Abstract
BCL-2 molecules are regulators of programmed cell death and defects in this pathway contribute to human diseases. One family member, MCL-1, is unique as its expression is tightly regulated and it is essential for promoting the survival of myriad cellular lineages. Additionally, MCL-1 promotes the maintenance of normal mitochondrial morphology and energy production. Dissection of these functions revealed recently that they depend on separate mitochondrial sub-localizations. MCL-1's anti-apoptotic activity is restricted to the outer mitochondrial membrane, whereas its function in mitochondrial physiology requires localization to the matrix. These findings provide an attractive model for how MCL-1's diverse functions may contribute to normal cell homeostasis and function. MCL-1 is highly amplified in human cancer suggesting that these functions may contribute to malignant cell growth and evasion of apoptosis.
Keywords: Apoptosis, MCL-1, homeostasis, mitochondrial function, cancer, development
The BCL-2 Family and Apoptosis
Apoptosis, or programmed cell death, regulates development and maintains tissue homeostasis in animals. Understanding this genetically encoded program is important, as when it is dysregulated it contributes to human pathologies including cancer, neurodegeneration, and autoimmunity [1]. The intrinsic cell death pathway is regulated by members of the BCL-2 family that share regions of homology known as BCL-2 homology (BH) domains. BH3-only molecules (including BID, BAD, BIM, PUMA, NOXA, etc.) are induced by cellular stress and death signals through transcriptional regulation and/or post-translational modification. Upon activation, BH3-only molecules promote the activation and oligomerization of the pro-apoptotic effectors BAX and BAK; a process antagonized by anti-apoptotic BCL-2 family members [2]. BAX and BAK oligomerization results in mitochondrial outer membrane permeabilization and facilitates the release of a variety of proteins, including cytochrome c, that promote caspase activation, catalyzing cellular destruction [3].
MCL-1: A Unique Anti-apoptotic BCL-2 Family Member
Myeloid cell leukemia sequence 1 (MCL-1) was identified as an immediate-early gene induced by TPA-mediated differentiation of a human myeloid leukemia cell line (ML-1) [4]. Structurally, its carboxy-terminal core resembles that of other anti-apoptotic BCL-2 family members, but it does feature some structural differences that dictate selectivity for binding BH3-only molecules [5]. MCL-1's amino-terminus is much longer than that of any other anti-apoptotic BCL-2 family member and is intrinsically unstructured and therefore excluded from structural analyses [6].
MCL-1's short protein half-life also distinguishes it from other anti-apoptotic BCL-2 family members [7] and its expression is regulated in response to a variety of growth factor and glucose signaling cascades [8, 9]. MCL-1 undergoes both ubiquitin-dependent and ubiquitin-independent degradation [10, 11]. To date, three E3 ubiquitin-ligases have been implicated in promoting the ubiquitinylation of MCL-1. MULE, a HECT-domain containing E3, has been reported to ubiquitinylate MCL-1 targeting it for degradation by the proteasome [10, 12]. However, it is unclear whether MULE-dependent ubiquitinylation of MCL-1 occurs under basal regulation or is only induced by specific death stimuli [10, 13]. The SKP1-cullin-1-F-box (SCF) complex E3 ligases, ß-TrCP and FBW7 have been shown to ubiquitinylate MCL-1 in a phosphorylation-dependent manner, indicating that cellular signaling can modulate degradation [14, 15]. Lastly, MCL-1 degradation is opposed by the action of the USP9X deubiquitinase that removes polyubiquitin chains from MCL-1 stabilizing MCL-1 protein expression leading to apoptotic resistance [16]. Therefore, MCL-1 expression can be rapidly changed in response to cellular stresses.
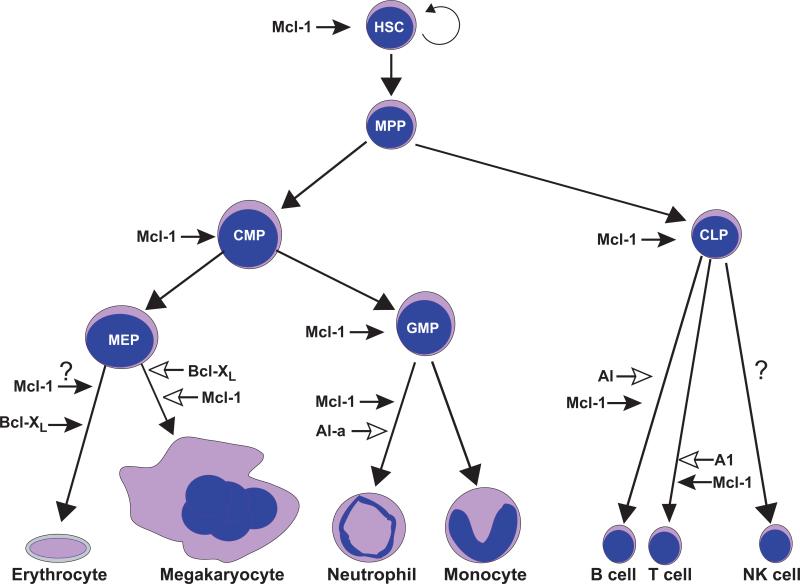
MCL-1 is also unique among pro-survival BCL-2 molecules in that it is essential for early (lethal at embryonic day 3.5) embryonic development [17] as well as for the survival of multiple cell lineages including lymphocytes [18, 19], hematopoietic stem cells [20], neutrophils [21, 22], and neurons [23] (Figure 1). Interestingly, many of these cell types concomitantly express other anti-apoptotic molecules in addition to MCL-1, demonstrating that endogenous levels of other anti-apoptotic molecules are insufficient to promote survival in the absence of MCL-1. In contrast, genetic ablation of BCL-2 results in a more selective loss of mature, activated lymphocytes and results in defective kidney development [24]. Loss of BCL-XL also results in embryonic lethality (embryonic day 13) due to failures in neuronal development and defects in red blood cell survival [25, 26]. In the megakaryocytic lineage, MCL-1 and BCL-XL play overlapping functions as loss of either perturbs development while loss of both has more profound effects [27, 28]. In chimeric mice, the loss of BCL-XL also results in defective T lymphocyte development [29]. BCL-w deficient mice are grossly normal with the exception of defective spermatogenesis [30, 31]. Genetic deletion of A1-a, a homolog of human BFL-1, renders neutrophils highly sensitive to death induced by lipopolysaccharide, but is not essential for their development [32]. An in vivo RNAi approach confirmed the importance of all 3 A1 isoforms (A1-a, b, d) on granulocyte development and also demonstrated a role for A1 isoforms in promoting lymphoid development [33]. Therefore, in contrast to other anti-apoptotic molecules, MCL-1 clearly plays a unique role as it is essential for the survival of so many cell lineages (Figure 1).
Figure 1. Role of Anti-Apoptotic Regulators during Hematopoiesis.
All blood cell lineages arise from a hematopoietic stem cell (HSC) that is capable of self-renewal and has an indefinite life-span. HSCs give rise to multi-potent progenitors (MPPs) which still retain the ability to give rise to all blood cell lineages, but lack long-term self-renewal capacity. MPPs can produce two progenitors, common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs). CLPs can produce the lymphoid lineages (B, T, and perhaps NK cells). CMPs produce at least two other oligopotent progenitor populations, the megakaryocyte erythroid progenitor (MEP) that produce red blood cells (erythrocytes) and megakaryocytes (generates platelets) and the granulocyte monocyte progenitor (GMP) that produces granulocytes (neutrophils) and monocytes (macrophages). Listed beside each differentiation step or progenitor population are the known anti-apoptotic regulators that promote the survival of the given population. Anti-apoptotic MCL-1 has multiple checkpoints as it has been illustrated to be critical for the survival of several multipotent and oligopotent progenitor populations (HSC, CMP, CLP, and GMP) and has been shown to be critical for the differentiation of granulocytes, but interestingly not the monocyte lineage. A1-a, a murine ortholog of BFL-1, and other A1 isoforms have also been shown to play an important role in promoting neutrophil survival in response to stress, but are not absolutely required for development, but primarily effect mature cell survival (indicated by open arrowhead). A1 isoforms also play a role, but are not essential in lymphoid development. In the erythroid lineage, it appears that anti-apoptotic BCL-XL is the essential survival molecule, but unpublished data indicates that MCL-1 may also play an essential role during early differentiation. In the megakaryocytic lineage it appears that while neither MCL-1 nor BCL-XL is solely responsible for survival, the two pro-survival molecules appear to have overlapping functions (indicated by open arrowheads) in promoting megakaryocyte survival. The critical anti-apoptotic regulators of some lineages including NK cells are still uncertain.
MCL-1 Regulates Mitochondrial Physiology from within the Mitochondria
MCL-1 protein appears as a doublet as detected by immunoblot in many cell types [34]; however, the origin of the doublet had been somewhat elusive [35, 36]. Over the last several years, several groups have independently demonstrated that full-length MCL-1 undergoes proteolytic processing on its amino-terminus to give rise to the differentially migrating forms [37-40]. Interestingly, the processing of MCL-1 from its full-length to the truncated form depends on a functional mitochondrial membrane potential [38, 39]. However, there is less agreement regarding the cellular site of the proteolytic processing and final localization of the truncated form. For example, it has been demonstrated that the processed form of MCL-1 resides on the outer mitochondrial membrane rendering it less susceptible to proteasome-mediated degradation [39]. These data imply that either MCL-1's amino-terminal domain gains transient access to the mitochondrial inner membrane or that a membrane potential-dependent protease on the outer mitochondrial membrane is responsible for the cleavage [39]. There exists precedent for such voltage-dependent cleavage as PINK1, a mitochondrial outer membrane protein, undergoes rapid cleavage by an unknown protease only in the presence of an intact membrane potential [41-43]. In contrast, another group has presented data that the processed form of MCL-1 actually resides within the mitochondrial matrix and identified that the matrix processing peptidase (MPP) is responsible for the amino-terminal cleavage of a mitochondrial targeting sequence suggesting that MCL-1 is cleaved during import into the mitochondria [40]. Similarly, our group also identified that MCL-1 underwent two separate cleavage events and that the truncated form of MCL-1 was imported into the mitochondrial matrix in a manner dependent on the translocases of the outer and inner membrane (TOM and TIM complexes) [37]. It will be important to identify whether cleavage at both sites occurs within the mitochondria and/or whether other cellular proteases beyond MPP are involved.
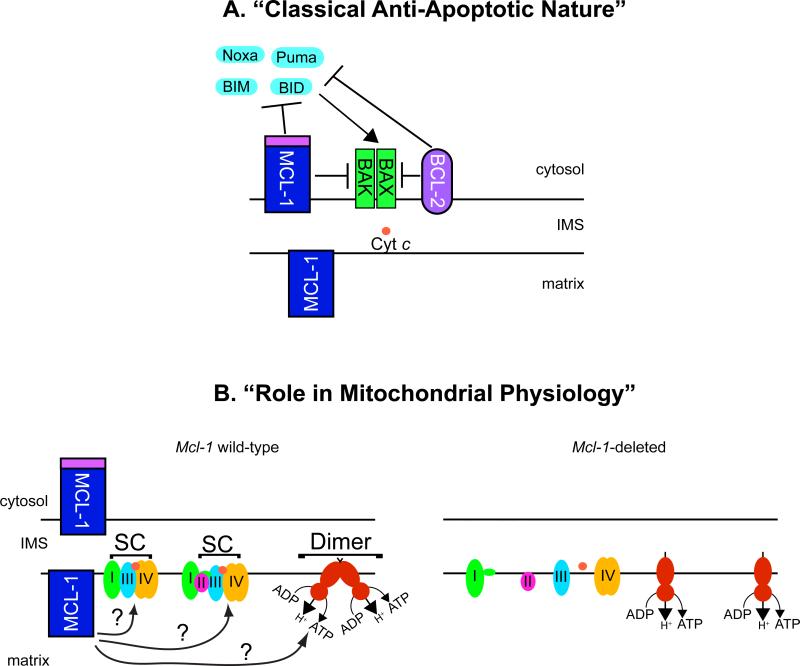
Recently, a role for the truncated form of MCL-1 in regulating mitochondrial metabolism and dynamics was revealed. This function is separate from full-length MCL-1's ability to antagonize cell death and requires its import into the mitochondrial matrix [37]. Functionally, the OMM-localized MCL-1 possesses anti-apoptotic activity inhibiting cell death and binding pro-apoptotic molecules (Figure 2a). In contrast, the matrix-localized MCL-1 does not possess anti-apoptotic function, but instead facilitates normal mitochondrial physiology and energy metabolism [37]. The matrix-localized MCL-1 promotes normal mitochondrial ultrastructure by maintaining cristae morphology as well as the dynamics of mitochondrial fusion (Figure 2b). Additionally, the MCL-1 matrix isoform supports oxidative phosphorylation, ATP production and maintenance of mitochondrial membrane potential (Figure 2b) [37]. Thus, MCL-1 appears to function both to directly oppose apoptosis and to promote mitochondrial physiology (Figure 2).
Figure 2. Model for MCL-1's Potential Functions at the Mitochondria.
MCL-1 possesses multiple functions at the mitochondria. (a) On the outer mitochondrial membrane (OMM), MCL-1 functions like other anti-apoptotic BCL-2 family members where it acts to prevent the activation of BAX and BAK to prevent cell death. MCL-1 can directly bind BH3-only family members, such as BIM, sequestering them away from the pro-apoptotic effectors BAX or BAK. Alternatively, MCL-1 may directly bind BAX and BAK and maintain them in an inactive conformation. (b) During mitochondrial importation, the full-length MCL-1 is proteolytically truncated on its amino-terminus. The truncated, matrix localized MCL-1 resides within the inner mitochondrial membrane where it functions to maintain mitochondrial cristae ultrastructure and promotes the assembly of the electron transport chain complexes into higher-order assemblies known as supercomplexes (SC). The assembly into supercomplexes has been shown to facilitate electron transport efficiency and reduce the production of deleterious reactive oxygen species. Additionally, matrix-localized MCL-1 facilitates the assembly of the higher-order assembly of the ATP synthase complexes into dimers and oligomers. Proper assembly of oligomeric ATP synthase has been implicated in being an important determinate of inner membrane cristae structure. Genetic ablation of Mcl-1 results in defects in both supercomplex and ATP synthase oligomer assembly. Whether MCL-1 acts directly or indirectly to facilitate these macromolecular assemblies of the electron transport supercomplexes or ATP synthase oligomers is still unclear.
Functional Perspective on MCL-1's Mitochondrial Function
While our data indicate that MCL-1 is required inside the mitochondrial matrix for normal mitochondrial cristae structure, fusion, and bioenergetics, including oxidative phosphorylation (OXPHOS), precisely how MCL-1 promotes this function is still uncertain [37]. Mitochondrial morphology, dynamics, and function are closely linked and the structure of the inner membrane is highly organized and dynamic adopting different structures according to the metabolic status of the cell. When ADP is low and ATP is abundant, the cristae are short and flat with fewer cristae junctions and an expanded matrix, often referred to as “orthodox” [44]. Whereas, under conditions of high ADP and low ATP, cristae have multiple tubular connections and a compacted matrix, called “condensed” [44]. Since cells lacking MCL-1 inside the mitochondrial matrix exhibit profound deficits in bioenergetics [37] it is possible that MCL-1 may directly modulate the function of one or more of the electron transport chain components. Thus, in the absence of matrix-localized MCL-1, the capacity for OXPHOS is diminished and ATP production decreases, thereby potentially affecting the cristae morphology and dynamics.
However, it is also possible that the dynamic transition between “orthodox” and “condensed” cristae requires an active fission and fusion process that may be impaired in the absence of MCL-1 [37]. Why is mitochondrial fusion defective in the absence of MCL-1? Perhaps MCL-1 influences the function of the fission/fusion machinery such as OPA1, a well-characterized regulator of inner mitochondria membrane (IMM) fusion. Furthermore, defects in mitochondrial fusion result in dysfunctional mitochondria as fusion incompetent cells generate less energy by OXPHOS and have increased ROS production in addition to fragmented mitochondria [45, 46]. Therefore, fusion is necessary to maintain a healthy mitochondrial population through the exchange of contents between mitochondria including mtDNA, which is organized into nucleoids and encodes for essential components of the respiratory chain. In cells that are incapable of mitochondrial fusion there is a loss of nucleoids [46, 47]. Consequently, a possible explanation for the decreased mtDNA content observed in Mcl-1-deleted cells may be due to incompetent mitochondrial fusion [37]; subsequently resulting in bioenergetic abnormalities such as inefficient respiration and decreased membrane potential. Moreover, mitochondrial membrane potential influences fusion efficiency [48] and, therefore could cause a feed-forward inhibition of fusion rate.
Alternatively, MCL-1 may regulate inner membrane structure by facilitating the dimerization and oligomerization of F1F0-ATP synthase [37]. ATP synthase dimers and oligomers constrain and curve the cristae membrane and this is required for maintaining the proper organization of the IMM [49-51]. The assembly of ATP synthase into dimers and oligomers is aberrant in mitochondria lacking MCL-1 (Figure 2b) [37]. Therefore, it is possible that MCL-1 acts to bridge either the F1 or F0 domains, stabilizing the complex. Thus, one possible model is that MCL-1 also may directly interact with ATP synthase. The concept that an anti-apoptotic BCL-2 family member may alter F1F0-ATP synthase function has already been observed for BCL-XL. Like MCL-1, BCL-XL has also been reported to localize within the mitochondrial inner membrane and appears to be important for the maintenance of the mitochondrial membrane potential [52, 53]. In the inner membrane, BCL-XL interacts with ATP synthase sub-units, including the β sub-unit of the F1 particle [52-54]. Indeed, genetic ablation or pharmacological inhibition of BCL-XL result in increased mitochondrial ion flux leading to decreased efficiency of ATP synthase enzymatic function [52, 53]. However, how the lost interaction perturbs ATP synthase function mechanistically is still unclear. Perhaps, the loss of BCL-XL also alters the oligomerization of the F1F0-ATP synthase as was observed for the genetic loss of MCL-1 [37]. However, unlike the loss of MCL-1, BCL-XL deletion does not appear to result in defective mitochondrial ultrastructure or morphology suggesting that the two anti-apoptotic molecules may have non-overlapping functions that remain to be revealed. It will be important to examine whether BCL-XL and MCL-1 can functionally compensate for each other inside the mitochondrial matrix.
MCL-1 may also indirectly support ATP synthase dimerization through another accessory protein such as inhibitory factor 1 (IF1). IF1 is a protein that binds to ATP synthase and prevents the hydrolysis of ATP [55] and also regulates mitochondrial ultrastructure by promoting ATP synthase dimerization [56]. The loss of ATP synthase oligomers in Mcl-1-deleted mitochondria may be responsible for the ultrastructural defects including disorganized cristae. Similar defects were seen in the assembly of electron transport chain (ETC) complexes into “supercomplexes” in Mcl-1-deficient mouse liver mitochondria (Figure 2b) [37]. It has been demonstrated that the assembly of the ETC complexes into supercomplexes promotes efficient energy production and decreases the production of harmful reactive oxygen species [57]. Since abnormalities in mitochondrial cristae ultrastructure can promote the breakdown of the reticular mitochondrial network [58] this could explain the observed mitochondrial fusion defect and subsequent bioenergetic abnormalities in Mcl-1-deficient cells.
Contribution of MCL-1's Functions in Normal Cells
Genetic studies have demonstrated that many different cell types depend on MCL-1 for survival, whereas other anti-apoptotic BCL-2 family members are more dispensable. However, these studies have induced ablation of both the anti-apoptotic and mitochondrial functions of MCL-1 making it difficult to assess the contribution of these roles to normal cell development and survival. For example, why the germline ablation of Mcl-1 leads to peri-implantation embryonic lethality is still unclear as no other anti-apoptotic molecule is similarly required at such an early embryonic developmental stage [59]. The Mcl-1-deficient blastocysts exhibit defective trophoectoderm, but surprisingly did not show evidence of increased apoptosis leading to the hypothesis that MCL-1 possesses a non-apoptotic role [17]. Mitochondrial function is important in the preimplantation embryo as aerobic metabolism is the primary source of ATP in the mouse blastocyst [60]. Mitochondria in the trophoectoderm of the blastocyst have a high inner membrane potential that has been shown to be important for early embryonic development [61]. Furthermore, mice deficient in Mitofusin 2, a critical regulator of mitochondrial fusion, are also embryonic lethal due to a defect in trophoblast cells [62]. It is conceivable that MCL-1 facilitates the mitochondrial function and energy production that is required for differentiation of cytotrophoblasts into trophoblasts. Failure of cytotrophoblast differentiation could result in a defective trophoectoderm that is unable to implant. To that end, it is plausible that MCL-1's role during embryonic development may not merely be to antagonize cell death, but may also be to promote normal mitochondrial function, assisting in differentiation and placental invasion of the trophoblast cells.
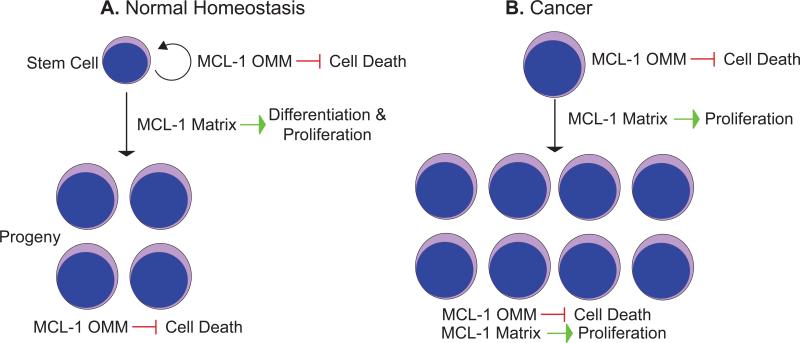
Hematopoietic stem cells (HSCs) give rise to a variety of mature cells including myeloid and lymphoid lineages and also maintain their populations through a balance of self-renewal and differentiation (Figure 1). Apoptosis has a clear role in the regulation of this balance as ectopic expression of BCL-2 can perturb HSC numbers [63]. Additionally, more committed lineages, such as lymphocytes are also regulated by apoptosis particularly through regulation by cytokines and growth factors (Figure 3a). During hematopoiesis, MCL-1 is unique because it is the essential survival factor for all early hematopoietic lineages and progenitor populations, but the reason for this requirement is unclear [20]. Accumulating evidence has shown that HSCs have a low mitochondrial membrane potential and are predominantly glycolytic in part due to the hypoxic niche in the bone marrow [64]. Furthermore, long-term hematopoietic stem cells (LTHSCs), which retain the capacity to self-renew, are quiescent with a lower metabolic rate [65]. In contrast, differentiation into the more committed progenitor cells is a high-energy demand process that requires mitochondrial function [66]. The profound requirement for Mcl-1 in HSCs and progenitors may result from a synergy of both the anti-apoptotic and mitochondrial functions (Figure 3a). It is possible that the signals for differentiation and proliferation are coupled such that the matrix-localized and OMM-localized MCL-1 species cooperate to provide both increased energy upon differentiation into progenitor cells and antagonize apoptosis in order to sustain the stem cell pool (Figure 3a). Nevertheless, Mcl-1 may have separate roles in distinct hematopoietic lineages. For example, matrix-localized MCL-1 may be important for early progenitors where proper mitochondrial function is required for differentiation whereas anti-apoptotic OMM-localized MCL-1 may be more important for promoting the survival of mature cells (Figure 3a).
Figure 3. Model for Possible MCL-1 Functions in Normal Homeostasis and Cancer.
MCL-1 possesses multiple functions in cells. On the outer mitochondrial membrane (OMM) MCL-1 inhibits cell death, similar to other anti-apoptotic BCL-2 family members. When targeted to the mitochondrial matrix (Matrix) the amino-terminal truncated MCL-1 also stimulates mitochondrial function including promoting normal ATP production, efficient oxidative phosphorylation, and reduced production of reactive oxygen species. These two separable functions may play critical roles in promoting normal and cancer cell survival. (a) In normal stem cells, it is possible that the homeostatic control on self-renewal is regulated primarily by MCL-1 OMM anti-apoptotic activity. In contrast, the proliferation and differentiation of stem cells into progenitors and terminally differentiated lineages may require efficient energy production promoted by the truncated MCL-1 targeted to the matrix. Lastly, proper homeostasis of terminally differentiated cells is likely controlled primarily by MCL-1 OMM's anti-apoptotic activity. (b) Cancer cells often have violated cellular checkpoints and become “addicted” to anti-apoptotic BCL-2 family members to counter the increased amounts of pro-apoptotic molecules expressed. Therefore, one prediction is that tumor cells may be highly dependent on MCL-1 OMM's anti-apoptotic function to “soak up” the pro-apoptotic expression and therefore inhibit the death of the malignant cell. However, cancer cells often proliferate rapidly and in addition to requiring a constant supply of ATP, they depend on mitochondrial byproducts as a source of macromolecular synthesis. MCL-1's matrix function may be necessary to help provide cancer cells with the building blocks to sustain rapid proliferation. Therefore, MCL-1's functional roles at the OMM and matrix may synergize to inhibit cell death and to promote cellular proliferation.
In the developing nervous system, ablation of Mcl-1 results in widespread neuronal apoptosis and embryonic lethality [23]. Since the development of the cerebral cortex is heavily dependent on apoptosis, MCL-1 may primarily maintain the balance between proliferation and cell death during cerebral cortex development. However, in addition to apoptosis, neurons are also extensively dependent on mitochondrial dynamics as an imbalance between healthy and dysfunctional mitochondria can be a determinant for human diseases such as neurodegeneration [46]. In support of this model, loss or inhibition of BCL-XL in hippocampal neurons negatively impacted their metabolic efficiency by increasing ion leak from mitochondria and decreasing the enzymatic function of ATP synthase [52, 53]. Thus, it is possible that the different functional roles of MCL-1 and BCL-XL on the OMM and within the inner mitochondrial membrane may have different spatial and temporal requirements for maintaining survival and health. For example, during development of the nervous system, while neurons are being established and eliminated, the anti-apoptotic activity may be the principal required function. In comparison in adult neurons, MCL-1 and BCL-XL may have an alternative function to promote normal mitochondrial fusion and energetics that is necessary for mitochondria to travel long distances from the neuronal cell body to the axonal termini where they generate ATP.
While the recent studies have revealed that anti-apoptotic molecules possess function beyond their classical anti-apoptotic function, it will be important to delineate the relative contributions of the anti-apoptotic function and effects on mitochondrial energetics on normal cell development and function in various cellular lineages.
Roles of MCL-1 in Cancer and Cancer Therapy
MCL-1 is one of the most highly amplified genes in a variety of human cancers [67], making it imperative to understand the contribution of MCL-1's functions to oncogenesis. Furthermore, its expression is often associated with chemotherapeutic resistance and relapse [68, 69]. In many malignancies, MCL-1 appears to be a critical survival molecule. For instance, MCL-1 is critical for the development and maintenance of acute myeloid leukemia [70, 71]. Moreover, MCL-1 overexpression dramatically accelerates Myc-induced lymphomagenesis [72]. Similar to studies in normal cells, the majority of investigations into the role of Mcl-1 in mouse models of cancer have genetically ablated both the anti-apoptotic and the mitochondrial functions of MCL-1 making it difficult to separate the functional contributions of MCL-1's anti-apoptotic and mitochondrial functions.
Cancer cells often violate key cellular checkpoints that would normally drive the cells to die by programmed cell death, as a result they need to overcome the apoptotic stress either by reducing the expression of pro-apoptotic factors or, more frequently by up regulating anti-apoptotic molecules such as BCL-2, BCL-XL, A1, and MCL-1 [73, 74]. For example, the amplification of Myc is one of the most frequent oncogenic occurrences observed in human cancers and drives carcinogenesis [67]. Oncogenic levels of Myc induce apoptosis that must be overcome in part by over-expression of anti-apoptotic molecules [75-78]. Accordingly, the anti-apoptotic function of MCL-1 and other anti-apoptotic family members may be obligatory to promote survival due to oncogenic stress (Figure 3b).
Nevertheless, cancer cells are often rapidly dividing and require increases in biomass to support abnormal proliferation. Although cancer cells often metabolize extremely high levels of glucose by glycolysis, glutamine is another essential metabolite that is excessively consumed through glutaminolysis [79, 80]. Glutamine is a mitochondrial-oxidizable substrate that is essential for macromolecular synthesis of nucleic acids, proteins, and lipids required for assembling new cells [81]. In particular, oncogenic Myc has been linked to increased glutaminolysis through activation of a transcriptional program that renders cancer cells glutamine dependent [82, 83].
It is conceivable that both the anti-apoptotic and mitochondrial functions of anti-apoptotic MCL-1 and BCL-XL may play important roles in promoting the survival of Myc-induced cancer. Myc transformation cause cells to become dependent on anti-apoptotic molecules in order to overcome apoptotic stress, therefore the anti-apoptotic activity may be indispensable (Figure 3b). Nevertheless, it is possible that the non-apoptotic function of MCL-1 and BCL-XL may help fulfill the requirements for rapid cell proliferation in Myc-cancer cells by facilitating the generation of byproducts from mitochondrial metabolism [81, 84] (Figure 3b).
There has been an increasing interest in developing BCL-2 family inhibitors as a cancer therapeutic strategy, focusing on antagonizing anti-apoptotic activity to foster cell death [85]. One such inhibitor, obatoclax, is a BH3-mimetic that binds to BCL-2, BCL-XL, and MCL-1 to disrupt their interaction with the pro-apoptotic molecules [86]. Although obatoclax exhibits single-agent activity and can kill certain types of cancer cells in-vitro, it also has non-specific effects in which non-apoptotic cell death occurs [87-89]. Additionally, ligands and modified peptides that are also BH3-mimetics modeled specifically for MCL-1's BH3-binding groove have been developed but to date appear to be only effective when combined with chemotherapeutic agents [90, 91]. One possibility for the lack of single-agent activity is that these BH3-mimetics only antagonize MCL-1's anti-apoptotic activity leaving its mitochondrial function unaffected; thus, they may be insufficient to promote cell death unless combined with another death-inducing agent. Therefore, studies will be necessary to define whether the mitochondrial function of MCL-1 requires functional domains needed for its anti-apoptotic function.
Genetic deletion of Mcl-1 has been shown to induce cell death in cancer cells regardless of complementary expression of other endogenous anti-apoptotic family members indicating that endogenous levels of other anti-apoptotic molecules is insufficient to promote cell survival [70, 71]. Only when other anti-apoptotic BCL-2 family members are ectopically expressed can loss of MCL-1 be tolerated in these cancer cells [70, 71]. It is possible that the non-apoptotic, mitochondrial function of MCL-1 may play an important role in promoting cancer cell survival and proliferation. One potential reason that overexpression can overcome the requirement for MCL-1 may be that other anti-apoptotic molecules also promote normal mitochondrial function, as has been identified for BCL-XL [52, 53]. Therefore, it will be important to identify whether BCL-XL's role in promoting mitochondrial energetics is also important for tumor cell survival. Clearly, in Myc-induced leukemia the endogenous levels of BCL-XL are insufficient to promote survival in the absence of MCL-1, but overexpression of BCL-XL can promote survival [70]. Is this because BCL-XL can also fulfill MCL-1's role in promoting mitochondrial energetics? To answer this question, it will be important to functionally separate BCL-XL's anti-apoptotic function from its role in mitochondrial energetics and examine whether BCL-XL's function in promoting mitochondrial ATP synthase function is important for cancer cells [52, 53].
In light of the potential for MCL-1 to promote both cancer cell apoptotic resistance and to support the high rate of proliferation often observed in cancer cells, it is possible that inhibition of both the anti-apoptotic and mitochondrial functions of MCL-1 may synergize by restricting cancer cell expansion and activating cell death leading to more effective therapies (Figure 3b). In this case, strategies to inhibit MCL-1's mitochondrial function may be equally important to those targeted at inhibiting its ability to bind and sequester pro-apoptotic molecules.
Concluding Remarks
Our recent findings regarding MCL-1-regulated mitochondrial physiology and its separable functions as an anti-apoptotic molecule at the OMM and regulator of mitochondrial bioenergetics inside the matrix, will likely have a major impact in our understanding of MCL-1 biology in normal cells, and represent a new clinical target. Undoubtedly, defining the mechanism by which MCL-1 regulates mitochondrial physiology as well as identifying MCL-1-interacting partners inside the mitochondrial matrix will be important to understanding its function. Future studies delineating the relative contributions of MCL-1's anti-apoptotic and metabolic functions in both normal and diseased cells may help us develop more effective MCL-1 inhibitors with reduced toxicity to normal cells.
Acknowledgments
We thank members of the Opferman laboratory, St. Jude Biochemistry Department, C. Sherr, J. Ihle, D. Green for helpful discussions. The project was supported by R01HL102175 from the National Institute of Heart, Lung and Blood (J.T.O); the American Cancer Society 119130-RSG-10-255-01-LIB (J.T.O); a Cancer Center Support Grant P30CA021765; and the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Heart, Lung and Blood or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they do not have any conflicts of interest.
References
- 1.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 2.Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JC, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 4.Kozopas KM, et al. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CL, et al. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 6.Day CL, et al. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J Biol Chem. 2005;280:4738–4744. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 7.Nijhawan D, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer U, et al. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, et al. Glycogen Synthase Kinase 3{alpha} and 3{beta} Mediate a Glucose-Sensitive Antiapoptotic Signaling Pathway To Stabilize Mcl-1. Mol. Cell. Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong Q, et al. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Stewart DP, et al. Ubiquitin-independent degradation of antiapoptotic MCL-1. Mol Cell Biol. 2010;30:3099–3110. doi: 10.1128/MCB.01266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warr MR, et al. BH3-ligand regulates access of MCL-1 to its E3 ligase. FEBS Lett. 2005;579:5603–5608. doi: 10.1016/j.febslet.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, et al. The E3 ubiquitin ligase Mule acts through the ATM-p53 axis to maintain B lymphocyte homeostasis. J Exp Med. 2012;209:173–186. doi: 10.1084/jem.20111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inuzuka H, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Q, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwickart M, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 17.Rinkenberger JL, et al. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- 18.Opferman JT, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 19.Dzhagalov I, et al. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–528. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opferman JT, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 21.Dzhagalov I, et al. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steimer DA, et al. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbour N, et al. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veis DJ, et al. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 25.Motoyama N, et al. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999;189:1691–1698. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motoyama N, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x- deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 27.Debrincat MA, et al. Mcl-1 and Bcl-xL coordinately regulate megakaryocyte survival. Blood. 2012;119:5850–5858. doi: 10.1182/blood-2011-12-398834. [DOI] [PubMed] [Google Scholar]
- 28.Kodama T, et al. Mcl-1 and Bcl-xL regulate Bak/Bax-dependent apoptosis of the megakaryocytic lineage at multistages. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma A, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci U S A. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Print CG, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci U S A. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AJ, et al. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 32.Hamasaki A, et al. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottina E, et al. Targeting antiapoptotic A1/Bfl-1 by in vivo RNAi reveals multiple roles in leukocyte development in mice. Blood. 2012;119:6032–6042. doi: 10.1182/blood-2011-12-399089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T, et al. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–1184. doi: 10.1083/jcb.128.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima S, et al. MCL-1V, a novel mouse antiapoptotic MCL-1 variant, generated by RNA splicing at a non-canonical splicing pair. Biochem Biophys Res Commun. 2010;391:492–497. doi: 10.1016/j.bbrc.2009.11.086. [DOI] [PubMed] [Google Scholar]
- 36.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–147. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 37.Perciavalle RM, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Biasio A, et al. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J Biol Chem. 2007;282:23919–23936. doi: 10.1074/jbc.M700938200. [DOI] [PubMed] [Google Scholar]
- 39.Warr MR, et al. Mitochondrion-dependent N-terminal processing of outer membrane Mcl-1 protein removes an essential Mule/Lasu1 protein-binding site. J Biol Chem. 2011;286:25098–25107. doi: 10.1074/jbc.M111.218321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang CR, Yang-Yen HF. The fast-mobility isoform of mouse Mcl-1 is a mitochondrial matrix-localized protein with attenuated anti-apoptotic activity. FEBS Lett. 2010;584:3323–3330. doi: 10.1016/j.febslet.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. Journal of neurochemistry. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda N, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narendra DP, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS biology. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, et al. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, et al. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Legros F, et al. Organization and dynamics of human mitochondrial DNA. J Cell Sci. 2004;117:2653–2662. doi: 10.1242/jcs.01134. [DOI] [PubMed] [Google Scholar]
- 48.Song Z, et al. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giraud MF, et al. Is there a relationship between the supramolecular organization of the mitochondrial ATP synthase and the formation of cristae? Biochim Biophys Acta. 2002;1555:174–180. doi: 10.1016/s0005-2728(02)00274-8. [DOI] [PubMed] [Google Scholar]
- 50.Thomas D, et al. Supramolecular organization of the yeast F1Fo-ATP synthase. Biol Cell. 2008;100:591–601. doi: 10.1042/BC20080022. [DOI] [PubMed] [Google Scholar]
- 51.Dudkina NV, et al. Structure of dimeric ATP synthase from mitochondria: an angular association of monomers induces the strong curvature of the inner membrane. FEBS Lett. 2005;579:5769–5772. doi: 10.1016/j.febslet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 52.Alavian KN, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen YB, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vento MT, et al. Praf2 is a novel Bcl-xL/Bcl-2 interacting protein with the ability to modulate survival of cancer cells. PloS one. 2010;5:e15636. doi: 10.1371/journal.pone.0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pullman ME, Monroy GC. A Naturally Occurring Inhibitor of Mitochondrial Adenosine Triphosphatase. J Biol Chem. 1963;238:3762–3769. [PubMed] [Google Scholar]
- 56.Campanella M, et al. Regulation of mitochondrial structure and function by the F1Fo-ATPase inhibitor protein, IF1. Cell Metab. 2008;8:13–25. doi: 10.1016/j.cmet.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Acin-Perez R, et al. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Velours J, et al. Mitochondrial F1F0-ATP synthase and organellar internal architecture. Int J Biochem Cell Biol. 2009;41:1783–1789. doi: 10.1016/j.biocel.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Ranger AM, et al. Mouse models of cell death. Nat Genet. 2001;28:113–118. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 60.Benos DJ, Balaban RS. Energy metabolism of preimplantation mammalian blastocysts. The American journal of physiology. 1983;245:C40–45. doi: 10.1152/ajpcell.1983.245.1.C40. [DOI] [PubMed] [Google Scholar]
- 61.Van Blerkom J, Davis P. High-polarized (Delta Psi m(HIGH)) mitochondria are spatially polarized in human oocytes and early embryos in stable subplasmalemmal domains: developmental significance and the concept of vanguard mitochondria. Reproductive biomedicine online. 2006;13:246–254. doi: 10.1016/s1472-6483(10)60622-0. [DOI] [PubMed] [Google Scholar]
- 62.Chen H, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Domen J, et al. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 66.Inoue S, et al. Mitochondrial respiration defects modulate differentiation but not proliferation of hematopoietic stem and progenitor cells. FEBS Lett. 2010;584:3402–3409. doi: 10.1016/j.febslet.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 67.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei G, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Wuilleme-Toumi S, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19:1248–1252. doi: 10.1038/sj.leu.2403784. [DOI] [PubMed] [Google Scholar]
- 70.Glaser SP, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang Z, et al. Mcl1 haploinsufficiency protects mice from Myc-induced acute myeloid leukemia. J Clin Invest. 2010;120:2109–2118. doi: 10.1172/JCI39964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell KJ, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116:3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Ni Chonghaile T, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evan GI, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, et al. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992;257:212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- 77.Eischen CM, et al. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zindy F, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 80.Reitzer LJ, et al. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 81.DeBerardinis RJ, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuneva M, et al. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deberardinis RJ, et al. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen M, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, et al. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 88.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 89.McCoy F, et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis. 2010;1:e108. doi: 10.1038/cddis.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee EF, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stewart ML, et al. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]