Abstract
Child hypothalamic-pituitary-adrenal (HPA) activity was investigated as a moderator of parental depressive symptom effects on child behavior in an adoption sample ( n =210 families). Adoptive parents’ depressive symptoms and child internalizing and externalizing were assessed at 18, 27, and 54 months, and child morning and evening HPA activity measured through salivary cortisol at 54 months. Children’s daily cortisol levels and day-to-day variability were tested as moderators of longitudinal associations between parent and child symptoms at within- and between-family levels. Mothers’ symptoms related directly to child internalizing, but child evening cortisol moderated effects of fathers’ symptoms on internalizing, and of both parents’ symptoms on externalizing. Different paths of within-family risk dynamics vs. between-family risk synergy were found for internalizing vs. externalizing outcomes.
Understanding the paths by which psychopathology is transmitted from parent to child is crucial for formulating effective prevention strategies. Although it is well established that parents suffering from psychological disturbance—particularly elevated depressive symptoms—tend to have children with higher rates of internalizing and externalizing problems (e.g., Connell & Goodman, 2002), the paths of transmission are far from clear. In particular, a recent meta-analytic review of risk transmission (Goodman et al., 2011) highlighted the need for 1) genetically sensitive designs to distinguish truly environmental parent symptom effects from passive gene-environment correlation, 2) attention to susceptibility moderators—particularly stress sensitivity measured through the hypothalamic-pituitary-adrenal (HPA) axis—of parent symptom effects, and 3) longitudinal examination of processes contributing to child internalizing and externalizing outcomes. The present study addresses each of these points by testing adopted children’s HPA activity as a moderator of adoptive mothers’ and fathers’ depressive symptom associations with children’s trajectories of internalizing and externalizing problems from 18 to 54 months. As part of clarifying environmental exposure paths for parental depressive symptom effects, this research further probes the moderating role of different HPA measures—morning vs. evening levels and variability—and different levels of parent-child association — within-family risk dynamics vs. between-family risk synergy.
Environmental Risk Transmission: Effects of Mothers vs. Fathers
Mechanisms for effects of parental depressive symptoms, as outlined by Goodman and Gotlib (1999), include both genetic and environmental exposure processes. The importance of the latter has been borne out by twin and adoption studies, which have demonstrated a substantial family environment component in relations between parental depressive symptoms and both internalizing and externalizing child problems, with particularly strong shared environment effects in younger children and adoption samples (Rice, Harold, & Thapar, 2002; Silberg, Maes, & Eaves, 2010). Meta-analytic findings (based largely on genetically-related families) suggest that mothers’ and fathers’ psychopathology have similar effects on children’s externalizing problems, but that mothers’ psychopathology—particularly elevated depressive symptoms—has a greater impact on children’s internalizing problems (Connell & Goodman, 2002). This may be due to a greater impact of depression on mothers’ compared to fathers’ parenting (Field, Hossain, & Malphurs, 1999; Lim, Wood, Miller, & Simmens, 2011) and/or a stronger association between mothers’ depressive symptoms and more generalized family stress that impacts child adjustment (e.g., Dehle & Weiss, 1998; Laurent, Kim, & Capaldi, 2009).
A more recent meta-analysis affirmed that mothers’ depressive symptoms affect both internalizing and externalizing child outcomes, but that these effects are relatively modest overall and moderated by a number of variables, including child age (i.e., stronger effects at younger ages; Goodman et al., 2011). Together, these studies suggest environmental mechanisms of risk transmission may best be approached with adoption designs beginning at young ages that can separate maternal and paternal symptom exposure effects from passive gene-environment correlation while attending to possible child moderating factors.
The Moderating Role of Child Stress Sensitivity
Part of the reason for heterogeneous effects of parents’ depressive symptoms across multiple studies may result from child factors that amplify or mitigate risk exposure. An emergent focus in developmental psychopathology research is differential susceptibility to adverse environments due to sensitivity of stress response systems, including the HPA axis (see Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011; Obradovic & Boyce, 2009). Several models of moderated risk effects have received research support. On the one hand, diathesis-stress models (Heim & Nemeroff, 1999; Zuckerman, 1999) construe elevated child stress system activity as a risk marker conferring vulnerability to adverse conditions, such as those created by parents’ depressive symptoms. On the other hand, biological sensitivity to context theory (BSCT; Boyce & Ellis, 2005) posits that stress system activity is neither helpful nor harmful in and of itself, but rather heightens the effects of both risky and supportive environments. As discussed by Ellis and colleagues (2011), whether one or the other model appears to fit may depend on the range of family conditions sampled and other methodological features of a given study, but the two theories converge in predicting heightened risk for stress-reactive children in adverse family environments.
For example, differential effects of family adversity on child adaptation outcomes were demonstrated in a community sample, where the magnitude of adversity effects varied by child indices of both autonomic nervous system (ANS) and HPA responses to challenge (Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010). “Adversity” indexed a combination of family-level (e.g., financial stress, family expressiveness) and parent-level (e.g., marital conflict, maternal depression) characteristics, and child outcomes included both positive (prosocial behavior, school engagement, academic competence) and negative (externalizing behavior) characteristics. Results were generally consistent with BSCT, although the expected crossover interaction pattern did not emerge consistently for child HPA reactivity. One potential barrier to detecting differential susceptibility may be combining measures across mother and father characteristics, particularly where effects of parental psychopathology on child problem behaviors are concerned.
Several studies investigating the impact of parental depression and related psychopathology on child adjustment have shown main effects for maternal symptoms, but stress physiology-moderated effects for paternal symptoms. One study of a low SES sample including a range of child internalizing and externalizing symptoms found that mothers’ melancholic depression reliably predicted elevated child symptoms, whereas fathers’ antisocial behavior predicted higher symptoms only among highly ANS-reactive children (Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007). Similarly, whereas maternal depressive symptoms predicted child internalizing and externalizing symptoms across 2 years in a community sample, paternal depressive symptom effects depended on high child ANS reactivity (Cummings, El-Sheikh, Kouros, & Keller, 2007). This apparent distinction between direct paths for maternal depression and moderated paths for paternal depression may be attributable to differing caregiving roles during early childhood. Because mothers typically spend more time directly interacting with and providing basic care to infants (e.g., Baildum et al., 2000; LaFlamme, Pomerleau, & Malcuit, 2002), maternal symptoms and associated parenting difficulties should be particularly salient for young children. On the other hand, dampened stress sensitivity could protect children from more limited exposure to paternal symptoms. In sum, there is currently more evidence to support differential effects of father (as opposed to mother) symptoms and ANS (as opposed to HPA) activity as a susceptibility moderator. Difficulty discerning HPA effects in a given study design may also arise from distinctions between correlates of acute stress vs. diurnal rhythms and variability.
HPA as a Measure of Sensitivity
Typically, elevated stress sensitivity—as indexed by higher cortisol levels and/or acute reactivity—is associated with child risk for internalizing and/or externalizing problems (e.g., Bagner, Sheinkopf, Vohr, & Lester, 2010; Hastings et al., 2011; Kestler & Lewis, 2009; Shirtcliff & Essex, 2008), though there is also evidence that suppressed cortisol signifies risk (see Gunnar & Vazquez, 2001). Some of the inconsistencies in this research likely have to do with the assessment window for HPA—acute stress vs. diurnal regulation in the morning or evening—and the parameters considered—stable individual tendencies vs. variability. Studies of HPA response to acute stressors and of diurnal output patterns have yielded some convergent findings but clearly measure different scales of regulation, and the latter may offer a better index of global sensitivity (Kudielka & Wüst, 2010).
A diurnal pattern of cortisol secretion—i.e., peak in the morning and decline over the course of the day—is typically established by 3 months of age, and the cortisol awakening response is found normatively in children as young as 2 years (Gribbin, Watamura, Cairns, Harsh, & LeBourgeois, 2011). At the same time, between-child variability in the establishment of diurnal cortisol rhythms has been found to be meaningful in predicting social and behavioral adjustment during the preschool period (see Turner-Cobb, 2005). Within diurnal output patterns, morning levels are thought to be more stable and genetically influenced, and evening levels thought to additionally reflect reactivity to and recovery from the day’s events (e.g., Bartels, deGeus, Kirschbaum, Sluyter, & Boomsma, 2003). Consistent with this idea, a number of studies have found elevated evening cortisol levels among children or youths showing problem behaviors, which has been interpreted as a failure in HPA efficiency and/or cumulative effects of daily stress (Corbett, Schupp, Levine, & Mendoza, 2009; Engert, Efanov, Dedovic, Dagher, & Pruessner, 2011; Van den Bergh & Van Calster, 2009). Findings for morning cortisol have been less consistent, with lower, higher, and nonsignificantly different levels in high- vs. low-risk samples reported (e.g., Dozier et al., 2006; Engert et al., 2011; Van den Bergh et al., 2009). In addition, there is some evidence for greater day-to-day variability in cortisol levels among individuals struggling with psychiatric symptoms (Corbett et al., 2009; Gonzalez et al., 2009). Thus, morning and evening cortisol levels and variability may offer different, non-redundant markers of sensitivity that complement acute stress response approaches.
Another source of discrepant findings for HPA risk involves distinctions between internalizing and externalizing symptoms. Whereas research more consistently implicates HPA hyperactivation in internalizing problems, externalizing problems have been associated with both heightened and lowered cortisol levels (e.g., Cicchetti & Rogosch, 2001; Smider et al., 2002). Externalizing correlates may depend on age, as suggested by a meta-analysis showing an overall negative association between externalizing and basal cortisol levels, but a positive association among preschool children (Alink et al., 2008). It may also be that findings of increased cortisol in high externalizers reflect overlap between internalizing and externalizing problems, and research approaches separating the two dimensions is needed.
Longitudinal Paths and Levels of Analysis
It is also possible that paths for parent risk effects operate at different levels in a longitudinal framework. On the one hand, one can examine effects of parental symptoms on fluctuations in child symptoms over time (within-family effect); on the other hand, one can examine differences in child symptom trajectories as a function of parents’ overall symptom levels (between-family effect). A stress sensitivity moderator could then act as a predictor of the strength of dynamic within-family associations between parent and child symptoms (i.e., heightening such associations), or it could interact with parents’ overall symptoms to predict child symptom trajectories (i.e., synergistic effect of high-risk parents paired with sensitive children predicting higher symptom trajectories). Here, we refer to the former process as “risk dynamics” and the latter as “risk synergy.” Although no studies that we know of have systematically tested these two possibilities, there is prior evidence that a particular risk factor (self-reported stressors) can predict both between-adolescent differences in depressive symptom trajectories and within-adolescent changes in depressive symptoms over a period of 11 years (Ge, Natsuaki, & Conger, 2006).
In the area of parent-child symptom transmission, there are studies supporting attention to both within- and between-family longitudinal effects. Treatment research relating improvement in maternal symptoms to improvement in child internalizing and externalizing outcomes over time provides evidence for a within-family effect of mothers’ depressive symptoms (e.g., Shaw, Connell, Dishion, Wilson, & Gardner, 2009; Weissman et al., 2006). There is also evidence for a direct between-family effect of mothers’ depressive symptoms on child internalizing trajectories, but not on externalizing trajectories (Leve, Kim, & Pears, 2005). Based on the available evidence, there may be more direct within- and between-family effects of maternal depressive symptoms on child internalizing outcomes, compared to moderated paternal symptom effects and child externalizing outcomes; however, each of these studies was limited to a specific level of analysis and cannot guide strong predictions regarding level of effects. Longitudinal multilevel models that consider both levels of analysis are needed to evaluate these effects in mothers vs. fathers and for internalizing vs. externalizing child outcomes. Better knowledge of whether risk synergy and/or dynamics are responsible for specific parent symptoms effects on child outcomes could provide importance guidance for prevention and intervention efforts.
The Current Study
The current study investigated the following question: Does child HPA activity moderate the effect of adoptive mother and father depressive symptoms on child internalizing and externalizing problems? Within this broad question, several more specific questions were posed: (1) Do transmission paths differ for maternal vs. paternal symptoms, or for internalizing vs. externalizing outcomes? (2) Is HPA sensitivity best captured through morning or evening cortisol levels, or through day-to-day variability? (3) Is there more evidence for transmission through within-family risk dynamics or between-family risk synergy? Based on the available evidence reviewed above, we hypothesized that child cortisol—particularly evening levels and variability—would interact with adoptive parent—particularly father—depressive symptoms to predict child problems. Specifically, higher child cortisol was predicted to amplify the effects of paternal depressive symptoms on child internalizing and externalizing outcomes, whereas maternal depressive symptoms were predicted to exert a main effect on child problems (especially internalizing). In the absence of prior models for within- vs. between-family risk processes, we made no specific hypotheses for level of effects, but investigated both.
Method
Participants
Participants were drawn from the Early Growth and Development Study, a longitudinal study of adopted children and their birth and adoptive parents. Recruitment of participants in the current study occurred between 2003 and 2006 (Cohort I), beginning with the recruitment of adoption agencies (N = 33 agencies in 10 states located in the Northwest, Mid-Atlantic, and Southwest regions of the United States). The participating agencies reflected the full range of adoption agencies operating in the United States: public, private, religious, secular, those favoring open adoptions, and those favoring closed adoptions. Agency staff identified participants who completed an adoption plan through their agency and met the following eligibility criteria: (a) the adoption placement was domestic, (b)SD the infant was placed within 3 months postpartum (M = 7.11 days postpartum, = 13.28; median = 2 days), (c) the infant was placed with a nonrelative adoptive family, (d) birth and adoptive parents were able to read or understand English at the eighth-grade level, and (e) the infant had no known major medical conditions such as extreme prematurity or extensive medical surgeries. The participants were representative of the adoptive parent population that completed adoption plans at the participating agencies during the same time period (Leve et al., 2007).
Of the families who met eligibility criteria, 68% ( n = 361) agreed to participate. These families were followed prospectively across the child’s postnatal development from 9 months to 54 months (additional assessment are ongoing). The sample included male (57%) and female (43%) children with a range of racial backgrounds (58.4% White, 11.1% Black/African American, 9.4% Latino, 20.2% multiracial, .3% American Indian/Alaskan Native, .6% unknown or not reported). Adoptive parents were predominantly White (over 90% of adoptive mothers and n fathers) and middle class. A comparison of cases included vs. not included revealed nonsignificant differences on all other study variables.
Measures
Parent depressive symptoms —Beck Depression Inventory (BDI; Beck & Steer, 1993)
Adoptive mothers and fathers completed this widely used measure of depressive symptoms during interviews when the child was 9 months, 18 months, 27 months, and 54 months old (measures starting at 18 months were used in the current study as that is when child behavior outcome assessment began). Parents rated 20 symptoms of depression in the past week on a 0-3 scale (the suicidal ideation item from the original 21-item scale was dropped to minimize situations requiring clinical follow-up), and a summed score was computed. Although the majority of parents reported minimal symptoms, a small proportion of observed scores (5% for adoptive mothers, 3% for adoptive fathers) fell in the clinical range, and the full range of clinical severity was represented (raw scores 0-39). See Table 1 for descriptive statistics. To aid in coefficient interpretability and computing interaction terms, Z-scores were computed for use in analyses.
Table 1.
Parent Depressive Symptoms and Child Symptoms over Time
| Assessment Time (Child Age) | |||
|---|---|---|---|
| Variable M (SD) | 18 months | 27 months | 54 months |
| Parental Depressive Symptoms (BDI) | |||
| Mother | 3.89 (3.96) | 3.79 (4.18) | 4.20 (4.42) |
| Father | 2.82 (3.16) | 2.62 (3.50) | 3.26 (3.57) |
| Child Symptoms (CBCL) | |||
| Internalizing T-score | 43.18 (8.22) | 44.74 (8.51) | 47.68 (9.62) |
| Externalizing T-score | 46.89 (8.26) | 47.70 (8.48) | 49.10 (8.83) |
Note. Although mother- and father-reported symptoms are averaged above for summary presentation, each parent’s report across the 3 assessments was modeled separately to test cross-reporter effects (i.e., father-reported CBCL served as outcome for effects of mothers’ BDI).
Child HPA activity—Salivary cortisol
Child morning (M time = 7:38 AM, SD = 43 minutes) and evening (M time = 8:12 PM, SD = 51 minutes) saliva samples were collected with the help of adoptive parents across 3 consecutive days as part of the 54-month assessment. Parents were instructed to collect the samples within 30 minutes after the child awoke in the morning (wake time range 5:00-10:15 AM) but before breakfast, and when the child was in bed for the night (sleep time range 6:55 PM – 12:30 AM). Study parents were trained in sample collection procedures in person, which involved saturating salivettes before placing them in prelabeled plastic vials. Samples were then mailed to the primary study site, at which point they were frozen and stored on site until all samples for all participants had been collected and could be mailed jointly to the analysis laboratory. Samples were stored at −5° F (−20° C) until assay using a competitive solid phase time-resolved fluorescence immunoassay (DELFIA; see Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992) with interassay coefficients of variation (CV) 7.1%-9.0%. Samples were assayed in duplicate, and all 6 scores were used in analyses morning cortisol (M morning cortisol = .63 μg/dl SD = .23; M evening cortisol = .07 μg/dl, SD = .12; M intraassay coefficient of variation = 6%, SD = 1.9).
Parents recorded the exact time of saliva collection and other information that could affect cortisol measurement, such as illness, medication use, and sleep time, in a collection diary. Standard data screening procedures (e.g., identifying and eliminating extreme outlying values, checks for implausible or contradictory time recording) were used. Such screening resulted in the deletion of 1-6 cortisol values (.5%-3% of the total) from each sampling period due to extreme values (> 2 μg/dl), reported sampling time before reported wake time or after sleep time, and inconsistency of 30 minutes or more between reported sampling time and time recorded on saliva vial. Fitted morning and evening levels and residual variability were computed using multilevel modeling (see below), and Z-scores were computed for use in analyses. Full information maximum likelihood estimation in HLM allowed for the computation of expected child cortisol values in the presence of missing data.
Child internalizing and externalizing outcomes—Child Behavior Checklist (CBCL 1½-5; Achenbach & Rescorla, 2000)
Child problem behaviors were assessed by adoptive parent-report on the CBCL, a well-validated measure of externalizing and internalizing difficulties. Both parents separately rated 99 behaviors on a scale from 0-2 at 18 months (mailed questionnaire), 27 months (mailed questionnaire), and 54 months (web-based questionnaire). Broadband internalizing and externalizing scales were computed according to each parent’s report, and T-scores (M = 50, T > 65 indicates borderline to clinical range) used to index child symptoms. Again, the majority of children showed behaviors in the normal range, but a range of severity was represented for internalizing (T-score range = 29-77, 6% in borderline-clinical range) and externalizing (T-score range = 28-82, 7% in borderline-clinical range). See Table 1 for descriptive statistics.
Demographic controls
Parents reported on a number of factors that could be important to control for such as child sex, age, prenatal and birth complications (birth mother report), their own ages at the child’s birth, and perceived openness of the adoption and contact between birth and adoptive families. These were considered as possible covariates in analyses.
Analysis Overview
A dependent data structure lent itself to multilevel modeling in HLM (Raudenbush & Bryk, 2002). This approach separates variance into within-family (Level 1) and between-family (Level 2) components, allowing for tests of parental symptom effects at both levels as proposed above. HLM has the additional benefit of allowing for missing data at Level 1 while using Full Information Maximum Likelihood Estimation to arrive at model parameters. Thus, families missing partial parent or child symptom data (approximately 5% of the total parent depressive symptom scores, 10% of the total child CBCL scores) were still included, but weighted less heavily, in analyses.
First, estimates of each child’s stable morning and evening cortisol levels and unexplained day-to-day variability were obtained by fitting a model with cortisol scores measured across the 3 days of collection as the Level 1 outcome, controlling for time of sample collection:
Level 1
Level 2
Each child’s unique intercept (β0) and residual error variability (e) across the 3 days for AM and PM sampling periods were extracted from the HLM Level 1 residual file to be used in subsequent explanatory models. Whereas the first measure taps the child’s typical morning or evening cortisol levels, the latter taps day-to-day variability not explained by these typical levels. Each of these measures—i.e., AM and PM cortisol fitted values and variability—was tested as a potential marker of stress sensitivity in family risk models.
The main explanatory models tested adoptive parents’ depressive symptoms and child cortisol values as predictors of child CBCL internalizing and externalizing scale outcomes across the 3 time points (18, 27, and 54 months). In order to avoid reporter bias in parent-child symptom associations, each parent’s depressive symptom scores were tested in relation to the opposite parent’s report of child externalizing and internalizing behavior. To constrain the number of predictors in each model and avoid overfitting the data, separate models were run for adoptive mother and father predictors (referred to generically as “parent”), and for fitted cortisol levels and residual variability (referred to as “cortisol”). As described above, parental symptom effects were tested at both within-family and between-family levels:
A) Within-Family Risk Dynamics
Level 1
Level 2
B) Between-Family Risk Synergy
Level 1
Level 2
For both conceptualizations of risk, child internalizing or externalizing symptoms across the 3 time points were modeled with an intercept (β0) and linear slope (β1), and child AM and PM cortisol measures were included as Level 2 predictors moderating the effect of parental depressive symptoms. The difference between the two conceptualizations lay in the level at which parent symptom effects were modeled.
To test dynamic risk, parent symptoms across the 3 time points were added as a time-varying covariate at Level 1 (β2). This effect tapped the relation between parent depressive symptoms and child symptoms over time, above and beyond the normative child adjustment trajectory (represented by the intercept and slope terms). Parent depressive symptom effects were allowed to vary across families, and this between-family variance was explained by adding child cortisol predictors—morning levels or variability (γ21) and evening levels or variability (γ22)—at Level 2. This allowed us to test the cross-level interaction effect showing differential strength of the parent depressive symptom effect on child symptom variation over time based on child cortisol measures.
To test risk synergy, mean parent symptoms (averaged across the 3 time points) were added as a Level 2 predictor of child symptom intercepts (γ03) and slopes (γ13). Child morning and evening cortisol levels or variability were also added as Level 2 predictors of child symptom intercepts (γ01 and γ02) and slopes (γ11 and γ12), as were interactions of parent symptoms x child morning and evening cortisol measures (γ04 and γ05 predicting intercepts, γ14 and γ15 predicting slopes). These interaction terms allowed us to test the differential effect of parents’ overall levels of depressive symptoms on child symptom trajectories as a function of child cortisol measures.
Results
Variables were checked for non-normality, and it was determined that untransformed scores were suitable for analysis; although adoptive parent depressive symptom scores and child cortisol fitted values tended toward positive skew, the same model effects were found using raw and natural log-transformed scores, so the more easily interpreted raw score results are reported. The CBCL internalizing and externalizing scales were positively correlated with one another (r = .63), suggesting a tendency for children with difficulties in one domain to show difficulties in the other. Given concerns about internalizing/externalizing overlap contributing to child HPA patterns, follow-up models probing uniqueness of risk effects for a particular problem dimension were tested, as described further below. Child morning and evening cortisol fitted values were not related to one another in this sample (r = .086, ns), nor were morning and evening cortisol variability (r = .093, ns), supporting their use as unique predictors. Within sampling periods, fitted values did relate positively to variability (r = .31, p < .05 morning, r = .81, p < .05 evening); this is not surprising, in that higher overall levels allow more room for deviation above and below, but suggests that effects of evening cortisol levels and variability may be difficult to separate. None of the demographic control or saliva collection diary variables were significantly related to child behavior or to cortisol and so these were not included in further testing. See Table 2 for correlations among all study variables.
Table 2.
Correlations among Study Measures (means across assessment time points)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. AM Depressive Symptoms | — | |||||||||
| 2. AF Depressive Symptoms | .16* | — | ||||||||
| 3. Child AM Cortisol Level | −.06 | .09 | — | |||||||
| 4. Child PM Cortisol Level | .02 | −.07 | .09 | — | ||||||
| 5. Child AM Cortisol Variability | .03 | .11 | .31** | .17* | — | |||||
| 6. Child PM Cortisol Variability | −.005 | −.09 | −.03 | .81** | .09 | — | ||||
| 7. Child Internalizing, AM Report | .18** | .03 | −.21** | −.04 | −.21** | −.04 | — | |||
| 8. Child Internalizing, AF Report | .11 | .23** | −.07 | .03 | −.05 | .02 | .40** | — | ||
| 9. Child Externalizing, AM Report | .20** | −.04 | −.09 | −.06 | −.13 | −.09 | .61** | .19** | — | |
| 10. Child Externalizing, AF Report | .11 | .20** | .02 | .10 | .02 | .05 | .27** | .70** | .62** | — |
Note. Cortisol Level = fitted cortisol, Variability = residual estimates from HLM models.
indicates p < .05
indicates p < .01.
Child Internalizing and Externalizing: Baseline Models
Before adding explanatory predictors, baseline models of child internalizing and externalizing outcomes were fit to obtain estimates of mean symptom patterns and variability across children, as well as to obtain measures of fit against which subsequent models could be compared. First, intercept-only models were fit to obtain an intra-class correlation (ICC) for each outcome, which offers information about the proportion of observed variance attributable to between-child (Level 2) true score variability. The ICC’s were .41 for internalizing and .36 for externalizing; this means that a substantial proportion of the variability in child CBCL scores could be attributed to stable between-child differences, but a greater proportion was attributable to within-child variability across time points. Linear slope terms were added to the models, centered at the final (54-month) time point; this means that intercept terms represented child CBCL outcome levels at the end of the study period, and slope terms represented change in CBCL outcomes from 18 to 54 months. Significant positive slopes for both outcomes meant that children tended, on average, to increase in both internalizing and externalizing problems across the study. At the same time, significant between-child variability (τ) in both intercepts and slopes supported the addition of explanatory predictors at Level 2, and significant within-child variability (δ2) supported the addition of explanatory predictors at Level 1.
Explanatory Models: Within-Family Dynamic Risk (Table 3; Figure 1)
Table 3.
Within-Family Dynamic Risk Models
| Internalizing Outcome | Externalizing Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Cortisol Level | Cortisol Variability | Cortisol Level | Cortisol Variability | ||||||||
| Coeff | p | τ | Coeff | p | τ | Coeff | p | τ | Coeff | p | τ | |
| A. Mothers’ Symptoms Model | ||||||||||||
| Intercept (level at 54 months) γ00 |
45.60 | < .001 | 45.21 | 45.62 | < .001 | 45.13 | 47.87 | < .001 | 39.70 | 47.86 | < .001 | 39.69 |
| Slope (rate of change 18-54 months) γ10 |
1.70 | < .001 | 1.70 | < .001 | .716 | .04 | .700 | .04 | ||||
| M Depressive Symptoms γ20 | .230 | .02 | .004 | .231 | .02 | .004 | .272 | .01 | .019 | .269 | .009 | .016 |
|
| ||||||||||||
| Child AM Cortisol γ21 | −.056 | .57 | .016 | .86 | −.025 | .79 | −.081 | .35 | ||||
| Child PM Cortisol γ22 | .058 | .82 | .115 | .56 | .126 | .65 | .141 | .58 | ||||
|
| ||||||||||||
| B. Fathers’ Symptoms Model | ||||||||||||
| Intercept (level at 54 months) γ00 |
48.52 | < .001 | 31.43 | 48.48 | < .001 | 31.43 | 49.71 | < .001 | 40.90 | 49.63 | < .001 | 41.13 |
| Slope (rate of change 18-54 months) γ10 |
2.45 | < .001 | 2.43 | < .001 | .795 | .02 | .756 | .02 | ||||
| F Depressive Symptoms γ20 | .155 | .19 | .163 | .136 | .28 | .162 | .033 | .77 | .016 | .019 | .86 | .014 |
|
| ||||||||||||
| Child AM Cortisol γ21 | −.060 | .58 | .017 | .87 | .113 | .25 | .193 | .06 | ||||
| Child PM Cortisol γ22 | .410 | < .001 | .398 | .04 | .029 | .75 | −.063 | .70 | ||||
Note. Child stress sensitivity-moderated effects indicated with double-outlined boxes. Parental depressive symptom and child cortisol predictors are Z-transformed scores. τ = random effect component.
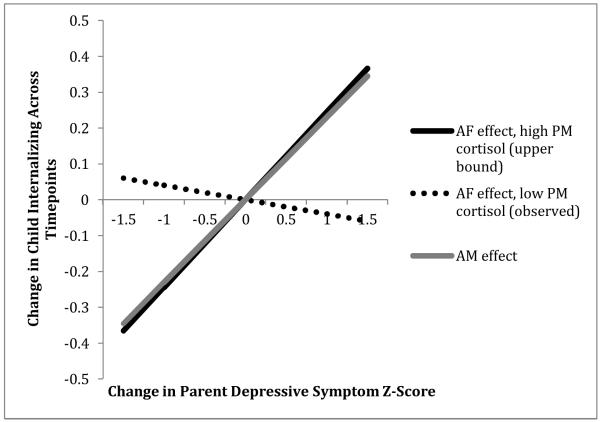
Figure 1.
Child evening cortisol moderates associations between change in father depressive symptoms and change in child internalizing over time (plotted at cortisol values representing upper bound for region of significance and lowest observed value).
Note. AF = Adoptive Father; AM = Adoptive Mother.
As described above, child AM and PM cortisol fitted values or variability were added as Level 2 predictors of the Level 1 parent depressive symptom covariate to test dynamic risk. Changes in adoptive mothers’ symptoms showed a significant positive association with both child internalizing and externalizing over time, but child cortisol measures (both fitted values and variability) failed to predict variability in these associations (see Table 3, panel A). In other words, changes in maternal symptoms tended to parallel changes in children’s symptoms, regardless of the child’s cortisol activity. In contrast, changes in adoptive fathers’ symptoms showed no main effect on child internalizing or externalizing over time, but the former effect was moderated by child PM cortisol fitted values and variability (see Table 3, panel B). Specifically, although changes in paternal depressive symptoms tended to be unrelated to changes in children’s internalizing symptoms, there was a positive association between the two for children with higher evening cortisol levels and/or variability (see Figure 1). Regions of significance for the interaction were probed using Preacher, Curran, and Bauer’s (2006) tool for multilevel modeling. This showed that a positive association between changes in paternal symptoms and child internalizing over time was expected for children with PM fitted cortisol Z-scores ≥ .22, and for PM cortisol variability Z-scores ≥ .36 (negative associations expected for cortisol Z-scores ≤ -2.14 and -3.93, respectively, which were outside the observed range of values). This effect was consistent with child stress sensitivity heightening within-family (father-child) internalizing risk dynamics.
A comparison of these models against the baseline model confirmed that each yielded a significant improvement in fit, according to change in the deviance statistic (χ2 [5] = 4089.23, p < .001 for father symptom-internalizing model; χ2 [5] = 3997.04, p < .001 for mother symptom-internalizing model; χ2 [5] = 4020.78, p < .001 for father symptom-externalizing model; χ2 [5] = 3968.46, p < .001 for mother symptom-externalizing model), though the incremental improvement associated with adding child cortisol predictors was significant for the father symptom-internalizing model only. The father symptom-internalizing model explained 29.5% of the Level 1 variance, and child cortisol predictors explained 8.4% of the variance in the father symptom predictor at Level 2. The mother symptom-internalizing and –externalizing models explained 21.7% and 23.1%, respectively, of the Level 1 variance. Reported comparisons are for the cortisol fitted value models; cortisol variability models yielded similar patterns, but weaker improvements in fit and variance explained.
Explanatory Models: Between-Family Risk Synergy (Table 4; Figures 2-3)
Table 4.
Between-Family Risk Synergy Models
| Internalizing Outcome | Externalizing Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Cortisol Level | Cortisol Variability | Cortisol Level | Cortisol Variability | ||||||||
| Coeff | p | τ | Coeff | p | τ | Coeff | p | τ | Coeff | p | τ | |
| A. Mothers’ Symptoms Model | ||||||||||||
| Intercept (level at 54 months) γ00 |
47.04 | < .001 | 36.73 | 47.04 | < .001 | 37.04 | 49.02 | < .001 | 34.57 | 48.98 | < .001 | 34.85 |
| Child AM Cortisol γ01 | −.784 | .18 | −.718 | .27 | .189 | .75 | −.093 | .89 | ||||
| Child PM Cortisol γ02 | −.747 | .18 | −.890 | .26 | .329 | .52 | −.640 | .53 | ||||
| M Depressive Symptoms γ03 | 1.06 | .05 | 1.01 | .05 | 1.31 | .03 | .989 | .06 | ||||
|
| ||||||||||||
| M Symptoms × AM Cortisol γ04 |
−.009 | .98 | .117 | .84 | .318 | .53 | .041 | .94 | ||||
| M Symptoms × PM Cortisol γ05 |
.989 | .36 | .265 | .77 | 2.61 | .03 | .774 | .48 | ||||
|
| ||||||||||||
| Slope (rate of change 18-54 months) γ10 |
2.10 | < .001 | 3.34 | 2.09 | < .001 | 3.64 | .879 | .001 | .785 | .850 | .001 | .839 |
| Child AM Cortisol γ11 | .140 | .63 | .194 | .51 | .393 | .13 | .311 | .26 | ||||
| Child PM Cortisol γ12 | −.235 | .45 | −.346 | .34 | .092 | .76 | −.599 | .09 | ||||
| M Depressive Symptoms γ13 | .042 | .88 | .115 | .67 | .0003 | .99 | −.181 | .48 | ||||
|
| ||||||||||||
| M Symptoms × AM Cortisol γ14 |
−.637 | .03 | −.369 | .18 | −.070 | .79 | .144 | .61 | ||||
| M Symptoms × PM Cortisol γ15 |
.516 | .29 | .320 | .38 | 1.27 | .02 | .275 | .43 | ||||
|
| ||||||||||||
| B. Fathers’ Symptoms Model | ||||||||||||
| Intercept (level at 54 months) γ00 |
47.01 | < .001 | 35.59 | 47.03 | < .001 | 36.33 | 48.99 | < .001 | 33.81 | 48.97 | < .001 | 33.87 |
| Child AM Cortisol γ01 | −1.10 | .05 | −.878 | .13 | −.072 | .89 | −.309 | .58 | ||||
| Child PM Cortisol γ02 | .730 | .49 | −.238 | .82 | 2.25 | .05 | .108 | .93 | ||||
| F Depressive Symptoms γ03 | 1.86 | .01 | 1.39 | .03 | 1.61 | .02 | .924 | .16 | ||||
|
| ||||||||||||
| F Symptoms × AM Cortisol γ04 |
−.164 | .69 | .123 | .80 | −.012 | .98 | .597 | .20 | ||||
| F Symptoms × PM Cortisol γ05 |
4.13 | .10 | 1.53 | .41 | 5.82 | .01 | 2.17 | .29 | ||||
|
| ||||||||||||
| Slope (rate of change 18-54 months) γ10 |
2.07 | < .001 | 3.78 | 2.09 | < .001 | 3.76 | .866 | .001 | .353 | .858 | .001 | .619 |
| Child AM Cortisol γ11 | .048 | .87 | .234 | .47 | .295 | .24 | .235 | .34 | ||||
| Child PM Cortisol γ12 | .300 | .58 | −.287 | .48 | 1.25 | .03 | −.065 | .89 | ||||
| F Depressive Symptoms γ13 | .418 | .22 | .130 | .69 | .732 | .02 | .280 | .38 | ||||
|
| ||||||||||||
| F Symptoms × AM Cortisol γ14 |
−.307 | .10 | −.108 | .66 | −.243 | .24 | .145 | .49 | ||||
| F Symptoms × PM Cortisol γ15 |
1.63 | .19 | .453 | .54 | 3.61 | .001 | 1.41 | .04 | ||||
Note. Child stress sensitivity-moderated effects indicated with double-outlined boxes. Parental depressive symptom and child cortisol predictors are Z-transformed scores. τ = random effect component.
Figure 2.
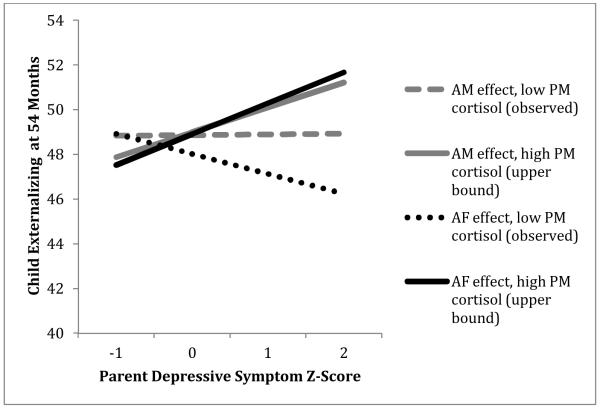
Child evening cortisol moderates associations between parents’ mean depressive symptoms and child externalizing at 54 months (plotted at cortisol values representing upper bound for region of significance and lowest observed value).
Note. AF = Adoptive Father; AM = Adoptive Mother.
Figure 3.
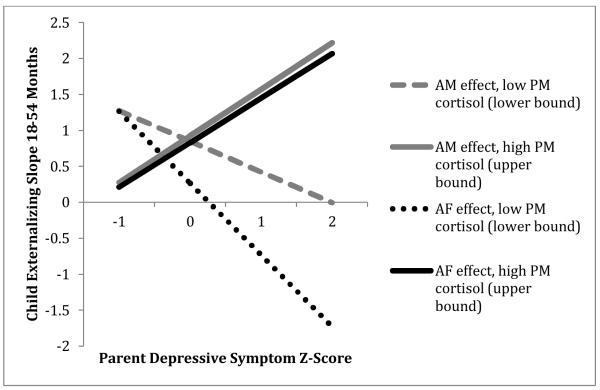
Child evening cortisol moderates associations between parents’ mean depressive symptoms and child externalizing slope from 18-54 months (plotted at cortisol values representing upper and lower bounds for region of significance).
Note. AF = Adoptive Father; AM = Adoptive Mother.
Child AM and PM cortisol levels or variability, mean parent depressive symptoms, and their interactions were entered as Level 2 predictors of children’s symptom intercepts and slopes to test between-family risk synergy. Adoptive mothers’ symptoms showed a positive main effect on child internalizing intercepts that was not moderated by cortisol, and the interaction of mothers’ symptoms and child AM fitted cortisol predicted child internalizing slopes (see Table 4, left panel A). This meant that children of mothers with higher mean levels of depressive symptoms exhibited more internalizing symptoms at 54 months. An examination of the interaction effect showed that even though maternal symptoms and child cortisol did not relate to child internalizing slopes on their own, in the presence of low child AM cortisol, maternal symptoms did predict more of an increase in child internalizing from 18 to 54 months (region of significance testing showed a positive association between maternal symptoms and child internalizing slopes for children with AM cortisol Z-scores ≤ -1.08, negative association for children with AM cortisol Z-scores ≥ 2.93, which represented the outer bound of the observed range).
Mothers’ symptoms also showed a positive main effect on child externalizing intercepts that was enhanced by child PM fitted cortisol, and the interaction of maternal symptoms and child PM fitted cortisol additionally predicted child externalizing slopes (see Table 4, right panel A). In other words, the combination of a mother with high mean depressive symptoms and a child with high evening cortisol predicted more steeply increasing externalizing symptoms and higher externalizing at 54 months (see Figures 2-3). Region of significance testing showed a positive association between maternal symptoms and child externalizing intercepts for children with PM cortisol Z-scores ≥ -.08 (negative association for children with PM cortisol Z-scores ≤ -3.28, outside of observed range), and a positive association with child externalizing slopes for children with PM cortisol Z-scores ≥ .51 (negative association for children with PM cortisol Z-scores ≤ -.34).
For adoptive fathers’ symptoms, a similar pattern of between-family risk effects emerged. As for mothers, fathers’ symptoms showed a positive main effect on child internalizing intercepts; in other words, fathers with higher mean levels of depressive symptoms had children with more internalizing symptoms at 54 months (see Table 4, left panel B). Fathers’ symptoms also showed a main effect on child externalizing intercepts and slopes, and both of these effects were enhanced by child PM fitted cortisol (see Table 4, right panel B). Child PM cortisol variability had a similar paternal symptom-enhancing effect on externalizing slopes. As for mothers, this meant that the combination of a father with high mean depressive symptoms and a child with high evening cortisol predicted more steeply increasing externalizing symptoms and higher externalizing at 54 months (see Figures 2-3). According to region of significance testing, a positive association between paternal symptoms and child externalizing intercepts was expected for children with PM cortisol Z-scores ≥ -.04 (negative association for children with PM cortisol Z-scores ≤ -1.20, outside of observed range), and a positive association with child externalizing slopes was expected for children with PM cortisol Z-scores ≥ -.03 and PM cortisol variability Z-scores ≥ .76 (negative associations for children with PM cortisol Z-scores ≤ -.48 and variability Z-scores ≤ -4.26—latter outside of observed range).
These models also yielded improvements in fit—significant for internalizing, marginally significant for externalizing—compared to baseline (χ2 [10] = 18.63, p < .05 for father symptom-internalizing model; χ2 [10] = 19.63, p < .05 for mother symptom-internalizing model; χ2 [10] = 17.86, p = .06 for father symptom-externalizing model; χ2 [10] = 15.52, p = .11 for mother symptom-externalizing model). The mother and father symptom-internalizing models respectively explained 6.2% and 9.1% of the variance in Level 2 intercepts and 16.8% and 5.5% of the variance in slopes. Mother and father symptom-externalizing models respectively explained 5.4% and 7.5% of the variance in intercepts and 39.1% and 72.6% of the variance in slopes.
Given the overlap between internalizing and externalizing problems in this sample, further models were tested to determine whether differing effects for internalizing vs. externalizing outcomes could be attributed to the unique components of each problem domain. One set of models tested CBCL total symptoms as the outcome, and another included internalizing or externalizing scores as a control in predicting the other problem outcome. The within-family risk dynamic effect was nonsignificant, as was the between-family risk synergy effect on child slopes, for the total symptom (as opposed to internalizing or externalizing) outcome. Controlling for the other problem scale resulted in slightly reduced coefficients, but the same pattern of significant effects in all explanatory models. Therefore, the burden of evidence pointed to unique risk processes explaining internalizing vs. externalizing problem domains.
Discussion
This study adds to knowledge about non-genetic mechanisms for psychopathology risk transmission by demonstrating direct and child HPA-moderated effects of adoptive parents’ depressive symptoms on children’s problem behaviors from 18 to 54 months. In particular, we found broad direct effects of adoptive mothers’ depressive symptoms on children’s internalizing problems, but HPA-moderated effects of adoptive fathers’ depressive symptoms on children’s internalizing, and of both parents’ symptoms on children’s externalizing problems. These findings help to explain stronger overall effects of maternal depressive symptoms on internalizing while validating the importance of stress sensitivity moderation for paternal symptom effects and for externalizing outcomes. They also highlight evening cortisol levels, and to a lesser extent evening variability in cortisol levels, as a stress sensitivity index. Finally, we found preliminary support for a distinction between within-family risk dynamics and between-family risk synergy as mechanisms for risk transmission. Below, we explore the implications of these findings for developmental psychopathology research.
The present results converge with prior work showing stronger effects of maternal depressive symptom on child internalizing but giving equal weight to maternal and paternal symptom effects for child externalizing (Connell & Goodman, 2002), at the same time shedding further light on why this might be so. Specifically, whereas the dynamic effect of paternal symptoms on child internalizing over time varied by child cortisol levels, the effect of maternal symptoms did not. As suggested by previous researchers (Cummings et al., 2007), these results are consistent with the premise that father dysphoria is less inherently distressing to children, affecting only those who are more dispositionally reactive. It may also reflect the degree or intensity of exposure children have to each parent, with a moderately sensitive “buffered” HPA profile protecting children from more limited exposure to fathers’ symptoms. More direct effects of maternal symptoms may further speak to stronger connections with negative parenting and/or broader family stress that fuels child internalizing problems (e.g., Laurent et al., 2009; Lim et al., 2011).
On the other hand, child externalizing related in a cortisol-dependent manner to both parents’ overall symptoms. It may be that early externalizing problems have a different set of influences less dependent on parental roles and depression-related interaction patterns. For example, a parent struggling with depression may create a family climate of diffuse and unpredictable limits to which children—especially those sustaining high levels of HPA activity throughout the day—react with attention-grabbing externalizing behaviors. These distinctions are somewhat complicated by the association between child externalizing and internalizing symptoms, though analyses separating the two problem dimensions confirmed unique risk paths to each outcome. Further multilevel investigations with samples including a broad range of both maternal and paternal symptoms and caregiving roles will be needed to tease apart these effects. However, the current findings underscore the importance of both parents’ depressive symptoms and the moderating role of daily HPA activity in children’s early adjustment.
Although preliminary, the finding that evening cortisol emerged as a unique moderator of parental depressive symptom effects may help to advance our understanding of stress sensitivity. Both morning and evening levels of HPA activity, as well as changes across the day, have been associated with adjustment, but our results suggest the combination of stable dispositional activity and ongoing response to daily events measured in evening cortisol best characterizes child susceptibility. As discussed by previous researchers finding elevated evening cortisol in high-risk groups, this pattern may signify a failure in HPA efficiency and/or a cumulative effect of daily stress the individual is unable to fully recover from. In particular, there is evidence that evening cortisol relates to observed sensitivity to stressors during the day (Corbett et al., 2009). Our results further suggest it contributes to child psychopathology risk by amplifying the effects of exposure to parents’ depressive symptoms. Some of these findings may also have to do with the developmental period under investigation; as suggested by previous researchers, elevated (as opposed to suppressed) cortisol may characterize externalizing, and the cortisol awakening response may be less pronounced, among younger children (Alink et al., 2008; Gribbin et al., 2011). Further investigation in older children or adolescents may reveal a more important role of hypocortisolism and/or morning cortisol levels in defining risk.
Consistent with BSCT and with diathesis-risk theories, the children with more active HPA systems may be especially permeable to the social threat cues in their environment, intensifying the impact of interacting with a depressed parent. At least under the conditions observed in this study, it was the child’s usual (stable) level of evening HPA activity, and to a lesser extent the degree of daily variability, that acted to intensify linkages with parental depressive symptoms. In part, this may have been an artifact of the high association between evening levels and variability, and more work needs to be done to determine the extent to which cortisol levels and variability can or should be distinguished at different points in the diurnal rhythm. For now, these results support attention not only to acute reactivity, but also to diurnal (evening) HPA activity as a marker of child sensitivity to parent symptoms. Furthermore, the present adoption design allows us to be more confident that enhanced parent symptom effects in children with higher cortisol reflect differential child susceptibility, and not simply heritable effects of parental risk characteristics.
An added layer of complexity comes from considering longitudinal paths of parent-child risk transmission at multiple levels. We have proposed separate consideration of within-family risk dynamics (i.e., child susceptibility moderating parent-child symptom associations over time) and between-family risk synergy (i.e., child susceptibility moderating overall parent symptom effects on child symptom trajectories), a distinction supported in this study. Specifically, whereas the former path applied to paternal symptom effects on child internalizing, the latter path was more relevant to associations between both parents’ depressive symptoms and child externalizing. It may be that young children’s internalizing problems are more directly sensitive to changing family conditions, whereas externalizing problems have more to do with overall compatibility of parent and child stress regulation resources.
This modeling approach also indicated that whereas within-family HPA-moderated effects of parental symptoms on child problem levels only yielded significant effects for children with higher cortisol, between-family effects of parental symptoms on child problem slopes yielded both positive effects for children with higher cortisol and negative effects for children with lower cortisol. The former pattern appears most consistent with BSCT, which predicts negligible effects of family context on low stress-sensitive children, whereas the latter pattern fits with diathesis-risk predictions that lower stress sensitivity actually confers benefits in risky environments. Further refinement of both diathesis-risk and BSCT models and information about how each operates may come from separating within- and between-family levels of longitudinal risk effects. Although further research in a variety of developmental contexts is needed to determine the practical importance of these distinctions, the current findings have important implications for intervention; adoptive parents of high-risk children should be offered support resources early on to avert externalizing growth, and additional support should be provided during high-stress periods (characterized by increasing parental depressive symptoms) to diminish child internalizing.
Limitations to the current study suggest future steps in this research. First, while we were able to investigate an important longitudinal slice of early child development (from 18-54 months), we were limited by availability of cortisol measures at the final time point only. Although evidence for stability of cortisol measures at this age and even earlier in development exists (Goldberg et al., 2003; Leung et al., 2011), we acknowledge that the measures in this study represent the endpoint of an individual response calibration process. A more comprehensive assessment of parent and child symptoms and cortisol over time, including tests of both concurrent and lagged effects, would allow researchers to more conclusively determine when and how daily cortisol levels and variability confer susceptibility to parental symptoms. The home-based cortisol collection method itself carries limitations; compared to a laboratory situation where influences on cortisol levels can be more tightly controlled, there are a multitude of factors reflecting both intrinsic child characteristics (i.e., tonic HPA activity, typical diurnal rhythms) and his or her ways of interacting with the environment (i.e., response to unknown daily experiences) contributing to the observed cortisol outcomes. On the other hand, this naturalistic assessment may offer a better picture of the child’s “sensitivity” endpoint once all these factors are combined. Our finding of more robust evening HPA-moderated effects than had been detected in prior studies examining acute stress response does not mean the latter is unimportant, and future HPA risk moderation research should combine attention to daily rhythms and stress reactivity and recovery features to describe susceptibility.
The relatively low-risk nature of this sample overall should also be considered when drawing conclusions, although previous meta-analytic findings suggest using a nonclinical community sample will either make no difference (for child externalizing outcomes) or underestimate effects (for child internalizing outcomes) of parental psychopathology (see Connell & Goodman, 2002; Goodman et al., 2011). Finally, while parental depression during early childhood is recognized as an important psychopathology risk marker, measuring a broader scope of parent symptomatology during early and later childhood would help to define which mother and father influences are most dependent on child HPA at which periods of development. Follow-up into later childhood might also reveal stronger effects of paternal, as opposed to maternal symptoms.
This study is distinguished by several strengths, including the use of an adoption sample to demonstrate paths of risk transmission that are not due to shared genes, consideration of morning and evening cortisol levels and variability as markers of stress sensitivity, and longitudinal multilevel modeling of mothers’, fathers’, and children’s adjustment across crucial periods of early development. It adds weight to the growing consensus that child physiological stress sensitivity modifies the impact of exposure to depressed parents while identifying new emphases—the importance of evening HPA, differing internalizing vs. externalizing and maternal vs. paternal susceptibility paths, and within-family vs. between-family levels of risk mechanisms—to be pursued in future research.
Acknowledgments
This project was supported by R01 HD042608, NICHD, NIDA, and OBSSR (the Office of the Director), NIH; U.S. PHS (PI Years 1–5: David Reiss; PI Years 6–10: Leslie D. Leve). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. Additional support was provided by P30 DA023920, R01 DA020585, and R01 MH092118.
We would like to thank the birth and adoptive parents who participated in this study and the adoption agencies who helped with the recruitment of study participants. Special gratitude is given to Rand Conger, John Reid, Xiaojia Ge, and Laura Scaramella who contributed to the larger study aims.
Contributor Information
Heidemarie K. Laurent, University of Wyoming
Leslie D. Leve, Oregon Social Learning Center
Jenae M. Neiderhiser, The Pennsylvania State University
Misaki N. Natsuaki, University of California, Riverside
Daniel S. Shaw, University of Pittsburgh
Philip A. Fisher, Oregon Social Learning Center, University of Oregon
Kristine Marceau, The Pennsylvania State University.
Gordon T. Harold, University of Leicester
David Reiss, Yale University Child Study Center.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. [Google Scholar]
- Alink LRA, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental Psychobiology. 2008;50:427–450. doi: 10.1002/dev.20300. doi:10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Sheinkopf SJ, Vohr BR, Lester BM. A preliminary study of cortisol reactivity and behavior problems in young children born premature. Developmental Psychobiology. 2010;52:574–582. doi: 10.1002/dev.20464. doi:10.1002/dev.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baildum EM, Hillier VF, Menon S, Bamford FN, Moore WMO, Ward BS. Attention to infants in the first year. Child: Care, Health and Development. 2000;26:199–216. doi: 10.1046/j.1365-2214.2000.00144.x. doi:10.1046/j.1365-2214.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJC, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavior Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. doi:10.1023/A:1025321609994. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Depression Inventory. Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. doi:10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. doi:10.1017/S0954579401003145. [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. doi:10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research. 2009;2:39–49. doi: 10.1002/aur.64. doi:10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings EM, El-Sheikh M, Kouros CD, Keller PS. Children’s skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. Journal of Child Psychology and Psychiatry. 2007;48:436–445. doi: 10.1111/j.1469-7610.2006.01713.x. doi:10.1111/j.1469-7610.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- Dehle C, Weiss R. Sex differences in prospective associations between marital quality and depressed mood. Journal of Marriage and the Family. 1998;60:1002–1011. doi:10.2307/353641. [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. doi:10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. doi:10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. doi:10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Engert V, Efanov SI, Dedovic K, Dagher A, Pruessner JC. Increased cortisol awakening response and afternoon/evening cortisol output in healthy young adults with low early life parental care. Psychopharmacology. 2011;214:261–268. doi: 10.1007/s00213-010-1918-4. doi:10.1007/s00213-010-1918-4. [DOI] [PubMed] [Google Scholar]
- Field T, Hossain Z, Malphurs J. Depressed fathers’ interactions with their infants. Infant Mental Health Journal. 1999;20:322–332. doi:10.1002/(SICI)1097-0355(199923)20:3<322::AID-IMHJ8>3.0.CO;2-T. [Google Scholar]
- Ge X, Natsuaki MN, Conger RD. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Development and Psychopathology. 2006;18:253–273. doi: 10.1017/S0954579406060147. doi:10.1017/S0954579406060147. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12- to 18-month-old infants: Stability over time, location, and stressor. Biological Psychiatry. 2003;54:719–726. doi: 10.1016/s0006-3223(03)00010-6. doi:10.1016/S0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology. 2009;34:76–86. doi: 10.1016/j.psyneuen.2008.08.012. doi:10.1016/j.psyneuen.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed parents: A developmental approach to the understanding of mechanisms. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. doi:10.1037/0033-295X.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. doi:10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gribbin CE, Watamura SE, Cairns A, Harsh JR, LeBourgeois MK. The cortisol awakening response (CAR) in 2- to 4-year-old children: Effects of acute nighttime sleep restriction, wake time, and daytime napping. Developmental Psychobiology. 2011 doi: 10.1002/dev.20599. epub ahead of print. doi:10.1002/dev.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. doi:10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Ruttle PL, Serbin LA, Mills RSL, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: Relations with parenting, temperament, and psychopathology. Developmental Psychobiology. 2011;53:694–710. doi: 10.1002/dev.20545. doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. doi:10.1016/S0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Kestler LP, Lewis M. Cortisol response to inoculation in 4-year-old children. Psychoneuroendocrinology. 2009;34:743–751. doi: 10.1016/j.psyneuen.2008.12.006. doi:10.1016/j.psyneuen.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wüst S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. doi:10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- LaFlamme D, Pomerleau A, Malcuit G. A comparison of fathers’ and mothers’ involvement in childcare and stimulation behaviors during free-play with their infants at 9 and 15 months. Sex Roles. 2002;47:507–518. doi:10.1023/A:1022069720776. [Google Scholar]
- Laurent HK, Kim HK, Capaldi DM. Longitudinal effects of conflict behaviors on depressive symptoms in young couples. Journal of Family Psychology. 2009;23:596–605. doi: 10.1037/a0015893. doi:10.1037/a0015893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Tasker SL, Atkinson L, Vaillancourt T, Schulkin J, Schmidt LA. Perceived maternal stress during pregnancy and its relation to infant stress reactivity at 2 days and 10 months of postnatal life. Clinical Pediatrics. 2011;49:158–165. doi: 10.1177/0009922809346570. doi:10.1177/0009922809346570. [DOI] [PubMed] [Google Scholar]
- Leve LD, Kim HK, Pears KC. Childhood temperament and family environment as predictors of internalizing and externalizing trajectories from ages 5 to 17. Journal of Abnormal Child Psychology. 2005;33:505–520. doi: 10.1007/s10802-005-6734-7. doi:10.1007/s10802-005-6734-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Ge X, Scaramella LV, Conger RD, Reid JB, Shaw DS, Reiss D. The Early Growth and Development Study: A prospective adoption design. Twin Research and Human Genetics. 2007;10:84–95. doi: 10.1375/twin.10.1.84. doi:10.1375/twin.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wood BL, Miller BD, Simmens SJ. Effects of paternal and maternal depressive symptoms on child internalizing symptoms and asthma disease activity: Mediation by interparental negativity and parenting. Journal of Family Psychology. 2011;25:137–146. doi: 10.1037/a0022452. doi:10.1037/a0022452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: Implications for development and adaptation. Developmental Neuroscience. 2009;31:300–308. doi: 10.1159/000216541. doi:10.1159/000216541. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. doi:10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. doi:10.3102/10769986031004437. [Google Scholar]
- Rice F, Harold G, Thapar A. The genetic aetiology of childhood depression: A review. Journal of Child Psychology and Psychiatry. 2002;43:65–79. doi: 10.1111/1469-7610.00004. doi:10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed Sage; Newbury Park, CA: 2002. [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperament predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. doi:10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Connell A, Dishion TJ, Wilson MN, Gardner F. Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Development and Psychopathology. 2009;21:417–439. doi: 10.1017/S0954579409000236. doi:10.1017/S0954579409000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. doi:10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: An extended Children of Twins study. Journal of Child Psychology and Psychiatry. 2010;51:734–744. doi: 10.1111/j.1469-7610.2010.02205.x. doi:10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73:75–92. doi: 10.1111/1467-8624.00393. doi:10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM. Psychological and stress hormone correlates in early life: A key to HPA-axis dysregulation and normalisation. Stress. 2005;8:47–57. doi: 10.1080/10253890500095200. doi:10.1080/10253890500095200. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. doi:10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, Hughes CW, Garber J, Malloy E, King CA, Cerda G, Sood AB, Alpert JE, Trivedi MH, Rush AJ, STAR*D-Child Team Remissions in maternal depression and child psychopathology: A STAR*D-child report. Journal of the American Medical Association. 2006;295:1389–1398. doi: 10.1001/jama.295.12.1389. doi:10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Vulnerability to psychopathology: A biosocial model. American Psychological Association; Washington, D. C.: 1999. doi:10.1037/10316-000. [Google Scholar]