Abstract
Pulmonary dendritic cells (DCs) are among the first responders to inhaled environmental stimuli such as ozone (O3), which has been shown to activate these cells. O3 reacts with epithelial lining fluid (ELF) components in an anatomically site-specific manner dictated by O3 concentration, airway flow patterns, and ELF substrate concentration. Accordingly, the anatomical distribution of ELF reaction products and airway injury are hypothesized to produce selective DC maturation differentially within the airways. To investigate how O3 affects regional airway DC populations, we utilized a model of O3-induced pulmonary inflammation, wherein C57BL/6 mice were exposed to 0.8 ppm O3 8 h/day for 1, 3, and 5 days. This model induced mild inflammation and no remarkable epithelial injury. Tracheal, but not more distant airway sites, and mediastinal lymph node (MLN) DC numbers were increased significantly after the third exposure day. The largest increase in each tissue was of the CD103+ DC phenotype. After 3 days of exposure, fewer DCs expressed CD80, CD40, and CCR7, and, at this same time point, total MLN T cell numbers increased. Together, these data demonstrate that O3 exposure induced site-specific and phenotype changes in the pulmonary and regional lymph node DC populations. Possibly contributing to ozone-mediated asthma perturbation, the phenotypic changes to DCs within pulmonary regions may alter responses to antigenic stimuli. Decreased costimulatory molecule expression within the MLN suggests induction of tolerance mechanisms; increased tracheal DC number may raise the potential for allergic sensitization and asthmatic exacerbation, thus overcoming O3-induced decrements in costimulatory molecule expression.
Keywords: cell trafficking, epithelial lining fluid, site specificity
the respiratory tract is continuously confronted by environmental challenges such as inhaled irritant gases, particulate matter, and antigens. Such challenges often result in an inflammatory response, but the specifics vary greatly depending on the inhaled agent, endogenous immune status, and preexisting disease states. Although immune responses have been extensively characterized across a spectrum of inhaled challenges and underlying pathologies, many of the regulatory mechanisms remain equivocal. Ozone (O3) is a ubiquitous air pollutant that is encountered at unhealthy levels by over a third of the US population. Moreover, studies indicate that O3 concentrations lower than the current National Ambient Air Quality Standard (75 ppb/8 h) (1) increase morbidity and mortality among children, the elderly, and those with preexisting lung disease (3, 18, 20, 29). Although O3 exposure-related inflammation has been characterized predominantly by neutrophilic infiltration (17), in the context of comorbidities (e.g., asthma), other innate immune cells provide crucial and/or required functions such as cytokine-mediated proinflammatory signaling (28) and linking innate immunity to an adaptive immune response (24).
Dendritic cells (DCs) act as respiratory tract sentinels by internalizing inhaled antigens through various mechanisms and presenting these antigens within the mediastinal lymph nodes (MLN). These events initiate pulmonary DC maturation eliciting a tolerogenic or effector immune response dependent on the types of antigen and stimuli such as pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), cytokines, and perhaps O3 reaction products present within the respiratory tract. Although O3 is not intrinsically antigenic, it reacts within the epithelial lining fluid (ELF) that covers the entire respiratory tract to generate a number of ELF constituent-derived products that likely account for its toxicity and proinflammatory potential. Increased asthma exacerbations, airway DC activation, and antigen presentation ability suggest that proinflammatory DC function may result from exposure (13, 22, 23). The generation of hyaluronan fragments and subsequent binding to Toll-like receptor 4 (16) may be one such DC activation pathway, but other ELF oxidative modifications and/or products should not be ruled out as possible DC-activation stimuli. Additionally, signals such as cytokines, chemokines, and DAMPs originating from other closely approximated cells can also be potent modulators of DC function.
DCs populate the entire respiratory tract but vary in both density and phenotype by location (14). In general, the DC population density is highest within the proximal conducting airways and decreases longitudinally toward the parenchyma. Conventional DCs, rather than plasmacytoid DCs, are the most populous within the conducting airways, and recent studies suggest that this population is comprised of subtypes with differing functions. CD11b−/CD103+ DCs (herein referred to as CD103+) are thought to reside and form a network within the epithelial cell layer, extending processes into the airway lumen (7); CD11b+/SIRPα+ DCs (herein referred to as CD11b+) lie within the lamina propria of the conducting airways (39), and CD11b+/Ly6c+ inflammatory DCs (iDCs) represent a population of newly recruited monocyte-like DCs (42). CD103+ and CD11b+ DCs are thought to sample antigen, traffic to lymph nodes, and present this antigen via major histocompatibility complex (MHC) I and MHC II, respectively, whereas iDCs may be a potent source of immunomodulatory cytokines within the airways (31).
Previous investigations suggest that epithelial injury occurs in site-specific locales due to the interplay among ELF physiochemical conditions, airway architecture and local airflow patterns, and the local intrapulmonary gas phase O3 concentrations (36). Thus we hypothesized that DC activation by O3 exposure depends on airway location and results in modulation of the DC subtypes. To investigate, we employed an exposure protocol, not dissimilar from urban exposure episodes, and conducted airway generation-specific and MLN analyses. The results suggest that exposure initiated a phenotype-specific accumulation of DCs within the trachea and MLN. Within the MLN, DCs demonstrated significantly lower expression of CD40 and CD80, but a significantly greater number of T cells were detected. These observations may partially explain how O3 affects adaptive immune responses by increasing the airway DC population capable of antigen presentation and altering the T cell effector responses by modulation of costimulatory molecule expression.
MATERIALS AND METHODS
Animals and O3 exposure protocol.
Male C57BL/6 mice (8–12 wk old; The Jackson Laboratory, Bar Harbor, ME) were housed in a pathogen-free containment facility and maintained and exposed under barrier conditions within the University of Alabama at Birmingham Environmental Exposure Facility. Mice were provided with food and water ad libitum except during exposure periods when food was withdrawn. All animal treatments were approved by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC) and were in accordance with the National Institutes of Health guidelines. Mice were continuously housed in exposure chambers. O3 exposures were conducted for 1, 3, or 5 days, each day consisting of an 8-h, 0.8 ppm exposure followed by 16 h of filtered air (FA). Although this concentration is higher than the National Ambient Air Quality Standards (0.075 ppm), mice are considered less susceptible than humans due to obligatory nose breathing. Architectural differences within the composite nasopharyngeal region enhance scrubbing, resulting in a relatively lower O3 concentration entering the lower respiratory tract (6). Additionally, mice were exposed during daytime hours when activity levels are low and breathing rates are reduced. Animals were exposed in 0.8-m3 stainless steel chambers within wire-topped cages employing 30 volume changes/hour turnover rates. Due to the rapid turnover rates and air mixing, O3 concentrations did not differ between the chamber bulk phase and breathing zone within the mouse cages. O3 was generated from medical-grade O2 using a model OZ1PCS-V/SW O3 Generator (Ozotech, Yreka, CA) and bled into the chamber inflow (∼22°C; 50% relative humidity) using mass flow controllers. Chamber supply air (O3 and FA chambers) was conditioned via sequential coarse filter, activated charcoal, and HEPA filter units. Chamber O3 concentrations were continuously monitored using a Thermo Scientific model 49 Photometric O3 Analyzer (Franklin, MA).
Tissue harvest and processing.
Immediately following exposure cessation, animals were anesthetized with 4% isofluorane (Vet One, Meridian, ID) followed by intraperitoneal administration of 50 mg/kg Na pentobarbital (Hospira, Lake Forest, IL) and euthanized via exsanguination. The chest cavity and trachea were exposed via a midline thoracotomy and the lungs resected en bloc. The lower respiratory tract was microdissected under a stereo dissecting microscope to differentially harvest anatomic regions of interest (trachea, mainstem bronchi, and mediastinal lymph nodes) and minimize parenchymal contamination.
To facilitate the select isolation of intrapulmonary airways and parenchyma, lungs were cannulated and then inflated with 1 ml of low-melting-point agarose solution at 37°C [1% SeaKem Low MP agarose (FMC BioProducts, Rockland, ME), RPMI 1640 medium (Mediatech, Manassas, VA)], resected, and immediately submerged into ice-cold RPMI 1640 for 5 min. Using forceps, the parenchyma was gently removed, leaving agarose-inflated pulmonary airways as discrete tissues, followed by incubation in 1 ml of digestion buffer [RPMI 1640, 5% fetal calf serum (Hyclone Laboratories, Logan, UT), 1 mg/ml Liberase TM (Roche, Indianapolis, IN), and 0.02 mg/ml DNase I (Sigma Aldrich, St. Louis, MO)] for 45 min at 37°C, and the resulting suspension passed through a 70-μm filter (BD Falcon, Bedford, MA). Red blood cells were removed via lysis buffer [10 mM KHCO3, 150 mM NH4Cl, 0.1 mM EDTA (Sigma Aldrich) pH 8.0] followed by centrifugation.
Flow cytometry.
Total dissociated cell counts were enumerated using a Neubauer hemacytometer (Hausser Scientific, Horsham, PA). CD32/CD16 binding was blocked with FC Block (BD PharMingen, San Diego, CA) for 15 min at room temperature and then incubated 15 min at room temperature with fluorochrome-conjugated antibodies listed in Table 1. Cells were washed, resuspended in 1% paraformaldehyde, and analyzed using a FACS Calibur or LRSII (BD PharMingen) and FlowJo (Treestar, Ashland, OR). Isotype controls to determine background staining and fluorescence-minus-one controls to generate a compensation matrix were used in each experimental run. DCs were defined by gating strategies as CD11c+/MHCII+/CD3ε−/CD45R− (herein referred to as CD11c+/MHCII+). The detection of DC subsets, CD103, CD11b, and Ly6c or activation maker expressing CCR7, CD40, CD80, and CD86 DCs were stained in combination in single experimental runs and enumerated as positive for their respective fluorescent markers after being selected as CD11c+/MHCII+.
Table 1.
Antibodies and clones
| Source | Antibody | Clone |
|---|---|---|
| BD | CD11b APC-Cy7 | M1/70 |
| EB | CD11c APC | N418 |
| EB | MHCII PE-Cy7 | M5/114.15.2 |
| EB | CD3ε FITC | 145-2C11 |
| EB | CD8α FITC | 53-6.7 |
| EB | CD103 PE | 2E7 |
| EB | SIRPa APC | P84 |
| EB | Ly6c eFluor 450 | HK1.4 |
| BD | CD80 FITC | 16-10A1 |
| BD | CD86 V450 | GL1 |
| EB | CD83 Biotin | Michel-17 |
| EB | CD40 PE | 1C10 |
| EB | IL-2 FITC | JES6-5H4 |
| EB | IFN-y FITC | XMG1.2 |
| EB | IL-4 PE | 11B11 |
| EB | IL-10 PE | JES5-16E3 |
BD, BD Pharmingen; EB, eBioscience.
Cell differentials.
Animals used for lavage were not used for other lung analyses. Cells were collected by bronchoalveolar lavage (BAL) using a single 0.8-ml aliquot of 310 mOsm phosphate-buffered saline (pH 7.0) (32), gently but rapidly instilled, and withdrawn three times. No differences in lavage recovery volumes across experimental groups were found. BAL cells were centrifuged onto slides and stained using the Hema 3 stain kit (Fisher, Kalamazoo, MI). At least 300 cells were counted per animal.
Statistical analysis.
Data are expressed as means ± SE. All data were tested for and found to be normally distributed by using normal quantile plots. Significant differences between groups evaluated were by one-way ANOVA and post hoc Tukey's test. A P value of <0.05 was considered statistically significant.
RESULTS
The cellular response to multi-day O3 exposure.
To investigate the response of respiratory tract DCs to O3 exposure, we utilized an exposure protocol that induced mild to moderate inflammation with minimal epithelial injury. Mice were exposed to 0.8 ppm O3 for 8 h/day, followed by 16 h of FA, for 1, 3, or 5 days, and killed for sample collection immediately following exposure cessation. After the first 8-h exposure period, BAL total cell counts increased significantly from 1 × 105 cells/ml in exposure period-matched control animals to 2.5 × 105 cells/ml in O3-exposed animals and remained significantly elevated thereafter. No differences were detected across the three O3 exposure groups. Neutrophils increased approximately threefold (1.8% ± 0.95 to 10.8% ± 0.94) after 1 day of exposure, returned to baseline at day 3, and increased significantly again at day 5 compared with day 3, as seen in Fig. 1. Histological assessment of the trachea, main stem bronchi, intrapulmonary airways, and parenchyma revealed no remarkable epithelial changes beyond modest shortening of cilia and epithelial height in the trachea after 3- and 5-day exposure (data not shown). These data provide evidence that the O3 exposure regimen caused moderate inflammation with only minimal epithelial damage as assessed by morphology.
Fig. 1.
Effect of multi-day O3 exposure on resident pulmonary immune cells. Mice were exposed to 0.8 ppm O3, 8 h per day for 5 days. Immediately following O3 exposure, lungs were lavaged and cells were counted via hemacytometer or after being spun onto slides and stained with the Hema-3 Stain kit. All control animals were exposed to filtered air (FA) by identical methods to experimental animals. A: total cell count within the bronchoalveolar lavage fluid (BALF) approximately doubled and remained elevated throughout exposure. B: neutrophil number increased after both 1 and 5 days of O3 exposure relative to the previous day. *P < 0.05 compared with FA, **P < 0.05 compared with day 3.
Throughout the composite respiratory tract, the phenotype, function, and density of DCs vary appreciably (14). Therefore, specific airway regions and the MLN were selectively isolated to determine exposure-mediated DC effects in discrete anatomic locations. There were no observable changes in total cells harvested from any region after enzymatic dissociation. Only within the trachea and mediastinal lymph nodes, seen in Fig. 2, were DC numbers significantly increased, whereas no exposure-related effects were detected in other locations. At day 3, DC numbers increased in both compartments compared with exposure period-matched FA controls and returned to baseline at day 5. As a consequence, subsequent experiments focused on the trachea and MLN after the third day of the O3 exposure protocol, when DC exposure effects were the greatest.
Fig. 2.
Flow cytometry of dendritic cells (DCs) over a multi-day O3 exposure. Immediately following exposure, regions of the respiratory tract and mediastinal lymph nodes (MLN) were isolated via microdissection, brought into a single cell suspension via collagenase digestion, and stained with known DC markers. A: gating strategy included selecting a broad range of cells based on size and granularity, excluding cells expressing B and T cells markers CD45R and CD3ε, respectively, then selecting the major histocompatibility complex (MHC)II/CD11c double-positive populations. B: only the trachea and mediastinal lymph node DC numbers were affected and increased due to O3 exposure. On day 3, DC numbers were significantly higher compared with FA or day 1 animals, and at day 5, these numbers dropped significantly from day 3. *P < 0.05 compared with FA, **P < 0.05 compared with day 1, #P < 0.05 compared with day 3. FSC, forward light scatter; SSC, side light scatter.
DC phenotype distribution after 3-day O3 exposure.
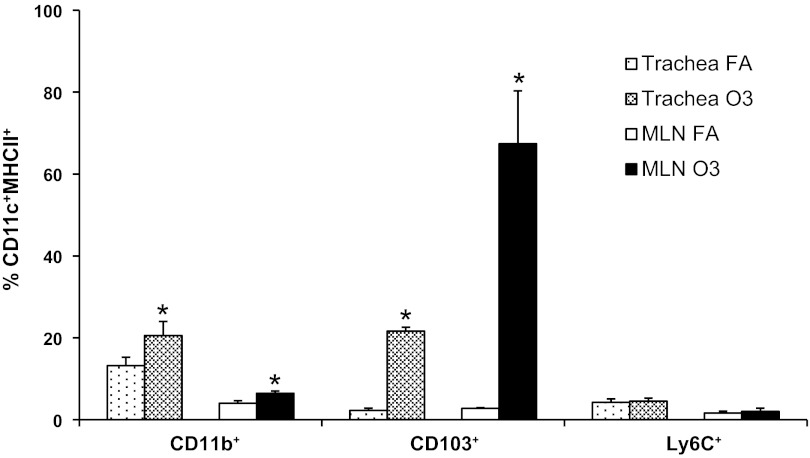
Pulmonary DC subsets may differentially control the immune response and initiate unique innate and/or adaptive responses. CD103+ and CD11b+ DCs are both possible targets of O3-induced activation due to their proximity to and/or direct contact with the ELF, respectively, and iDCs are recruited during times of inflammation. Within the trachea (Fig. 3), the percentage of both CD11b+ and CD103+ DC phenotypes significantly increased after exposure although not all CD11c+MCHII+ DCs were of either phenotype. Similarly, the percentage of both DC phenotypes significantly increased within the lymph nodes, but the increase in CD103+ cells was much greater compared with any other phenotype. No significant change was found in iDC numbers. These shifts in DC populations may translate to alterations in DC functionality in both the airway and regional lymph nodes.
Fig. 3.
Specific phenotypes of DCs increased within the trachea and MLNs after 3-day O3 exposure. Following dissection, digestion, and staining of cells, a significant increase in CD11b+ CD11c+/MHCII+ DCs was observed in the trachea and mediastinal lymph nodes after 3-day O3 exposure. *P < 0.05 compared with FA.
Markers of DC activation.
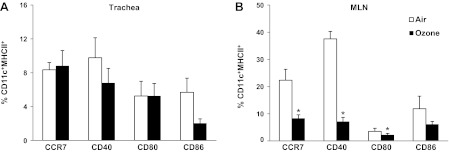
The increased tracheal and MLN DC numbers suggest enhanced DC trafficking and recruitment. To clarify migrating cell functional characteristics, markers of activation on CD11c+MHCII+ DC were analyzed. DCs within the trachea basally expressed low levels of CD80, CD86, CD40, and CD83 (all important during the formation of a T cell effector response), as well as CCR7 (receptor for CCL19 DC chemokine). Figure 4 shows that, after 3-day exposure, no change in marker expression was observed on tracheal DCs. Although an increase in the number of DCs within the MLN could suggest increased DC maturation, unexpectedly, MLN DCs showed significant decreases in all of these markers with the exception of CD86. These data indicate that O3 exposure decreased markers of activation and trafficking on DCs residing in regional lymph nodes.
Fig. 4.
DC activation status after 3-day O3 exposure. Immediately following the 3rd O3 exposure day, cells were collected from the trachea and MLN and stained for DC markers CD11c and MHCII and activation markers. Within the trachea (A), there was no significant change, but, within the MLN (B), CCR7, CD40, and CD80 levels were significantly decreased. *P < 0.05 compared with FA.
T cell characterization.
The ultimate role of DC trafficking to lymph nodes is to initiate either a T cell effector response or induce tolerance. To ascertain whether DC trafficking to the MLN as a consequence of O3 exposure induced such an effect, the number of T cells was quantified after day 3 of exposure. Exposure caused a significant increase in the percentage of T cells, gated as CD3ε+ cells, (Fig. 5) within the lymph nodes.
Fig. 5.
T cell numbers in pulmonary lymph nodes following 3-day O3 exposure. After cells were collected from MLN, the cells were stained with CD3ε to identify all T cells. The percentage of T cells within the lymph nodes increased after 3-day O3. *P < 0.05 compared with FA.
DISCUSSION
During inflammatory events, pulmonary DCs migrate toward lymph nodes after acquiring antigen and/or receiving stimuli (e.g., cytokines, PAMPs, DAMPs) and mature into efficient antigen presenters capable of eliciting an adaptive immune response. Although O3 is generally thought to stimulate DC activity, the spectrum of experimental conditions employed across investigations (e.g., O3 concentration, time courses, coexposures) does not facilitate ready discernment of the mechanistic bases that govern DC functions after activation. However, studies combining O3 as an exogenous stressor either before or directly after ovalbumin administration do suggest that, within the complexities of exposure-related sequelae, O3 may create an adjuvant-like stimulatory signal (12, 16). Although the exposure-related factors that modulate DC maturation also remain equivocal, human and experimental animal studies (22, 23, 27) have linked exposures to altered activation marker expression. Furthermore, even in the absence of other biological stimuli, O3 is able to induce airway hyperreactivity (12, 33, 34), a hallmark of asthmatic exacerbations. Nonetheless, it is important that exposure conditions are carefully considered because high concentrations may lead to airway injury, resulting in necrotic cell death, a breakdown of epithelial barrier integrity, and enhanced accessibility to subepithelial strata. Thus, in compilation, the precise mechanisms (e.g., ELF-derived oxidant/bioactive species, cell damage, inflammation) that drive the compendium of DC pathophysiological sequelae remain poorly understood.
The goal of the exposure paradigm employed herein was to facilitate investigating site-specific DC activation, trafficking, and recruitment while minimizing potential confounding stemming from overt airway epithelial damage. Histological analyses (data not shown, no remarkable pathology) provided support that the exposure protocol did not induce appreciable airway injury but, based on BAL differentials, was sufficient to initiate inflammatory cascades. The increases in total cell number and neutrophil percentages seen after the first exposure period (day 1) were similar to previous studies (34) that employed a slightly higher O3 concentration but shorter exposure times. The exposure regimen may have induced a cyclic pattern of neutrophil recruitment, demonstrated by a waning of neutrophil percentages from day 1 to day 3, followed by a significant increase from day 3 to day 5. However, there were no differences in total lavage cell counts among exposure groups. Therefore, this model permitted direct investigation into O3-mediated DC biological sequelae concomitant with mild inflammation, as might occur as a consequence of environmentally relevant human exposures experienced during air pollution episodes (multiple sequential days of elevated ambient O3).
To optimize analysis of phenotypic and possible functional differences, we conducted time course studies to guide sample acquisition. Significant increases in DC numbers were observed within the trachea and MLN following the third exposure period (day 3). Previous studies indicate that DC lymph node homing is initiated ∼8 h after stimuli (4), consistent with the fact that DC numbers were not increased within the lymph nodes until our second sampling time point. Thus we focused our DC analyses after the third exposure period (day 3; 2nd sample time). The overall change in tracheal DC numbers represented a net balance between airway recruitment and airway to lymph node trafficking, wherein, under these study conditions, recruitment rates likely exceeded trafficking. Because we did not directly evaluate tracheal DC movement to the MLN and did not observe statistically significant alterations in distal DC populations, we were unable to discern whether DCs originating from distal regions contributed to the absolute DC population changes observed in the MLN. Moreover, because MLN DC turnover rates were not investigated, the extent of MLN DC accumulation due to possible prosurvival signaling (5) also remained undefined.
The observation that only tracheal DCs displayed significant numeric changes may be attributable to the confluence of site-specific differences in DC populations and regional O3 dosimetry. DC densities decrease longitudinally from trachea to parenchymal space, and, furthermore, the phenotypic and functional properties of airway mucosal DCs exhibit greater potential for antigen presentation and more rapid turnover rates (44) than more distal lung DCs. In concert is the longitudinal distribution of O3 reactive uptake, wherein luminal gas phase concentrations decline due to removal at the airway walls as inhaled O3 transits distally. Greater flux rates in more proximal regions potentially enhance generation of ELF-derived products (e.g., bioactive lipids, DAMPs) that initiate DC trafficking and maturation (21). Thus, considering O3 intrapulmonary dispersion, its coupled biological chemistry, and the anatomic distribution and biology of airway DCs, observations herein support that predominate effects on DC functional outcomes should occur within the proximal airways. The influence of O3, however, may not be isolated to the upper airways. Although we were unable to note statistical differences in DC numbers within parenchymal regions, recent studies suggest that alveolar DC antigen uptake within the parenchyma influences DC activity and phenotype distribution within proximal airway regions (40, 43). Therefore, the observed proximal DC trafficking and phenotype distribution may, in part, be due to exposure related influences on parenchymal DCs.
The development of more precise pulmonary DC phenotyping has helped identify functional differences among CD103+, CD11b+, and iDCs. Although resident pulmonary DC subset functionality remains to be fully elucidated, diverse disease states such as asthma and viral infection illustrate that differences in antigen presentation and chemokine production occur (15, 25). During the studies herein, CD103+ and CD11b+ DCs had increased in both trachea and MLN by the second sampling period (day 3), but these increases did not significantly change the ratio of CD103+/CD11b+ in either compartment. Both CD103+ and CD11b+ DCs have been implicated as important for the priming of Th1/Th17 and Th2 responses. Reports indicate that an increase in either CD103+ (29) cells or CD11b+ cells (12) within the lymph nodes may result in an ability to skew T cell responses toward a Th2 phenotype. Additionally, CD103+ cells are crucial to asthma pathogenesis and perturbations (39). However, CD11b+ cells may also play pivotal roles in the development and exacerbation of asthmatic symptoms (30) and the known neutrophilic response to O3 by influencing proinflammatory cytokine production (12). Consequently, the influence of O3 exposure on these DC subsets may not exclusively shape immune responses toward Th1/Th17 or Th2 phenotypes.
The increase in CD103+ and CD11b+ DCs found within the lung tissue and MLN have been previously observed across pathological states, in which antigen is a major component of the immunological response, such as atopic asthma and respiratory syncytial virus infection (19, 41). Thus increases in both DC phenotypes due to O3 exposure with no exogenous antigen could potentiate atopic responses by enhancing the number of available antigen-presenting cells. Notably, significant changes in iDC number were not observed within any compartment, suggesting that O3-mediated inflammation and/or stimuli were insufficient to elicit detectable iDC recruitment in this murine exposure model.
The current paradigm regarding DCs, once within the lymph nodes, involves antigen presentation followed by induced T cell expansion. Due to the exposure-related DC migration, T cell numbers within the MLN were analyzed to ascertain whether DC homing could affect T cell population changes as a consequence of O3 exposure. After 3 days, there was a significant increase in MLN T cell numbers, which may suggest that O3, as the sole challenge agent, can induce dynamic changes in T cell populations. DC activation markers, which function as costimulators of T cell expansion, provide insight into the nature of T cell responses generated. Consequently, CD80, C86, CD40, and CCR7 expression on DCs were analyzed within the trachea and MLN at the same sampling point (day 3). Tracheal CD80, C86, CD40, and CCR7 DC levels were unchanged following exposure. However, within the MLN, CD80, CD40, and CCR7, but not CD86 DC levels were decreased. Increases in CD86 have been reported consequent to O3 exposure (16), but these previous studies incorporated sampling and exposure conditions different from those employed herein. CD80, CD86, and CD40 are necessary for effective adaptive immune responses via T cell costimulation (5). Decreased expression of these proteins, relative to basal levels, may result in lowered T cell stimulatory capacity. T regulatory cells (TReg) have been suggested, however, to mediate CD80, CD86, and CD40 expression decrements within peripheral lymph nodes (38). Although we did not note a statistical change in DCs expressing CD86, this protein could influence T cell-DC interactions.
Nonetheless, the observed increase in the T cell population may have been predominately TReg phenotypes initiated by O3-dependent DC stimulation and subsequent MLN migration without exogenous antigen. To garner insights regarding this issue, preliminary studies evaluated Fox3p expression [a marker of TRegs (9)] in MLN CD4+CD25+ T cells by flow cytometry. Observations made at the 3-day sampling period suggested increased Foxp3 expression among these T cells (unpublished observations). Therefore, TRegs may have expanded during the 3-day exposure period and actively downregulated MLN DC CD80 and CD40 expression. Because only the net change in cell population numbers were quantified, we cannot rule out the possibility that DC activation marker expression decreased during their migration to the MLN or that T cell numbers increased due to T cell MLN trafficking from an unknown origin. However, TReg expansion and costimulatory molecule downregulation could prevent aberrant failure of self vs. nonself discrimination of the adaptive immune system after O3-induced inflammation.
Further preliminary experiments were conducted to determine whether the observed DC costimulatory molecule expression pattern could be due to direct interaction with O3-ELF reaction products. In select, but limited, in vitro studies, murine bone marrow-derived DC (BMDC) were cultured and exposed to O3 while covered by a thin film of model ELF, as previously described (2). The apical fluid compartment (model ELF) contained reduced glutathione and ascorbate, uric acid, glutathione disulfide, egg phosphatidyl choline liposomes, and immunoglobin/fatty acid-free bovine serum albumin, and the cultures were exposed with intermittent tilting to 0.8 ppm O3 (in warmed, humidified 95% air-5% CO2) for 120 min followed by exposure media removal and incubation with conditioned cell culture media for 24 h. Preliminary results from these nascent investigations suggest that O3 exposure, in the presence of ELF substrates, induced CD80 and CD86 expression decrements in BMDCs. Notably, in the absence of apical fluid reactive substrates, no exposure-induced impacts were noted, which is consistent with previous observations (8). Therefore, we speculate that O3-ELF-derived reaction product(s) may be responsible, in part, for the altered expression levels of DC activation markers in our in vivo model, which is internally consistent with our overall observations and insights regarding O3 reaction/diffusion and bioactive product formation within the ELF (26, 36, 37). However, moieties derived from injured and/or otherwise perturbed cells may also play a role, as a recent report has shown that CD103+ DCs present cellular autophagy products (10).
In summary, we report that acute intermittent O3 exposure of a murine model induces accumulation of CD103+ DCs and CD11b+ within the trachea and MLN but not analogous DC perturbations in more distal airway regions. Additionally, MLN DCs had decreased expression of the lymph node-homing chemokine receptor CCR7 and the activation markers CD80 and CD40 while T cell numbers increased. Numerous reports indicate that O3 increases asthmatic exacerbations in humans and, when combined with allergen, allergic airway disease in animal models. Our results provide additional background to understanding how O3 exposure alone may prime the pulmonary innate immune system for increased asthma pathology. The decrease in T cell costimulatory molecules suggests that regulatory mechanisms that dampen adaptive immune responses may take place in the context of environmental oxidant exposure. By increasing the number of airway DC, however, the potential for antigen presentation increases within the MLN. This could lead to an increase in immune responsiveness during coexposure or O3 preexposure and therefore partially explain the suggested additive effect of O3 on asthmatic sensitization and exacerbations. Whether or not such biology would occur during human environmental exposures (often occurring as complex mixtures) remains unknown; nonetheless, we believe that data presented herein highlight the need for further investigation into O3-mediated changes in DC biology and thus integral control over both innate and adaptive immune responses.
GRANTS
This work was supported by grant ESP0111617 from the National Institute of Environmental Health Sciences (E. Postlethwait, M. Fannuchi, J. Brand, K. Tuggle) and T32HL007918 from the National Heart, Lung, and Blood Institute (J. Brand). Work done at the Division of Rheumatology and Immunology FACS Core Facility was supported by grant NAHT30AR48311 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.B., C.A.B., L.M.S., and E.M.P. conception and design of research; J.D.B. and K.L.T. performed experiments; J.D.B., C.A.B., K.L.T., M.V.F., and E.M.P. analyzed data; J.D.B., C.A.B., K.L.T., M.V.F., L.M.S., and E.M.P. interpreted results of experiments; J.D.B. and E.M.P. prepared figures; J.D.B. and E.M.P. drafted manuscript; J.D.B., C.A.B., K.L.T., M.V.F., L.M.S., and E.M.P. edited and revised manuscript; J.D.B., C.A.B., K.L.T., M.V.F., L.M.S., and E.M.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Enid Keyser for help conducting flow cytometry.
REFERENCES
- 1. Agency USEP, US EPA. Air Quality Criteria for Ozone and Related Photochemical Oxidants (2006 Final). Washington, DC: Agency USEP, 2006 [Google Scholar]
- 2. Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 38: 515–526, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292: 2372–2378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byersdorfer CA, Chaplin DD. Visualization of early APC/T cell interactions in the mouse lung following intranasal challenge. J Immunol 167: 6756–6764, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol 25: 655–658, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Carey SA, Minard KR, Trease LL, Wagner JG, Garcia GJ, Ballinger CA, Kimbell JS, Plopper CG, Corley RA, Postlethwait EM, Harkema JR, Einstein DR. Three-dimensional mapping of ozone-induced injury in the nasal airways of monkeys using magnetic resonance imaging and morphometric techniques. Toxicol Pathol 35: 27–40, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Condon TV, Sawyer RT, Fenton MJ, Riches DW. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol 90: 883–895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connor LM, Ballinger CA, Albrecht TB, Postlethwait EM. Interfacial phospholipids inhibit ozone-reactive absorption-mediated cytotoxicity in vitro. Am J Physiol Lung Cell Mol Physiol 286: L1169–L1178, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30: 626–635, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Depuydt PO, Lambrecht BN, Joos GF, Pauwels RA. Effect of ozone exposure on allergic sensitization and airway inflammation induced by dendritic cells. Clin Exp Allergy 32: 391–396, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Furuhashi K, Suda T, Hasegawa H, Suzuki Y, Hashimoto D, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Shibata K, Nakamura H, Chida K. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am J Respir Cell Mol Biol 46: 165–172, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 13. Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 290: 1859–1867, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy 66: 579–587, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Hao X, Kim TS, Braciale TJ. Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol 82: 4908–4919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollingsworth JW, Free ME, Li Z, Andrews LN, Nakano H, Cook DN. Ozone activates pulmonary dendritic cells and promotes allergic sensitization through a Toll-like receptor 4-dependent mechanism. J Allergy Clin Immunol 125: 1167–1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc 4: 240–246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology 16: 446–457, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur J Immunol 40: 1042–1052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katsouyanni K, Zmirou D, Spix C, Sunyer J, Schouten JP, Ponka A, Anderson HR, Le Moullec Y, Wojtyniak B, Vigotti MA, Bacharova L, Schwartz J. Short-term effects of air pollution on health: a European approach using epidemiological time-series data The APHEA project: background, objectives, design. Eur Respir J 8: 1030–1038, 1995 [PubMed] [Google Scholar]
- 21. Keshavarzi B, Ultman J, Borhan A. Numerical prediction of the focal sites of ozone-induced tissue injury in the respiratory tract. Model Med Biol 13: 352, 2009 [Google Scholar]
- 22. Koike E, Kobayashi T. Ozone exposure enhances antigen-presenting activity of interstitial lung cells in rats. Toxicology 196: 217–227, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Koike E, Watanabe H, Kobayashi T. Exposure to ozone enhances antigen-presenting activity concentration dependently in rats. Toxicology 197: 37–46, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kool M, Lambrecht BN. Dendritic cells in asthma and COPD: opportunities for drug development. Curr Opin Immunol 19: 701–710, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 31: 412–424, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Langford SD, Bidani A, Postlethwait EM. Ozone-reactive absorption by pulmonary epithelial lining fluid constituents. Toxicol Appl Pharmacol 132: 122–130, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, Hazucha MJ, Devlin RB, Peden DB. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. Allergy Clin Immunol 120: 719–722, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Matangkasombut P, Pichavant M, Dekruyff RH, Umetsu DT. Natural killer T cells and the regulation of asthma. Mucosal Immunol 2: 383–392, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Medina-Ramon M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol 163: 579–588, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103+ dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol 5: 53–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 10: 394–402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielson DW, Goerke J, Clements JA. Alveolar subphase pH in the lungs of anesthetized rabbits. Proc Natl Acad Sci USA 78: 7119–7123, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, McConville G, Allen CB, Sfyroera G, Shultz LD, Lambris JD, Giclas PC, Holers VM, Gelfand EW. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med 169: 726–732, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med 205: 385–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Postlethwait EM, Cueto R, Velsor LW, Pryor WA. O3-induced formation of bioactive lipids: estimated surface concentrations and lining layer effects. Am J Physiol Lung Cell Mol Physiol 274: L1006–L1016, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Postlethwait EM, Joad JP, Hyde DM, Schelegle ES, Bric JM, Weir AJ, Putney LF, Wong VJ, Velsor LW, Plopper CG. Three-dimensional mapping of ozone-induced acute cytotoxicity in tracheobronchial airways of isolated perfused rat lung. Am J Respir Cell Mol Biol 22: 191–199, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Postlethwait EM, Langford SD, Bidani A. Determinants of inhaled ozone absorption in isolated rat lungs. Toxicol Appl Pharmacol 125: 77–89, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Shevach EM. Biological functions of regulatory T cells. Adv Immunol 112: 137–176, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176: 2161–2172, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med 209: 1183–1199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201: 981–991, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Rijt LS, Prins JB, Leenen PJ, Thielemans K, de Vries VC, Hoogsteden HC, Lambrecht BN. Allergen-induced accumulation of airway dendritic cells is supported by an increase in CD31(hi)Ly-6C(neg) bone marrow precursors in a mouse model of asthma. Blood 100: 3663–3671, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Veres TZ, Voedisch S, Spies E, Tschernig T, Braun A. Spatiotemporal and functional behavior of airway dendritic cells visualized by two-photon microscopy. Am J Pathol 179: 603–609, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol 175: 1609–1618, 2005 [DOI] [PubMed] [Google Scholar]