Abstract
Intrinsic limits on cellular proliferation in human somatic tissue serves as a tumor suppressor mechanism by restricting cell growth in aged cells with accrued pre-cancerous mutations. This is accompanied by the potential cost of restricting regenerative capacity and contributing to cellular and organismal aging. Emerging data support a model where telomere erosion controls proliferative boundaries through the progressive change of telomere structure from a protected state, through two distinct states of telomere deprotection. In this model telomeres facilitate a controlled permanent cell cycle arrest with a stable diploid genome during differentiation and may serve as an epigenetic sensor of general stress in DNA metabolism processes.
Introduction
Fifty years after Leonard Hayflick’s seminal discovery we are still working to understand the molecular mechanisms that limit cell proliferation and their contributions to cellular and organismal aging [1,2]. There is a consensus that various cellular stressors in primary human cells induce “senescence”: a permanent state of cell cycle arrest where cells remain viable and metabolically active [3]. In the absence of exogenous insult or oncogene activation, senescence in human somatic cells is associated with increasing total accumulated cell divisions (i.e. replicative aging). Inactivation of the p53 and Retinoblastoma (Rb) tumor suppressor pathways confers insensitivity to senescence, allowing for a short period of proliferative lifespan extension and continued cell division. This ends at “crisis” when cells exhibit a marked increase in genomic instability and cell death. It has been known for sometime that telomeres, the protective terminal structures at eukaryotic chromosome ends, contribute to the regulation of proliferative boundaries. Recent studies have established a model where progressive telomere deprotection through two different structural states facilitates first the orderly removal of viable diploid cells from the cell cycle at senescence and if this is unsuccessful, kills precancerous cells at crisis. In this article we discuss the molecular mechanisms underlying telomere-dependent control of cellular proliferation in human tissues and postulate that telomeres may serve as an epigenetic sensor of general stress in DNA metabolism to regulate a controlled proliferative arrest at senescence.
Telomeres and replicative aging
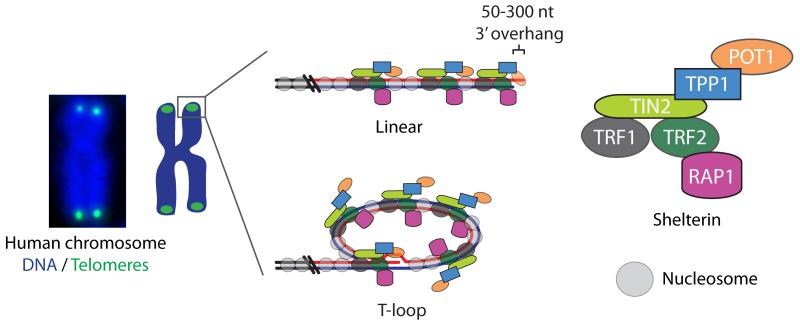
Telomeres delineate naturally occurring chromosome ends from double-strand breaks. This is an essential function as illicit telomere ligation results in dicentric chromosomes that if pulled to opposite spindle poles during mitosis can result in genomic instability and aneuploidy. Human telomeres are 4-15 kb of chromatinized 5′-TTAGGG-3′ repeats that typically terminate in a 50-300 nt single-strand 3′ overhang of the G-rich sequence, which are bound extensively by a specific six-subunit protein complex termed “shelterin” (Figure 1) [4,5]. Telomeres can adopt a higher-order telomere-loop (t-loop) structure where the 3′ overhang strand invades a proximal region of the same chromosome end [6]. In vitro studies suggest t-loop formation and protection is controlled by the shelterin protein TRF2 [6,7] and likely assisted by other shelterin and accessory factors [8,9]. T-loops are known to function in a mechanism that negatively regulates telomere length at overextended telomeres by resolving the t-loop junction into a free telomeric DNA circle and a shortened chromosome end [10,11]. While the percentage of looped telomeres is unclear to date, the requirement for sequestration of the 3′ overhang within the duplex telomeric DNA and the conservation of t-loops in a diverse variety of species strongly support a putative role for t-loops in chromosome end protection [6,12-14].
Figure 1.
Graphical representation of human telomeres arranged in both linear and t-loop configuration. The shelterin components are expanded on the right and a micrograph of a metaphase chromosome with the DNA counterstained blue and the telomeres stained in green is shown on the left.
The majority of data pertaining to shelterin function were generated in a series of studies where individual or multiple shelterin components were deleted in murine models lacking p53 activity. In general, deleting shelterin components results in acute phenotypes where the chromosome ends elicit either an ATM- or ATR-dependent DNA damage response (DDR) and the telomeres are subjected to homologous recombination or fusion via non-homologous (NHEJ) or alternative end joining (alt-NHEJ) [4,15,16]. TRF2 is the shelterin component most responsible for inhibiting end fusions, as its deletion results in NHEJ-dependent ligation of almost every chromosome end [17]. RAP1, which localizes to telomeres by binding TRF2, may also contribute to NHEJ prevention, though the exact mechanisms remain unresolved [18,19]. The invaluable experiments performed in mouse models have established the evolved protective functions of shelterin proteins. However, murine models of acute telomere deprotection do not recapitulate the process of spontaneous telomere deprotection resulting from replicative aging in human cells.
Spontaneous telomere deprotection in aging human cells coincides with erosion of the telomeric DNA without concomitant loss or mutation of shelterin components. A small amount of terminal DNA sequence is lost with each round of DNA replication and most human somatic tissues lack the mechanisms to maintain telomere length homeostasis. Human telomeres, therefore, progressively erode until senescence, or in the absence of p53 and Rb, until crisis. Cells that emerge from a telomere length crisis in mouse models invariably suffer from fusion-bridge-breakage cycle driven genome instability, which is a major hallmark of cancer [20]. Upregulation of telomerase, which catalytically adds telomere repeats to chromosome ends, or ALT, which maintains telomere length via homologous recombination, stabilizes telomere lengths and confers an indefinite replicative potential (i.e. cellular immortality). The correlations between shortening telomeres and proliferative arrest, and the observations that immortalized human stem and cancer cells actively maintain their telomere lengths via telomerase or ALT, indicates telomere-length is a contributing factor to replicative aging [21,22]. Telomere deprotection resulting from replicative aging in mice also appears to contribute to organismal aging though the activation of p53, which leads to compromised mitochondrial and metabolic function [23-25].
Telomere length, however, can ultimately be dissociated from senescence in human cells by altering shelterin function [26,27]. Disconnecting telomere length from telomere-dependent growth arrest implies that it is eventually the alteration of a telomere structure, which is dependent on sufficient telomere length and shelterin function, that is the causative factor driving telomere-dependent senescence. The outcome of telomere structural change at senescence is the activation of a localized DNA damage response (DDR) at a subset of chromosome ends and ATM and/or ATR-activation of p53-dependent growth arrest [28].
Spontaneous telomere deprotection in human cells
Deprotected chromosome ends subjected to a DDR are cytologically visible as colocalizations between telomeric proteins or DNA and DDR markers, such as phosphorylated histone H2AX (γ-H2AX) or 53BP1 in “telomere-deprotection induced foci (TIFs)” [29]. While it is difficult to accurately quantify absolute TIF numbers in interphase nuclei this can be overcome by visualizing metaphase-TIFs on cytocentrifuged metaphase spreads stained for telomeric DNA and γ-H2AX [30,31]. Metaphase-TIFs are present in small numbers in young primary human cells and increase in number with accumulated cell divisions [32]. Whereas, some cancer and immortalized human cell lines lacking p53 activity carry an excessive burden of DDR(+) telomeres without any apparent negative consequences on cell growth [30]. Consistent with the telomeric DDR being suppressed by a telomere length dependent structure, direct comparison of telomere lengths at DDR(+) and DDR(-) chromosome ends revealed that while the total number of DDR(+) telomeres in a cell population was inversely proportional to telomere length, it was not uncommon for individual DDR(+) telomeres to be of equal or greater length than other DDR(-) telomeres in the same metaphase [30,31]. The probability that a telomere will form a protective structure, therefore, increases with telomere length but it is not an absolute correlation.
Despite numerous DDR(+) chromosome ends, spontaneous end-to-end chromosome fusions were not observed in aged primary cells preceding senescence [32]. Moreover, > 98% of DDR(+) chromosome ends were fusion resistant in immortalized cell lines with numerous DDR(+) telomeres indicating most spontaneously occurring DDR(+) telomeres are resistant to end joining [30]. When senescence was prevented in primary cells by inhibiting p53 and Rb, spontaneous fusions occurred only after extensive telomere erosion as the cells approached crisis [32,33].
A three-state model of chromosome-end protection
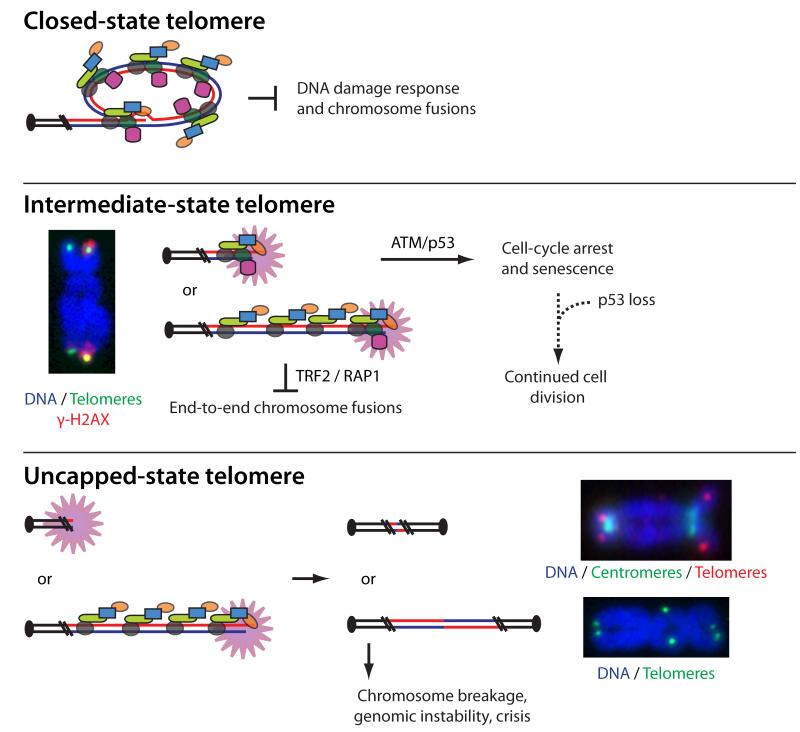
Observations of spontaneous telomere deprotection during replicative aging and terminal differentiation are consistent with a three-state model of chromosome end protection (Figure 2) [30]. In this model “closed-state” telomeres are a structure-dependent solution that protect chromosome ends from a DDR. Failure to adopt the closed-state structure results in “intermediate-state” telomeres where the chromosome end is exposed as a substrate to the DDR. However, retention of TRF2 at intermediate-state telomeres prevents NHEJ at the DDR(+) chromosome ends. Spontaneous chromosome end-to-end fusions result from “uncapped-state” telomeres that retain insufficient levels of TRF2 to repress end joining.
Figure 2.
Graphical representation of closed-state, intermediate-state and uncapped-state human telomeres as predicted to occur during replicative aging or following experimental disruption of TRF2. The closed-state telomere is shown as a t-loop though this remains hypothetical. Intermediate-state telomeres are depicted as the result of excessive telomere shortening where steric constraints prevent formation of the protective closed state, and at an elongated telomere that has failed to form a closed-state due to partial depletion of TRF2. The activated DDR is indicated by a starburst. To the left is an example of a human metaphase chromosome with the DNA counterstained blue, the telomeres stained green, and γ-H2AX stained red. Chromatid-type metaphase-TIFs are evident on both arms of this chromosome. Uncapped-state telomeres are shown as the result of excessive shortening that has eroded all TRF2 binding sites, consistent with spontaneous fusions at crisis, and at elongated telomeres following experimental TRF2 deletion. The predicted fusion events are demonstrated in the middle. To the right are examples of fused chromosomes from human cells during lifespan extension that do not retain telomere repeats, and fused chromosomes in cells lacking TRF2 where telomeric repeats are evident within the dicentric chromosome.
The molecular identity of closed-state telomeres remains enigmatic. A t-loop fits the model, though this remains to be determined experimentally. Chromatin marks, telomeric chromatin compaction or some other structural feature may also serve to protect the chromosome end from the DDR. If the closed-state telomere structure is a t-loop, this implicates TRF2 in separate end-protection functions regulating: 1) protection against the DDR by promoting t-loop structure and 2) prevention of NHEJ at DDR(+) telomeres by directly binding to the telomeric DNA. The shelterin protein TIN2 may also contribute to closed-state telomere structure as TRF2 overexpression in TIN2 null cells results in a phenotype consistent with intermediate-state telomeres [34].
Spontaneous telomere deprotection during the cell cycle
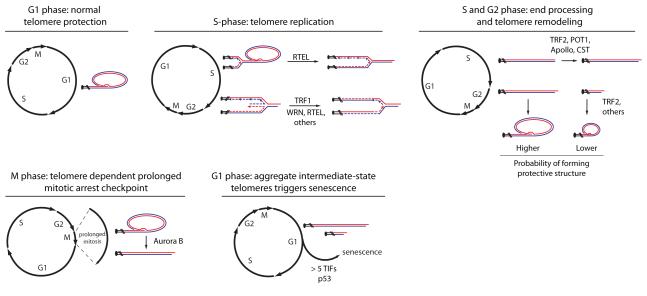
In order to conceptualize how telomere deprotection results from replicative aging it is important to consider the DNA metabolic activities at chromosome ends during progression through the cell cycle (Figure 3). Protected telomeres are arranged into closed-state structures in G1 phase cells. During the process of DNA replication the closed-state structure is expected to unfold, either as a passive response to the passage of the replicative DNA polymerase [9,35] or through active unwinding by the RTEL helicase [36]. It is common for replication forks to encounter difficulties when replicating repetitive G-rich telomeric DNA and as a result, proteins that restart stalled replication forks or unwind structural impediments to replication commonly associate with the telomeric DNA during S or G2-phase [9,35-42]. TRF1 is the shelterin member that is primarily responsible for regulating efficient replication through the mammalian telomeric sequence [38,43]. Because telomeres are unidirectionally replicated from mostly subtelomeric origins [44], resolution of stalled telomeric DNA replication is necessary to prevent loss of telomeric sequences distal to the stalled replication fork. Telomeres are replicated throughout S-phase [45] and late-replicating intermediate-structures in the telomeric DNA are resolved by the BLM helicase [46]. Telomeric chromatin is also established during DNA replication. After replication is complete, the shelterin proteins POT1 and TRF2 direct processing of the 3′-overhangs into their mature form in coordination with Apollo and the CST complex [39,47-50]. Finally, the telomeres are re-packaged into the protective closed-state by TRF2 and potentially other shelterin and recombination proteins [9]. When the process of reestablishing the closed-state fails, we anticipate that the chromosome end will be exposed to the DDR as an intermediate-state telomere in G2 and/or M-phase and visible as a metaphase-TIF.
Figure 3.
Graphical representation of the DNA metabolic activities that occur normally at human telomeres in the context of the cell division cycle as described throughout the article text.
Most spontaneous intermediate-state telomeres affect only one sister chromatid of a metaphase chromosome, consistent with spontaneous DDR(+) chromosome ends resulting from a replicative or post-replicative failure in chromosome end protection [30,32]. In aged primary human cells there is no bias for metaphase-TIFs on the leading- or lagging-strand replicated telomere, suggesting that the telomere refolding process occurs independently on sister-chromatid telomeres after DNA replication is complete [32].
However, while the majority of telomeric DNA metabolism occurs during S and G2, replicative senescence arrests cell growth in G1, suggesting that spontaneous intermediate-state telomeres are passed from S, G2 and/or M through cell division, before eliciting cell cycle arrest in the subsequent G1-phase daughter cells. Because some intermediate-state telomeres are present in young primary cells and cancer cells with wild type p53 without initiating cell cycle arrest, it appears an aggregate number of deprotected telomeres are necessary to arrest cell growth. Calculations of the expected number of G1-phase intermediate-state telomeres at replicative senescence is consistent with around five DDR(+) chromosome ends [32]. Lack of p53 activity represses the G1/S checkpoint, which is what presumably allows cells in the lifespan extension period (or cancer and immortalized cells without p53 function) to propagate with impunity despite having > 5 G1-phase intermediate-state telomeres. This is until the telomeres shorten sufficiently to enter the uncapped-state and the resulting chromosome fusions lead to cell death at crisis.
Intermediate- and uncapped-state telomeres thus appear to independently regulate the senescence and crisis proliferative boundaries, with replicative senescence being an active response that protects genomic stability by arresting growth in viable cells with a diploid genome, while crisis is a passive response that kills cells as a result of genomic instability induced by end-to-end chromosome fusions. The importance of the tumor suppressive functions of telomere-dependent proliferative boundaries was reiterated in recent work showing that telomere-dependent mechanisms drive aneuploidy during lifespan extension [51,52] or in p53 deficient mouse models with acute telomere deprotection [53], and that conditionally re-activating telomerase in ATM or p53 deficient late-generation telomerase knockout mice with accrued genomic instability results in highly aggressive tumors [54,55].
A mitotic duration checkpoint regulated by intermediate-state telomeres
A surprising recent observation was the discovery of a telomere-dependent prolonged mitotic arrest checkpoint in human cells regulated by the transition of closed- to intermediate-state telomeres [56]. For sometime it had been known that a prolonged mitotic arrest causes p53-dependent cell cycle arrest through an unknown mechanism [57]. Our laboratory discovered that during prolonged mitotic arrest, an Aurora B dependent mechanism results in an ATM-dependent telomeric DDR in the absence of telomere shortening which induces a p53-dependent G1-phase cell cycle arrest. During this process some TRF2 is dissociated from the chromosome ends but enough protein remains to prevent end joining, consistent with intermediate-state telomeres. In agreement with a threshold number of deprotected telomeres being required to arrest the cell cycle, the duration of a prolonged mitosis necessary to induce p53-activity coincides with the appearance of around 12 DDR(+) telomeric chromatids that when separated by random segregation would correspond to 6 DDR(+) telomeres in the subsequent G1-phase daughter cells.
Telomeres as epigenetic sensors of cellular stress
Unlike double strand breaks, which must be repaired for cell viability, it is deleterious to “repair” intermediate-state telomeres by end joining. Through its putative dual functions, TRF2 establishes the unique environment of the chromosome end by allowing activation of a DDR to arrest the cell cycle at senescence, but preventing fusions and genomic instability. This is evident in the persistent DDR foci that go unrepaired in senescent cells, consistent with their likely telomeric origin. Moreover, when DNA breaks are introduced by irradiation in senescent cells, DDR foci at genomic sequences disappear with time as the chromatin is repaired, while breaks induced in telomeric sequences remain DDR(+), owing to TRF2 dependent repression of end joining [58,59]. In the three-state model of telomere protection discussed here, the DDR at shortened telomeres does not occur because telomeres are damaged per se, but because the telomeres are no longer arranged into protective structures resulting in exposure of the chromosome ends.
In this view, it is a possibility that telomeres also serve as a general sensor of genomic health. The process of constructing and maintaining closed-state telomeres requires several consecutive DNA metabolic activities: efficient telomeric DNA replication, resolution of late-replicating structures, establishment of the telomeric chromatin, processing of the chromosome end into a 3′ overhang, shelterin and potentially recombination-dependent formation of a closed-state structure, and efficient progression through mitosis. Disturbance at any of these steps could result in intermediate-state telomeres. These DNA metabolic functions are not exclusive to the telomeres, but unlike the telomeric sequence, genomic sequences must be efficiently repaired for cellular viability. When disturbances in the above-mentioned processes are manifested in genomic regions, compensatory mechanisms likely function to minimize genomic instability. Whereas in the telomeres, disturbances in DNA metabolic processes may result in chromosome ends that fail to form a protective closed-state and register a DDR.
The telomere-dependent prolonged mitotic arrest checkpoint is a pertinent example. Here difficulties in mitotic progression, a problem seemingly unrelated to telomere protection, result in the transition of closed- to intermediate-state telomeres inducing cell cycle arrest. Telomeres in this context serve as an epigenetic sensor for a larger cell-wide disturbance, and become deprotected to ensure growth arrest without the risk of genome instability. Similarly, activated oncogene-induced senescence was previously shown to result from a hyper DNA replication-dependent DDR [60] and recent evidence suggests this might be primarily manifested in telomeric regions [61]. As DDR(+) telomeres accumulate through various means the aggregate stress signals emitted intermediate-state telomeres drive global changes in histone production and chromatin architecture that enforce the senescence program [62].
It will be interesting to determine if other apparently telomere-independent cellular stresses exert similar telomere-dependent mechanisms to restrict cell growth, and whether telomeres influence other pan-nuclear responses as during aging.
Intermediate-state telomeres in stem cells
The dynamics of spontaneous intermediate-state telomeres in immortalized, cancer and somatic cells suggest that an intermediate-state telomere in one cell cycle may be closed-state in the next [30,32,63]. The aggregate nature of around five DDR(+) telomeres being necessary to induce p53 dependent arrest in fibroblast cultures suggest some tolerance in somatic tissues to the spurious occurrence of small numbers of DDR(+) chromosome ends. However, it is unclear how the telomeric DDR is exerted in stem cells. While stem cells maintain telomere length by telomerase activity, perturbation in other DNA metabolic processes might lead to deprotected chromosome ends. The sensitive nature of the stem niche may be less tolerant to DDR(+) chromosome ends and quickly upregulate p53 activity with fewer intermediate-state telomeres. This may explain why p53 is a boundary to induced pluripotent stem cell generation [64]. This also may explain why disease mutations in Dyskeratosis congenita that affect telomere elongation by telomerase [65] and disease mutations in Coats Plus that affect efficient telomeric DNA replication and 3′ overhang processing [41,66-69], result in over-lapping pathogenic manifestations effecting highly proliferative tissues .
Conclusions
In this article we have chosen to describe DDR(+) telomeres arising from normal biological processes as “deprotected” instead of “dysfunctional”. Dysfunction suggests an inappropriate or absent function. Whereas, spontaneous telomere deprotection is a vital and normal tumor suppressive function of human telomeres that removes aged or damaged cells with a diploid genome from the cell cycle to prevent genomic instability. In concordance with a telomere-structure dependent solution to prevent a DDR, we suggest a strict interest in telomere length is short sighted when considering the contribution of telomeres to human aging and disease. We speculate that putative variations in the expression of shelterin components and the efficiency of DNA metabolic activities between individuals may result in different telomere lengths being protective against a DDR from person to person. While telomere length is undoubtedly the major underlying regulator of telomere-dependent proliferative boundaries and therefore for differentiation, it is necessary to remember that it is one of many factors contributing to telomere protection. A more systemic view of telomere health is likely a better measure of telomere function than a singular view of telomere length.
Highlights.
Human telomeres are protected from the DDR by a higher-order structure
Intermediate-state telomeres are susceptible to a DDR and regulate senescence
Uncapped-state telomeres are susceptible to fusions and induce crisis
Intermediate-state telomeres regulate the prolonged mitosis checkpoint
Telomeres may serve as epigenetic sensors of cellular stress
Acknowledgements
We apologize to authors whose work has not been cited due to space restrictions or for being outside of review period and for not always citing the primary literature. Dr. Zeenia Kaul is thanked for providing images of mitotic chromosomes. A.J.C. is supported by a training grant from the US National Institutes of Health (NIH) (5T32CA009370-29). The author’s lab is supported by the Salk Institute Cancer Center Core Grant (P30 CA014195-38) the NIH (GM087476).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Giaimo S, d’Adda di Fagagna F. Is cellular senescence an example of antagonistic pleiotropy? Aging Cell. 2012;11:378–383. doi: 10.1111/j.1474-9726.2012.00807.x. [DOI] [PubMed] [Google Scholar]
- 3.Larsson LG. Oncogene- and tumor suppressor gene-mediated suppression of cellular senescence. Semin Cancer Biol. 2011;21:367–376. doi: 10.1016/j.semcancer.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 4.de Lange T. How Shelterin Solves the Telomere End-Protection Problem. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2010.75.017. [DOI] [PubMed] [Google Scholar]
- 5.Oganesian L, Karlseder J. Mammalian 5′ C-rich telomeric overhangs are a mark of recombination-dependent telomere maintenance. Mol Cell. 2011;42:224–236. doi: 10.1016/j.molcel.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 7.Poulet A, Buisson R, Faivre-Moskalenko C, Koelblen M, Amiard S, Montel F, Cuesta-Lopez S, Bornet O, Guerlesquin F, Godet T, et al. TRF2 promotes, remodels and protects telomeric Holliday junctions. Embo J. 2009;28:641–651. doi: 10.1038/emboj.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith J, Bianchi A, de Lange T. TRF1 promotes parallel pairing of telomeric tracts in vitro. J Mol Biol. 1998;278:79–88. doi: 10.1006/jmbi.1998.1686. [DOI] [PubMed] [Google Scholar]
- 9.Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–720. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Pickett HA, Cesare AJ, Johnston RL, Neumann AA, Reddel RR. Control of telomere length by a trimming mechanism that involves generation of t-circles. Embo J. 2009;28:799–809. doi: 10.1038/emboj.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickett HA, Henson JD, Au AY, Neumann AA, Reddel RR. Normal mammalian cells negatively regulate telomere length by telomere trimming. Hum Mol Genet. 2011;20:4684–4692. doi: 10.1093/hmg/ddr402. [DOI] [PubMed] [Google Scholar]
- 12.Cesare AJ, Groff-Vindman C, Compton SA, McEachern MJ, Griffith JD. Telomere loops and homologous recombination-dependent telomeric circles in a Kluyveromyces lactis telomere mutant strain. Mol Cell Biol. 2008;28:20–29. doi: 10.1128/MCB.01122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cesare AJ, Quinney N, Willcox S, Subramanian D, Griffith JD. Telomere looping in P. sativum (common garden pea) Plant J. 2003;36:271–279. doi: 10.1046/j.1365-313x.2003.01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Raices M, Verdun RE, Compton SA, Haggblom CI, Griffith JD, Dillin A, Karlseder J. C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell. 2008;132:745–757. doi: 10.1016/j.cell.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Rai R, Zheng H, He H, Luo Y, Multani A, Carpenter PB, Chang S. The function of classical and alternative non-homologous end-joining pathways in the fusion of dysfunctional telomeres. EMBO J. 2010;29:2598–2610. doi: 10.1038/emboj.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •16.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012;336:593–597. doi: 10.1126/science.1218498. This manuscript describes combinatorial deletion of TRF1 and TRF2 in mouse cells and complements previous studies to identify the specific shelterin components that inhibit ATM- or ATR-signaling, NHEJ, ALT-NHEJ, end resection and homologous recombination at chromosome ends.
- 17.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 18.Sarthy J, Bae NS, Scrafford J, Baumann P. Human RAP1 inhibits non-homologous end joining at telomeres. EMBO J. 2009;28:3390–3399. doi: 10.1038/emboj.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sfeir A, Kabir S, van Overbeek M, Celli GB, de Lange T. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327:1657–1661. doi: 10.1126/science.1185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 21.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 22.Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Lett. 2010;584:3819–3825. doi: 10.1016/j.febslet.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••23.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. This study used telomerase deficient mice to show how telomere deprotection-dependent p53-activation represses PGC-1α and PGC-1β, thereby impairing mitochondria biogenesis and negatively impacting metabolism. These results directly connected telomere deprotection to diminishing organismal fitness.
- 24.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- 27.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 28.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 29.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 30.Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol. 2009;16:1244–1251. doi: 10.1038/nsmb.1725. [DOI] [PubMed] [Google Scholar]
- 31.Thanasoula M, Escandell JM, Martinez P, Badie S, Munoz P, Blasco MA, Tarsounas M. p53 prevents entry into mitosis with uncapped telomeres. Curr Biol. 2010;20:521–526. doi: 10.1016/j.cub.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •32.Kaul Z, Cesare AJ, Huschtscha LI, Neumann AA, Reddel RR. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 2012;13:52–59. doi: 10.1038/embor.2011.227. This study accurately measured spontaneous telomere deprotection in primary human cells from isolation to senescence and during lifespan extension. It provided evidence that intermediate- and uncapped-state telomeres independently control senescence and crisis and described how an aggregate of around five intermediate-state telomeres are required to induce senescence.
- 33.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai KK, Kibe T, Donigian JR, Frescas D, de Lange T. Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell. 2011;44:647–659. doi: 10.1016/j.molcel.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verdun RE, Crabbe L, Haggblom C, Karlseder J. Functional Human Telomeres Are Recognized as DNA Damage in G2 of the Cell Cycle. Mol Cell. 2005;20:551–561. doi: 10.1016/j.molcel.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Vannier JB, Pavicic-Kaltenbrunner V, Petalcorin MI, Ding H, Boulton SJ. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell. 2012;149:795–806. doi: 10.1016/j.cell.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Crabbe L, Verdun RE, Haggblom CI, Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- 38.Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye J, Lenain C, Bauwens S, Rizzo A, Saint-Leger A, Poulet A, Benarroch D, Magdinier F, Morere J, Amiard S, et al. TRF2 and apollo cooperate with topoisomerase 2alpha to protect human telomeres from replicative damage. Cell. 2010;142:230–242. doi: 10.1016/j.cell.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 40.Uringa EJ, Lisaingo K, Pickett HA, Brind’amour J, Rohde JH, Zelensky A, Essers J, Lansdorp PM. RTEL1 contributes to DNA replication and repair and telomere maintenance. Mol Biol Cell. 2012;23:2782–2792. doi: 10.1091/mbc.E12-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu P, Min JN, Wang Y, Huang C, Peng T, Chai W, Chang S. CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J. 2012;31:2309–2321. doi: 10.1038/emboj.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla R, Redon S, Raftopoulou C, Wischnewski H, Gagos S, Azzalin CM. Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. EMBO J. 2011;30:4047–4058. doi: 10.1038/emboj.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drosopoulos WC, Kosiyatrakul ST, Yan Z, Calderano SG, Schildkraut CL. Human telomeres replicate using chromosome-specific, rather than universal, replication programs. J Cell Biol. 2012;197:253–266. doi: 10.1083/jcb.201112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnoult N, Schluth-Bolard C, Letessier A, Drascovic I, Bouarich-Bourimi R, Campisi J, Kim SH, Boussouar A, Ottaviani A, Magdinier F, et al. Replication timing of human telomeres is chromosome arm-specific, influenced by subtelomeric structures and connected to nuclear localization. PLoS Genet. 2010;6:e1000920. doi: 10.1371/journal.pgen.1000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barefield C, Karlseder J. The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu P, van Overbeek M, Rooney S, de Lange T. Apollo contributes to G overhang maintenance and protects leading-end telomeres. Mol Cell. 2010;39:606–617. doi: 10.1016/j.molcel.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam YC, Akhter S, Gu P, Ye J, Poulet A, Giraud-Panis MJ, Bailey SM, Gilson E, Legerski RJ, Chang S. SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair. EMBO J. 2010;29:2230–2241. doi: 10.1038/emboj.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow TT, Zhao Y, Mak SS, Shay JW, Wright WE. Early and late steps in telomere overhang processing in normal human cells: the position of the final RNA primer drives telomere shortening. Genes Dev. 2012;26:1167–1178. doi: 10.1101/gad.187211.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu P, Takai H, de Lange T. Telomeric 3′ Overhangs Derive from Resection by Exo1 and Apollo and Fill-In by POT1b-Associated CST. Cell. 2012;150:39–52. doi: 10.1016/j.cell.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pampalona J, Frias C, Genesca A, Tusell L. Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells. PLoS Genet. 2012;8:e1002679. doi: 10.1371/journal.pgen.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davoli T, de Lange T. Telomere-driven tetraploidization occurs in human cells undergoing crisis and promotes transformation of mouse cells. Cancer Cell. 2012;21:765–776. doi: 10.1016/j.ccr.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81–93. doi: 10.1016/j.cell.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •54.Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, et al. Telomerase Reactivation following Telomere Dysfunction Yields Murine Prostate Tumors with Bone Metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••55.Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, et al. Antitelomerase Therapy Provokes ALT and Mitochondrial Adaptive Mechanisms in Cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. References 54 and 55 utilized mice with an inducible telomerase allele to show that reactivating telomerase in animals with accrued genomic instability due to telomere deprotection results in highly aggressive tumors. These studies unequivocally show that telomeres function in a tumor suppressive role.
- ••56.Hayashi MT, Cesare AJ, Fitzpatrick JA, Lazzerini-Denchi E, Karlseder J. A telomere-dependent DNA damage checkpoint induced by prolonged mitotic arrest. Nat Struct Mol Biol. 2012;19:387–394. doi: 10.1038/nsmb.2245. This manuscript details the discovery of a novel prolonged mitotic arrest checkpoint and shows that p53 dependent cell cycle arrest following prolonged mitotic arrest is the result of an Aurora B dependent mechanism that induces intermediate-state telomeres.
- 57.Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- •58. Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. This manuscript details how induction of genomic breaks within the telomeric DNA in mammalian cells results in a persistent DDR due to TRF2 dependent suppression of repair, similar to intermediate-state telomeres.
- 59.Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, Anderson R, Taschuk M, Mann J, Passos JF. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, Schurra C, Garre M, Nuciforo PG, Bensimon A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 61.Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, Fumagalli M, Di Micco R, Mirani N, Gurung RL, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •62.O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17:1218–1225. doi: 10.1038/nsmb.1897. This manuscript describes a study of the global and telomeric chromatin landscape in primary human cells during replicative aging. It details how histone biogenesis is impaired with aging resulting in changes in both the telomeric and global chromatin architecture that lead to senescence.
- 63.Price CM. Telomere flip-flop: an unfolding passage to senescence. EMBO Rep. 2012;13:5–6. doi: 10.1038/embor.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menendez S, Camus S, Izpisua Belmonte JC. p53: guardian of reprogramming. Cell Cycle. 2010;9:3887–3891. doi: 10.4161/cc.9.19.13301. [DOI] [PubMed] [Google Scholar]
- 65.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keller RB, Gagne KE, Usmani GN, Asdourian GK, Williams DA, Hofmann I, Agarwal S. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr Blood Cancer. 2012;59:311–314. doi: 10.1002/pbc.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, Urquhart JE, Daly SB, Dickerson JE, O’Sullivan J, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet. 2012;44:338–342. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- 68.Polvi A, Linnankivi T, Kivela T, Herva R, Keating JP, Makitie O, Pareyson D, Vainionpaa L, Lahtinen J, Hovatta I, et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. Am J Hum Genet. 2012;90:540–549. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyake Y, Nakamura M, Nabetani A, Shimamura S, Tamura M, Yonehara S, Saito M, Ishikawa F. RPA-like mammalian Ctc1-Stn1-Ten1 complex binds to single-stranded DNA and protects telomeres independently of the Pot1 pathway. Mol Cell. 2009;36:193–206. doi: 10.1016/j.molcel.2009.08.009. [DOI] [PubMed] [Google Scholar]