivision; Coa, anterior cortical nucleus; Ld, lateral nucleus of amygdala, dorsal subdivision; Lv, lateral nucleus of amygdala, ventral subdivision; OC, optic chiasm; PAC, periamygdaloid cortex. For other abbreviations see Figure 3.
The ubiquitous effects of retinotopic position are in keeping with the retinotopic biases in input apparent in the intrinsic connectivity (Fig. 3A), and it has been argued that retinotopic information could be useful in the representation of objects (see[82] for an alternate model that incorporates retinotopy). Shared retinotopic information may also provide a common language in which regions with different selectivities can communicate. Further, the continuing discovery of retinotopic maps within object-selective cortex in human IT suggests that retinotopy may be a general organizing principle in the ventral visual pathway.
Clustering
One of the most consistent findings regarding the intrinsic function of the occipitotemporal network to emerge in both human and monkey (e.g.[83, 84]) is the presence of functional clustering in cortex with selectivity for particular object categories (e.g. body parts[85], faces[85], scenes[86], objects[76], tools[87], written words[73], color[88, 89]). Beyond the prominence of clustering in physiological measurements, the observation of category-biased deficits/enhancements following lesions (e.g.[73, 90, 91]) and transcranial magnetic stimulation (TMS) of these clusters in human (e.g.[92]), as well as direct stimulation in monkey[93], suggests that the clusters are behaviorally significant. The nature and origin of these clusters remain unclear (see [94, 95] for recent proposals); they were not anticipated in the original formulation of the ventral pathway, which makes no predictions about differences within a putative level of the hierarchy. Yet, such clustering can be easily incorporated into the proposed framework, emerging naturally from an interaction between large-scale connectivity and experience.
Broadly, these cluster might emerge at either a phylogenetic or developmental timescale. Phylogenetically, large numbers of interconnected neurons might have genetically specified selectivity for evolutionarily important categories. Clustering minimizes biologically expensive long-distance connections between these neurons (see[96] for a review), conveying an evolutionary advantage[97]. However, this explanation cannot account for the origin of the visual word form area (VWFA), which is selective for orthography, a category too recent to have undergone evolutionary selection[98]. The strongest version of the alternative account is that the clusters emerge exclusively through experience, a proposal that is strongly challenged by the striking consistency in the location of these clusters across individuals.
The proposed framework offers an explanation for this apparent contradiction without positing distinct mechanisms for the origin of different clusters (e.g.[97]). Since connectivity among the areas of the occipitotemporal network is heterogrenous, information about particular stimuli or aspects of those stimuli is more strongly available in particular areas (e.g. Retinotopic biases section above). Clusters then emerge from an interaction between this constraint and visual experience (see[99] for an example in monkey). Categories that require a large population of neurons (e.g. those requiring extensive recurrent processing) will naturally exhibit clusters at those locations where the connectivity affords the necessary information (see[100–102] for related proposals). Thus, the large-scale connectivity is an innate phylogenetic constraint that defines the availability of information to particular areas of cortex, creating a likely set of cluster locations that will be common across individuals given similar experience (e.g. exposure to faces). Importantly, although this set of locations may provide the most efficient wiring for representing a category, they are not the only possible set. In cases of cortical brain damage or unique experience, particularly early in development, entirely different clusters might arise, explaining the relatively spared abilities even of individuals with hemispherectomies (e.g. [103]).
Recovery of function following damage to the large-scale connectivity may be much more difficult, even if the damage occurs early in life, as appears to be the case in individuals with congenital prosopagnosia who demonstrate normal cortical activation in IT to presented faces but a weakened projection between posterior and aIT as shown by diffusion tensor imaging (DTI)[104].
The simplest account of clustering in a high-level cortical area is that it reflects large-scale connectivity. For example, the parahippocampal gyrus, which contains the scene-selective PPA and extends from the the peripheral visual field in humans (Fig. 6B), is known to receive strong projections from V4v in monkey (Fig. 2A) and from the parietal cortex via the parieto-medial temporal pathway[105], which presumably conveys spatial information. On the basis of these connections it is not surprising that the region predominantly represents the peripheral visual-field aspects of scenes[71, 72]. In contrast, the portions of IT with a foveal bias contain regions selective for stimuli requiring fine discrimination like faces and words. It has been reported that the location of the ventral face-selective cluster in individual human participants can be predicted from large-scale connectivity as revealed by DTI[106] and the patterns of response to faces and houses are more similar in monozygotic than dizygotic twins[107].
The neuroanatomy also aligns to some degree with the specific within-category aspects of stimuli processed within particular regions. For example, in humans, major face-selective clusters have been identified in the banks of the STS and on the lateral (Occipital Face Area -- OFA) and ventral (Fusiform Face Area -- FFA) surfaces of IT[101]. These three areas mirror three of the parallel routes we identified in the preceding sections (through STSv/f, TEOd, and TEOv; Fig. 2A). There is also evidence that the type of face processing performed within each route is related to its primary input. Thus, the STS, which receives strong input from the MT/MST complex, shows strong sensitivity to expression and other dynamic aspects of faces in both human and monkey (e.g.[108]). In contrast, the other clusters (OFA and FFA) seem more sensitive to identity and other more stable aspects of faces[101]. Further, in monkey, there is evidence from direct stimulation that the various face-selective clusters are strongly connected to one another[109], though more direct evidence from anatomical studies using tracers would help to determine what other systems might also be connected to these regions.
In sum, the prominence of functional clusters with different selectivities within the same putative level of the hierarchy represents a challenged to both the original formulation of the ventral visual pathway and the current conception. The proposed framework provides some traction on the genesis and stereotypical position of these clusters by considering the large-scale connectivity (particularly the propagation of retinotopic biases) as a constraint on the regions within the occipitotemporal network where certain types of information are available to form the stimulus-selective clusters.
Recurrent Processing
The importance of recurrent processing (i.e. feedback and lateral interactions) has not been emphasized sufficiently, on the assumption that the feedforward processing of visual information along the central route can be understood in isolation. While there may be an initial response to stimuli in aIT that occurs too quickly to incorporate stimulus-driven recurrent signals[110], those signals play a critical role in processing beyond the initial response[111] and top-down signals (e.g. attention, goals) contribute to the state of the entire network prior to stimulus onset. Enforcing a division between feedforward and recurrent processing makes it very difficult to incorporate, for example, attentional, contextual, and masking effects. Indeed, rigid adherence to the concept of serial-order processing is a stumbling block to understanding the function and nature of representations in the occipitotemporal network.
Consider that the function in area TE most directly affected by extensive damage to area TEO is not the basic response to stimuli (Fig. 4A) but the attentional filtering of distracters[39]. This result highlights the fact that many connections within the occipitotemporal network, perhaps especially those in the central route, might exist to enable functions more complex than simple selectivity. Visual attention, whether directed to spatial locations[112], objects[113], or features[114], is known to affect processing directly within areas of the occipitotemporal network responsible for perceptual processing (see[115] for reviews and models). Further, selection along any of these dimensions interacts with the other dimensions (e.g.[113, 116]) to create a pattern of facilitation across the entire visual scene[117, 118]. The dense bidirectional connections along the central route likely contribute to the complex interactions necessary to generate attentional effects that span both the entire visual field and multiple perceptual dimensions.
Recurrent processing probably underlies a number of psychological and neural phenomena that depend on processing beyond the initial visual response (see[111] for a review). For example, change blindness, in which a change in a visual scene goes undetected (e.g.[119]), often occurs when the change does not alter either the gist of the scene[120] or a currently relevant feature[121]. Interestingly, large, low-level changes in the input that are undetected evoke reduced activity throughout temporal and parietal vision-related cortex compared tothe activity evoked by similar but detected changes[122]. Recurrent processing likely also underlies the well-studied effects of context and attention in even V1 (e.g.[116, 123–126]).
Finally, backward masking, in which the presentation of a mask shortly after a stimulus impairs both behavioral performance[127] and activity in the occipitotemporal network in human[128] and monkey[129], also emphasizes the importance of recurrent processing. In a purely feedfoward system, there should be no effect of a mask presented after a stimulus evokes the initial neural response, yet the mask profoundly impairs both performance and awareness. Whether backward masking depends on the interruption of ongoing processing within a region (e.g.[130–132]) or the disruption of feedback signals[133], it clearly demonstrates the importance of recurrent connections and of processing beyond the initial neural response for even basic visual object perception.
Output pathways: Anatomy and Function
We turn next to the six major subcortical and cortical output pathways from the occipitotemporal network. In each section we detail the neuroanatomical connection and any relationships that exist between the connection, the likely information it carries from the occipitotemporal network, and the functional properties of the target structure. All of the output structures are critical for various forms of learning and memory, either in the formation of associations with the visual stimuli (e.g. response, reward, affect, valence), in creating visual memories, or in the online utilization and manipulation of visual information (see[134] for a recent review). In combination, the functions subserved by these output structures provide the raison d’etre for the occipitotemporal network (see also Text Box 1), which has little direct connectivity with motor output.
Cortico-subcortical output pathways
There are three major output pathways to subcortical structures, which are all critical in forming associations between visual stimuli and non-visual information. First, contrary to the single output region that is commonly assumed, the unidirectional occipitotemporo-neostriatal pathway arises from every subregion of the occipitotemporal network except V1 (Fig. 7A) and supports the formation of links between stimuli and responses. Second, the occipitotemporo-ventral striatum pathway arises in aIT and supports the association with and processing of stimulus valence. Third, and in contrast to the occipitotemporo-neostriatal pathway, the projections from the occipitotemporal network to the amygdala arise primarily in aIT, but projections from the amygdala target every subregion of the network (occipitotemporo-amygdaloid pathway; Fig. 7B). This pathway is critical to a broad range of affective processing of stimuli.
Occipitotemporo-neostriatal pathway
Projections to the neostriatum from the occipitotemporal network, like those from the cerebral cortex generally, are largely organized topographically[17, 36, 135–141]. In particular, as illustrated in Fig. 7A, the neostriatal projection zones of areas V2 and V4 lie within the most caudal part of the body of the caudate nucleus (i.e. the genu), and each of the more rostral cortical areas (TEO, subregions of TE, TGv granular, and STSv/f) have overlapping but progressively more rostral projection zones in the tail of the caudate nucleus and the caudoventral parts of the putamen[137, 140]. Neostriatal processing can affect motor output and cortical processing via projections to the globus pallidus and the substantia nigra pars reticulata (SNr). These structures project in turn to subregions of the thalamus (VA, VL, and MD)[142], which then project to different parts of the frontal cortex, forming several corticostriatocortical loops (see[142, 143] for reviews). These loops have been implicated in a number of functions, including categorization[143, 144], working memory (e.g.[145]), as well as learning and selecting rewarded actions[146, 147], and even directing information transfer amongst cortical regions[148].
However, the function most directly related to the inputs from the occipitotemporal network concerns visual discrimination learning based on the reinforcement versus extinction of stimulus-response associations, i.e. habit formation or procedural learning[149]. In human, these associations are typically implicit and not verbalizable (e.g.[150]), and though the importance of the pathway for visual habit formation is well documented[149, 151–153], the precise role of the neostriatum in this implicit learning process is still unknown. Recent single-cell recording studies have reported neurons in the tail of the caudate nucleus that show strong selectivity for complex visual stimuli[154, 155], and these neural representations are extremely stable over time [Kim & Hikosaka, 2011; Society for Neuroscience]. These characteristics contrast with neurons in the head of the caudate nucleus, which demonstrate coding for explicit rules[156], change their representations quickly[157], and whose stimulation can increase the rate of rule learning[158]. Determining whether these findings imply that the head and tail of the caudate nucleus serve fundamentally different functions, or whether this difference simply reflects the different sources of the cortical input into these areas of the caudate nucleus (e.g.[151, 159]) awaits further study.
Occipitotemporo-ventral striatum pathway
Subregions within aIT (TEav, TGv granular, and rostral STSv/f) give rise to unidirectional projections which target the ventral striatum (shell and core regions of the nucleus accumbens and olfactory tubercule)[17, 140, 141] (see also[135, 136]). The ventral striatum is also heavily connected with orbitofrontal cortex (an area closely associated with both taste and gustation[160]), lateral prefrontal cortex, the hippocampus, and the amygdala[161–164].
The functional properties of the ventral striatum have not been extensively studied in primates, though they have in rodents (see[165] for a review). The region is thought to participate in the assignment of value (positive and negative) to particular stimuli. In monkey, single-cell recording studies have reported neurons in the region that respond to anticipated or received reward[166] and to the magnitude of that reward[167]. In contrast to the neural activity in the neostriatum[168], activity in the ventral striatum appears to be completely independent of the particular action that the monkey executes to receive the reward[168–170] (see also[171]). However, neurons in the ventral striatum of rats[172] and monkeys[173] do appear to encode the general effort or cost required to receive a reward. Lesions of the ventral striatum in rats impair the assignment[174] or reassignment[175] of value to particular stimuli and the vigor of responses to them[176], but not response accuracy[176] (see[177] for analogous results in monkey). In human fMRI, the ventral striatum is generally activated by stimuli with an affective valence, whether positive or negative (see[178] for a review), in both social and non-social domains[179]. The area may also be generally activated by unexpected or novel stimuli[166, 180] (but see[181]). These results, combined with its dense limbic and medial prefrontal cortex connectivity suggest the region may serve to integrate the output of reward based systems for the purpose of motivating or prioritizing actions. Differentiating the contribution of the occipitotemporal inputs to its function from those arising from the hippocampus, amygdala, and prefrontal cortices may prove challenging.
Occipitotempo-amygdaloid pathway
In contrast to the neostriatum, which receives input from nearly every area in the occipitotemporal network but projects directly back to none, the amygdala receives the majority of its input from aIT but projects back to almost every area in the occipitotemporal network[17, 138, 140, 182, 183] (Fig. 7B). The primary projections arise from TEav and TGv granular and target the dorsal subregion of the lateral nucleus (Ld), and the magnocellular, intermediate, and parvicellular regions of the basal nucleus (Bmc, Bi, and Bpc, respectively), as well as the accessory basal nucleus (AB). Secondary projections arise from TEad to Ld, with weaker projections to the ventral subregion of lateral nucleus (Lv) and AB[33, 140] and from STSv/f to Ld[184]. Finally, there are strong projections from TEpd[182, 184] and weak projections from TEOd to Ld[33, 182]. Efferents from the amygdala to the occipitotemporal network are exceptionally widespread[183, 185], with connections arising from the Bi and Bmc nuclei to virtually every region in the network, including primary visual cortex (V1). There are also strong reciprocal connections arising from the Bpc, Bi, and Ld nuclei to TEav (Fig. 7B). Evidence from functional connectivity in humans likewise indicate a strong link between the basolateral nuclei and the occipitotemporal network[186].
The projections from the occipitotemporal network likely provide the input necessary for the visually-dependent emotional regulation for which the amygdala is critical (see[187, 188] for reviews). Some have suggested that there is also a subcortical route through which the amygdale receives visual information in order to enable fast responses to emotional stimuli. However, the plethora of bypass connections (Fig. 3A) in the occipitotemporal network and the details of the neuroanatomy of the likely subcortical route suggest that the cortical projections provide the vast majority of the visual input to the amygdala (see[189] for a review of these issues). The basolateral nuclei of the amygdala contain many neurons that are visually responsive[190] and broadly selective for the content of images[191], including facial identity and expression (e.g.[192]). Further, the lateral nucleus of the amygdala is activated when face-selective areas in the STS are electrically stimulated in monkey[109].
The amygdala participates in, though is not necessarily critical for, a number of different forms of visual learning. For example, the amygdala signals the current reward value of a stimulus, rapidly changing its value representations during reversal learning[193]. However, lesions of the amygdala in either adult[194] or infant[195] monkeys do not seem to impair reversal learning. Such lesions do cause mild impairments in paired associate learning[196], but the most striking deficit is a generally reduced fear response to aversive stimuli (e.g[197]), leading to the suggestion that the amygdala might be more important for linking stimuli to instinctive (i.e. fear-induced) avoidance responses than for learning associations between stimuli and negative outcomes generally[198].
The functional consequences of the projections from the amygdala to the occipitotemporal network are poorly understood. These projections are very diffuse (Fig. 7B), suggesting a role in orienting attention or awareness towards particular stimuli (see[188, 199] for reviews), rather than in fundamentally altering specific representations. For example, lesions of the amygdala reduce the response of face-selective areas of the fusiform cortex to fearful faces, but do not change the fundamental selectivity of the region for faces generally[200]. The precise nature of the modulatory signals arising from the amygdala remains unknown, but such diffusely distributed signals likely serve to direct attentional resources to the processing of visual stimuli signaling potential danger or other emotionally intense events.
Cortico-cortical output pathways
There are three major output pathways to cortical structures, which are all critical in long- and short-term visual memory. In contrast to the subcortical pathways, the cortical outputs are all bidirectional and originate in aIT, though from different subregions within it. First, the occipitotemporo-medial temporal pathway arises from every region in aIT as well as TEpv (Fig. 8) and supports the formation of long-term cognitive visual memories. Second, the occipitotemporo-orbitofrontal pathway arises primarily from STSv/f, TEav, and TGv granular and supports the association of visual stimuli with reward. Third, the occipitotemporo-ventrolateral prefrontal pathway arises primarily from STSv/f with only a minor projection from TEad and supports object-based working memory.
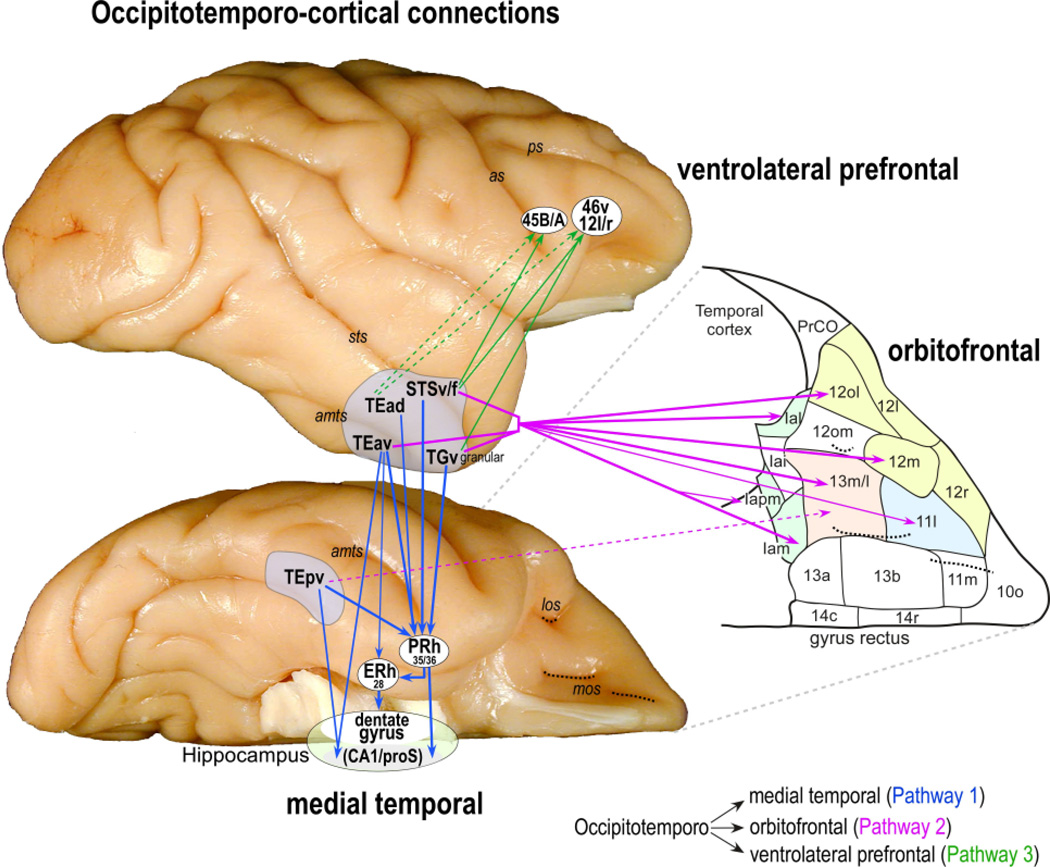
Figure 8. Corticocortical pathways.
The corticocortical pathways on the lateral and ventral views of the rhesus monkey brain. The orbitofrontal and part of the temporal pole are redrawn from the original image (gray dashed lines) to show the different cytoarchitectonic areas (subdivisions of areas 11, 12, 13, and 14, and caudal orbital areas Iai, Ial, Iam and Iapm; See[231, 313]). The corresponding location of medial and lateral orbital sulci (mos and los, respectively; black dashed lines) is also indicated in the drawing. Note that only part of the orbitofrontal areas (those shaded in the drawing) receive direct projections from the inferotemporal cortex is shaded in the drawing. For example, the lateral part of area 12o (12ol) receives a direct projection from the inferotemporal cortex but not the medial part of area 12o (12om).
Three major reciprocal corticocortical pathways (indicated by single arrowheads) originate from the rostral inferotemporal areas TEad, TEav, TEpv, TGv granular, and rostral STSv/f. These pathways target different areas in the medial temporal lobe (blue arrows), ventrolateral prefrontal cortex (green arrows), and orbitofrontal cortex (purple arrows). The heavy solid lines indicate dense projections, the thin solid lines moderate to dense projection, and the dashed lines sparse to moderate projections. Abbreviations: 12l-12r-45A-45B-46v, ventrolateral prefrontal areas; as, arcuate sulcus; CA1, CA1 subfield of hippocampus; Iai, Intermediate agranular insular area; Ial, lateral agranular insular area; Iam, medial agranular insular area; Iapm, posteromedial agranular insular area; los, lateral orbital sulcus; mos, medial orbital sulcus; PrCO, precentral opercular area, proS, prosubiculum; ps, principal sulcus. For other abbreviations see Figure 3.
Occipitotemporo-medial temporal pathway
This pathway consists of both direct and indirect projections arising from TEad, TEav, TEpv, STSv/f and the granular portion of TGv targeting various structures within the medial temporal lobe (MTL) (Fig. 8), particularly the perirhinal cortex (PRh; areas 35/36)[17, 33, 34, 201–203], which projects in turn to both the entorhinal cortex (ERh; area 28)[37, 204, 205], and CA1/pros (prosubiculum) regions of the hippocampus[206] [Saleem and Hashikawa 1998, SFN Abstract 24:898]. The ERh projects directly to the dentate gyrus[207, 208]. The projections from aIT into the rhinal cortices carry visual information used in the encoding of long-term memory of object quality, explaining the numerous findings indicating complex visual selectivity in these regions[209]. It has also been proposed that the rhinal cortices contribute to basic perception (e.g.[210]), but the empirical evidence for any non-memory related role of the rhinal cortices in perception remains unclear (e.g.[211]). Importantly, area TEav[212, 213] and TEpv[212] also project directly to CA1/proS[Saleem and Hashikawa 1998, SFN Abstract 24:898] (see also[214] for the reverse projection). This projection likely contributes object information useful in generating selectivity for particular landmarks, places, and views of the environment found in CA1/proS[5].
There is clear functional dissociation between the types of long-term memory subserved by the MTL and the neostriatum (see section above), exemplified by patient H.M., who after bilateral surgical removal of the MTL was unable to form new long-term memories of events or facts (i.e. episodic and semantic memory, respectively), yet was relatively unimpaired at learning new stimulus-response associations (i.e. habits and motor skills)[215]. Importantly, while H.M. could not form new memories, his memories formed prior to the surgery were less impacted (but see[216]), suggesting that the MTL is more critical for encoding and storing new information than for retrieving information that has already been encoded (e.g. [217–219]). However, the exact mnemonic functions subserved by subareas of the MTL are still being debated.
One prominent theory holds that PRh supports familiarity-based recognition of a stimulus divorced from ‘where’ and ‘when’ the item was encountered, whereas the hippocampus support the recollection of just such associated context (e.g.[220, 221]). An alternative proposal is that the apparent functional dissociation between PRh-dependent familiarity and hippocampus-dependent recollection is artifactual and, instead, both structures contribute to both familiarity and recollection (e.g.[222–225]). Resolving this debate by examining the effects of selective lesions has proved elusive for two reasons. First, PRh is a critical source of visual input into the hippocampus, such that PRh lesions necessarily have an impact on the mnemonic functions of both structures regardless of any functional specialization they might have. Second, and even more problematic, no index of context recollection has yet been established in the monkey. Without this index, it is difficult to use lesions in the monkey as a model to test the necessity of the hippocampus for recollection.
Although a resolution of this debate is therefore not within sight, the sharp neuroanatomical distinctions between the PRh and hippocampus argue strongly in favor of their having distinct mnemonic functions. For example, it has been proposed that the unique internal structure of the hippocampus is optimized to perform pattern separation – a computational process whereby similar patterns are made more distinct from one another – enabling differential encoding of very similar events for selective recollection (e.g.[226–228]; see[229, 230] for reviews). Further, the neuropathology in cases of developmental amnesia, in which damage appears to be limited to the hippocampus, seems to result in impairment only of recollection for specific events, leaving familiarity based recognition largely intact. These findings suggest that while familiarity and recollection may lie along a continuum[222], and may not capture perfectly the functional distinction between the hippocampus and PRh, the hippocampus does appear to perform a fundamentally distinct computation, and thus is likely to mediate a different function from that served by PRh.
Occipitotemporo-orbitofrontal pathway
Like the occipitotemporo-medial temporal pathway, the occipitotemporo-orbitofrontal pathway arises from areas TEav, TGv granular, and STSv/f, which project to the central (areas 11lateral, 13m/l), lateral (12m, 12ol) and caudal (Iam, Iapm, and Ial) orbitofrontal cortex (Fig. 8), with a weaker projection arising from rostral portion of TEad and TEpv targeting central orbitofrontal cortex[231] (see also[232, 233]). The orbitofrontal cortex has been implicated in the processing of both primary and secondary reward, and this pathway may enable both by providing the visual input to (i) the medial frontal affective processing system resulting in object-primary reward association, and (ii) olfactory and gustatory processing areas resulting in object-secondary reward association[234–237]. There is an extensive literature regarding orbitofrontal cortex function in monkey, human, and rat (see[238–242] for recent reviews). Here we highlight and contrast findings related to ventral pathway projections the lateral and medial orbitofrontal cortices (lOFC and mOFC, respectively) (Fig. 8).
Object reversal learning, in which animals must learn and then reverse the association between reward and one of a pair of objects, is impaired (slower learning of the reversal) with lesions of the orbitofrontal cortex (both lateral and medial) in both monkey (e.g.[243, 244]) and human[245, 246]. Further, a recent single-cell recording study reported that orbitofrontal neurons encode the distribution of the expected relative to actual rewards[247], a useful representation for updating stimulus-reward associations. However, deficits in object reversal learning do not differentiate between the subareas of OFC, as lesions of neither lOFC[248, 249] nor mOFC[249] are sufficient to impair this function. This pattern of results might arise from either region being sufficient to support reversal learning, from damage to nearby structures caused by extensive OFC lesions[238], or alternatively, from a critical mid-region within the OFC being completely removed when both partial lesions are combined but not when each partial lesion is performed separately.
There are some functional dissociations between lOFC and mOFC that are well predicted by their differential visual connectivity with the occipitotemporal network. lOFC, in keeping with its strong input from the occipitotemporal network, appears more closely involved in updating particular stimulus-reward associations[238, 239, 241]. For example, in monkey, lesions of the lOFC impair the ability to assign a causal relationship between the choice of a particular stimulus and reward[239, 250, 251]. Lesions[249] or inactivation[252] of the lOFC but not mOFC[249] impair reinforcer devaluation (i.e. decreased stimulus preference after satiation of food). In human, lOFC shows strong activation whenever the expected value of a stimulus choice is updated[253].
In contrast, mOFC seems more strongly involved in fine-grained comparisons between reward values[238, 239]. Lesions of mOFC but not lOFC impair extinction[249] (i.e. cessation of responding to a stimulus that is no longer positive) and also impair decisions amongst a set of stimuli with only slightly differing reward values[251]. In human, mOFC also consistently signals the current level of reward regardless of whether expected values have changed[253].
Occipitotemporo-ventrolateral prefrontal pathway
Unlike the other corticocortical projections, the occipitotemporo-ventrolateral prefrontal pathway (see also Fig. 1A) originates primarily in the anterior portions of STSv/f and it projects to areas 45A/B, 46v, and 12r/l in the ventrolateral prefrontal cortex (VLPFC); a weaker projection also exists from TEad to these cortical areas[231, 254, 255] [Saleem et al., 2008b, SFN abstract 465.12] (see also[232, 233]) (Fig. 8). The precise distribution of the dorsal and ventral visual pathways’ terminals in the lateral prefrontal cortex as a whole may help resolve the conflict between two competing theories of its functional organization: complete convergence vs. domain specificity.
Broadly, but strictly within the domain of vision, the entire lateral prefrontal cortex is thought to be involved in the maintenance and manipulation (e.g. attention[256], working memory[257–259], switching task set[260]) of task-relevant information represented in the posterior cortices (see[261–263] for reviews). However, the lateral prefrontal cortex is not thought to contribute to basic processing of that information, as even complete removal of this area does not impair basic perception or categorization[264].
On one account, the lateral prefrontal cortex is the ultimate site of integration between all forms of information, representing the final stage of the putative processing hierarchy[265]. Under this view, the necessity of integration implies that there should be no strict division between different forms of information (e.g. between spatial and object vision[266]) (see Text Box 3 for a discussion of areas where the dorsal and ventral visual pathways converge). However, there is strong anatomical and functional evidence for some domain-specificity (see also[261, 267–269] for related proposals). The division between visuospatial and stimulus quality information[270, 271] (see[261, 272] for others) has the strongest anatomical evidence, with clearly distinct projections from the dorsal and ventral pathways to the VLPFC and DLPFC, respectively[105] (Fig. 8). There is also direct evidence for functional specialization. In monkey, there is strong spatial selectivity in dorsolateral[273, 274] and a bias for object selectivity in ventrolateral neurons[274], and this evidence is complemented by a dissociation of deficits in spatial and object working memory after dorsolateral and ventrolateral lesions, respectively (e.g.[275]). Likewise in human, lesions of the dorsolateral portion are associated with selective deficits in spatial working memory[258, 259], whereas ventrolateral lesions lead to deficits in working memory for faces[259] (but see[257]).
Text Box 3: Connections between the dorsal and ventral visual pathways.
In both this review of the ventral pathway in vision and the previous review of the dorsal visual pathway[5] we have focused on the processing and connectivity unique to each one. However, there are many connections and points of convergence between them (in addition to the neostriatum and lateral prefrontal cortex) that might contribute to many different forms of visual processing.
Beyond, the early visual areas (V1,V2,V3) the pathways are directly connected via a direct projection between LIP and TEOd/TEd[232] as well as by their common inclusion of the MT/MST/FST complex. This complex has connections with every region within the occipitoparietal network[5] as well as with V4d, TEOd, and the caudal portion of STSv/f in the occipitotemporal network (Fig 3A). The MT/MST/FST complex is sensitive to many aspects of motion (see[300] for a review) and depth[301] and contains many neurons selective for shapes defined by motion (e.g.[302]). It is likely that this complex, in concert with areas in intraparietal sulcal cortex, provide the depth and motion information needed to generate the selectivity for 3D (e.g.[303]) and motion-defined shapes (e.g.[304]) observed in IT.
The bidirectional connection between areas LIP and TEOd/TEd might also be important for the generation of the object selectivity sometimes observed in the occipitoparietal network even for 2D shapes not currently being acted upon (e.g.[305]). These results have been interpreted as evidence of independent shape analysis in the dorsal visual pathway, but damage to the ventral pathway reduces object-related activity in the parietal cortex[306], indicating that at least some of the object processing in the occipitoparietal network is dependent on the ventral pathway. Whether this connection is important for the generation of the 3D shape selectivity observed in the occipitoparietal network (e.g.[307]) remains to be tested.
The MTL (hippocampus together with the rhinal and parahippocampal cortices), which receives input from the dorsal pathway via the parieto-medial temporal pathway[5] and from the ventral pathway via V4v and aIT, is clearly a critical region of convergence. Damage to regions along the parieto-medial temporal pathway lead to various deficits in navigation and the coordination of egocentric and allocentric reference frames[5, 308]. The parahippocampal cortex, which receives direct projections from the inferior parietal lobule and V4V evidences sensitivity to both object and spatial information[309]. Damage to the posterior portions of the parahippocampal cortex (TFO; Fig 3A), which receives direct input from V4v can lead to agnosia for familiar landmarks[308]. Damage to the mid-anterior portion (posterior to the rhinal cortices), which has strong connectivity with the hippocampus and posterior parietal cortex, leads to a deficit in learning routes through new environments (e.g.[310]).
Finally, it should be noted that information from the two pathways can also be integrated in early visual areas by virtue of extensive feedback connections[311]. Recurrent signals from each pathway could affect processing in the other pathway by modulating processing in these early visual areas. Both pathways are also connected with the frontal eye field (albeit with far denser connection to the dorsal than to the ventral pathway) so eye movements initiated by one stream might also impact the other.
There is also some evidence in monkey for stimulus-specific domains within the VLPFC in the form of three discrete “patches” of cortex that are selective for faces (e.g[276]). Face selectivity has also been observed with single cell recording[268, 277] and with fMRI in an analogous region in human[278]. Two of these patches, PL and PA, overlap 46v/12r/l and 45B, respectively, the two regions receiving direct input from STSv/f. The third patch, PO, is within the lOFC, suggesting that the location of face selectivity in the prefrontal cortex aligns with anatomical connectivity.
Regardless of the need for integration between different forms of information, communication between regions and the limited channels available necessitate some level of functional specialization (see also[261]). The resolution between the homogenous and heterogenous viewpoints of lateral prefrontal function is to propose a gradient of functional specialization[261, 274] based on the relative strength of the inputs from the dorsal and ventral pathways. Such a gradient could also explain the occasionally conflicting reports of distinct and intermixed selectivity within the region[257, 259, 266, 274] based on the precise location of recording or damage (see[262] for a contrasting proposal of dorsal-ventral organization).
Discussion
Here we have proposed an expanded neural framework for the processing of object quality, in which the intrinsic connectivity of the ventral visual pathway is characterized as a recurrent network that provides visual information to at least six distinct cortical and subcortical areas, each mediating different forms of learning and memory. Characterizing the occipitotemporal pathway as a recurrent network parsimoniously accounts for a number of its functional properties, and specification of its output targets provides insight into the function not only of those targets but also of the pathway itself (Text Box 1). In this final section, we highlight new empirical and theoretical considerations that arise from this expanded framework.
One question concerns the functional consequences of the complex intrinsic connectivity beyond providing multiple routes for the flow of visual information, though this redundancy almost certainly underlies the striking recovery of function observed following lesions of the network but not of its output targets (e.g.[197, 279]). In particular, the connectivity enables distinct areas to carry out specialized processing of distinct aspects of stimuli (e.g. motion, shape) that are eventually synthesized into unified representations within neuronal populations in aIT and/or the medial temporal lobe[209]. This leads to a developmental theory of the functions of the alternate and central routes. Early in development the central route might be very important for creating representations of complex stimuli in aIT via the iterative aggregation prominent in some extant models of the central route (e.g.[13]). However, later in development, as a result of learned associations with representations in the alternate routes, these routes alone might be sufficient to drive complex representations in aIT even in the absence of the central route (e.g.[38]).
We have highlighted the likely contribution of visual information from the occipitotemporal network to the function of the target structures, but the proposed framework also raises questions about how signals from the target structures constrain and modify stimulus representations within the occipitotemporal network. First, the framework helps to explain why learning and task effects within the occipitotemporal network are generally small (see[280] for a review). In contrast to the occipitoparietal network[5], much of the occipitotemporal network does not receive direct feedback from the output targets. While many regions receive efferents from the amygdala, only a subset of the more rostral regions receive efferents from the other output targets (excepting striatum). There are projections from all visual areas to the frontal eye-field, but the projections from the occipitotemporal network are generally much sparser than those from the occipitoparietal network[281]. Thus, the early and intermediate regions of the network receive largely indirect feedback about the utility of their representations for processing by the target regions, let alone for guiding adaptive action. This lack of direct feedback might allow these areas to more faithfully reflect the statistics of visual experience, creating stimulus representations that are more general and capable of contributing to adaptive action in many different contexts. But it also implies that feedback-dependent changes (e.g. learning) within these areas will be smaller and take longer to develop than in areas with more direct feedback[280].
The extrinsic connectivity also raises interesting questions about how feedback contributes to its early ontogeny. The major projection targets with feedback connections (amygdala, medial temporal, orbitofrontal, and ventrolateral prefrontal) receive the majority of their visual input from the most rostral regions of the occipitotemporal network. Given that visual responses in these rostral regions are likely undeveloped early in life (e.g.[282]), the informativeness of any visual feedback from the output targets is questionable. However, the orbitofrontal and anterior insula cortex are strongly tied to gustation, olfaction, and visceral sensation (see[160, 235] for reviews), senses that are likely well formed early in life. These cortices might provide multimodal information that constrains the early development of the occipitotemporal pathway, reinforcing visual representations associated with strongly positive or negative stimuli in these modalities.
Finally, although this review and our previous review of the dorsal visual pathway[5] have focused exclusively on vision, the split we have described between dorsal and ventral processing (Text Box 1) may also apply to other sensory modalities. There is strong evidence that many areas within the parietal cortex may play critical roles in both auditory and somatosensory processing in addition to vision. A comparison of the analogous dorsal/ventral divisions in other senses with those in vision may well lead to new insights into the functions served by ventral and dorsal pathways within and across modalities.
Outstanding Questions Box.
What is the homology or lack thereof in the visual properties of the aIT in human and monkey?
What are the visual properties of the major occipitotemporal output targets in human?
How do the major occipitotemporal output targets constrain the functional organization and processing within the ventral visual pathway?
How do the necessity and sufficiency of the components of the ventral visual pathway for its function change over the course of development?
Do homologous splits between dorsal and ventral processing occur in sensory modalities other than of vision?
Acknowledgements
We wish to thank B. Averbeck, M. Behrmann, M. Goodale, A. Harel, O. Hikosaka, D. Leopold and A. Martin for their extremely helpful comments. This research was supported by the Intramural Research Program of the US National Institutes of Health (NIH), National Institute of Mental Health (NIMH), US National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ungerleider LG, Mishkin M. Two Cortical Visual Systems. In: Ingle DJ, et al., editors. Analysis of Visual Behavior. The MIT Press; 1982. pp. 549–586. [Google Scholar]
- 2.Mishkin M, et al. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- 3.Macko KA, et al. Mapping the primate visual system with [2-14C]deoxyglucose. Science. 1982;218:394–397. doi: 10.1126/science.7123241. [DOI] [PubMed] [Google Scholar]
- 4.Goodale MA, et al. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr Biol. 1994;4:604–610. doi: 10.1016/s0960-9822(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz DJ, et al. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Haan EH, Cowey A. On the usefulness of 'what' and 'where' pathways in vision. Trends Cogn Sci. 2011;15:460–466. doi: 10.1016/j.tics.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Serre T, et al. A feedforward architecture accounts for rapid categorization. Proc Natl Acad Sci U S A. 2007;104:6424–6429. doi: 10.1073/pnas.0700622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. Eur J Neurosci. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- 9.Rousselet GA, et al. How parallel is visual processing in the ventral pathway? Trends in Cognitive Sciences. 2004;8:363–370. doi: 10.1016/j.tics.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Yau JM, et al. Curvature Processing Dynamics in Macaque Area V4. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brincat SL, Connor CE. Underlying principles of visual shape selectivity in posterior inferotemporal cortex. Nat Neurosci. 2004;7:880–886. doi: 10.1038/nn1278. [DOI] [PubMed] [Google Scholar]
- 12.DiCarlo JJ, et al. How does the brain solve visual object recognition? Neuron. 2012;73:415–434. doi: 10.1016/j.neuron.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2:1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- 14.Rousselet GA, et al. How parallel is visual processing in the ventral pathway? Trends Cogn Sci. 2004;8:363–370. doi: 10.1016/j.tics.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- 16.Distler C, et al. Cortical connections of inferior temporal area TEO in macaque monkeys. J Comp Neurol. 1993;334:125–150. doi: 10.1002/cne.903340111. [DOI] [PubMed] [Google Scholar]
- 17.Kondo H, et al. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- 18.Rockland KS, Virga A. Organization of individual cortical axons projecting from area V1 (area 17) to V2 (area 18) in the macaque monkey. Vis Neurosci. 1990;4:11–28. doi: 10.1017/s095252380000273x. [DOI] [PubMed] [Google Scholar]
- 19.Rockland KS. Configuration, in serial reconstruction, of individual axons projecting from area V2 to V4 in the macaque monkey. Cereb Cortex. 1992;2:353–374. doi: 10.1093/cercor/2.5.353. [DOI] [PubMed] [Google Scholar]
- 20.Saleem KS, et al. Specific and columnar projection from area TEO to TE in the macaque inferotemporal cortex. Cereb Cortex. 1993;3:454–464. doi: 10.1093/cercor/3.5.454. [DOI] [PubMed] [Google Scholar]
- 21.Kuypers HG, et al. Occipitotemporal Corticocortical Connections in the Rhesus Monkey. Exp Neurol. 1965;11:245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- 22.Zeki SM. The cortical projections of foveal striate cortex in the rhesus monkey. J Physiol. 1978;277:227–244. doi: 10.1113/jphysiol.1978.sp012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 24.Van Essen DC, et al. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- 25.Ungerleider LG, et al. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- 26.Baizer JS, et al. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shipp S. Structure and function of the cerebral cortex. Curr Biol. 2007;17:R443–R449. doi: 10.1016/j.cub.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H, et al. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaques. J Neurosci. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- 31.Shipp S, Zeki S. The Organization of Connections between Areas V5 and V1 in Macaque Monkey Visual Cortex. Eur J Neurosci. 1989;1:309–332. doi: 10.1111/j.1460-9568.1989.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 32.Sincich LC, Horton JC. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J Neurosci. 2003;23:5684–5692. doi: 10.1523/JNEUROSCI.23-13-05684.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster MJ, et al. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem KS, et al. Connections between anterior inferotemporal cortex and superior temporal sulcus regions in the macaque monkey. J Neurosci. 2000;20:5083–5101. doi: 10.1523/JNEUROSCI.20-13-05083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boussaoud D, et al. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J Comp Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- 36.Webster MJ, et al. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J Comp Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertini G, et al. Visual responses to targets and distracters by inferior temporal neurons after lesions of extrastriate areas V4 and TEO. Neuroreport. 2004;15:1611–1615. doi: 10.1097/01.wnr.0000134847.86625.15. [DOI] [PubMed] [Google Scholar]
- 39.Buffalo EA, et al. Impaired filtering of distracter stimuli by TE neurons following V4 and TEO lesions in macaques. Cereb Cortex. 2005;15:141–151. doi: 10.1093/cercor/bhh117. [DOI] [PubMed] [Google Scholar]
- 40.Mishkin M. Cortical visual areas and their interactions. In: Karczmar AG, Eccles JC, editors. Brain and Human Behavior. Springer-Verlag; 1972. [Google Scholar]
- 41.Hegde J, Van Essen DC. A comparative study of shape representation in macaque visual areas v2 and v4. Cereb Cortex. 2007;17:1100–1116. doi: 10.1093/cercor/bhl020. [DOI] [PubMed] [Google Scholar]
- 42.Yamane Y, et al. A neural code for three-dimensional object shape in macaque inferotemporal cortex. Nat Neurosci. 2008;11:1352–1360. doi: 10.1038/nn.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kravitz DJ, et al. High-level object representations are constrained by position. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afraz A, et al. Spatial heterogeneity in the perception of face and form attributes. Curr Biol. 2010;20:2112–2116. doi: 10.1016/j.cub.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan AW, et al. Cortical representations of bodies and faces are strongest in commonly experienced configurations. Nat Neurosci. 2010;13:417–418. doi: 10.1038/nn.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kravitz DJ, et al. How position dependent is visual object recognition? Trends Cogn Sci. 2008;12:114–122. doi: 10.1016/j.tics.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Lavidor M, Walsh V. The nature of foveal representation. Nature reviews. 2004;5:729–735. doi: 10.1038/nrn1498. [DOI] [PubMed] [Google Scholar]
- 48.Wandell BA, et al. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Fize D, et al. The retinotopic organization of primate dorsal V4 and surrounding areas: A functional magnetic resonance imaging study in awake monkeys. J Neurosci. 2003;23:7395–7406. doi: 10.1523/JNEUROSCI.23-19-07395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewer AA, et al. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J Neurosci. 2002;22:10416–10426. doi: 10.1523/JNEUROSCI.22-23-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orban GA, et al. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Kolster H, et al. The retinotopic organization of the human middle temporal area MT/V5 and its cortical neighbors. J Neurosci. 2010;30:9801–9820. doi: 10.1523/JNEUROSCI.2069-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winawer J, et al. Mapping hV4 and ventral occipital cortex: the venous eclipse. J Vis. 2010;10:1. doi: 10.1167/10.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen KA, et al. Topographic organization in and near human visual area V4. J Neurosci. 2007;27:11896–11911. doi: 10.1523/JNEUROSCI.2991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewer AA, et al. Visual field maps and stimulus selectivity in human ventral occipital cortex. Nat Neurosci. 2005;8:1102–1109. doi: 10.1038/nn1507. [DOI] [PubMed] [Google Scholar]
- 56.Hemond CC, et al. A preference for contralateral stimuli in human object-and face-selective cortex. PLoS ONE. 2007;2:e574. doi: 10.1371/journal.pone.0000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niemeier M, et al. A contralateral preference in the lateral occipital area: sensory and attentional mechanisms. Cereb Cortex. 2005;15:325–331. doi: 10.1093/cercor/bhh134. [DOI] [PubMed] [Google Scholar]
- 58.Swisher JD, et al. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boussaoud D, et al. Visual topography of area TEO in the macaque. The Journal of comparative neurology. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- 60.Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral-cortex. Journal of Neurophysiology. 1994;71:856–867. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka K, et al. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. Journal of Neurophysiology. 1991;66:170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- 62.Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain research. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- 63.Op De Beeck H, Vogels R. Spatial sensitivity of macaque inferior temporal neurons. Journal of Comparative Neurology. 2000;426:505–518. doi: 10.1002/1096-9861(20001030)426:4<505::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 64.Merigan WH, Saunders RC. Unilateral deficits in visual perception and learning after unilateral inferotemporal cortex lesions in macaques. Cerebral Cortex. 2004;14:863–871. doi: 10.1093/cercor/bhh045. [DOI] [PubMed] [Google Scholar]
- 65.Cichy RM, et al. Encoding the identity and location of objects in human LOC. Neuroimage. 2011;54:2297–2307. doi: 10.1016/j.neuroimage.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 66.Sayres R, Grill-Spector K. Relating retinotopic and object-selective responses in human lateral occipital cortex. J Neurophysiol. 2008;100:249–267. doi: 10.1152/jn.01383.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larsson J, Heeger DJ. Two retinotopic visual areas in human lateral occipital cortex. J Neurosci. 2006;26:13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hasson U, et al. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- 69.Levy I, et al. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- 70.Sato N, Nakamura K. Visual response properties of neurons in the parahippocampal cortex of monkeys. J Neurophysiol. 2003;90:876–886. doi: 10.1152/jn.01089.2002. [DOI] [PubMed] [Google Scholar]
- 71.Park S, et al. Disentangling scene content from spatial boundary: complementary roles for the parahippocampal place area and lateral occipital complex in representing real-world scenes. J Neurosci. 2011;31:1333–1340. doi: 10.1523/JNEUROSCI.3885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kravitz DJ, et al. Real-world scene representations in high-level visual cortex -- it's the spaces not the places. 2010 doi: 10.1523/JNEUROSCI.4588-10.2011. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Konkle T, Oliva A. A real-world size organization of object responses in occipito-temporal cortex. Neuron. doi: 10.1016/j.neuron.2012.04.036. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor JC, Downing PE. Division of labor between lateral and ventral extrastriate representations of faces, bodies, and objects. J Cogn Neurosci. 2011;23:4122–4137. doi: 10.1162/jocn_a_00091. [DOI] [PubMed] [Google Scholar]
- 76.Malach R, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci U S A. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwarzlose RF, et al. The distribution of category and location information across object-selective regions in human visual cortex. Proc Natl Acad Sci U S A. 2008;105:4447–4452. doi: 10.1073/pnas.0800431105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arcaro MJ, et al. Retinotopic organization of human ventral visual cortex. J Neurosci. 2009;29:10638–10652. doi: 10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown LE, et al. Peripheral vision for perception and action. Exp Brain Res. 2005;165:97–106. doi: 10.1007/s00221-005-2285-y. [DOI] [PubMed] [Google Scholar]
- 80.Khan MA, Lawrence GP. Differences in visuomotor control between the upper and lower visual fields. Exp Brain Res. 2005;164:395–398. doi: 10.1007/s00221-005-2325-7. [DOI] [PubMed] [Google Scholar]
- 81.Danckert J, Goodale MA. Superior performance for visually guided pointing in the lower visual field. Exp Brain Res. 2001;137:303–308. doi: 10.1007/s002210000653. [DOI] [PubMed] [Google Scholar]
- 82.Edelman S, Intrator N. (Coarse coding of shape fragments) + (retinotopy) approximately = representation of structure. Spat Vis. 2000;13:255–264. doi: 10.1163/156856800741072. [DOI] [PubMed] [Google Scholar]
- 83.Bell AH, et al. Relationship between functional magnetic resonance imaging-identified regions and neuronal category selectivity. J Neurosci. 2011;31:12229–12240. doi: 10.1523/JNEUROSCI.5865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsao DY, et al. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci U S A. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Downing PE, et al. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- 86.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 87.Mahon BZ, et al. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conway BR, et al. Specialized color modules in macaque extrastriate cortex. Neuron. 2007;56:560–573. doi: 10.1016/j.neuron.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphey DK, et al. Perception matches selectivity in the human anterior color center. Curr Biol. 2008;18:216–220. doi: 10.1016/j.cub.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 90.Fox CJ, et al. Perceptual and anatomic patterns of selective deficits in facial identity and expression processing. Neuropsychologia. 2011;49:3188–3200. doi: 10.1016/j.neuropsychologia.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 91.Barton JJ, et al. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58:71–78. doi: 10.1212/wnl.58.1.71. [DOI] [PubMed] [Google Scholar]
- 92.Pitcher D, et al. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr Biol. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Afraz SR, et al. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 94.Op de Beeck HP, et al. Interpreting fMRI data: maps, modules and dimensions. Nat Rev Neurosci. 2008;9:123–135. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kourtzi Z, Connor CE. Neural representations for object perception: structure, category, and adaptive coding. Annu Rev Neurosci. 2011;34:45–67. doi: 10.1146/annurev-neuro-060909-153218. [DOI] [PubMed] [Google Scholar]
- 96.Chklovskii DB, Koulakov AA. Maps in the brain: what can we learn from them? Annu Rev Neurosci. 2004;27:369–392. doi: 10.1146/annurev.neuro.27.070203.144226. [DOI] [PubMed] [Google Scholar]
- 97.Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proc Natl Acad Sci U S A. 2010;107:11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker CI, et al. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci U S A. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Srihasam K, et al. Behavioral and anatomical consequences of early versus late symbol training in macaques. Neuron. 2012;73:608–619. doi: 10.1016/j.neuron.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- 101.Haxby JV, et al. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 102.Plaut DC, Behrmann M. Complementary neural representations for faces and words: a computational exploration. Cogn Neuropsychol. 2011;28:251–275. doi: 10.1080/02643294.2011.609812. [DOI] [PubMed] [Google Scholar]
- 103.Werth R. Visual functions without the occipital lobe or after cerebral hemispherectomy in infancy. Eur J Neurosci. 2006;24:2932–2944. doi: 10.1111/j.1460-9568.2006.05171.x. [DOI] [PubMed] [Google Scholar]
- 104.Thomas C, et al. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kravitz DJ, et al. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011 doi: 10.1038/nrn3008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saygin ZM, et al. Anatomical connectivity patterns predict face selectivity in the fusiform gyrus. Nat Neurosci. 2011;15:321–327. doi: 10.1038/nn.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Polk TA, et al. Nature versus nurture in ventral visual cortex: a functional magnetic resonance imaging study of twins. J Neurosci. 2007;27:13921–13925. doi: 10.1523/JNEUROSCI.4001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitcher D, et al. Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. J Neurosci. 2008;28:8929–8933. doi: 10.1523/JNEUROSCI.1450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moeller S, et al. Patches with links: a unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu H, et al. Timing, timing, timing: fast decoding of object information from intracranial field potentials in human visual cortex. Neuron. 2009;62:281–290. doi: 10.1016/j.neuron.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 112.Gandhi SP, et al. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proc Natl Acad Sci U S A. 2006;103:11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muller NG, Kleinschmidt A. Dynamic interaction of object- and spacebased attention in retinotopic visual areas. J Neurosci. 2003;23:9812–9816. doi: 10.1523/JNEUROSCI.23-30-09812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kravitz DJ, Behrmann M. Space-, object-, and feature-based attention interact to organize visual scenes. Atten Percept Psychophys. 2011;73:2434–2447. doi: 10.3758/s13414-011-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kravitz DJ, Behrmann M. The space of an object: object attention alters the spatial gradient in the surround. J Exp Psychol Hum Percept Perform. 2008;34:298–309. doi: 10.1037/0096-1523.34.2.298. [DOI] [PubMed] [Google Scholar]
- 119.Simons DJ, et al. Change blindness in the absence of a visual disruption. Perception. 2000;29:1143–1154. doi: 10.1068/p3104. [DOI] [PubMed] [Google Scholar]
- 120.Levin DT, Simons DJ. Failure to detect changes to people in a real-world interaction. Psychon. Bull. Rev. 1998;5:644–649. [Google Scholar]
- 121.Triesch J, et al. What you see is what you need. J Vis. 2003;3:86–94. doi: 10.1167/3.1.9. [DOI] [PubMed] [Google Scholar]
- 122.Beck DM, et al. Neural correlates of change detection and change blindness. Nat Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- 123.Murray SO, et al. The representation of perceived angular size in human primary visual cortex. Nat Neurosci. 2006;9:429–434. doi: 10.1038/nn1641. [DOI] [PubMed] [Google Scholar]
- 124.Fang F, et al. Attention-dependent representation of a size illusion in human V1. Curr Biol. 2008;18:1707–1712. doi: 10.1016/j.cub.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee TS, et al. Neural activity in early visual cortex reflects behavioral experience and higher-order perceptual saliency. Nat Neurosci. 2002;5:589–597. doi: 10.1038/nn0602-860. [DOI] [PubMed] [Google Scholar]
- 126.Zipser K, et al. Contextual modulation in primary visual cortex. J Neurosci. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bacon-Mace N, et al. The time course of visual processing: backward masking and natural scene categorisation. Vision Res. 2005;45:1459–1469. doi: 10.1016/j.visres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Grill-Spector K, et al. The dynamics of object-selective activation correlate with recognition performance in humans. Nat Neurosci. 2000;3:837–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- 129.Rolls ET, et al. The neurophysiology of backward visual masking: information analysis. J Cogn Neurosci. 1999;11:300–311. doi: 10.1162/089892999563409. [DOI] [PubMed] [Google Scholar]
- 130.Super H, et al. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- 131.Kovacs G, et al. Cortical correlate of pattern backward masking. Proc Natl Acad Sci U S A. 1995;92:5587–5591. doi: 10.1073/pnas.92.12.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keysers C, Perrett DI. Visual masking and RSVP reveal neural competition. Trends Cogn Sci. 2002;6:120–125. doi: 10.1016/s1364-6613(00)01852-0. [DOI] [PubMed] [Google Scholar]
- 133.Fahrenfort JJ, et al. Masking disrupts reentrant processing in human visual cortex. J Cogn Neurosci. 2007;19:1488–1497. doi: 10.1162/jocn.2007.19.9.1488. [DOI] [PubMed] [Google Scholar]
- 134.Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 135.Van Hoesen GW, et al. Temporal cortical projections to the olfactory tubercle in the rhesus monkey. Brain Res. 1976;109:375–381. doi: 10.1016/0006-8993(76)90537-0. [DOI] [PubMed] [Google Scholar]
- 136.Van Hoesen GW, et al. Widespread corticostriate projections from temporal cortex of the rhesus monkey. J Comp Neurol. 1981;199:205–219. doi: 10.1002/cne.901990205. [DOI] [PubMed] [Google Scholar]
- 137.Saint-Cyr JA, et al. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol. 1990;298:129–156. doi: 10.1002/cne.902980202. [DOI] [PubMed] [Google Scholar]
- 138.Baizer JS, et al. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Vis Neurosci. 1993;10:59–72. doi: 10.1017/s0952523800003229. [DOI] [PubMed] [Google Scholar]
- 139.Yeterian EH, Pandya DN. Corticostriatal connections of extrastriate visual areas in rhesus monkeys. J Comp Neurol. 1995;352:436–457. doi: 10.1002/cne.903520309. [DOI] [PubMed] [Google Scholar]
- 140.Cheng K, et al. Organization of corticostriatal and corticoamygdalar projections arising from the anterior inferotemporal area TE of the macaque monkey: a Phaseolus vulgaris leucoagglutinin study. J Neurosci. 1997;17:7902–7925. doi: 10.1523/JNEUROSCI.17-20-07902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung Y, Hong S. Ventral striatal connections of unimodal and multimodal cortex of the superior temporal sulcus in the Macaque monkeys. Korean J Biol Sci. 2004;8:319–328. [Google Scholar]
- 142.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 143.Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neurosci Biobehav Rev. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seger CA, Miller EK. Category learning in the brain. Annu Rev Neurosci. 2010;33:203–219. doi: 10.1146/annurev.neuro.051508.135546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci U S A. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shohamy D. Learning and motivation in the human striatum. Curr Opin Neurobiol. 2011;21:408–414. doi: 10.1016/j.conb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 147.Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stocco A, et al. Conditional routing of information to the cortex: a model of the basal ganglia's role in cognitive coordination. Psychol Rev. 2010;117:541–574. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fernandez-Ruiz J, et al. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proc Natl Acad Sci U S A. 2001;98:4196–4201. doi: 10.1073/pnas.061022098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ashby FG, Maddox WT. Human category learning. Annu Rev Psychol. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- 151.Divac I, et al. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- 152.Buerger AA, et al. Effects of ventral putamen lesions on discrimination learning by monkeys. J Comp Physiol Psychol. 1974;86:440–446. doi: 10.1037/h0036142. [DOI] [PubMed] [Google Scholar]
- 153.Teng E, et al. Contrasting effects on discrimination learning after hippocampal lesions and conjoint hippocampal-caudate lesions in monkeys. J Neurosci. 2000;20:3853–3863. doi: 10.1523/JNEUROSCI.20-10-03853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brown VJ, et al. Responses of cells in the tail of the caudate nucleus during visual discrimination learning. J Neurophysiol. 1995;74:1083–1094. doi: 10.1152/jn.1995.74.3.1083. [DOI] [PubMed] [Google Scholar]
- 155.Yamamoto S, et al. What and where information in the caudate tail guides saccades to visual objects. J Neurosci. 2012;32:11005–11016. doi: 10.1523/JNEUROSCI.0828-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muhammad R, et al. A comparison of abstract rules in the prefrontal cortex, premotor cortex, inferior temporal cortex, and striatum. J Cogn Neurosci. 2006;18:974–989. doi: 10.1162/jocn.2006.18.6.974. [DOI] [PubMed] [Google Scholar]
- 157.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 158.Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. 2006;9:562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- 159.Levy R, et al. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 161.Haber SN, et al. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 163.Ferry AT, et al. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 164.Freedman LJ, et al. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 165.Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 166.Williams GV, et al. Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res. 1993;55:243–252. doi: 10.1016/0166-4328(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 167.Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- 168.Apicella P, et al. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- 169.Hassani OK, et al. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol. 2001;85:2477–2489. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- 170.Schultz W, et al. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cai X, et al. Heterogeneous coding of temporally discounted values in the dorsal and ventral striatum during intertemporal choice. Neuron. 2011;69:170–182. doi: 10.1016/j.neuron.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Day JJ, et al. Nucleus accumbens neurons encode predicted and ongoing reward costs in rats. Eur J Neurosci. 2011;33:308–321. doi: 10.1111/j.1460-9568.2010.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shidara M, et al. Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. J Neurosci. 1998;18:2613–2625. doi: 10.1523/JNEUROSCI.18-07-02613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cardinal RN, et al. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- 175.Christakou A, et al. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- 177.Stern CE, Passingham RE. The nucleus accumbens in monkeys (Macaca fascicularis). III. Reversal learning. Exp Brain Res. 1995;106:239–247. doi: 10.1007/BF00241119. [DOI] [PubMed] [Google Scholar]
- 178.Liu X, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Britton JC, et al. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 180.Axmacher N, et al. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65:541–549. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 181.Sabatinelli D, et al. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- 182.Iwai E, Yukie M. Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata, M. mulatta, and M. fascicularis) J Comp Neurol. 1987;261:362–387. doi: 10.1002/cne.902610304. [DOI] [PubMed] [Google Scholar]
- 183.Amaral DG, et al. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 184.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 185.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comp Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 186.Bzdok D, et al. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Morrison SE, Salzman CD. Re-valuing the amygdala. Curr Opin Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pessoa L. Emotion and cognition and the amygdala: from "what is it?" to "what's to be done?". Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a 'low road' to 'many roads' of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nishijo H, et al. Topographic distribution of modality-specific amygdalar neurons in alert monkey. J Neurosci. 1988;8:3556–3569. doi: 10.1523/JNEUROSCI.08-10-03556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mosher CP, et al. Response characteristics of basolateral and centromedial neurons in the primate amygdala. J Neurosci. 2010;30:16197–16207. doi: 10.1523/JNEUROSCI.3225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gothard KM, et al. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 193.Paton JJ, et al. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kazama AM, Bachevalier J. Preserved stimulus-reward and reversal learning after selective neonatal orbital frontal areas 11/13 or amygdala lesions in monkeys. Dev Cogn Neurosci. 2012;2:363–380. doi: 10.1016/j.dcn.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murray EA, et al. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bliss-Moreau E, et al. Neonatal amygdala lesions alter responsiveness to objects in juvenile macaques. Neuroscience. 2011;178:123–132. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murray EA, Wise SP. Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors. Curr Opin Neurobiol. 2010;20:212–220. doi: 10.1016/j.conb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Duncan S, Barrett LF. The role of the amygdala in visual awareness. Trends Cogn Sci. 2007;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vuilleumier P, et al. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 201.Saleem KS, Tanaka K. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 1996;16:4757–4775. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kondo H, et al. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- 203.Yoshida M, et al. Anatomical organization of forward fiber projections from area TE to perirhinal neurons representing visual long-term memory in monkeys. Proc Natl Acad Sci U S A. 2003;100:4257–4262. doi: 10.1073/pnas.0736457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Insausti R, et al. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- 205.Insausti R, Amaral DG. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. J Comp Neurol. 2008;509:608–641. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Leonard BW, et al. Transient memory impairment in monkeys with bilateral lesions of the entorhinal cortex. J Neurosci. 1995;15:5637–5659. doi: 10.1523/JNEUROSCI.15-08-05637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Witter MP, et al. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- 209.Quiroga RQ, et al. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 210.Barense MD, et al. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Knutson AR, et al. Visual discrimination performance, memory, and medial temporal lobe function. Proc Natl Acad Sci U S A. 2012;109:13106–13111. doi: 10.1073/pnas.1208876109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yukie M. Connections between the medial temporal cortex and the CA1 subfield of the hippocampal formation in the Japanese monkey (Macaca fuscata) J Comp Neurol. 2000;423:282–298. doi: 10.1002/1096-9861(20000724)423:2<282::aid-cne7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 213.Zhong YM, Rockland KS. Connections between the anterior inferotemporal cortex (area TE) and CA1 of the hippocampus in monkey. Exp Brain Res. 2004;155:311–319. doi: 10.1007/s00221-003-1728-6. [DOI] [PubMed] [Google Scholar]
- 214.Ichinohe N, Rockland KS. Zinc-enriched amygdalo- and hippocampocortical connections to the inferotemporal cortices in macaque monkey. Neurosci Res. 2005;53:57–68. doi: 10.1016/j.neures.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 215.Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 216.Moscovitch M, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meunier M, et al. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- 219.Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 220.Eichenbaum H, et al. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sauvage MM, et al. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci. 2008;11:16–18. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wixted JT, Squire LR. The role of the human hippocampus in familiarity-based and recollection-based recognition memory. Behav Brain Res. 2010;215:197–208. doi: 10.1016/j.bbr.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Smith CN, et al. The hippocampus supports both recollection and familiarity when memories are strong. J Neurosci. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bakker A, et al. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee I, Solivan F. Dentate gyrus is necessary for disambiguating similar object-place representations. Learn Mem. 2010;17:252–258. doi: 10.1101/lm.1678210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aimone JB, et al. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Saleem KS, et al. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 232.Webster MJ, et al. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 233.Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- 234.Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- 235.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 236.Schultz W, et al. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- 237.Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- 238.Rudebeck PH, Murray EA. Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values. Ann N Y Acad Sci. 2011;1239:1–13. doi: 10.1111/j.1749-6632.2011.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Walton ME, et al. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci. 2011;1239:14–24. doi: 10.1111/j.1749-6632.2011.06257.x. [DOI] [PubMed] [Google Scholar]
- 240.Young JJ, Shapiro ML. The orbitofrontal cortex and response selection. Ann N Y Acad Sci. 2011;1239:25–32. doi: 10.1111/j.1749-6632.2011.06279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Balleine BW, et al. The orbitofrontal cortex, predicted value, and choice. Ann N Y Acad Sci. 2011;1239:43–50. doi: 10.1111/j.1749-6632.2011.06270.x. [DOI] [PubMed] [Google Scholar]
- 242.Fellows LK. Orbitofrontal contributions to value-based decision making: evidence from humans with frontal lobe damage. Ann N Y Acad Sci. 2011;1239:51–58. doi: 10.1111/j.1749-6632.2011.06229.x. [DOI] [PubMed] [Google Scholar]
- 243.Rygula R, et al. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Clarke HF, et al. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hornak J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 246.Tsuchida A, et al. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–16875. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kennerley SW, et al. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kazama A, Bachevalier J. Selective aspiration or neurotoxic lesions of orbital frontal areas 11 and 13 spared monkeys' performance on the object discrimination reversal task. J Neurosci. 2009;29:2794–2804. doi: 10.1523/JNEUROSCI.4655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Walton ME, et al. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.West EA, et al. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci. 2011;31:15128–15135. doi: 10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Noonan MP, et al. Distinct roles of three frontal cortical areas in rewardguided behavior. J Neurosci. 2011;31:14399–14412. doi: 10.1523/JNEUROSCI.6456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gerbella M, et al. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb Cortex. 2010;20:141–168. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- 255.Borra E, et al. Anatomical evidence for the involvement of the macaque ventrolateral prefrontal area 12r in controlling goal-directed actions. J Neurosci. 2011;31:12351–12363. doi: 10.1523/JNEUROSCI.1745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rushworth MF, et al. Attentional selection and action selection in the ventral and orbital prefrontal cortex. J Neurosci. 2005;25:11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Muller NG, et al. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J Cogn Neurosci. 2002;14:673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- 258.du Boisgueheneuc F, et al. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- 259.Volle E, et al. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cereb Cortex. 2008;18:2460–2469. doi: 10.1093/cercor/bhn010. [DOI] [PubMed] [Google Scholar]
- 260.Rossi AF, et al. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27:11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.O'Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 264.Minamimoto T, et al. Monkeys quickly learn and generalize visual categories without lateral prefrontal cortex. Neuron. 2010;66:501–507. doi: 10.1016/j.neuron.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 265.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 266.Rao SC, et al. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- 267.Romanski LM. Domain specificity in the primate prefrontal cortex. Cogn Affect Behav Neurosci. 2004;4:421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- 268.Scalaidhe SP, et al. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 1999;9:459–475. doi: 10.1093/cercor/9.5.459. [DOI] [PubMed] [Google Scholar]
- 269.Nelissen K, et al. Observing others: multiple action representation in the frontal lobe. Science. 2005;310:332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- 270.Wilson FA, et al. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 271.Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- 272.Passingham RE, et al. Specialisation within the prefrontal cortex: the ventral prefrontal cortex and associative learning. Exp Brain Res. 2000;133:103–113. doi: 10.1007/s002210000405. [DOI] [PubMed] [Google Scholar]
- 273.Genovesio A, et al. Prefrontal cortex activity during the discrimination of relative distance. J Neurosci. 2011;31:3968–3980. doi: 10.1523/JNEUROSCI.5373-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Meyer T, et al. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci. 2011;31:6266–6276. doi: 10.1523/JNEUROSCI.6798-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mishkin M, Manning FJ. Non-spatial memory after selective prefrontal lesions in monkeys. Brain Res. 1978;143:313–323. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- 276.Tsao DY, et al. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Romanski LM, Diehl MM. Neurons responsive to face-view in the primate ventrolateral prefrontal cortex. Neuroscience. 2011;189:223–235. doi: 10.1016/j.neuroscience.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chan AW, Downing PE. Faces and eyes in human lateral prefrontal cortex. Front Hum Neurosci. 2011;5:51. doi: 10.3389/fnhum.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Malkova L, et al. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behav Neurosci. 1995;109:212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- 280.Op de Beeck HP, Baker CI. The neural basis of visual object learning. Trends Cogn Sci. 2010;14:22–30. doi: 10.1016/j.tics.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schall JD, et al. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kourtzi Z, et al. Development of visually evoked cortical activity in infant macaque monkeys studied longitudinally with fMRI. Magn Reson Imaging. 2006;24:359–366. doi: 10.1016/j.mri.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 283.Weisberg J, et al. A neural system for learning about object function. Cereb Cortex. 2007;17:513–521. doi: 10.1093/cercor/bhj176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dehaene S. The Number Sense: How the Mind Creates Mathematics. Oxford University Press; 2011. [Google Scholar]
- 285.Harvey CD, et al. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rogalsky C, et al. Functional anatomy of language and music perception: temporal and structural factors investigated using functional magnetic resonance imaging. J Neurosci. 2011;31:3843–3852. doi: 10.1523/JNEUROSCI.4515-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kristensen LB, et al. The Interface Between Language and Attention: Prosodic Focus Marking Recruits a General Attention Network in Spoken Language Comprehension. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs164. [DOI] [PubMed] [Google Scholar]
- 288.Olman CA, et al. Distortion and signal loss in medial temporal lobe. PLoS One. 2009;4:e8160. doi: 10.1371/journal.pone.0008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Visser M, et al. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- 290.McIntosh AR, et al. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Skipper LM, et al. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49:3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Anzellotti S, et al. Differential activity for animals and manipulable objects in the anterior temporal lobes. J Cogn Neurosci. 2011;23:2059–2067. doi: 10.1162/jocn.2010.21567. [DOI] [PubMed] [Google Scholar]
- 293.Rajimehr R, et al. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci U S A. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Simmons WK, et al. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kriegeskorte N, et al. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Williams MA, et al. Abnormal configural face perception in a patient with right anterior temporal lobe atrophy. Neurocase. 2006;12:286–291. doi: 10.1080/13554790601026379. [DOI] [PubMed] [Google Scholar]
- 297.Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia. 2007;45:1591–1607. doi: 10.1016/j.neuropsychologia.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 298.Snowden JS, et al. Famous people knowledge and the right and left temporal lobes. Behav Neurol. 2012;25:35–44. doi: 10.3233/BEN-2012-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Rotshtein P, et al. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 300.Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- 301.Anzai A, DeAngelis GC. Neural computations underlying depth perception. Curr Opin Neurobiol. 20:367–375. doi: 10.1016/j.conb.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mysore SG, et al. The selectivity of neurons in the macaque fundus of the superior temporal area for three-dimensional structure from motion. J Neurosci. 2010;30:15491–15508. doi: 10.1523/JNEUROSCI.0820-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Orban GA, et al. Extracting 3D structure from disparity. Trends Neurosci. 2006;29:466–473. doi: 10.1016/j.tins.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 304.Farivar R, et al. Dorsal-ventral integration in the recognition of motiondefined unfamiliar faces. J Neurosci. 2009;29:5336–5342. doi: 10.1523/JNEUROSCI.4978-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nat Neurosci. 2008;11:224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- 306.Konen CS, et al. The functional neuroanatomy of object agnosia: a case study. Neuron. 2011;71:49–60. doi: 10.1016/j.neuron.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Srivastava S, et al. A distinct representation of three-dimensional shape in macaque anterior intraparietal area: fast, metric, and coarse. J Neurosci. 2009;29:10613–10626. doi: 10.1523/JNEUROSCI.6016-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Aguirre GK, D'Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(Pt 9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- 309.Harel A, et al. Deconstructing Visual Scenes in Cortex: Gradients of Object and Spatial Layout Information. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Barrash J, et al. The neuroanatomical correlates of route learning impairment. Neuropsychologia. 2000;38:820–836. doi: 10.1016/s0028-3932(99)00131-1. [DOI] [PubMed] [Google Scholar]
- 311.Deco G, Lee TS. The role of early visual cortex in visual integration: a neural model of recurrent interaction. Eur J Neurosci. 2004;20:1089–1100. doi: 10.1111/j.1460-9568.2004.03528.x. [DOI] [PubMed] [Google Scholar]
- 312.Hikosaka K. Responsiveness of neurons in the posterior inferotemporal cortex to visual patterns in the macaque monkey. Behav Brain Res. 1997;89:275–283. doi: 10.1016/s0166-4328(97)00073-9. [DOI] [PubMed] [Google Scholar]
- 313.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]