Abstract
The best method to monitor anticoagulation during extracorporeal membrane oxygenation (ECMO) is unknown. We conducted a prospective observational study in a tertiary pediatric intensive care unit. Anti-factor Xa (anti-FXa), antithrombin (AT), and factor VIII activity (FVIII) were measured in blood samples collected at 6, 12, and every 24h of ECMO.
We enrolled 34 children who underwent 35 ECMO runs from April 2008–September 2010. ACT and heparin doses were higher, whereas anti-FXa levels were lower in neonates compared to infants/children. Median anti-FXa was 0.4 IU/mL, median AT was 60%, and median FVIII was 67%. Heparin infusion rate, anti-FXa, and AT increased, FVIII was stable, and ACT decreased with each day on ECMO. ACT had poor agreement with anti-FXa (42%). AT was inversely correlated with ACT (r=−0.33), even after adjusting for heparin dose, and positively correlated with anti-FXa (r=0.57).
This study emphasizes the age differences as well as the variability over days of coagulation monitoring assays during ECMO. ACT is poorly correlated with anti-FXa and AT modifies the relationship between ACT and heparin dose, indicating that results should be interpreted with caution when managing anticoagulation on ECMO. Additional studies are warranted to determine optimal ECMO anticoagulation monitoring.
Keywords: anticoagulation, antithrombin, ECMO, extracorporeal membrane oxygenation, heparin assay
Introduction
Extracorporeal membrane oxygenation (ECMO) is a well-established method of support in pediatric and adult patients with severe cardiac and/or respiratory failure 1, 2. Despite ECMO having been used in pediatric patients for more than 30 years, appropriate measurement and intensity of anticoagulation of patient and circuit remain controversial. Although specific protocols vary from center to center, most ECMO programs use a continuous infusion of unfractionated heparin and monitor anticoagulation by point-of-care testing of the activated clotting time (ACT), in conjunction with blood cell counts and coagulation studies performed in a central laboratory 3. These results guide the intensity of anticoagulation therapy and help clinicians to balance the risk of bleeding complications with the need to maintain appropriate circuit flow and avoid thrombosis. However, disorders of coagulation, including life-threatening hemorrhage and thrombosis, still occur frequently 1, 2.
Only limited evaluations have been made of changes in the coagulation system occurring during ECMO. In addition, the methods used to control and monitor anticoagulation have been limited to global measures of coagulation, except within the confines of small studies 4–7. Several measures may be used to monitor the state of the coagulation system including ACT, anti-factor Xa activity (heparin level), antithrombin (AT) activity, factor VIII activity, activated partial thromboplastin time (aPTT), and thromboelastography (TEG). The objective of this study was to compare global (ACT, aPTT) and specific (anti-factor Xa activity) measures of anticoagulation used in clinical practice and determine the agreement among them and potential confounding by low AT or high factor VIII activity.
Methods
Study Design
This is a prospective observational cohort study of children who initiated ECMO from April 2008 to September 2010 in a 26-bed pediatric intensive care unit of an academic tertiary pediatric center. Patients younger than 18 years who required ECMO for any indication were eligible for this study. Exclusion criteria were history of heparin-induced thrombocytopenia and use of direct thrombin inhibitors for anticoagulation during ECMO. No patient met these criteria. Informed consent from parents or legal guardians was sought after patient stabilization within the first 6 h after ECMO cannulation. If parents were not present in the intensive care unit, consent was deferred. Demographic, clinical, laboratory, imaging, and survival data were collected prospectively for each enrolled subject. The ECMO circuit consisted of custom-packed 1/4- or 3/8-inch flexible polyvinylchloride tubing (Medtronic, Minneapolis, MN) with a silicone reservoir, a bladderbox (Johns Hopkins Hospital, Baltimore, MD), a 0.8–4.5 m2 membrane oxygenator (Medtronic), a heat exchanger (Medtronic), and a roller pump (Sorin Cardiovascular U.S.A., Arvada, CO). The rate of heparin infusion was adjusted based on the ACT, with a goal of 180 to 220 seconds, according to institutional protocols. The ECMO specialists adjust the infusion rate by 0–5 U/kg/h no more frequently than every two hours to maintain ACT within parameters. The institutional transfusion protocol calls for transfusion of packed red blood cells (PRBC) to maintain hematocrit ≥35%, platelet transfusion to maintain platelet count ≥100,000 cells/microL, fresh frozen plasma (FFP) administration if aPTT > 100 seconds, even if ACT was within goal range, and cryoprecipitate to maintain fibrinogen ≥100 mg/dL. During the study period, pooled antithrombin was administered at clinician’s discretion, based on manufacturer’s recommendations (ATIII dose in units [IU] = [desired − current] × weight [kg]/1.4). This study was approved by the Johns Hopkins Institutional Review Board.
Blood sampling and analysis
Venous blood samples (5 mL in sodium citrate 3.2%) were collected at 6 h, 12 h, 24 h, and then daily after initiation of ECMO until ECMO discontinuation. All samples were collected from a designated circuit port (pre-bladder and pre-pump) at the same time that regular clinical samples were collected, with a 2–3 mL “waste.” After being separated by centrifugation within 1 h, platelet-poor plasma was processed for anti-factor Xa activity with supplementation of AT (Berichrom Heparin, Siemens Healthcare Diagnostics Products GmbH, Germany), AT (Berichrom Antithrombin III (A), Siemens Healthcare Diagnostics Products GmbH), fibrinogen and factor VIII activity (Siemens Healthcare Diagnostics Products GmbH). aPTT was measured using Dade® Actin® (Siemens Healthcare Diagnostics Products GmbH), d-dimers were measured using Innovance (Siemens Healthcare Diagnostics Products GmbH), and the ACT test was carried out using the Hemochron Signature (ITC Corporation, Edison, NJ). For factor VIII measurements, heparin was neutralized with Dade Hepzyme (Siemens Healthcare Diagnostics Products GmbH). All assays except the ACT (measured at point of care) were conducted in the Johns Hopkins Coagulation Laboratory in the Department of Pathology. Routine hematologic and coagulation assays, blood products administered, hourly ACT, and heparin infusion rates were recorded longitudinally.
Statistical Analysis
Descriptive data analysis was conducted to examine patient and ECMO course characteristics and to describe the distribution of heparin infusion rates, coagulation assays, and blood product use among subjects. The Mann-Whitney U test was used to compare these parameters between age groups (neonates ≤30 days and infants and children >30 days). Each patient’s ECMO course was divided into 1-h time periods, and each laboratory value or intervention was recorded within its corresponding time period(s). Data were analyzed using longitudinal linear regression to model association between coagulation markers. A p-value of 0.05 was considered significant. Statistical analysis was conducted using STATA 11.0 (StataCorp, College Station, TX, 2009).
Results
We screened 71 patients and enrolled 34 patients within the 6-h consent window from April 2008 to September 2010. One patient had two ECMO runs for different indications 2 years apart that were analyzed separately. Demographic and ECMO course characteristics are presented in Table 1.
Table 1.
Patient and ECMO Characteristics
| Variable | All (n=35 ECMO runs) | Age ≤30 days (n=21 ECMO runs) | Age >30 days (n=14 ECMO runs) | p |
|---|---|---|---|---|
|
| ||||
| Age, median (IQR) | 10d (2d – 10y) | 3d (1d – 9d) | 11y (5m – 15y) | <0.001 |
|
| ||||
| Male, n(%) | 16 (44) | 9 (43) | 10 (71) | 0.096 |
|
| ||||
| Race | ||||
| Caucasian, n(%) | 16 (46) | 9 (43) | 7 (50) | 0.538 |
| African American, n(%) | 14 (40) | 9 (43) | 5 (36) | |
| Other, n(%) | 5 (14) | 3 (14) | 2 (14) | |
|
| ||||
| ECMO indications, n(%) | ||||
| Respiratory failure | 18 (51) | 17 (81) | 1 (7) | <0.001 |
| Cardiac failure | 9 (26) | 3 (14) | 6 (43) | |
| ECPR | 7 (20) | 1 (5) | 6 (43) | |
| Sepsis | 1 (3) | 0 | 1 (7) | |
|
| ||||
| ECMO mode | ||||
| VA-ECMO | 29 (83) | 16 (76) | 13 (93) | 0.311 |
| VV-ECMO | 3 (9) | 3 (14) | 0 | |
| VV- to VA-ECMO | 3 (9) | 2 (10) | 1 (7) | |
|
| ||||
| ECMO duration, median(IQR) | 7d (3d – 14d) | 12d (6d – 15d) | 3.5d (1d – 5d) | 0.006 |
|
| ||||
| Neurologic injury during ECMO, n(%)* | 12 (34) | 7 (33) | 5 (36) | 0.884 |
|
| ||||
| Survival to discharge, n(%) | 24 (69) | 18 (86) | 6 (43) | 0.007 |
|
| ||||
| Circuit clotting requiring circuit replacement, n(%) | 14 (40) | 12 (57) | 2 (14) | 0.011 |
|
| ||||
| Oxygenator failure, n(%) | 6 (17) | 5 (24) | 1 (7) | 0.200 |
|
| ||||
| ε-aminocaproic acid use, n(%)† | 9 (26) | 5 (24) | 4 (29) | 0.752 |
|
| ||||
| Hemorrhagic complications, n(%) | ||||
| ICH | 7 (20) | 5 (24) | 2 (14) | 0.490 |
| Pulmonary hemorrhage | 7 (20) | 5 (24) | 2 (14) | 0.490 |
| Other (hemothorax, adrenal hemorrhage, retroperitoneal hemorrhage) | 4 (11) | 2 (10) | 2 (14) | 0.664 |
Neurologic injury during ECMO is defined as: intracranial hemorrhage, ischemic stroke, cerebral edema or brain death.
No antifibrinolytic agents other than ε-aminocaproic acid were used in this cohort of patients.
Median heparin infusion rate was 34 U/kg/h (IQR: 22–48 U/kg/h), median ACT was 210 s (IQR: 195–227 s), and median aPTT was 91.5 s (IQR: 66.4–128.3 s). Median anti-factor Xa was 0.4 IU/mL (IQR: 0.2–0.6 IU/mL), median AT was 60% (IQR: 48–72%), and median factor VIII was 67% (IQR: 38–94%). Given differences in plasma proteins seen between newborns and infants and children8, 9, coagulation and blood transfusion requirement data are presented in Table 2 for the entire cohort as well as by age category. Significant differences were found between newborns vs. infants and children. Newborns had higher heparin infusion rates with higher ACTs, lower anti-factor Xa, lower AT levels, lower factor VIII levels, and higher weight-adjusted daily transfusion volumes of packed red blood cells, platelets, FFP, and cryoprecipitate.
Table 2.
Coagulation and Blood Product Requirement Characteristics*
| Variable | All (n=35 ECMO runs) | Age ≤30 days (n=21 ECMO runs) | Age >30 days (n=14 ECMO runs) | p-value |
|---|---|---|---|---|
| Heparin (U/kg/h) | 34 (22–48) | 35 (25–47) | 25 (18–52) | <0.001 |
| ACT (s) | 210 (195–227) | 213 (198–228) | 203 (190–221) | <0.001 |
| PT (s) | 11.9 (11.1–13.6) | 12 (11.3–13.6) | 11.3 (10.7–13.7) | <0.001 |
| aPTT (s) | 91.5 (66.4–128.3) | 92.7 (67.5–126.8) | 87.1 (64.1–131.4) | 0.604 |
| Fibrinogen (mg/dL) | 287 (214–365) | 263 (211–345) | 336 (218–472) | <0.001 |
| d-dimer (mg/L) | 6.7 (3.4–14.1) | 7.1 (3.6–14.7) | 6.2 (2.6–12.4) | 0.013 |
| Anti-factor Xa (IU/mL) | 0.4 (0.2–0.6) | 0.35 (0.2–0.5) | 0.5 (0.35–0.7) | <0.001 |
| AT (%) | 60 (48–72) | 57 (47–71) | 64 (55–76) | 0.007 |
| Factor VIII (%) | 67 (38–94) | 58 (34–79) | 114 (83–145) | <0.001 |
| Platelet count (K/mm3) | 113 (96–131) | 116 (102–134) | 102 (83–121) | <0.001 |
| Hemoglobin (g/dL) | 12.5 (12.0–13.2) | 12.6 (12.1–13.2) | 12.3 (11.6–13.3) | <0.001 |
| PRBC (mL/kg/day) | 28 (15–57) | 29 (15–61) | 24 (12–45) | <0.001 |
| Platelets (mL/kg/day) | 29 (16–58) | 33 (18–63) | 18 (12–44) | <0.001 |
| FFP (mL/kg/day) | 0 (0–16) | 0 (0–17) | 0 (0–15) | <0.001 |
| Cryoprecipitate (mL/kg/day) | 0 (0–1.4) | 0 (range 0–30) | 0 (range 0–12) | <0.001 |
ACT, activated clotting time; aPTT, activated partial thromboplastin time; AT, antithrombin III; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; PRBC, packed red blood cells; PT, prothrombin time.
Data are presented as median (interquartile range) for each group of patients after the median value for each variable was calculated longitudinally for each patient.
Data are presented as median (interquartile range) for each group of patients after the median value for each variable was calculated longitudinally for each patient.
Heparin infusion rate, ACT, and aPTT
On average, heparin infusion rate increased with each day on ECMO by 0.9 U/kg/h (95% CI: 0.7–1.1 U/kg/h, p<0.001), whereas ACT and aPTT decreased with each day on ECMO by 0.7 s (95% CI: 0.4–1.0 s, p=0.001) and 1.4 s (95% CI: 0.7–2.1 s, p<0.001), respectively, with an abrupt decrease in the first 48 h post-cannulation and a slower decrease thereafter (Fig. 1). By patient, simultaneously recorded heparin infusion rates and ACT showed that for each 10 U/kg/h increase in heparin infusion rate, the ACT was prolonged by 3 s (95% CI: 2.3–3.3 s, p=0.005). Within each patient, ACT correlated closely with heparin infusion rates over time (r=0.77).
Fig. 1.
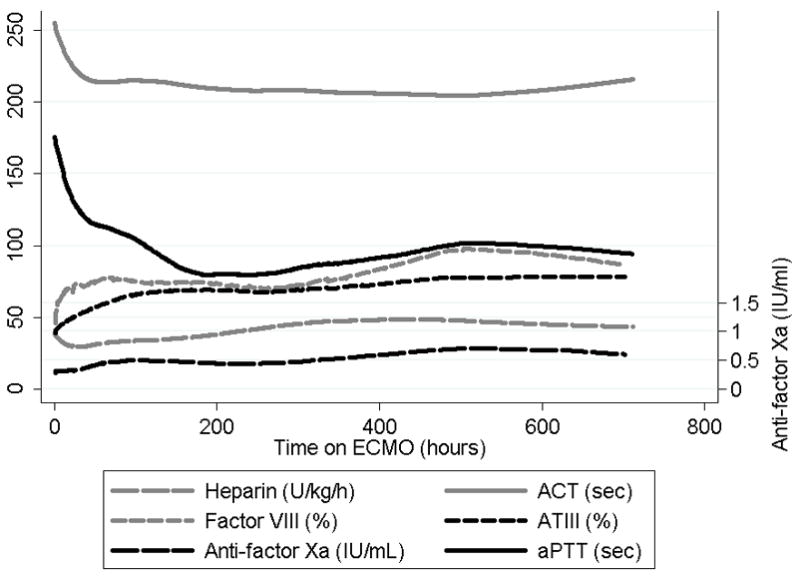
Trends of coagulation markers by time in patients on ECMO.
ACT, activated clotting time; AT, antithrombin; aPTT, activated partial thromboplastin time.
Anti-factor Xa
Median anti-factor Xa was 0.4 IU/mL (IQR: 0.2–0.6 IU/mL). Of 352 anti-factor Xa measurements, 215 (61%) fell within the previously proposed target of 0.3–0.7 IU/mL during ECMO6, 7. Anti-factor Xa increased with ECMO duration by 0.01 IU/mL (95% CI: 0.005–0.014 IU/mL, p=0.001) daily (Fig. 1). Anti-factor Xa was positively correlated with heparin dose when simultaneously measured values were compared (r=0.33). For each 10 U/kg/h increase in heparin, the anti-factor Xa was higher by 0.07 IU/mL (95% CI: 0.06–0.09 IU/mL, p<0.001). This association remained significant after adjustment for AT levels (p<0.001).
We tested the agreement between target anti-factor Xa and target ACT in our population and found poor agreement (42%) for simultaneously measured anti-factor Xa and ACT (Fig. 2). For anti-factor Xa values between 0.3 and 0.7 IU/mL, corresponding median ACT was 214 s (IQR: 198–229 s, range: 160–349 s). ACT and anti-factor Xa remained poorly correlated when they were analyzed longitudinally and when time on ECMO and intra-patient correlation of serial measurements were taken into account (r=0.02). The association between ACT and anti-factor Xa became significant only after adjusting for potential confounders (age, fibrinogen, d-dimer, AT and factor VIII deficiency, platelet count, and heparin dose; p<0.001).
Fig. 2.
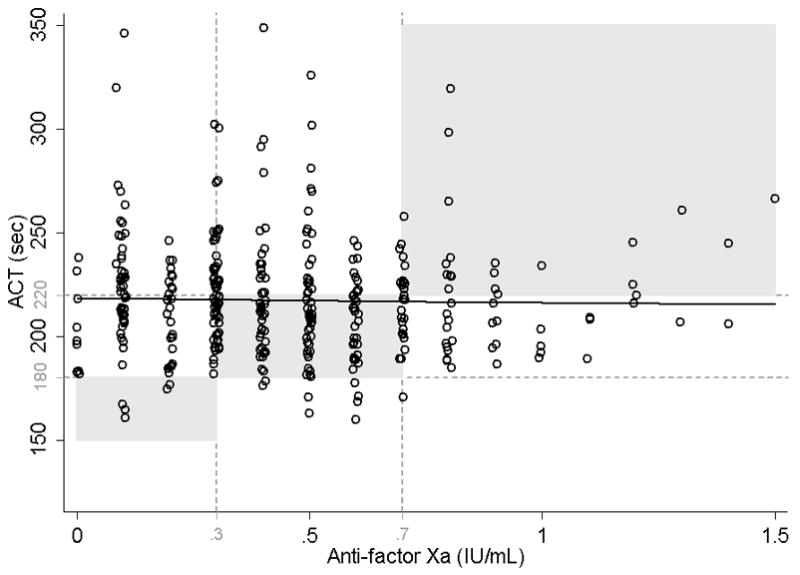
Correlation of simultaneously measured activated clotting time (ACT) and anti-factor Xa.
Agreement for clinically meaningful values was defined as: ACT <180 s corresponding to anti-factor Xa <0.3 IU/mL, ACT 180–220 s corresponding to anti-factor Xa 0.3–0.7 IU/mL and ACT >220 s corresponding to anti-factor Xa >0.7 IU/mL. Shaded areas represent agreement between ACT and anti-factor Xa, seen in only 42% of measurements. Longitudinal linear regression: y = 218.5+7.2x, p = 0.251, r = −0.02.
aPTT was also weakly correlated with anti-factor Xa, although somewhat better than ACT (r=0.17). The aPTT values corresponding to anti-factor Xa between 0.3–0.7 IU/mL had a median of 89 s (IQR: 69–122 s, range: 37–200 s).
Antithrombin
AT also increased with ECMO duration by an average of 1% (95% CI: 0.8–1.3%, p<0.001) daily (Fig. 1). This increase was not related to daily volume of FFP transfusions (p=0.399). Nine of 35 patients (26%) received 1 to 6 doses of plasma-derived AT concentrate, a median of 40 U/kg/dose (IQR: 29–45 U/kg/dose). Median AT activity in these 9 patients was 65% (IQR: 58–74%), a value that was significantly higher than that in the remainder of the cohort (56%; IQR: 46–69%, p<0.001). Our ECMO program does not have a set target for plasma AT, and all AT concentrate was administered at the clinicians’ discretion. The median baseline AT activity for which patients were treated was 43%, with a range of 38–64%.
AT was inversely correlated with ACT. For each 1% increase in AT, ACT was shorter by 0.6 s (95% CI: 0.4–0.7%, p<0.001, r=−0.33), even after adjusting for the heparin dose at the time of the ACT and AT measurements. AT showed a weak positive correlation with heparin infusion rate (r=0.15) and strong positive correlation with anti-factor Xa (r=0.57). For a 10% increase in AT activity, anti-factor Xa increased by 0.08 IU/mL (95% CI: 0.07–0.1 IU/mL, p<0.001). The shorter ACT and higher heparin infusion rates and higher anti-factor Xa in patients with higher AT levels may reflect less consumptive coagulopathy (a cause of low AT levels and prolongation of the ACT despite low rates of heparin infusion).
Factor VIII
On average, factor VIII activity increased by 14% in the first 48 h of ECMO (95% CI: 2–26%, p=0.024), after which it remained stable throughout the ECMO course (0.14% average daily increase, 95% CI: −0.5–1%, p=0.643; Fig. 1). The initial increase may have resulted from a higher volume of FFP transfusions given in the first 1–2 days post-ECMO cannulation. Thirty-one of 35 patients received FFP in the first 48 h of ECMO for a median of 26 mL/kg/day, whereas only 24 of 35 patients received FFP at some point after 48 h, for a median of 12 mL/kg/day. Another possibility is that consumptive coagulopathy decreased during the first 48 h.
The average heparin infusion rate, ACT, aPTT and anti-factor Xa were similar (p>0.05) for patients with high and low factor VIII activity, with cutoffs of 100% and 150%.
Thrombotic and hemorrhagic outcomes
There were 14 patients (40%) who required a circuit change due to thrombus formation in the circuit that extended to the arterial side of the circuit, post-oxygenator, or was deemed by the clinical team to be too extensive to be tolerated. There were 6 oxygenator failures (17%) thought to be due to clotting. The percentage of discordant ACT to anti-factor Xa values did not differ between patients who required a circuit change vs those who did not: 60% (IQR: 53%–83%) vs 55% (IQR: 40%–90%), p=0.82. The most discordant values that could potentially indicate inadequate anticoagulation (i.e., ACT>220 sec and concomitant anti-factor Xa <0.3 IU/mL) also showed no difference between patients who required a circuit change vs those who did not, p=0.13.
There were 15 patients (33%) who experienced a hemorrhagic complication (see Table 1). The percentage of discordant ACT to anti-factor Xa values that could indicate excessive anticoagulation by high anti-factor Xa yet low ACTs (i.e., ACT<180 sec and concomitant anti-factor Xa >0.7 IU/mL) was significantly higher in patients who had a hemorrhagic complication compared to those who did not: 4% (IQR: 0%–14%, range 0%–40%) vs 0% (IQR: 0%–0%, range 0%–75%), p=0.01.
Discussion
Newly available assays that may better describe the state of the coagulation system in patients on ECMO than traditional assays are being increasingly used in ECMO centers internationally and reported in the literature4, 5, 7, 10–13. Our study describes differences by age and by duration of ECMO as well as comparisons among these coagulation assays in a cohort of 34 pediatric patients on ECMO. We found significant differences by age: ACT and heparin doses were higher, whereas anti-factor Xa levels were lower in neonates than in infants/children. This finding may result from higher heparin needs in neonates secondary to faster heparin clearance 14 or a relatively higher circuit-volume–to–patient-blood-volume ratio in smaller patients. AT activity was also lower in neonates compared to older infants and children, mirroring trends previously described in large cohorts of healthy children 9, 13. Overall, however, median AT levels were higher in our newborn patients compared to values previously reported for neonatal ECMO 13.
ACT remains the preferred point-of-care measure of anticoagulation for most ECMO centers 3. In the absence of heparin, ACT is prolonged with elevated d-dimers, low platelet count or platelet dysfunction, low fibrinogen, other coagulation factor deficiency, hypothermia or hemodilution 15 and can be shortened with the opposite or in hypercoagulable states such as increased levels of fibrinogen or factor VIII. Further, different ACT devices may yield different results, potentially confusing clinicians on adequacy of anticoagulation 4, 15–17. In our study, ACT showed poor correlation with anti-factor Xa and may not be an adequate measure of anticoagulation in patients on ECMO who have a high prevalence of thrombocytopenia, platelet dysfunction, elevated d-dimers, and/or coagulation factor deficiencies. Although it is not clear what the anti-factor Xa target should be in patients on ECMO, when using the most commonly reported target of 0.3–0.7 IU/mL 6, 7, 11, we found poor agreement between the most common ACT target of 180–220 s and anti-factor Xa. In our patients, the association between ACT and anti-factor Xa was confounded by factors that may affect the ACT (age, fibrinogen, d-dimer, AT and factor VIII deficiency, platelet count, and heparin dose) and became significant only after adjusting for these factors. Importantly, our results suggest that those patients who have low ACTs but high concomitant anti-factor Xa levels suggesting excessive anticoagulation could experience higher rates of hemorrhagic complications.
In this study, heparin dose, anti-factor Xa, and AT increased with time on ECMO, while ACT and aPTT decreased. Prior studies suggested that increasing anti-factor Xa activity with time on ECMO may result from a decrease in AT, an increase in circulating heparin that is released from the circuit’s surface, and/or decreased clearance of heparin over time on ECMO 4. However, the anti-factor Xa assay used in this study was supplemented with additional AT to correct for potential AT deficiency. Although we could not test the latter hypothesis, we tested the former and found that in our patient population, AT levels increased, rather than decreased, with ECMO duration. One might expect this increase in the minority of patients who received plasma-derived AT concentrate, but the steady daily increase was also seen in patients without AT replacement therapy and may have been related to decreased consumptive coagulopathy after stabilization and resolution of inciting events leading to ECMO cannulation.
We found that AT was negatively correlated with ACT and remained so after adjusting for heparin infusion rate, suggesting that ACT values may be inaccurate in ECMO patients with AT deficiency. Some ECMO centers have begun replacing AT with plasma-derived or recombinant AT, but the target AT and cutoff for replacement in ECMO patients remain unclear 5, 10–12. In a single center case series, investigators attempted continuous AT infusion and found it to be associated with a lower incidence of hemorrhage in six postcardiotomy patients 10. However, significant suppression of thrombin with high-dose or continuous AT infusions may be deleterious in ECMO patients; therefore, this practice will need additional investigation. Routine daily AT monitoring was begun recently at our center, but AT replacement remains at the clinician’s discretion.
Factor VIII activity was remarkably stable throughout the ECMO course in our study group after an initial increase that may have been related to FFP transfusions in the first 48 h after cannulation or elevation secondary to the inflammation associated with acute illness 18. High factor VIII activity can be associated with apparent heparin resistance, with shortened aPTT but stable antithrombotic effect of heparin as measured by anti-factor Xa 18, 19. We tested for apparent factor VIII-induced heparin resistance in patients whose factor VIII was above 100% and 150%, but our patients did not show evidence for heparin resistance.
Our study was limited by a small sample size and heterogeneity of indications for ECMO. However, to our knowledge, this is the largest prospective study to compare coagulation monitoring assays that are increasingly used in clinical practice, such as anti-factor Xa, AT, and factor VIII. We also plan to evaluate the association between coagulation abnormalities and hemorrhagic and thrombotic outcomes in this study patient population. Future research is needed to 1) describe the role that other coagulation monitoring methods (e.g., TEG, anti-factor IIa) may play in the management of ECMO patients; 2) further describe the complicated relationships between anticoagulation and the risk of thrombosis and hemorrhage in critically ill children on ECMO, and 3) examine via rigorous prospective methodologies the safety and efficacy of interventions that are becoming popular, such as intermittent or continuous administration of recombinant or pooled AT.
Conclusions
This study describes the age differences as well as the variability over days of coagulation system monitoring tests such as ACT, anti-factor Xa or AT during ECMO. ACT remains the most popular method of anticoagulation monitoring during ECMO because it is a rapid assay available at the point of care. On the downside, ACT results are affected by multiple physiologic and equipment-related factors and therefore may not be an accurate measure of anticoagulation by heparin. Anti-factor Xa has recently been introduced in clinical practice as an alternative to anticoagulation monitoring during ECMO. However, processing of anti-factor Xa takes longer than processing for ACT, the test is more expensive and not readily available at all centers. In our study, ACT was poorly correlated with anti-factor Xa, indicating that results should be interpreted with caution when managing anticoagulation on ECMO. Anti-factor Xa results had a direct relation to heparin dose and were not affected by coagulopathy or age. AT did not confound this association between anti-factor Xa and heparin dose but did confound the association between ACT and heparin dose. Based on our results, anti-factor Xa has potential as a marker of anticoagulation for ECMO patients, but additional validation studies are required.
Abbreviations
- ACT
activated clotting time
- aPTT
activated partial thromboplastin time
- AT
antithrombin
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- FFP
fresh frozen plasma
- IQR
interquartile range
- OR
odds ratio
- PICU
pediatric intensive care unit
Footnotes
Disclaimer: The authors have no disclaimers relevant to this article.
Disclosures: The project described was supported by Grants Number UL1 RR 025005 and 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. (MMB).
References
- 1.Conrad SA, Rycus PT, Dalton H. Extracorporeal life support registry report 2004. ASAIO J. 2005;51(1):4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 2.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A. Extracorporeal life support registry report 2008: Neonatal and pediatric cardiac cases. ASAIO J. 2009;55(1):111–116. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 3.Lawson DS, Lawson AF, Walczak R, et al. North american neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of extracorporeal life support organization (ELSO) centers. J Extra Corpor Technol. 2008;40(3):166–174. [PMC free article] [PubMed] [Google Scholar]
- 4.Nankervis CA, Preston TJ, Dysart KC, et al. Assessing heparin dosing in neonates on venoarterial extracorporeal membrane oxygenation. ASAIO J. 2007;53(1):111–114. doi: 10.1097/01.mat.0000247777.65764.b3. [DOI] [PubMed] [Google Scholar]
- 5.Urlesberger B, Zobel G, Zenz W, et al. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. J Pediatr. 1996;129(2):264–268. doi: 10.1016/s0022-3476(96)70252-4. [DOI] [PubMed] [Google Scholar]
- 6.Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs. 1999;23(11):979–983. doi: 10.1046/j.1525-1594.1999.06451.x. [DOI] [PubMed] [Google Scholar]
- 7.Khaja WA, Bilen O, Lukner RB, Edwards R, Teruya J. Evaluation of heparin assay for coagulation management in newborns undergoing ECMO. Am J Clin Pathol. 2010;134(6):950–954. doi: 10.1309/AJCPGVD62LKKVDLH. [DOI] [PubMed] [Google Scholar]
- 8.Ignjatovic V, Lai C, Summerhayes R, et al. Age-related differences in plasma proteins: How plasma proteins change from neonates to adults. PLoS One. 2011;6(2):e17213. doi: 10.1371/journal.pone.0017213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95(2):362–372. doi: 10.1267/THRO06020362. [DOI] [PubMed] [Google Scholar]
- 10.Agati S, Ciccarello G, Salvo D, Turla G, Undar A, Mignosa C. Use of a novel anticoagulation strategy during ECMO in a pediatric population: Single-center experience. ASAIO J. 2006;52(5):513–516. doi: 10.1097/01.mat.0000242596.92625.a0. [DOI] [PubMed] [Google Scholar]
- 11.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13(3):154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 12.Sievert A, Uber W, Laws S, Cochran J. Improvement in long-term ECMO by detailed monitoring of anticoagulation: A case report. Perfusion. 2011;26(1):59–64. doi: 10.1177/0267659110385513. [DOI] [PubMed] [Google Scholar]
- 13.Arnold P, Jackson S, Wallis J, Smith J, Bolton D, Haynes S. Coagulation factor activity during neonatal extra-corporeal membrane oxygenation. Intensive Care Med. 2001;27(8):1395–1400. doi: 10.1007/s001340100991. [DOI] [PubMed] [Google Scholar]
- 14.McDonald MM, Jacobson LJ, Hay WW, Jr, Hathaway WE. Heparin clearance in the newborn. Pediatr Res. 1981;15(7):1015–1018. doi: 10.1203/00006450-198107000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Perry DJ, Fitzmaurice DA, Kitchen S, Mackie IJ, Mallett S. Point-of-care testing in haemostasis. Br J Haematol. 2010;150(5):501–514. doi: 10.1111/j.1365-2141.2010.08223.x. [DOI] [PubMed] [Google Scholar]
- 16.Fleming GM, Gupta M, Cooley E, Remenapp R, Bartlett RH, Annich GM. Maintaining the standard: A quality assurance study for new equipment in the michigan ECMO program. ASAIO J. 2007;53(5):556–560. doi: 10.1097/MAT.0b013e31810c082f. [DOI] [PubMed] [Google Scholar]
- 17.Colby CE, Sheehan A, Benitz W, Van Meurs K, Halamek LP, Moss RL. Maintaining adequate anticoagulation on extracorporeal membrane oxygenation therapy: Hemochron junior low range versus hemochron 400. J Extra Corpor Technol. 2003;35(1):35–38. [PubMed] [Google Scholar]
- 18.Levine MN, Hirsh J, Gent M, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med. 1994;154(1):49–56. [PubMed] [Google Scholar]
- 19.Olson JD, Arkin CF, Brandt JT, et al. College of american pathologists conference XXXI on laboratory monitoring of anticoagulant therapy: Laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782–798. [PubMed] [Google Scholar]


