Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease without effective therapeutics. Periostin has been reported to be elevated in IPF patients relative to controls, but its sources and mechanisms of action remain unclear. We confirm excess periostin in lungs of IPF patients and show that IPF fibroblasts produce periostin. Blood was obtained from 54 IPF patients (all but 1 with 48 wk of follow-up). We show that periostin levels predict clinical progression at 48 wk (hazard ratio = 1.47, 95% confidence interval = 1.03–2.10, P < 0.05). Monocytes and fibrocytes are sources of periostin in circulation in IPF patients. Previous studies suggest that periostin may regulate the inflammatory phase of bleomycin-induced lung injury, but periostin effects during the fibroproliferative phase of the disease are unknown. Wild-type and periostin-deficient (periostin−/−) mice were anesthetized and challenged with bleomycin. Wild-type mice were injected with bleomycin and then treated with OC-20 Ab (which blocks periostin and integrin interactions) or control Ab during the fibroproliferative phase of disease, and fibrosis and survival were assessed. Periostin expression was upregulated quickly after treatment with bleomycin and remained elevated. Periostin−/− mice were protected from bleomycin-induced fibrosis. Instillation of OC-20 during the fibroproliferative phase improved survival and limited collagen deposition. Chimeric mouse studies suggest that hematopoietic and structural sources of periostin contribute to lung fibrogenesis. Periostin was upregulated by transforming growth factor-β in lung mesenchymal cells, and periostin promoted extracellular matrix deposition, mesenchymal cell proliferation, and wound closure. Thus periostin plays a vital role in late stages of pulmonary fibrosis and is a potential biomarker for disease progression and a target for therapeutic intervention.
Keywords: fibroblast, lung, bleomycin, mesenchymal
idiopathic pulmonary fibrosis (IPF) is a chronic pulmonary disease associated with progressive scarring of the lungs, loss of lung function, and eventual death (1). Despite an increase in knowledge of the profibrotic pathways leading to pathological fibrosis, no therapies, other than lung transplant, have proven efficacious, and morbidity and mortality remain high.
Periostin is a matricellular protein that binds to matrix proteins and cellular receptors to affect cell function (15). Periostin is integral to wound healing and cardiac fibrosis after myocardial infarction by stabilizing collagen cross-linking (17, 25). Periostin also impacts tumorigenesis by promoting cell proliferation and migration, decreasing apoptosis, and increasing epithelial-mesenchymal transition (33, 38). In the lung, periostin plays a role in subepithelial fibrosis associated with bronchial asthma (31, 34). Okamoto et al. (26) identified periostin in the lungs of Japanese patients with IPF and showed serum periostin levels to be increased compared with controls. More recently, Uchida et al. (35) demonstrated that bleomycin injury induced expression of periostin and that periostin-deficient (periostin−/−) mice were protected from bleomycin-induced lung fibrosis (35). Mechanistic studies by this group suggest that periostin is necessary for appropriate chemokine expression and recruitment of macrophages and neutrophils in response to bleomycin challenge (35).
In the present study, we examined periostin expression in a cohort of North American patients with IPF and studied the role of periostin in the postinflammatory fibrotic phase of bleomycin-induced lung fibrosis.
METHODS
Prospective human cohort.
Correlating Outcomes With Biochemical Markers to Estimate Time Progression in Idiopathic Pulmonary Fibrosis (COMET) is a multicenter, observational cohort study of well-defined IPF patients followed prospectively at 16-wk intervals up to 80 wk (clinicaltrials.gov, clinical trial ID no. NCT01071707). All subjects underwent baseline assessment, including demographics, patient-reported descriptors, spirometry, diffusing capacity of the lung for CO (DlCO), 6-min walk testing, and high-resolution computed tomography. All subjects underwent fiber-optic bronchoscopy with bronchoalveolar lavage, transbronchial biopsy, and blood sampling at baseline. The primary outcome was progression-free survival as determined by the time until any of the following: death, acute exacerbation of IPF, lung transplant, or relative change in forced vital capacity (FVC, liters) of ≥10% or DlCO (ml·min−1·mmHg−1) of 15% (13). All human studies were approved by the University of Michigan Institutional Review Board. Table 1 provides demographic information about IPF patients. Table 2 provides demographic information about control subjects utilized for in vitro studies.
Table 1.
Demographics of IPF patients
| All |
Nonprogressor by 48 wk† |
Progressor by 48 wk |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Age, yr | 54 | 64.27 | 8.19 | 32 | 63.89 | 9.24 | 21 | 64.38 | 6.36 |
| Baseline FVC, %predicted | 53 | 68.47 | 15.84 | 32 | 68.79 | 15.78 | 20 | 68.82 | 16.28 |
| Baseline FEV1, %predicted | 53 | 71.34 | 16.79 | 32 | 72.16 | 17.98 | 20 | 70.91 | 15.03 |
| Baseline DlCO, %predicted | 50 | 40.77 | 14.31 | 29 | 42.74 | 12.33 | 20 | 39.14 | 16.26 |
| Baseline periostin, μg/ml | 54 | 21.49 | 116.97 | 32 | 2.23 | 6.46 | 21 | 51.86 | 186.04 |
| n | No. | % | n | No. | % | n | No. | % | |
|---|---|---|---|---|---|---|---|---|---|
| Male | 54 | 39 | 72.22 | 32 | 24 | 75.00 | 21 | 15 | 71.43 |
| History of smoking* | 54 | 43 | 79.63 | 32 | 25 | 78.13 | 21 | 18 | 85.71 |
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; DlCO, diffusion capacity of the lung for CO.
Patient was a past or current smoker vs. never smoker.
One patient with only 6 mo of follow-up was excluded from progressor and nonprogressor demographic summary, since classification was unknown past 6 mo.
Table 2.
Demographics for normal samples used for in vitro analysis
| Age, yr | Sex |
|---|---|
| Monocyte flow cytometry | |
| 59 | F |
| 68 | F |
| 56 | F |
| Avg 61 ± 6.3 | |
| Fibrocyte mRNA analysis | |
| 74 | M |
| 68 | F |
| 50 | F |
| 62 | M |
| 62 | F |
| Avg 63.2 ± 8.9 | |
F, female; M, male.
Immunohistochemistry on human lung sections utilized rabbit anti-periostin (Abcam, Cambridge, MA), biotinylated anti-rabbit IgG-avidin horseradish peroxidase, and diaminobenzidine detection system (Vector Labs, Burlingame, CA). Blocks for immunohistochemical analysis were given to us in an anonoymous fashion with no demographic information other than diagnosis.
Human lung fibroblasts from control or IPF lungs were provided in an anonymous fashion with no demographic information other than diagnosis and were cultured as described elsewhere (37). Normal fibroblasts were obtained from clean margins of lung cancer resections.
Mice.
B6;129-Postntm1Jmol/J mice (Jackson Laboratories, Bar Harbor, ME) were bred to C57Bl/6 mice to generate heterozygotes. These heterozygotes were mated, and the periostin−/− and littermate periostin+/+ controls were used at 6–8 wk of age. Genotyping was performed by Transnetyx (Cordova, TN). In some in vitro experiments, fibroblasts were purified from C57Bl/6 mice purchased from Jackson Laboratories. For generation of chimeric animals, recipient mice were irradiated at 13 Gy (split dose) and infused with 5 × 106 donor bone marrow cells. Mice were maintained on acidified water for 3 wk and used for experiments at 5 wk posttransplant, when donor cell reconstitution in the lung is maximal (16). Animal work was approved by the University Committee on the Use and Care of Animals.
Flow cytometry.
Flow cytometry was performed on peripheral blood mononuclear cells (PBMCs) isolated by Ficoll-Hypaque density centrifugation (GE Healthcare, Pittsburgh, PA). Purified cells were first blocked for 30 min at 4°C in mouse IgG (Jackson Immunoresearch, West Grove, PA) diluted 1:50 in fluorescein-activated cell sorting (FACS) buffer and then stained with FITC mouse anti-human CD45 for 30 min in darkness (BD Pharmingen, San Diego, CA). After incubation, 100 μl of Cytofix/Cytoperm (BD Biosciences) solution was added, and cells were incubated at 4°C for 20 min. After wash steps and centrifugation, cells were stained with anti-human periostin rabbit polyclonal Ab (1 μg/ml; Biovendor, Chandler, NC) or irrelevant rabbit IgG control Abs (Southern Biotech, Birmingham, AL) diluted 1:850 in Perm/Wash buffer (BD Biosciences) for 30 min. Periostin expression was detected with goat anti-rabbit IgG phycoerythrin (Southern Biotech) diluted 1:400 in Perm/Wash buffer. Cells were examined by forward vs. side scatter for live cells followed by gating on CD45 and forward scatter differentiating the monocyte population by size. A BD Biosciences LSR II flow cytometer was used, and the analysis was performed using WinList (Verity Software House, Topsham, ME).
Fibrocyte culturing.
PBMCs purified as described above were cultured for 14 days in complete medium containing 20% fetal calf serum. At this time, the adherent cell population was >95% CD45- and collagen I-positive. Total RNA was collected from adherent cells and analyzed by quantitative RT-PCR (qRT-PCR) for periostin expression.
qRT-PCR.
For human samples, total RNA was extracted using the RNeasy Plus Mini kit (Qiagen, Valencia, CA) and transcribed to first-strand cDNA using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). First-strand cDNA was used to quantify the expression of periostin and GAPDH using SYBR green technology. The following primers were used: human periostin (POSTN) forward (5′-GCGCTTTAGCACCTTCCT-3′) and reverse (5′-GCACAAATAATGTCCAGTCTCC-3′) primers and human GAPDH forward (5′-CGACCACTTTGTCAAGCTCA-3′) and reverse (5′AGGGGTCTACATGGCAACTG-3′) primers. For murine studies, total RNA was prepared using TRIzol (Invitrogen, Carlsbad, CA). One-step RT-PCRs were then carried out using the following primers and probes labeled with 6-carboxyfluorescein (FAM)/tetramethylrhodamine (TAMRA): collagen type I forward (5′-TGACTGGAAGAGCGGAGAGTACT-3′) and reverse (5′-GCTGTGGGCATATTGCACAA-3′) primers and probe (5′-TGCCCCAACCCAGAGATCCCATTT-3′), periostin forward (5′-GGGGTTGTCACTGTGAACTG-3′) and reverse (5′-CGGCTGCTCTAAATGATGAA-3′) primers and probe (5′-CGTGTCCTGACACAAATTGG-3′), and β-actin forward (5′-CCGTGAAAAGATGACCCAGATC-3′) and reverse (5′-CACAGCCTGGATGGCTACGT-3′) primers and probe (5′-TTTGAGACCTTCAACACCCCCAGCCA-3′). All primers were obtained from IDT (Coralville, IA) or Sigma (St. Louis, MO). Reactions were carried out using the Step One Plus real-time PCR machine (Applied Biosystems, Carlsbad, CA).
Fibrosis and survival studies.
Periostin−/− and littermate control mice were injected with bleomycin (0.025 U; Sigma) intratracheally on day 0. Lungs were collected on days 0, 3, 7, 14, and 21 for RNA isolation and on day 14 for histology and determination of collagen content by hydroxyproline assay. For survival studies, mice were injected with 0.05 U of bleomycin and then with OC-20 (200 μg) or control Ab (200 μg) on days 10 and 15 and analyzed by day 19 (27). For studies of fibrosis following OC-20 administration as described above, mice were injected with 0.025 U of bleomycin and analyzed on day 21.
Mouse histology.
Lungs were perfused with saline and inflated with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Sections were stained with hematoxylin-eosin and Masson's trichrome. Additionally, tissue sections were probed with Cy3-labeled mouse anti-α-smooth muscle actin (α-SMA; clone 1A4, Sigma-Aldrich) and Alexa Fluor 488-labeled rabbit anti-periostin (Abcam). Nuclei were stained with Hoescht 33258. Slides were coverslipped in Prolong Antifade medium (Invitrogen). Abs for immunohistochemistry were rabbit anti-periostin (Abcam) and unlabeled mouse anti-α-SMA (clone 1A4, Sigma-Aldrich).
Hydroxyproline assays.
Total lung collagen was quantified by the hydroxyproline assay (24).
Mesenchymal cells.
Mesenchymal cells isolated as described previously (19) were cultured in serum-free medium for 24 h with or without a dose response of periostin before isolation of RNA or collection of supernatants.
Periostin ELISA.
Cell supernatants and human plasma were analyzed for periostin by ELISA (Adipo Bioscience, Santa Clara, CA).
PAI-1 concentrations.
Plasminogen activator inhibitor (PAI)-1 concentrations in cell supernatants were measured using a carboxylated microsphere-based ELISA (Luminex) (8).
Proliferation assay.
For proliferation assay, 5 × 103 cells were cultured in serum-free medium + a dose response of periostin for 48 h, with the addition of 10 μl of [3H]thymidine for the last 16 h.
PAI-1 luciferase assays.
For PAI-1 luciferase assay, PAIL cells (a gift from Dr. D. Rifkin, Department of Cell Biology, New York University) were used as previously described (19). Cells were cultured at 1 × 106 cells/well in a six-well plate in serum-free medium + G418 for 24 h with or without 2 ng/ml TGF-β, 100 ng/ml periostin, or 1 μg/ml periostin. Luciferase activity in cell lysates was then measured using a single-tube luminometer.
Wound confluence assay.
For wound confluence assay, fibroblasts were plated at 1 × 105 in a 96-well Essen ImageLock plate, allowed to grow overnight, and then loaded into the 96-pin WoundMaker device (Essen BioScience, Ann Arbor, MI). The medium was aspirated from each well, and wells were washed twice with PBS to prevent settling and reattachment of dislodged cells. Next, serum-free medium with or without a dose response of periostin supplemented with or without OC-20 Ab was added, and the plate was placed inside an incubator imaging system (IncuCyte, Essen BioScience). Wound images were automatically acquired at 3-h intervals for a total of 96 h and registered by IncuCyte from within the incubator. The data were analyzed for wound confluence calculated using a customized algorithm provided by the IncuCyte program. This program measures the density of the scratch wound at baseline and over time and subtracts the baseline background.
Reagents.
Complete medium consists of DMEM (Lonza, Walkersville, MD) with 10% fetal bovine serum (Fisher, Pittsburgh, PA), 1% penicillin-streptomycin (GIBCO/Invitrogen, Carlsbad, CA), 1% l-glutamine (Fisher), and 0.1% amphotericin B (Lonza). Serum-free medium consists of DMEM (for IncuCyte wound assay) with 1% bovine serum albumin (Sigma), 1% penicillin-streptomycin, 1% l-glutamine, and 0.1% amphotericin. The activin receptor-like kinase (ALK5) inhibitor was A83-01 (Tocris Bioscience, R & D, Minneapolis, MN).
Statistical analyses.
Statistical significance of differences in group means for murine studies and human fibroblast studies was measured by ANOVA (≥3 groups) or two-sample t-tests. The log-rank test was used for two-sample comparisons of survival. Cox proportional hazards regression models were used in univariate and multivariate models estimating the association between baseline periostin and time to clinical progression; SAS version 9.2 software (SAS, Cary, NC) was used for computations. P < 0.05 was considered significant.
RESULTS
Periostin expression is increased in IPF patients and localizes to areas of active fibrosis.
Lung tissue from IPF patients and normal controls was stained for periostin. While periostin was present in control lungs, far more periostin was found in lungs from IPF patients (Fig. 1A). Periostin localized to areas of active fibrosis, the fibroblastic foci, and subepithelial and subendothelial regions within IPF lung. Fibroblasts from IPF lungs produced ∼3.5 times the amount of periostin mRNA made by normal fibroblasts (Fig. 1B). Flow cytometry analysis verified intracellular staining with anti-periostin Abs (not shown).
Fig. 1.
Increased periostin production in patients with idiopathic pulmonary fibrosis (IPF). A: immunohistochemistry for periostin was performed on lung biopsies that had previously been pathologically classified as usual interstitial pneumonia (the pathological diagnosis for IPF). Periostin is present in large amounts and localizes to fibroblastic foci as well as subendothelial and subepithelial regions (representative of 3 normal and IPF specimens examined). Immunohistochemistry for periostin performed on normal lungs shows only a small amount of periostin. B: fibroblasts derived from lung biopsies performed on IPF patients and control patients were plated in equal numbers. RNA was collected and converted to cDNA (n ≥ 4/group). Real-time PCR was performed using periostin and GAPDH primers. **P < 0.01.
Plasma periostin levels are increased in IPF patients with clinical progression at 48 wk.
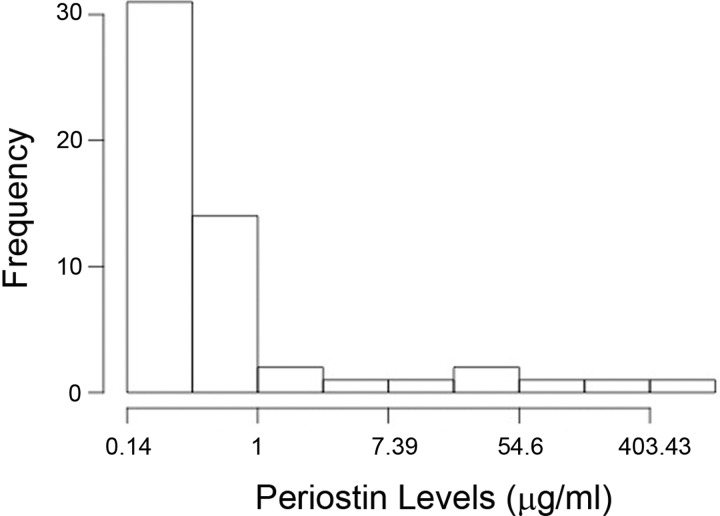
Periostin levels were measured in plasma of IPF patients at baseline and correlated with clinical progression at 48 wk. Tables 3 and 4 show the unadjusted and adjusted Cox regression models assessing the hazard of disease progression associated with periostin level in plasma of patients with IPF at baseline (both P < 0.05). In particular, the adjusted Cox model demonstrates that, for each standard deviation increase of baseline periostin (corresponding to an increase in periostin of 116.97 μg/ml), the hazard ratio for disease progression was 1.47 (95% confidence interval = 1.03–2.10, P = 0.033) when adjusted for age, sex, smoking history, baseline FVC, and DlCO %predicted. The distribution of periostin expression within the IPF patients is shown in Fig. 2.
Table 3.
Unadjusted Cox regression model
| HR | 95% CI | P Value | AIC | |
|---|---|---|---|---|
| Baseline periostin | 1.36 | 1.03–1.81 | 0.030 | 157.34 |
Hazard ratio (HR) can be interpreted as risk of progression increasing 1.4-fold for an increase of 1 SD in baseline periostin levels. CI, confidence interval; AIC, Akaike information criterion. n = 54, 21 events.
Table 4.
Adjusted Cox regression model
| HR | 95% CI | P Value | AIC | |
|---|---|---|---|---|
| Baseline periostin (SD units) | 1.47 | 1.03–2.10 | 0.033 | 154.27 |
| Age | 1.00 | 0.95–1.05 | 0.876 | |
| Male | 0.96 | 0.35–2.67 | 0.943 | |
| History of smoking* | 2.32 | 0.52–10.29 | 0.268 | |
| Baseline FVC %predicted | 1.01 | 0.98–1.04 | 0.546 | |
| Baseline DLCO %predicted | 0.99 | 0.95–1.03 | 0.673 |
SD, SD (of baseline periostin). HR can be interpreted as risk of progression increasing 1.5-fold for an increase of 1 SD in baseline periostin levels, when age, sex, history of smoking, and baseline pulmonary physiology tests were held constant. n = 50 [54–50 = 4 patients had missing values for predictors in multivariate model and were removed (3 removed from model because of missing DlCO and 1 removed because of missing FVC, FEV1, and DlCO)], 20 events.
Patient was a past or current smoker vs. never smoker.
Fig. 2.
Distribution of periostin values in IPF patients.
Patients with IPF have increased percentages of periostin-producing fibrocytes and monocytes.
Fibroblasts from IPF patients produce more periostin than control cells (Fig. 1). Since fibroblasts are resident lung cells, we hypothesized that other cell types secrete periostin in the circulation of IPF patients. We performed flow cytometry on PBMCs from IPF patients and normal controls. Monocytes (gated by CD45 expression and forward-scatter profile) were analyzed for periostin production. We found a higher percentage of monocytes that secrete periostin in IPF patients than in normal controls (Fig. 3A). This difference was not seen in lymphocyte- or granulocyte-sized cells (not shown). Additionally, periostin mRNA was expressed by fibrocytes cultured from the peripheral blood of IPF patients, but not controls (Fig. 3B). In this smaller subgroup, we did not see differences in periostin-producing monocyte or fibrocyte populations in progressive vs. stable IPF patients at 48 wk. However, as noted in our animal studies (see below), periostin production by hematopoietic cells can influence fibrotic progression.
Fig. 3.
Fibrocytes and monocytes are sources of periostin in circulation. Peripheral blood samples from IPF patients and normal controls were obtained, leukocytes were isolated on Ficoll-Hypaque, and cells were stained for flow cytometry analysis. A: monocytes were identified by CD45 expression and forward-scatter profiles. Percentage of periostin-expressing monocytes was significantly larger in IPF patients (n = 22) than in normal controls (n = 3). B: fibrocytes were cultured from peripheral blood of IPF patients and normal controls. Total RNA was collected and converted to cDNA. Real-time PCR was performed using periostin and GAPDH primers. While periostin was essentially undetectable in normal fibrocytes from normal controls (n = 5), periostin expression was significant in fibrocytes from 7 of 8 IPF patients.
Bleomycin induces periostin production.
Balb/c mice accumulate periostin in the lung following intratracheal administration of the fibrotic agent bleomycin (35). We determined the kinetics of periostin expression after bleomycin administration in the more susceptible C57Bl/6 strain (23). We harvested lungs at different times after bleomycin or saline administration and performed quantitative RT-PCR. We found that periostin was upregulated by 3 days after bleomycin challenge and remained elevated, although not significantly, for up to 21 days posttreatment (Fig. 4A). To determine if increased transcription of periostin after bleomycin administration was accompanied by increased protein, we performed immunofluorescence on lung sections from PBS- or bleomycin-treated mice. At day 14, we found significant increases in periostin deposition in the extracellular matrix of bleomycin-treated mice, along with the expected increase in α-SMA (a myofibroblast marker; Fig. 4C), compared with saline-treated mice (Fig. 4B).
Fig. 4.
Bleomycin-treated mice have increased periostin expression and protein production in vivo. Mice were injected intratracheally with 0.025 U of bleomycin or 50 μl of saline (controls). Lungs were harvested 3, 7, 14, and 21 days after bleomycin or saline treatment. RNA was isolated from lungs and converted to cDNA, and real-time PCR was performed using periostin and GAPDH primers. A: periostin expression in lungs increased dramatically by 3 days after bleomycin treatment but remained elevated up to 21 days later (n = 4 per group). *P < 0.05. B and C: lungs were also harvested for fluorescence microscopy at 14 days after bleomycin treatment. Images are representative of 3 sections examined per group. B: normal saline control lung with minimal periostin staining (green) and α-smooth muscle actin [α-SMA (red)] localized to myocytes around vessels only. Nuclei stained blue with 4′,6-diaminido-2-phenylindole. C: bleomycin-treated lung with increased periostin staining and increased α-SMA staining in interstitium.
Periostin promotes bleomycin-induced fibrosis.
To determine whether periostin was critical for development of bleomycin-induced fibrosis in this strain, we administered bleomycin or saline intratracheally to periostin−/− mice and littermates on the C57Bl/6 background. To assess collagen content, we harvested lungs at 14 days posttreatment for histology and hydroxyproline concentration. We found that periostin−/− mice treated with bleomycin intratracheally had decreased collagen content compared with wild-type littermates (Fig. 5A) and improved lung architecture (Fig. 5B). To determine the contribution of structural cell- vs. hematopoietic cell-derived periostin to fibrogenesis, chimeric mice were created by bone marrow transplantation in all combinations between wild-type and periostin−/− mice. Figure 6 demonstrates that chimeric mice without the ability to generate periostin from structural cells (B6→KO) or hematopoietic cells (KO→B6) were protected compared with the wild-type controls (B6→B6) and not significantly different from the KO→KO mice.
Fig. 5.
Periostin-deficient (periostin−/−) mice are protected from bleomycin-induced fibrosis after 14 days. Wild-type (WT) littermates and periostin−/− mice were injected intratracheally with 0.025 U of bleomycin or 50 μl of saline. At 14 days, lungs were harvested for hydroxyproline assay and histology. A: hydroxyproline levels in wild-type mice treated with bleomycin are increased, but periostin−/− mice are protected from increased collagen production (n ≥ 4/group representative of 2 experiments). *P < 0.05, **P < 0.01. B: histology showing WT mice treated with saline or bleomycin and periostin−/− mice treated with bleomycin. H&E, hematoxylin-eosin. Images are representative sections from 3 mice/group.
Fig. 6.
Chimeric mouse studies demonstrate that hematopoietic and structural sources of periostin promote fibrosis. Chimeric mice were created by transplantation of C57Bl/6 marrow into C57Bl/6 recipients (B6→B6) or periostin−/− recipients (B6→KO). Complementary chimeras were created by transplantation of KO→B6 or KO→KO. Chimeric mice were then treated with saline (n = 3/group) or bleomycin (BLM; n = 5/group), and hydroxyproline analyses were done at day 21. There was no statistically significant difference between any of the groups of saline-treated mice. Sal, data for all groups treated with saline. As expected, B6→B6 chimeras developed a significant increase in lung collagen content over saline-treated mice. Collagen contents in B6→KO and KO→B6 chimeras were similar to those in protected KO→KO mice. *P < 0.05, **P < 0.01, ***P < 0.001.
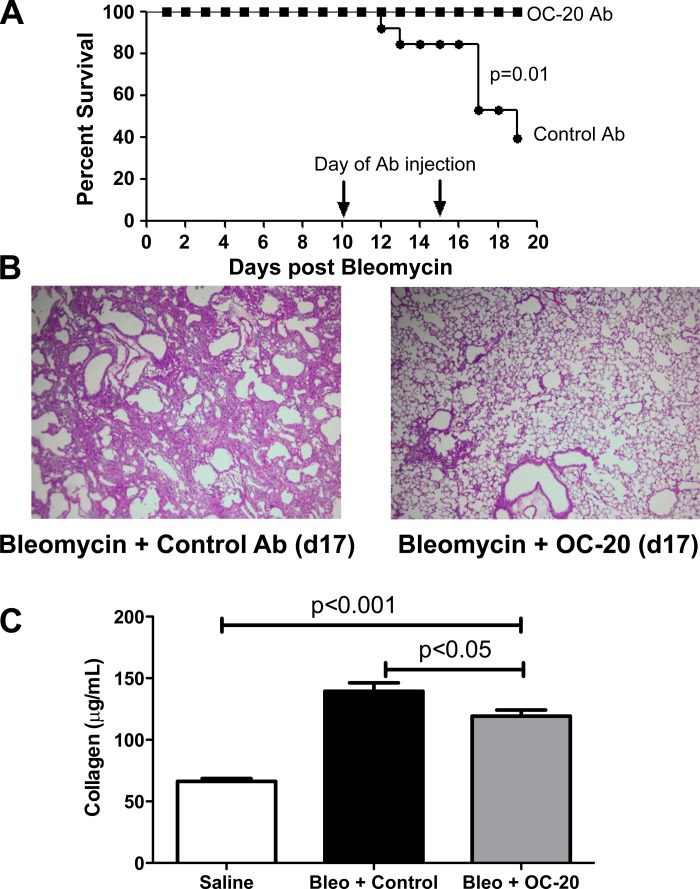
Periostin blockade after day 10 improves survival and fibrosis.
After bleomycin injury, development of fibrosis can be described in three phases: acute lung injury (days 1–5), inflammation (days 7–10), and fibroproliferation and extracellular matrix deposition (days 10–28) (23). Uchida et al. (35) suggested that periostin promotes fibrosis via regulation of chemokine production and recruitment of inflammatory cells by 7 days after bleomycin treatment. To determine the effects of periostin blockade during the fibroproliferative phase on survival, we injected mice with a higher dose of bleomycin (0.05 U) on day 0. On days 10 and 15, mice were injected intraperitoneally with a control Ab or OC-20, a MAb that blocks periostin's interaction with cellular integrins (27). Periostin blockade during the fibroproliferative phase of fibrosis improved survival through day 19 (Fig. 7A). Hematoxylin-eosin staining demonstrated preservation of lung architecture at day 17 in the OC-20-treated mice compared with control Ab-treated animals following high-dose bleomycin (Fig. 7B). To determine whether OC-20 treatment could limit collagen deposition, we chose to use the 0.025-U dose of bleomycin, which permits long-term survival of the mice, and determined whether OC-20 given in a therapeutic regimen at 10 and 15 days after 0.025 U of bleomycin would protect from fibrosis at day 21. Figure 7C demonstrates that therapeutic dosing with OC-20 protects from lung fibrosis.
Fig. 7.
Late neutralization of periostin function improves survival and fibrosis. Wild-type mice were injected with 0.05 U of bleomycin on day 0. On days 10 and 15, mice were injected intraperitoneally with 200 μg of OC-20 Ab or 200 μg of irrelevant Ab control. A: survival was monitored over a 19-day period (n = 10 mice/group). B: lungs were collected for histology, representative of 2 experiments. C: mice were injected with 0.025 U of bleomycin (Bleo) prior to treatment with OC-20 or control Ab as described above, and lungs were harvested for hydroxyproline analysis on day 21 (n = 8–11/group combined from 2 separate experiments).
Periostin promotes secretion of extracellular matrix from mesenchymal cells.
To further define periostin's role in fibrosis, we investigated its effect on lung mesenchymal cells. Mesenchymal cells were isolated from lungs of C57Bl/6 mice and treated with escalating concentrations of periostin for 24 h in serum-free medium. RNA was isolated, and qRT-PCR was performed. Fibroblasts treated with periostin upregulated collagen I expression in a dose-dependent manner (Fig. 8A).
Fig. 8.
Recombinant periostin increases collagen expression from mesenchymal cells, and transforming growth factor (TGF)-β increases periostin production from mesenchymal cells in vitro. A: lung mesenchymal cells from wild-type mice were isolated and plated at 4 × 105 cells/well in 6-well plates. Each well was then treated with recombinant mouse periostin at 0, 100, 250, or 500 ng/ml in serum-free medium. After 24 h, RNA was isolated and converted to cDNA. Real-time RT-PCR was performed with collagen and β-actin primers (n = 3/group, representative of 2 experiments). B: lung mesenchymal cells from wild-type mice were isolated and plated at 4 × 105 cells/well. One set of wells was left untreated as a control group in serum-free medium, and the other set was treated with 2 ng/ml TGF-β. After 24 h, supernatant was collected from the wells, and periostin ELISA was performed (n = 4/group representative of 2 experiments). *P < 0.05, **P < 0.01.
TGF-β induces periostin secretion from mesenchymal cells.
TGF-β is overexpressed in IPF patients and plays a critical role in development of pulmonary fibrosis by upregulating collagen (5, 32, 39). To determine whether TGF-β upregulates periostin expression, we treated murine lung mesenchymal cells from wild-type mice with 2 ng/ml TGF-β for 24 h and used ELISA to compare periostin production with that in untreated cells. Treatment of wild-type mesenchymal cells with TGF-β nearly tripled periostin production (Fig. 8B).
Periostin affects PAI-1.
TGF-β promotes production of PAI-1, a protease inhibitor consistently found in IPF patients that also increases the severity of fibrosis in several different animal models (3, 11, 28, 36). The ability of PAI-1 to inhibit extracellular matrix degradation, inhibit PGE2 synthesis, and bind vitronectin has been suggested as a plausible mechanism for the effects of PAI-1 to worsen fibrosis (2, 4, 8). To investigate the effect of periostin on PAI-1 production by mesenchymal cells, we treated mesenchymal cells isolated from wild-type mice with 0 or 100 ng/ml periostin for 24 h and found an increase in total and active PAI-1 in periostin-treated mesenchymal cells (Fig. 9A).
Fig. 9.
Periostin induces plasminogen activator inhibitor (PAI)-1 expression in vitro by mesenchymal cells and epithelial cells. A: mesenchymal cells from wild-type mice were isolated and plated at 4 × 105 cells/well. One set of wells was left untreated as a control group in serum-free medium, and the other set was treated with 100 ng/ml recombinant mouse periostin. After 24 h, supernatant was collected from the wells, and total PAI-1 and active PAI-1 ELISAs were run (n = 5/group). B: PAIL cells (mink lung epithelial cells with a PAI-1 promoter driving luciferase) were plated at 4 × 105 cells/well. One set of wells remained untreated in serum-free medium as a negative control, and another set of wells was treated with 2 ng/ml TGF-β as a positive control. The remaining sets of wells were treated with 100 ng/ml recombinant mouse periostin or 1 μg/ml periostin. After 24 h, cells were harvested, and luminescence was measured using the luciferase reporter assay as a measure of PAI-1 transcription (n = 3 wells/group representative of 2 experiments). **P < 0.01, ***P < 0.001.
We also investigated periostin's effect on PAI-1 transcription in epithelial cells. PAIL cells are mink lung epithelial cells stably transfected with a PAI-1 promoter driving luciferase. PAIL cells were treated with 100 ng/ml or 1 μg/ml periostin or 2 ng/ml TGF-β (positive control) or left untreated. After 24 h, PAI-1 transcriptional activity was assessed by luciferase assay. Addition of periostin to PAIL cells increased PAI-1 transcription as measured by luminescence in a dose-dependent manner (Fig. 9B). To test if periostin's induction of PAI-1 was TGF-β-dependent, we repeated this assay using 500 ng/ml periostin in the presence or absence of 20 μM A83-01, an ALK5 inhibitor that blocks TGF-β signaling, or DMSO control. Periostin + vehicle gave 103 ± 5 units of luminescence, whereas periostin in the presence of A83-01 produced 0.5 ± 0.01 unit (n = 3, P = 0.002).
Periostin promotes mesenchymal cell proliferation and wound closure.
Given the ability of periostin to promote cell proliferation and migration in tumorigenesis (33), we investigated its effect on lung mesenchymal cell proliferation. We cultured mesenchymal cells from wild-type mice in serum-free medium containing varying doses of periostin for 48 h. Proliferation was assessed by [3H]thymidine incorporation during the final 16 h. Periostin stimulated a dose-dependent increase in lung mesenchymal cell proliferation (Fig. 10A).
Fig. 10.
Periostin promotes mesenchymal cell proliferation and wound closure in murine cells. A: mesenchymal cells from wild-type mice were isolated and plated at 5 × 103 cells/well in 96-well plates in serum-free medium (SFM) in the presence of a dose response of recombinant periostin (0, 50, 100, 500, and 1,000 ng/ml). Cells were allowed to grow for 48 h, and [3H]thymidine was added during the final 16 h (n = 12 wells/group representative of 3 experiments). Similar results were seen in mesenchymal cells from Balb/c mice (not shown). ***P < 0.001. B: mesenchymal cells were plated on 96-well plates at 1 × 105 cells/well and allowed to adhere overnight in complete medium. On the following day, a scratch was introduced on the bottom of each well with the Essen BioScience WoundMaker, and wells were washed to remove nonadhered cells and changed to serum-free medium with a dose response containing 0, 25, 50, 100, and 200 ng/ml recombinant periostin (n = 12 wells/condition). Plates were loaded into the IncuCyte incubator. Wells were photographed every 3 h for a total of 96 h, and relative wound density was calculated at each time point. C: for ease of visualization, only 0 and 200 ng/ml doses of periostin (POSTN) are graphed as means ± SE at each time point. Data are representative of 3 experiments.
We used a kinetic imaging program to analyze the rate of closure of a scratch wound in confluent monolayers of wild-type murine mesenchymal cells cultured with periostin in serum-free medium. Periostin stimulated closure and increased wound density in a dose-dependent manner (Fig. 10B). Figure 10C shows wound density for untreated cells and cells treated with 200 ng/ml periostin. Periostin significantly increased wound density over the 96-h period.
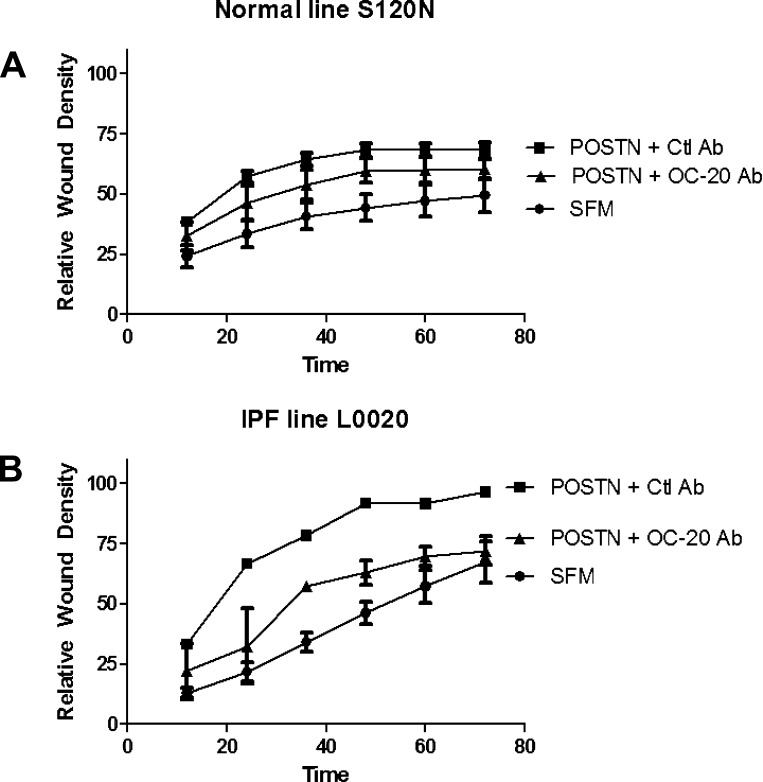
The effects of periostin on wound closure were verified in normal and IPF human mesenchymal cells as well. Figure 11 provides a representative example for each cell type. Periostin increased the closure rate for IPF and control lines, and OC-20 Ab was able to block a portion of that increase in both cell types.
Fig. 11.
Periostin promotes wound closure in human fibroblasts that is mitigated by treatment with OC-20. Fibroblasts were obtained from normal and IPF patients and tested as described in Fig. 10 legend for ability to close a scratch wound in the presence of serum-free medium, 400 ng/ml recombinant human periostin + 2 μg/ml control Ab, or 400 ng/ml periostin + 2 μg/ml OC-20. A: normal fibroblasts representative of 2 tested. B: IPF fibroblasts representative of 3 tested. IPF fibroblasts closed the wound at baseline much faster than did control cells, but this was only true for 2 of 3 IPF lines tested. Periostin stimulated wound closure of all lines, and this was partially blocked by OC-20 in all lines.
DISCUSSION
IPF likely results from dysregulated repair of unknown alveolar injury, leading to fibrocyte recruitment, fibroblast accumulation, myofibroblast differentiation, overproduction of extracellular matrix proteins, and, ultimately, destruction of lung architecture. Our results demonstrate that periostin is highly upregulated in fibroblasts from IPF patients, and immunohistochemistry shows increased localization to areas of active fibrosis in IPF lungs. These findings are consistent with the work of Okamoto and colleagues (26), who also showed elevated periostin in the serum of IPF patients and suggested its value as a potential biomarker. Our results agree with these observations, as we demonstrate the utility of periostin measurements at baseline in predicting IPF disease progression within 48 wk, when adjusted for age, sex, smoking history, FVC %predicted, and DlCO %predicted. These results suggest that measurement of baseline periostin may prognosticate disease course and allow stratification of patients for future clinical trials. Furthermore, we provide insight into potential cellular sources of periostin, and murine modeling confirms the pathogenic potential of periostin during the fibroproliferative phase of the disease.
IPF disease course is heterogeneous, with some patients experiencing slowly progressive disease over years and others experiencing rapid progression within months or an acute exacerbation with a fall in lung function (21). There is no accepted way to predict IPF disease course. We and others have demonstrated the value of longitudinal change in pulmonary physiology in predicting IPF mortality (6, 9, 13, 14, 18). We have also demonstrated that some IPF patients experience acute deteriorations and mortality that are independent of change in pulmonary physiology (7, 22). In COMET, a progressor was defined as experiencing a combined end point of death, transplant, acute exacerbation, or a 10% change in FVC or a 15% change in DlCO 48 wk later. The time frame was chosen to facilitate clinical decision making and for efficiency in designing future clinical trials.
The source of plasma periostin was not obvious, since periostin has previously been shown to be produced by resident lung cells (31, 34). Monocytes and fibrocytes isolated from peripheral blood of IPF patients express this matricellular protein and likely contribute to the pool of circulating periostin in IPF patients. Our chimeric mouse studies suggest that hematopoietic-derived periostin, as well as periostin derived from structural cells, contributes similarly to fibrogenesis. However, the fact that there was no difference in the percentage of monocytes and fibrocytes expressing periostin from rapid progressors vs. nonprogressors may indicate that lung-specific production (most likely from fibroblasts or epithelial or endothelial cells) may be the trigger for rapid progression and that periostin may be leaking from the lungs in the setting of increased injury in these patients. Another possibility is that the total amount of periostin produced by the monocytes and fibrocytes in rapid progressors is increased compared with nonprogressors, something that we did not formally test.
To further study the role of periostin in lung fibrosis, we examined effects of bleomycin in periostin−/− mice. Periostin−/− mice on a C57Bl/6 background were protected from bleomycin-induced fibrosis compared with littermate controls, corroborating the earlier studies of Uchida et al. (35) with mice on a Balb/c background. In wild-type mice, periostin mRNA increased dramatically during the inflammatory phase of fibrosis (up to day 7) but remained elevated even until day 21. Furthermore, periostin protein was clearly evident in the lungs at day 14 (Fig. 3C). This time course is consistent with a role for periostin in the postinflammatory fibroproliferative phase of bleomycin-induced fibrosis. To test this, we administered OC-20 to mice after bleomycin injury, during the fibroproliferative phase of the disease. This Ab neutralizes periostin interactions with cellular integrins (27). OC-20 given 10 days after injury decreased bleomycin-associated mortality and fibrosis, suggesting that periostin plays important roles early and late in fibrosis. Because IPF patients are typically identified after they have established scarring of the lung, the possibility that periostin regulates the fibroproliferative phase implies that this matricellular protein could represent a viable therapeutic target for late-stage fibrosis, as well as a biomarker for progressive disease.
The mechanism(s) by which periostin promotes fibrosis 10 days after bleomycin treatment is likely via mesenchymal cell effects. Our data show that recombinant periostin increases collagen I expression in lung mesenchymal cells in a dose-dependent manner. Periostin may cross-link collagen and stiffen the matrix created by fibroblasts, thereby activating the cells for further extracellular matrix production (10). Periostin may also directly activate mesenchymal cells or induce TGF-β expression from these cells. Our results in the epithelial PAIL cells suggest that periostin effects are mediated via TGF-β in this cell type.
We have shown that TGF-β promotes periostin production and that many effects of periostin appear to be similar to effects of TGF-β. Periostin treatment increases PAI-1 expression, an activity known to be TGF-β-dependent. Additionally, previous work by Sidhu et al. (31) in human bronchial epithelial cells showed that periostin promotes TGF-β1, TGF-β2, and TGF-β3 production and that periostin-induced collagen expression and epithelial-mesenchymal transition are TGF-β-dependent. Therefore, it appears that the effects of TGF-β and periostin are interdependent. On the other hand, results of genetic knockout studies show that deletion of these two genes has very different outcomes. TGF-β-deficient mice die shortly after birth due to overwhelming autoimmune inflammation (20, 30), whereas periostin−/− mice show only modest periodontal bone and ligament defects (29). Thus these results suggest that periostin may be a much safer therapeutic target than TGF-β.
Another difference between periostin and TGF-β appears to be their effects on mesenchymal cell proliferation. Mesenchymal cells from our wild-type mice proliferate in response to periostin, and the addition of periostin increases the rate of wound closure, which is dependent on cell proliferation and/or migration in murine and human fibroblasts. Typically, TGF-β is not proliferative for fibroblasts (12). Chemotaxis assays are needed to fully test periostin effects on migration in the absence of proliferation. However, taken together, these results suggest that heterogeneously expressed periostin production in patients may contribute to proliferation, activation, and/or migration of fibroblasts to fibroblastic foci. This is consistent with the observation of profound periostin staining within fibrotic foci of IPF lungs (Fig. 1A).
In summary, we have shown that periostin is produced by structural and inflammatory cells and is upregulated during fibrotic responses in mice and humans. In addition, plasma periostin may be a useful biomarker to predict early progression of disease. Blockade of periostin function during the fibroproliferative phase of bleomycin-induced lung fibrosis improves mortality, and periostin−/− mice are protected from experimental fibrosis. Our results demonstrate direct effects of periostin, including upregulation of extracellular matrix, increased proliferation, and stimulation of wound closure, on lung mesenchymal cells. Periostin and TGF-β appear to function as a positive-feedback loop, with both factors inducing the other, but periostin may be a more amenable target for therapeutic intervention in IPF patients. Future studies will be aimed at determining the efficacy of targeting this molecule as therapy or as a biomarker in humans. In addition, a better understanding is needed to determine how TGF-β and periostin signaling pathways may diverge.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-091745 and HL-087846 (to B. B. Moore), RC2 HL-101740 (to F. J. Martinez), and HL-079339 (to M. B. Hershenson).
DISCLOSURES
K. R. Flaherty serves as a consultant for Boerhingher Ingelheim, GlaxoSmithKlein (GSK), Gilead, Fibrogen, and Medimmune and has been paid as a speaker for GSK, Pfizer, and Boehringer Ingelheim; he obtains royalties from UpToDate. B. B. Moore has a grant from Centecor. F. J. Martinez has participated in Advisory Boards in chronic obstructive pulmonary disease (COPD) and/or IPF development for Actelion, Almirall, American Institutes for Research, Astra Zeneca, Bayer, BoomComm, Cardiomems, Center for Health Care Education, Elan, Forest, GSK, HCRC, IntraMed, Ikaria, Janssens, JK Associates, MedImmune, Merck, Merion, Novartis, Nycomed/Takeda, Pearl, Pfizer, Schering, Sudler and Hennessey, and UBC; he has been a member of Steering Committee for COPD or IPF studies sponsored by Actelion, Centocor, Gilead, GSK, Forest, MPex, and Nycomed and has participated in US Food and Drug Administration mock panels for Boehringer Ingelheim and Forest. The University of Michigan received funds from Boehringer Ingelheim for a COPD study. F. J. Martinez has served on Speaker's Bureaus or in continuing medical education activities sponsored by ACCP, American Lung Association, Almirall, Astra Zeneca, William Beaumont Hospital, Boehringer Ingelheim, Center for Healthcare Education, CME Incite, ePocrates, Forest, France Foundation, GSK, Lovelace, MedEd, NACE, Nycomed/Takeda, Potomac, Prescott, Sanofi Aventis, St. Luke's Hospital, University of Virginia, and UpToDate; he has received royalties from Associates in Medical Marketing, Informa, and Castle Connolly.
AUTHOR CONTRIBUTIONS
P.K.N., P.D.B., J.K.B., A.P.P., C.M.B., C.A.W., C.D.F., E.S.W., and T.H.S. performed the experiments; P.K.N., P.D.B., T.H.S., N.T., K.R.F., M.B.H., S.M., F.J.M., and B.B.M. analyzed the data; P.K.N., P.D.B., J.K.B., A.P.P., B.C., K.R.F., M.B.H., S.M., F.J.M., and B.B.M. interpreted the results of the experiments; P.K.N., P.D.B., N.T., and B.B.M. prepared the figures; P.K.N. drafted the manuscript; P.K.N., P.D.B., J.K.B., A.P.P., C.M.B., C.A.W., C.D.F., E.S.W., T.H.S., N.T., B.C., P.O., K.R.F., M.B.H., S.M., F.J.M., and B.B.M. approved the final version of the manuscript; P.D.B., J.K.B., E.S.W., N.T., M.B.H., S.M., F.J.M., and B.B.M. edited and revised the manuscript; B.C., P.O., M.B.H., F.J.M., and B.B.M. are responsible for conception and design of the research.
REFERENCES
- 1. American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement American Thoracic Society (ATS) and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bauman KA, Wettlaufer SH, Okunishi K, Vannella KM, Stoolman JS, Huang SK, Courey AJ, White ES, Hogaboam CM, Simon RH, Toews GB, Sisson TH, Moore BB, Peters-Golden M. The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice. J Clin Invest 120: 1950–1960, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chapman HA, Allen CL, Stone OL. Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis 133: 437–443, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Chuang-Tsai S, Sisson TH, Hattori N, Tsai CG, Subbotina NM, Hanson KE, Simon RH. Reduction in fibrotic tissue formation in mice genetically deficient in plasminogen activator inhibitor-1. Am J Pathol 163: 445–452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor-β1 and -β3 mRNA transcripts in normal and fibrotic human lung. Thorax 56: 549–556, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 168: 538–542, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Muller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176: 636–643, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 118: 2313–2321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, Szwarcberg J, Thomeer M, Valeyre D, King TE., Jr Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 184: 459–466, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, Krieg T. Mechanical tension and integrin-α2β1 regulate fibroblast functions. J Invest Dermatol Symp Proc 11: 66–72, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 97: 232–237, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fine A, Goldstein RH. The effect of transforming growth factor-β on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem 262: 3897–3902, 1987 [PubMed] [Google Scholar]
- 13. Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, Martinez FJ. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 174: 803–809, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, Travis WD, Flint A, Toews GB, Lynch JP, 3rd, Martinez FJ. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 168: 543–548, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor-β. J Bone Miner Res 14: 1239–1249, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hubbard LL, Ballinger MN, Wilke CA, Moore BB. Comparison of conditioning regimens for alveolar macrophage reconstitution and innate immune function post bone marrow transplant. Exp Lung Res 34: 263–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal 3: 125–133, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jegal Y, Kim DS, Shim TS, Lim CM, Do Lee S, Koh Y, Kim WS, Kim WD, Lee JS, Travis WD, Kitaichi M, Colby TV. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 171: 639–644, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, Wilke CA, Chrisman CJ, Moore BB. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 172: 4068–4076, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni AB, Karlsson S. Transforming growth factor-β1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143: 3–9, 1993 [PMC free article] [PubMed] [Google Scholar]
- 21. Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183: 431–440, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, Brown KK. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 142: 963–967, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Moore BB, Paine R, 3rd, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368–4377, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, Ohta S, Kato S, Izuhara K, Aizawa H. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 37: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Orecchia P, Conte R, Balza E, Castellani P, Borsi L, Zardi L, Mingari MC, Carnemolla B. Identification of a novel cell binding site of periostin involved in tumour growth. Eur J Cancer 47: 2221–2229, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Osterholzer JJ, Christensen PJ, Lama V, Horowitz JC, Hattori N, Subbotina N, Cunningham A, Lin Y, Murdock BJ, Morey RE, Olszewski MA, Lawrence DA, Simon RH, Sisson TH. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol 228: 170–180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25: 11131–11144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 107: 14170–14175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100: 768–776, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis 26: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118: 98–104, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 46: 677–686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vayalil PK, Iles KE, Choi J, Yi AK, Postlethwait EM, Liu RM. Glutathione suppresses TGF-β-induced PAI-1 expression by inhibiting p38 and JNK MAPK and the binding of AP-1, SP-1, and Smad to the PAI-1 promoter. Am J Physiol Lung Cell Mol Physiol 293: L1281–L1292, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin-α4β1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 168: 436–442, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan W, Shao R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J Biol Chem 281: 19700–19708, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 282: L585–L593, 2002 [DOI] [PubMed] [Google Scholar]