Abstract
Here, we investigated whether pharmacological PPARγ activation modulates key early events in brown adipose tissue (BAT) recruitment induced by acute cold exposure with the aim of unraveling the interrelationships between sympathetic and PPARγ signaling. Sprague-Dawley rats treated or not with the PPARγ ligand rosiglitazone (15 mg·kg−1·day−1, 7 days) were kept at 23°C or exposed to cold (5°C) for 24 h and evaluated for BAT gene expression, sympathetic activity, thyroid status, and adrenergic signaling. Rosiglitazone did not affect the reduction in body weight gain and the increase in feed efficiency, V̇o2, and BAT sympathetic activity induced by 24-h cold exposure. Rosiglitazone strongly attenuated the increase in serum total and free T4 and T3 levels and BAT iodothyronine deiodinase type 2 (D2) and PGC-1α mRNA levels and potentiated the reduction in BAT thyroid hormone receptor (THR) β mRNA levels induced by cold. Administration of T3 to rosiglitazone-treated rats exacerbated the cold-induced increase in energy expenditure but did not restore a proper activation of D2 and PGC-1α, nor further increased uncoupling protein 1 expression. Regarding adrenergic signaling, rosiglitazone did not affect the changes in BAT cAMP content and PKA activity induced by cold. Rosiglitazone alone or in combination with cold increased CREB binding to DNA, but it markedly reduced the expression of one of its major coactivators, CREB binding protein. In conclusion, pharmacological PPARγ activation impairs short-term cold elicitation of BAT adrenergic and thyroid signaling, which may result in abnormal tissue recruitment and thermogenic activity.
Keywords: brown adipose tissue recruitment, sympathetic activity, thyroid status, adrenergic signaling, thiazolidinediones
a possible utilization of brown adipose tissue (BAT) nonshivering thermogenesis as an alternative to treat obesity has gained renewed interest with the recent confirmation that a substantial number of adult humans possesses active BAT (6, 21, 31, 32, 37). Although current knowledge about the impact of BAT on human energy balance is still scarce, it is plausible to envisage that the efficacy of BAT-targeted therapies will depend upon the recruitment (increase in tissue mass and thermogenic capacity) of BAT in humans and the development of strategies to safely switch thermogenesis on and off.
In addition to chronic canonical sympathetic activation (5), BAT recruitment can be induced in rodents by administration of synthetic, specific ligands of peroxisome proliferator-activated receptor γ (PPARγ). This nuclear receptor is highly expressed in BAT, where it acts as a master transcriptional regulator of brown adipocyte differentiation required for tissue development, function, and survival (1, 8, 15–17, 22). Similarly to chronic sympathetic activation, treatment of rodents with the PPARγ ligand rosiglitazone is associated with a marked increase in BAT mass, content of the thermogenic uncoupling protein 1 (UCP1), and key enzymes involved in fatty acid oxidation and lipolysis (13, 29, 35). In a standard thermal environment, PPARγ-induced upregulation of BAT lipolytic, oxidative, and thermogenic machineries does not, however, translate into higher thermogenesis and energy expenditure in vivo due to a rosiglitazone-induced down-regulation of BAT sympathetic activity and thyroid status, the major neurohormonal regulators of BAT function (14).
Further evaluation of BAT recruitment indicates that despite a similar increase in tissue mass and content of some thermogenic proteins, chronic sympathetic and PPARγ activations induce somewhat divergent morphological and metabolic phenotypes in BAT. Indeed, whereas sympathetic stimulation results in an increase in the number of multilocular brown adipocytes with enhanced rates of glucose uptake and fatty acid oxidation, PPARγ activation is associated with an increase in unilocular brown adipocytes with reduced glucose uptake and unaltered oxidative rates (11).
It has been previously shown that, even in the face of a reduced thyroid status, the high thermogenic, oxidative, and lipolytic potential induced by PPARγ activation (i.e., mRNA and protein content and concomitant increase in related processes when assessed in vitro) is actuated at the functional level in vivo by pharmacological β3-adrenergic stimulation (4, 26, 30). Because thyroid status was not evaluated in these studies, it was not possible to establish whether this actuation of BAT function induced by β3-adrenergic activation in PPARγ ligand-treated animals was associated with a reestablishment of normal thyroid function.
One interesting aspect of adrenergic elicitation of BAT thermogenic potential in PPARγ-treated animals was the absence of effect of the β3-adrenergic CL316,243 agonist on the expression of the adrenergically regulated genes UCP1, lipoprotein lipase (LPL), and PPARγ coactivator 1α (PGC-1α) (26), which otherwise are direct targets of β3 stimulation. Supporting these findings, 24 h of cold exposure did not additively increase BAT UCP1 expression further than that induced by rosiglitazone alone in rats (10). The absence of additive effects of simultaneous PPARγ and adrenergic activation on BAT gene expression was quite unexpected in the face of both the reduced sympathetic drive to BAT found upon PPARγ ligand treatment of rats living in a standard thermal environment (14), as well as our recent findings indicating that the optimal upregulation of UCP1 by PPARγ activation depends upon the presence of intact BAT sympathetic innervation and maintenance of minimal basal sympathetic tone (10).
Thus, in the present study, in an attempt to further characterize the inter-relationships between sympathetic and PPARγ signaling in the regulation of key early events in BAT recruitment elicited by cold exposure, control, and rosiglitazone-treated rats maintained at 23°C or exposed to cold (5°C) for 24 h were evaluated for determinants of energy balance, serum metabolites, lipids and hormone levels, thyroid status, sympathetic activity, BAT gene expression profile of thermogenic proteins, and BAT intracellular adrenergic signaling. Our main findings indicate a major failure of cold, in the presence of pharmacological PPARγ activation, to upregulate the thyroid status and expression of some specific adrenergic genes in BAT, which, in turn, might affect tissue recruitment and thermogenic function.
MATERIALS AND METHODS
Animals and treatment.
Animal care and handling were performed in accordance with the Canadian and Brazilian Guides for the Care and Use of Laboratory Animals. All experimental procedures received prior approval of the Institute of Biomedical Sciences and Laval University animal care committees. Male Sprague-Dawley rats (Charles River Laboratories, St. Constant, Canada or Institute of Biomedical Sciences Animal Facility) were individually housed in stainless-steel cages in a room kept at 23 ± 1°C with a light-dark cycle of 12:12-h (lights on at 0800). After a 4-day adaptation period, rats were matched by weight and divided into control and rosiglitazone-treated groups that were fed a nonpurified powdered rodent diet (Charles River Rodent Diet no. 5075; digestible energy content: 12.9 kJ/g) alone (control), or supplemented with the PPARγ agonist rosiglitazone (Avandia) at a dose of 15 mg·kg−1·day−1 for 7 days. This dose was chosen on the basis of preliminary studies that showed its effectiveness to increase BAT thermogenic proteins in a short period of treatment (e.g., 7 days). Ground rosiglitazone was mixed with the powdered chow diet, and the desired dose was achieved by adjusting the amount of drug to the average food consumption and body weight of rats every other day. At the seventh day of treatment, half of control and rosiglitazone-treated rats were transferred to a cold room (5°C) for 24 h with a similar light-dark cycle and free access to water and food containing or not containing rosiglitazone. At the end of the 24-h cold exposure, rats were killed by decapitation, and trunk blood and tissues were collected. A similar protocol was performed in which an extra group of rats treated with rosiglitazone received an intraperitoneal injection of triiodothyronine (T3) at a dose of 10 μg/100 g body wt before exposure to cold. This dose of T3 was previously shown to induce maximal saturation of thyroid hormone receptors in BAT (2).
Energy expenditure.
O2 consumption was determined in an open circuit system with an O2 analyzer (Applied Electrochemistry, S-3A1). Energy expenditure measurements were carried out during 24 h after a 24-h adaptation period to the new cages. Data are presented as milliliters per oxygen per minute.
Norepineprine turnover.
Norepinephrine turnover rate (NETO), a reliable index of sympathetic activity in a given tissue, was estimated from the decline in tissue NE content after inhibition of catecholamine synthesis with dl-α-methyl-tyrosine ester (α-MT; Sigma, St. Louis, MO), as previously described (14). Rats kept at 23°C or exposed to cold (5°C) for 20 h were killed by anesthetic (ketamine/xylazine) overdose before or 4 h after intraperitoneal injection of α-MT (350 mg/kg body weight). Interscapular BAT was rapidly removed, weighed, frozen in liquid nitrogen, and stored at −80°C for later determination of NE content. Rates of NETO were calculated as the product of the fractional turnover rate (k) and the endogenous NE content at time 0, as previously described (3). Fractional turnover rate, k, was calculated by the formula: k = (log [NE]0 − log [NE]4)/(0.434 × 4), where [NE]0 and [NE]4 are the NE content at times 0 and 4 h, respectively.
Tissue NE content.
Tissue NE content was measured as previously described (20). Briefly, tissues were homogenized in 0.2 N perchloric acid, 1 mM of EDTA, and 1% sodium metabisulfite containing dihydroxybenzylamide as an internal standard, and then centrifuged. The supernatant destined for catecholamine quantification was extracted with alumina. Catecholamines and dihydroxybenzylamide (internal standard) were eluted from alumina with the above homogenization solution and assayed by HPLC.
In situ hybridization for cocaine- and amphetamine-related transcript.
Hypothalamic mRNA levels of cocaine- and amphetamine-related transcript (CART) were measured by in situ hybridization, essentially as previously described (14). Briefly, hypothalamic sections were mounted onto poly-l-lysine-coated slides, dehydrated in ethanol, fixed in paraformaldehyde, digested with proteinase K (10 μg/ml), acetylated with 0.25% acetic anhydride, and dehydrated in ethanol gradient. Sections were incubated overnight with antisense 35S-labeled cRNA probe (107 cpm/ml) for CART at 60°C. Slides were rinsed with SSC, digested with RNAse-A, washed in descending concentrations of SSC, and dehydrated in ethanol gradient. Slides were defatted in toluene, dipped in NTB2 nuclear emulsion (Eastman Kodak), and exposed for 7 days before being developed. Slides were examined by darkfield microscopy using an Olympus BX51 microscope (Olympus America, Melville, NY). Images were acquired with an Evolution QEi camera and analyzed with ImagePro plus v5.0.1.11 (Media Cybernetics, Silver Spring, MD). The system was calibrated for each set of analyses to prevent saturation of the integrated signal. Mean pixel densities were obtained by taking measurements from both hemispheres of one to four brain sections and subtracting background readings taken from areas immediately surrounding the region analyzed.
RNA isolation and quantification.
RNA was isolated from BAT and hypothalamus using QIAzol and the RNeasy lipid tissue kit (Qiagen). For cDNA synthesis, expand reverse transcriptase (Invitrogen) was used following manufacturer's instructions, and cDNA was diluted in DNase-free water (1:25) before quantification by real-time PCR. mRNA transcript levels were measured in duplicate samples using a Rotor Gene 3000 system (Montreal Biotech, Montreal, QC, Canada). The primers used for the PCR reactions are presented in Table 1. Chemical detection of the PCR products was achieved with SYBR Green I (Molecular Probes). At the end of each run, melt curve analyses were performed, and a few samples representative of each experimental group were run on agarose gel to ensure the specificity of the amplification. Results are expressed as the ratio between the expression of the target gene and the housekeeping gene ARBP/36B4 (NM_022402), which was selected because no significant variation in its expression was observed between treatments.
Table 1.
Pairs of primers used for PCR
| Gene | Accession # | 5′ Primer (5′-3′) | 3′ Primer (5′-3′) |
|---|---|---|---|
| 36B4 | NM_022402 | TAAAGACTGGAGACAAGGTG | GTGTAGTCAGTCTCCACAGA |
| CBP | NM_133381 | CTGCTGGAAGAGGAAGGGGA | GGCACAGTGGTGACTGAAGTATTC |
| CIDEA | XM_214551 | ACACCCTGCTCGTCCTTTCC | GGTGGCTTTGACATTGAGACAG |
| D2 | NM_031720 | ATGGGACTCCTCAGCGTAGA | GCACAGGCAAAGTCAAGAAG |
| GyK | NM_024381 | CCTGTCCATTGAAATGTGTCATCC | GCCATGAAGCCATGACAATTAGTG |
| PGC-1α | NM_031347 | TCCTGTTACTATTATGAATCAAGCC | AAACCATAGCTGTCTCCATCATCC |
| PRDM16 | NM_027504 | GCAGACCCTGTGGGAGTCCTGAAA | GCTCCCCTGTGTGTGTCCTCAGAT |
| SHP | NM_057133 | CCTCTCTTCCTGCTTGGGTT | ACACAATGCCCAGTGAGCCT |
| THRα1 | NM_001017960 | AAGTGGCTCTGCTGCAGGCT | TTGTCCCTTCTCTCCAAGCTG |
| THRβ | NM_012672 | GAATGGGAGCTCATCAAGACAGTCA | GGACATGATCTCCATGCAGCA |
| TRH | NM_013046 | GGTCAGGAGACCCTGGTGAA | TCTTGGCCAGTGCTGAAGGG |
| UCP1 | NM_012682 | TGGTGAGTTCGACAACTTCC | GTGGGCTGCCCAATGAATAC |
| UCP3 | NM_013167 | GAAGCACTTTCGACAAGGCC | TGCAGGTGAAGCTGGTCAGG |
Western blot analyses.
BAT was homogenized in lysis buffer (50 mM HEPES, pH 7.4, 40 mM NaCl, 2 mM EDTA, 10 mM β-glycerophosphate, 10 mM Na4P2O7, 1% Triton 100×, 1% sodium deoxycholate 50 mM NaF, 1.5 mM Na3VO4, 0.1% SDS, and a cocktail of protease inhibitors), subjected to SDS-PAGE, transferred to nitrocellulose membranes, blocked for 1 h and incubated overnight at 4°C with primary antibodies (Cell Signaling Technologies). After washing, membranes were incubated with immunoglobulin G conjugated to horseradish peroxidase and washed again. The immunoreactive bands were detected by the enhanced chemiluminescence method. Densitometric analysis was performed with ImageQuant TL software (GE Healthcare).
CREB binding.
Nuclear extracts of BAT of rats treated or not with rosiglitazone maintained either at 23°C or exposed to cold for 24 h were analyzed for CREB binding with a CREB (Phospho-Ser133) transcription factor assay (Cayman Chemicals). Briefly, 50 μg of protein from whole BAT homogenates prepared for Western blot analysis were used for the CREB DNA binding activity assay, according to the manufacturer's instructions (CREB, Phospho-Ser133). The specificity of the method was verified by the use of a wild-type consensus oligonucleotide as a competitor for CREB binding, which decreased the signal dramatically, and data were expressed as a percentage of positive control.
Serum determinations.
Plasma glucose concentration was measured by the glucose oxidase method with the YSI 2300 STAT plus glucose analyzer. Plasma insulin, leptin, adiponectin (Linco Research, St. Charles, MO), total and free T3 and T4 (Coat-A-Count, DPC), and thyroid-stimulating hormone (TSH) (Biotrak rat TSH; Amersham Biosciences) were determined by radioimmunoassay. Plasma triacylglycerol and nonesterified fatty acid levels were measured by enzymatic methods (Roche Diagnostics and Wako Chemicals, respectively).
Statistical analysis.
Results are expressed as means ± SE. Multifactorial ANOVA followed by the Newman-Keuls procedure were used to compare the effects of cold exposure and thyroid hormone treatment in control and rosiglitazone-treated rats. P < 0.05 was taken as the threshold of significance.
RESULTS
As shown in Table 2, rosiglitazone did not affect the reductions in body weight gain and feed efficiency or the increases in food intake and V̇o2 induced by 24 h of cold exposure. Rosiglitazone significantly increased BAT and inguinal adipose tissue masses, an effect that was attenuated by cold exposure in the former but not the latter. Retroperitoneal white tissue mass was not affected by any of the treatments. Regarding serum parameters, rosiglitazone potentiated the reduction in insulin and triacylglycerol levels and attenuated the increases in nonesterified fatty acid induced by cold exposure. Furthermore, rosiglitazone alone significantly reduced glycemia and increased serum adiponectin levels, such effects being completely blocked and attenuated by cold exposure, respectively. Finally, rosiglitazone, in combination with cold, significantly reduced serum leptin levels in comparison to other treatments.
Table 2.
Effect of a 24-h cold exposure on body weight gain, food intake, feed efficiency, mass of white and brown adipose tissues, and serum concentrations of insulin, metabolites, adiponectin, and leptin in rats treated or not with rosiglitazone
| Control, 23°C | Control, 5°C | RSG, 23°C | RSG, 5°C | |
|---|---|---|---|---|
| Body weight gain, g | 10.03 ± 0.8a | 2.59 ± 1.5b | 8.45 ± 0.9a | −0.47 ± 1.37b |
| Food intake, g | 28.7 ± 0.5a | 37.2 ± 1.3b | 31.8 ± 0.4a | 38.1 ± 0.9b |
| Feed efficiency, %e | 34.8 ± 2.6a | 3.68 ± 3.7b | 29.8 ± 3a | −3.1 ± 3b |
| V̇o2, ml·min−1·kg body wt0.75−1 | 12.2 ± 0.3a | 16.9 ± 0.6b | 13.0 ± 0.2a | 17.7 ± 0.5b |
| BAT, mg | 399 ± 17a | 421 ± 20a | 871 ± 36b | 770 ± 40c |
| Inguinal WAT, g | 1.85 ± 0.09a | 1.91 ± 0.1a | 2.7 ± 0.13b | 2.5 ± 0.07b |
| Retroperitoneal WAT, g | 1.27 ± 0.09a | 1.23 ± 0.07a | 1.39 ± 0.74a | 1.37 ± 0.12a |
| Insulin, pM | 234.8 ± 22.7a | 134.8 ± 13b | 115.6 ± 9b | 75.5 ± 13c |
| Glucose, mM | 10.1 ± 1.4a | 12.4 ± 1.1a | 6.1 ± 0.5b | 9.7 ± 1.04a |
| NEFA, Eq/l | 96 ± 8a | 168 ± 10b | 52 ± 10c | 97 ± 10a |
| TAG, mM | 2.34 ± 0.2a | 1.61 ± 0.3b | 0.95 ± 0.12c | 0.49 ± 0.05d |
| Adiponectin, μg/ml | 3.85 ± 0.4a | 4.38 ± 0.32a | 16.77 ± 0.78b | 14.39 ± 0.80c |
| Leptin, ng/ml | 4.56 ± 0.44a | 3.98 ± 0.36a | 5.24 ± 0.48a | 2.22 ± 0.25b |
Data are expressed as means ± SE of 6-12 rats. WAT, white adipose tissue; BAT, brown adipose tissue; NEFA, nonesterified fatty acid; TAG, triacylglycerol; RSG, rosiglitazone.
Means not sharing a common superscript letter are significantly different from each other, P < 0.05.
Calculated as gram body wt gain/100 g food ingested.
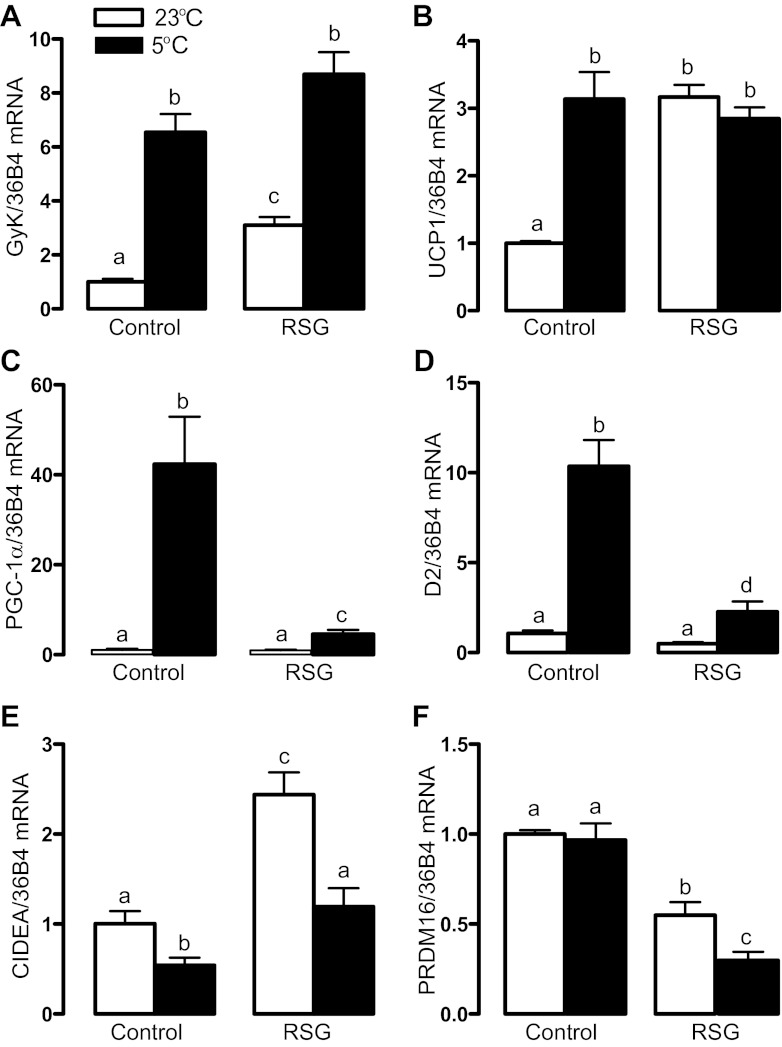
As depicted in Fig. 1, A and B, pharmacological PPARγ activation did not affect the cold-induced increase in BAT glycerokinase (GyK), an enzyme that synthesizes glycerol 3-phosphate for triacylglycerol synthesis, and UCP1 mRNA levels. In contrast to those genes, rosiglitazone markedly attenuated the increase in iodothyronine deiodinase type 2 (D2), which converts T4 into biologically active T3, that of PGC-1α, an important regulator of BAT uncoupling protein 1 (UCP1) transcription, and the reduction in cell death-inducing DFF45-like effector A (CIDEA, an attenuator of UCP1 activity) mRNA levels induced by cold exposure (Fig. 1, C–E). Finally, rosiglitazone reduced mRNA levels of the positive regulatory domain containing 16 (PRDM16), a zinc-finger protein involved in brown adipogenesis that binds PPARγ and increases its transcriptional activity (25), an effect that was potentiated by cold exposure (Fig. 1F).
Fig. 1.
Brown adipose tissue (BAT) gene expression profile in rats treated or not with rosiglitazone (RSG) and exposed or not to cold for 24 h. a,b,c,dMeans not sharing a common superscript letter are significantly different from each other, P < 0.05; n = 12.
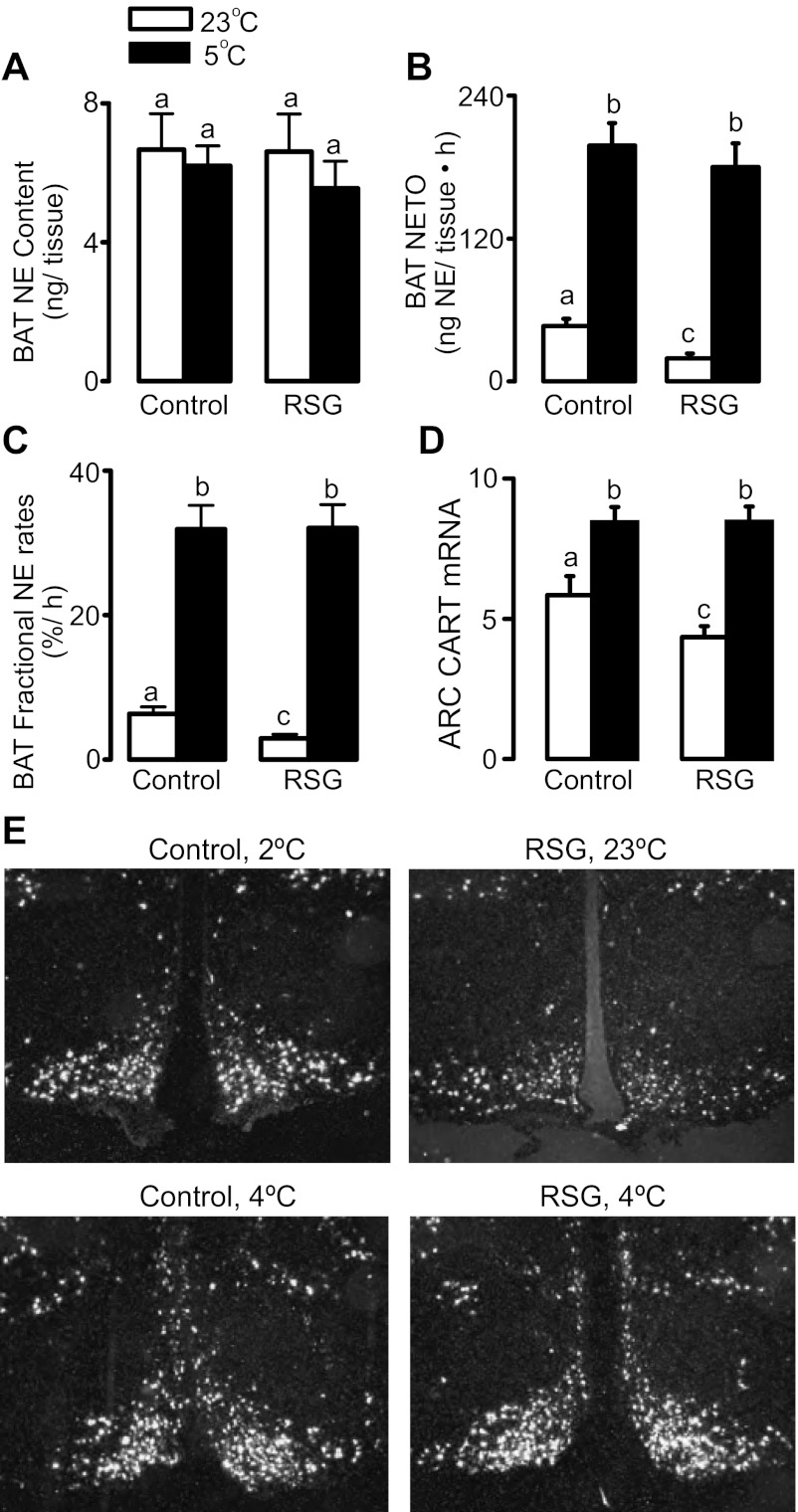
The inability of cold exposure to maximally activate BAT expression of the adrenergically regulated genes PGC-1α and D2 associated with PPARγ activation prompted us to investigate a possible effect of rosiglitazone modulating cold activation of BAT sympathetic activity. There were no changes in BAT norepinephrine (NE) content, an index of the degree of sympathetic innervation rather than activity, by any of the treatments (Fig. 2A). As depicted in Fig. 2, B and C, rosiglitazone did not affect the increase in BAT NETO or BAT k (Fig. 2, B and C) and arcuate nucleus CART mRNA levels (Fig. 2, D and E), a neuropeptide that has been previously demonstrated to be anatomically linked to and modulate BAT sympathetic nerves (9, 18).
Fig. 2.
BAT norepinephrine (NE) content (A), NE turnover rates (NETO; B), NE fractional turnover rate (C), and arcuate hypothalamic nucleus mRNA levels of CART (D and E) in rats treated or not with RSG and exposed or not to cold for 24 h. Optical density of CART mRNA hybridization signal is presented together with representative pictures of the in situ hybridization. a,b,cMeans not sharing a common superscript letter are significantly different from each other, P < 0.05; n = 6.
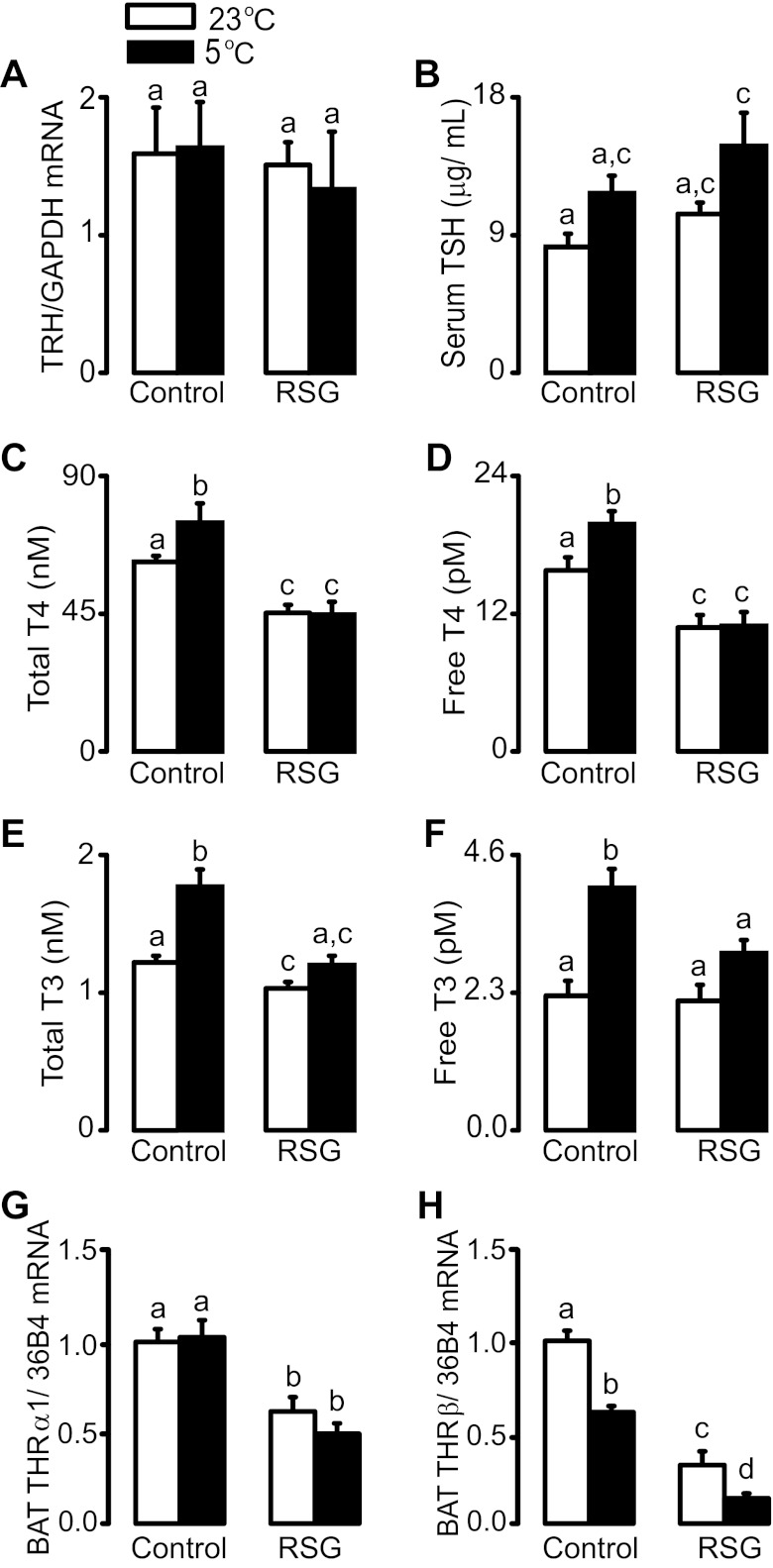
Because thyroid hormones exert an important role in the amplification of intracellular adrenergic signaling in BAT, we next investigated whether rosiglitazone affects the activation of the hypothalamic-pituitary-thyroid axis associated with cold exposure. As depicted in Fig. 3A, neither intervention altered hypothalamic mRNA levels of thyrotropin-releasing hormone (TRH). Serum TSH levels were not significantly changed by rosiglitazone, but the combination of both cold and rosiglitazone induced a significant increase in TSH levels in comparison to control rats maintained at 23°C (Fig. 3B). On the other hand, rosiglitazone completely blocked the increases in serum total and free T4 and T3 and potentiated the decrease in THRβ mRNA levels induced by cold exposure (Fig. 3, C–F, and H). Finally, rosiglitazone significantly reduced BAT THRα1 mRNA levels, an effect that was not modulated by cold exposure (Fig. 3G).
Fig. 3.
Hypothalamic TRH mRNA levels (A), serum thyroid-stimulating hormone (TSH; B), total (C) and free (D) T4, total (E) and free (F) T3, and brown adipose tissue mRNA levels of THRα1 and THRβ (G and H) in rats treated or not with RSG and exposed or not to cold for 24 h. a,b,c,dMeans not sharing a common superscript letter are significantly different from each other, P < 0.05; n = 12.
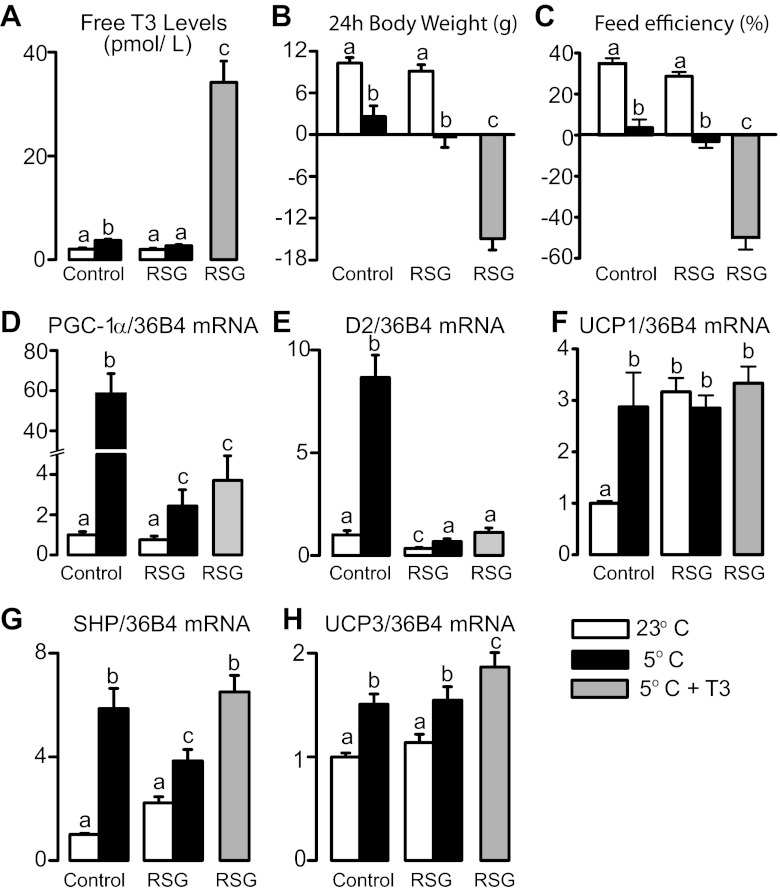
The marked attenuation of thyroid axis activation by cold exposure induced by rosiglitazone prompted us to investigate more specifically whether their relative hypothyroid state was involved in the inability of cold to stimulate the expression of BAT PGC-1α and D2 (D2-generated T3 potentiates adrenergic signaling toward D2 expression in a feed-forward fashion). To this end, rats treated with rosiglitazone received, prior to cold exposure, an intraperitoneal injection of T3. As depicted in Fig. 4, T3 administration markedly increased serum levels of free T3 (Fig. 4A), exacerbated the weight loss (Fig. 4B) and the reduction in feed efficiency (Fig. 4C), while it amplified the cold-induced increase in the mRNA levels of the T3 target genes small heterodimer protein [SHP, a negative regulator of PGC-1α expression and energy expenditure in brown adipocytes (33)] and UCP3 in rats treated with rosiglitazone (Fig. 4, G and H). Despite these effects, hormone therapy was not able to restore in rosiglitazone-treated rats the ability of cold exposure to increase BAT PGC-1α and D2 mRNA levels (Fig. 4, D and E). Surprisingly, the strong increase in UCP1 mRNA levels brought by rosiglitazone alone was maximal and suggestive of a ceiling effect associated with PPARγ agonism, as neither cold exposure alone nor in combination with T3, further increased UCP1 in rosiglitazone-treated rats (Fig. 4F).
Fig. 4.
Serum free T3 levels (A), body weight gain (B), feed efficiency (C), and BAT gene expression (D–H) in control and rosiglitazone-treated rats exposed or not to cold for 24 h in association or not with an intraperitoneal injection of T3 (10 μg/100 g body weight). a,b,cMeans not sharing a common superscript letter are significantly different from each other, P < 0.05; n = 12.
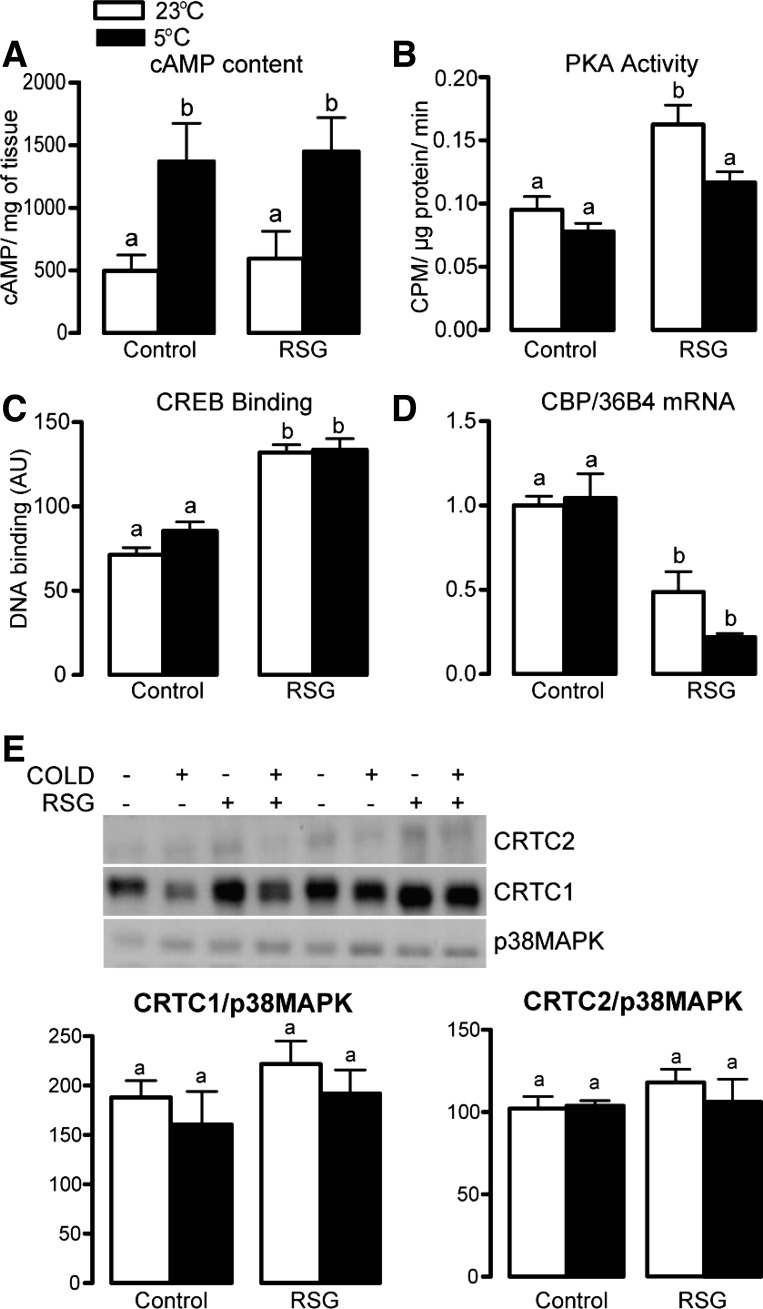
In an attempt to elucidate the mechanisms underlying the failure of the sympathetic nervous system to increase the expression of the adrenergically regulated genes PGC-1α and D2 in BAT of rosiglitazone-treated rats, we investigated the status of key steps of the intracellular adrenergic signaling pathway in this tissue, including cAMP content, PKA, CREB DNA binding, and the expression and content of some coregulators of its transcriptional activity. As depicted in Fig. 5A, rosiglitazone did not affect the increase in BAT cAMP content induced by cold exposure. Rosiglitazone alone significantly increased BAT PKA activity and CREB binding activity to its consensus DNA binding sequence and reduced CREB coactivator histone acetyltransferase CREB binding protein (CBP) mRNA levels (Fig. 5, B–D). Among these effects, only the increase in PKA activity was attenuated by cold exposure. Finally, neither rosiglitazone nor cold nor their combination affected BAT protein content of the CREB-regulated transcription coactivators (CRTC) 1 and 2 (Fig. 5E) and mRNA levels of other coactivators involved in the regulation of PGC-1α transcription (SIRT3, CHOP10, and ATF2; data not shown).
Fig. 5.
cAMP content (A), PKA activity (B), cyclic AMP-responsive element binding protein (CREB) binding (C), CREB binding protein mRNA levels (D), and CREB-regulated transcription coactivator 1 and 2 protein content in BAT of control and rosiglitazone-treated rats exposed or not to cold for 24 h (E). a,bMeans not sharing a common superscript letter are significantly different from each other, P < 0.05; n = 4–12.
DISCUSSION
Our main findings indicate that despite having higher BAT mass and UCP1 content, rosiglitazone-treated rats displayed upon an acute cold exposure similar body weight gain, feed efficiency, V̇o2, and BAT sympathetic activity than control, untreated rats. Despite those similarities, rosiglitazone markedly impaired cold's ability to stimulate the thyroid axis and to properly increase BAT levels of the thermogenic genes D2 and PGC-1α, an effect associated with a marked reduction in the mRNA levels of THRβ and the CREB coactivator CBP.
Rosiglitazone-treated rats were challenged here with a short period of cold exposure (24 h) and evaluated for very early events involved in BAT recruitment aiming to unveil the interactions between PPARγ and adrenergic signaling and a possible potentiation of this process through tissue “priming” by PPARγ activation. The markedly increased BAT mass (10, 11) and UCP1 content induced by rosiglitazone did not lead to higher energy expenditure upon cold exposure, as estimated by body weight gain, feed efficiency, and V̇o2. These findings are in contrast with the additive increase in energy expenditure found upon concomitant treatment of mice with a PPARγ ligand and the β3 adrenergic receptor agonist CL-316243 (26). Such discrepancy between pharmacological vs. physiological elicitation of thermogenesis could be due to the duration of adrenergic activation (24 h of cold vs. 2 wk of CL-316243), species differences (rats vs. mice), and the preferential recruitment of different thermogenic processes associated with pharmacological β3-adrenergic activation (nonshivering thermogenesis only) and acute cold exposure (shivering thermogenesis mainly) (5).
In contrast to energy expenditure, however, rosiglitazone markedly reduced cold ability to properly upregulate BAT mRNA levels of the adrenergically regulated genes PGC-1α (23) and D2 (28) in BAT. Interestingly, this effect seems to be specific to some, but not all adrenergically regulated genes, as the expression of GyK, a gene positively regulated by the sympathetic nervous system and PPARγ in BAT (11, 12), was, as expected, upregulated by cold in rosiglitazone-treated rats.
On the basis of our previous findings of a reduced BAT sympathetic tone and arcuate nucleus CART levels in rosiglitazone-treated rats (14), we tested the hypothesis that the impairment in cold-induced upregulation of D2 and PGC-1α by rosiglitazone was due to an impairment in the cold-induced activation of BAT sympathetic outflow due to a central action of rosiglitazone on arcuate CART levels. This hypothesis proved false, as rosiglitazone did not impair cold activation of BAT sympathetic drive and upregulation of arcuate CART levels. Of note, the close association between arcuate CART levels and BAT sympathetic activity upon rosiglitazone and cold strongly supports a major involvement of CART in BAT regulation. Accordingly, arcuate CART-expressing neurons are anatomically linked to BAT sympathetic nerves (9), and their positive modulation increases BAT sympathetic tone (18).
The absence of impact of rosiglitazone on cold-mediated induction of BAT sympathetic tone led us to test whether the failure of cold to induce D2 and PGC-1α was due to a defect in intracellular adrenergic signaling. Because adrenergic signaling in brown adipocytes is strongly modulated by T3 (7, 27), an extensive analysis of thyroid status was performed. Cold exposure not only failed to increase serum total and free T4 and T3, but further reduced the already low BAT mRNA levels of THRβ in rosiglitazone-treated rats. The mechanisms by which rosiglitazone abolishes the upregulation of serum thyroid hormone by cold are unknown, but the absence of change in TRH and TSH indicates an action of the ligand on thyroid function. Furthermore, rosiglitazone impaired cold-induced upregulation of both BAT D2, a local generator of T3 from T4 needed for the amplification of BAT adrenergic signaling (7), and PGC-1α, an important coactivator of THRβ activation of UCP1 transcription (23). These findings suggest an involvement of the thyroid status in the failure of cold to properly activate the expression of PGC-1α and D2 in rosiglitazone-treated rats.
To test the above hypothesis, we acutely administered T3 to rosiglitazone-treated rats prior to cold exposure at a dose shown to induce maximal saturation of thyroid hormone receptors in BAT (2) in an attempt to bypass the inability of cold to stimulate BAT D2 and thus local production of T3. In spite of the marked increase in energy expenditure (body weight loss and reduced feed efficiency) and the expected increase in the expression of T3 target genes (UCP3 and SHP) upon cold exposure, T3 did not restore the ability of cold to increase BAT D2 and PGC-1α, which may be due to a defect in T3 activation of BAT THRβ. Accordingly, expression of THRβ, which is involved not only in T3 regulation of UCP1 and UCP3 (24) but also in the adrenergic activation of D2 expression (19), was markedly reduced in rosiglitazone-treated rats exposed to cold. A similar modulation may also apply to PGC-1α, whose expression in the liver is upregulated by T3 (34); however, whether such regulation occurs in BAT and involves THRβ remains to be established.
To gain further insight into the mechanisms underlying the inability of cold to increase BAT D2 and PGC-1α in rosiglitazone-treated rats, we evaluated several aspects of BAT adrenergic intracellular signaling. The absence of effect of rosiglitazone on the increase in BAT cAMP induced by cold and the absence of major negative changes in maximal PKA activity exclude their involvement in the failure of cold to properly stimulate BAT D2 and PGC-1α under PPARγ activation. Rates of CREB DNA binding, on the other hand, were significantly increased by rosiglitazone in rats at 23°C or 5°C, indicating an exacerbation of CREB phosphorylation, translocation to the nucleus, and interaction with its consensus DNA-binding sequence. Although such an increase in CREB binding seems counterintuitive to the failure of cold to upregulate BAT D2 and PGC-1α in rosiglitazone-treated rats, it does, in fact, indicate that PPARγ activation is perhaps affecting steps downstream of CREB binding to DNA. The binding of transcription factors, such as CREB onto a promoter region, can result in stimulation or inhibition of gene transcription, depending on the recruitment and interaction with coactivators or corepressors, respectively. Evaluation of BAT content of several CREB coactivators, including CBP, CRTC-1, and CRTC-2, revealed a major effect of rosiglitazone decreasing mRNA levels of CBP, a protein lysine acetyltransferase that binds to phosphorylated CREB and acetylates histones, loosening up chromatin structure for transcription initiation. Such reduction in CBP is compatible with reduced CREB efficiency; however, further experiments are required to establish whether reduced CBP is involved in the failure of cold to stimulate D2 and PGC-1α in BAT of rosiglitazone-treated rats.
In conclusion, pharmacological PPARγ activation is associated with an abnormal response of BAT thyroid and adrenergic signaling despite normal sympathetic activation induced by cold exposure and/or correction of relative hypothyroidism by T3 treatment. Because of the importance of thyroid and adrenergic signaling for the proper activation of BAT thermogenesis, it is very likely that the cold induction of a functional BAT thermogenic response may be partially impaired by rosiglitazone, a possibility that remains to be experimentally tested through the measurement of BAT temperature. Identification of the mechanisms underlying BAT abnormal response in rosiglitazone-treated rats to cold could help define better strategies to recruit BAT without affecting its thermogenic efficiency.
Perspectives and Significance
The identification of BAT in adult humans has opened up the opportunity to develop strategies that take advantage of its unique thermogenic ability to treat obesity. For this, strategies not only to safely recruit BAT (i.e., increase functional mass and thermogenic capacity) in humans but also to turn thermogenesis on and off, will have to be developed. PPARγ activation has recently been recognized as a pharmacological alternative to adrenergic stimulation for BAT recruitment (22). Here, however, we report strong evidence indicating that BAT recruited by pharmacological PPARγ activation has an impaired thermogenic response upon an acute cold challenge. Whether such reduced BAT thermogenic ability persists following long-term cold exposure and whether it is dependent upon sustained continuation of rosiglitazone administration need to be investigated. Pharmacological PPARγ activation increases BAT mass by stimulating hyperplasia and lipid-associated hypertrophy. The fact that BAT recruitment is associated with a reduction in important markers of brown adipocytes, such as PRDM16 and D2, along with an increase in the number of unilocular adipocytes, despite increased UCP1 levels, raises the possibility that PPARγ may be either inducing white adipocyte features in brown adipocytes or acting on the recently characterized “brite/beige” adipocytes (36). Characterization of the mechanisms underlying these phenotypes and the development of novel selective PPARγ modulators may help optimize PPARγ-mediated BAT recruitment at the functional level.
GRANTS
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC) to Y. Deshaies. W. T. Festuccia is a recipient of a Young Scientist Fellowship and Grant from the São Paulo Research Foundation (FAPESP 2009/15354-7 and 2010/10909-8). P.-G. Blanchard was the recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship-Doctoral Award from CIHR.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: W.T.F., D.R., and Y.D. conception and design of research; W.T.F., P.-G.B., T.B.O., J.M., and V.A.P. performed experiments; W.T.F., P.-G.B., T.B.O., J.M., and V.A.P. analyzed data; W.T.F., D.R., and Y.D. interpreted results of experiments; W.T.F. prepared figures; W.T.F. drafted manuscript; W.T.F., P.-G.B., T.B.O., J.M., V.A.P., D.R., and Y.D. edited and revised manuscript; W.T.F., P.-G.B., T.B.O., J.M., V.A.P., D.R., and Y.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are very grateful for the invaluable professional assistance of Yves Gélinas.
REFERENCES
- 1. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4: 585–595, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 79: 295–300, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brodie BB, Costa E, Dlabac A, Neff NH, Smookler HH. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther 154: 493–498, 1966 [PubMed] [Google Scholar]
- 4. Burkey BF, Dong M, Gagen K, Eckhardt M, Dragonas N, Chen W, Grosenstein P, Argentieri G, de Souza CJ. Effects of pioglitazone on promoting energy storage, not expenditure, in brown adipose tissue of obese fa/fa Zucker rats: comparison to CL 316,243. Metabolism 49: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Celi FS. Brown adipose tissue—when it pays to be inefficient. N Engl J Med 360: 1553–1556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duan SZ, Ivashchenko CY, Whitesall SE, D'Alecy LG, Duquaine DC, Brosius FC, 3rd, Gonzalez FJ, Vinson C, Pierre MA, Milstone DS, Mortensen RM. Hypotension, lipodystrophy, and insulin resistance in generalized PPARγ-deficient mice rescued from embryonic lethality. J Clin Invest 117: 812–822, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21: 1375–1385, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Festuccia WT, Blanchard PG, Richard D, Deshaies Y. Basal adrenergic tone is required for maximal stimulation of rat brown adipose tissue UCP1 expression by chronic PPAR-γ activation. Am J Physiol Regul Integr Comp Physiol 299: R159–R167, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, Richard D, Deshaies Y. The PPARγ agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am J Physiol Regul Integr Comp Physiol 296: R1327–R1335, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Festuccia WT, Guerra-Sa R, Kawashita NH, Garofalo MA, Evangelista EA, Rodrigues V, Kettelhut IC, Migliorini RH. Expression of glycerokinase in brown adipose tissue is stimulated by the sympathetic nervous system. Am J Physiol Regul Integr Comp Physiol 284: R1536–R1541, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Festuccia WT, Laplante M, Berthiaume M, Gelinas Y, Deshaies Y. PPARγ agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 49: 2427–2436, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Festuccia WT, Oztezcan S, Laplante M, Berthiaume M, Michel C, Dohgu S, Denis RG, Brito MN, Brito NA, Miller DS, Banks WA, Bartness TJ, Richard D, Deshaies Y. Peroxisome proliferator-activated receptor-γ-mediated positive energy balance in the at is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 149: 2121–2130, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Gray SL, Dalla Nora E, Backlund EC, Manieri M, Virtue S, Noland RC, O'Rahilly S, Cortright RN, Cinti S, Cannon B, Vidal-Puig A. Decreased brown adipocyte recruitment and thermogenic capacity in mice with impaired peroxisome proliferator-activated receptor (P465L PPARγ) function. Endocrinology 147: 5708–5714, 2006 [DOI] [PubMed] [Google Scholar]
- 16. He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100: 15712–15717, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W, Chambon P, Metzger D. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA 101: 4543–4547, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kong WM, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, Connoley I, Ghatei M, Stephens D, Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J 17: 1688–1690, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Martinez-deMena R, Hernandez A, Obregon MJ. Triiodothyronine is required for the stimulation of type II 5'-deiodinase mRNA in rat brown adipocytes. Am J Physiol Endocrinol Metab 282: E1119–E1127, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Migliorini RH, Garofalo MA, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol Regul Integr Comp Physiol 272: R656–R661, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Petrovic N, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARγ agonist. Am J Physiol Endocrinol Metab 295: E287–E296, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Ribeiro MO, Bianco SD, Kaneshige M, Schultz JJ, Cheng SY, Bianco AC, Brent GA. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-beta isoform specific and required for adaptive thermogenesis. Endocrinology 151: 432–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sell H, Berger JP, Samson P, Castriota G, Lalonde J, Deshaies Y, Richard D. Peroxisome proliferator-activated receptor γ agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145: 3925–3934, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86: 435–464, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Silva JE, Larsen PR. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305: 712–713, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Teruel T, Hernandez R, Rial E, Martin-Hidalgo A, Lorenzo M. Rosiglitazone up-regulates lipoprotein lipase, hormone-sensitive lipase and uncoupling protein-1, and down-regulates insulin-induced fatty acid synthase gene expression in brown adipocytes of Wistar rats. Diabetologia 48: 1180–1188, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Thurlby PL, Wilson S, Arch JR. Ciglitazone is not itself thermogenic but increases the potential for thermogenesis in lean mice. Biosci Rep 7: 573–577, 1987 [DOI] [PubMed] [Google Scholar]
- 31. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD. The orphan nuclear receptor SHP regulates PGC-1α expression and energy production in brown adipocytes. Cell Metab 2: 227–238, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Weitzel JM, Radtke C, Seitz HJ. Two thyroid hormone-mediated gene expression patterns in vivo identified by cDNA expression arrays in rat. Nucleic Acids Res 29: 5148–5155, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366–376, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113–3120, 2009 [DOI] [PubMed] [Google Scholar]