Abstract
Akt is a serine/threonine kinase that plays a key role in numerous cellular functions including metabolism, growth, protein synthesis, apoptosis, and cell proliferation. The most consistent and robust effect of moderate calorie restriction (CR; ∼60% of ad libitum, AL, food consumption) on insulin signaling in rodent muscle has been enhanced insulin-induced phosphorylation of Akt (pAkt). However, there is limited knowledge regarding the mechanism for this enhancement and its consequences in predominantly slow-twitch muscle. Accordingly, in soleus muscle of 9-mo-old rats, we analyzed the effect of CR and insulin on important signaling events that are proximal to Akt activation including: pIRTyr1162/1163, pIRS1Tyr, pIRS1Ser312, IRS1-associated phosphatidylinositol 3-kinase activity, or pPTENSer380. In addition, we analyzed the effect of CR and insulin on Akt substrates that have established or putative roles in glucose metabolism, cellular growth, maintenance of muscle structure, or protein synthesis including pGSK3αSer21, pGSK3βSer9, pTSC2Ser939, pP70S6KThr412, pAS160Thr642, and pFLNcSer2213. The current study demonstrated that the CR-induced increase in pAkt in isolated soleus muscles from 9-mo-old rats can occur without concomitant enhancement of several important insulin signaling events that are proximal to Akt activation. These results suggest that the greater pAkt in the soleus muscles from CR rats was attributable to an alternative mechanism. We also observed that the effects of CR were not uniform for phosphorylation of six insulin-regulated Akt substrates in the soleus. The differential response in phosphorylation by Akt substrates likely has important implications for explaining the complex effect of CR diverse cellular functions.
Keywords: Rab-GAP glucose uptake, dietary restriction, insulin sensitivity, glucose transport, skeletal muscle
Akt (also known as protein kinase B) is a serine/threonine kinase that plays a central role in regulating a number of cellular functions including metabolism, growth, apoptosis, autophagy, angiogenesis, protein synthesis, and cell proliferation (18, 22, 38). Moderate calorie restriction (CR; ∼60% of ad libitum, AL, food consumption) has been demonstrated to cause a striking increase in the insulin-stimulated phosphorylation of Akt in the skeletal muscle of various species (21, 37, 40). In rodents, the most consistent and robust result on isolated muscles has been an elevated insulin-induced phosphorylation of Akt attributed to CR (25–27, 34). The precise molecular mechanism for enhancement of CR of insulin-stimulated Akt phosphorylation is unclear, but we did not find diet-induced differences in key upstream elements of the insulin signaling pathway in isolated rat soleus muscles incubated with a physiological insulin dose (33, 34). Because it seemed possible that CR might alter Akt activation via phosphorylation of other signaling proteins that were not evaluated in these earlier studies, our first aim was to determine in isolated soleus muscles from 9-mo-old rats the influence of CR on insulin-stimulated tyrosine phosphorylation of insulin receptor substrate 1 (IRS1), serine phosphorylation of IRS1, and serine phosphorylation of phosphatase and tensin homologue (PTEN).
In addition to elucidating the specific upstream signaling processes that account for CR's enhancement of Akt phosphorylation, it would be important to identify which of Akt's many protein substrates are influenced by CR. We previously demonstrated that CR enhanced insulin-stimulated threonine phosphorylation of Akt substrate of 160 kDa (AS160; also known as TBC1D4) in the predominantly fast-twitch epitrochlearis muscle (34). AS160 is a Rab GTPase activating protein that is a mediator of insulin's activation of glucose transport (4, 5, 31). We also recently reported that CR resulted in elevated serine phosphorylation of Filamin C (FLNc) in the epitrochlearis of 9-mo-old rats (35). FLNc, an actin-binding protein, has been identified as an Akt substrate that becomes phosphorylated in response to insulin (15, 29). The functional consequences of phosphorylation of FLNc is uncertain, but it is interesting that insulin-regulated remodeling of actin filaments has been implicated in the control of both the spatial localization of insulin signaling proteins and translocation of GLUT4 glucose transporter vesicles (43). However, the effect of CR on FLNc phosphorylation in slow-twitch skeletal muscle has not been studied. Accordingly, our second aim was to determine the influence of CR on insulin-stimulated phosphorylation of multiple Akt substrates, including AS160, FLNc, glycogen synthase kinase-α and -β (GSK-α and GSK-β), tuberous sclerosis complex-2 (TSC2), and the 70-kDa S6 protein kinase (P70S6K), in isolated soleus muscles from 9-mo-old rats.
Finally, our group has previously found that CR effects on the isolated soleus muscle (predominantly slow-twitch) are not identical to effects on predominantly fast-twitch skeletal muscles of rats (33, 34). For example, CR increased insulin-stimulated pAS160Thr642 in the predominantly fast-twitch epitrochlearis but not in the soleus from 9-mo-old rats (34). Whereas a large number of studies have characterized CR effects on predominantly fast-twitch skeletal muscles of rats (3, 7–10, 16, 17, 26, 27, 34, 35), a great deal less information is available for predominantly slow-twitch muscles of rats (33, 34). Therefore, our third aim was to measure the effects of CR on other important insulin signaling events (pIRTyr1162/1163, IRS1-associated PI3K activity, pAS160Thr642) and glucose uptake by isolated soleus muscles from 9-mo-old rats.
EXPERIMENTAL PROCEDURES
Materials.
Unless otherwise noted, all chemicals were purchased from Fisher Scientific (Hanover Park, IL) or Sigma Chemical (St. Louis, MO). Reagents and apparatus for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Tissue Protein Extraction Reagent (TPER; no. 78510), bicinchoninic acid (BCA) protein assay (no. 23225), and West Dura Extended Duration Substrate (no. 34075) were from Fisher Scientific (Rockford, IL). MILLIPLEXMAP cell signaling buffer and detection Kit (no. 48-602), MILLIPLEX phospho-MAPmates for IRS1panTyr(no. 46-627), AktThr308 (no. 46-645), and MILLIPLEX MAP Akt/mTOR Phosphoprotein Panel (no. 48-611; AktSer473, GSK3αSer21, GSKβSer9, IRTyr1162/1163, IRS1Ser312, P70S6 kinaseThr412, PTENSer380, and TSC2Ser939), and anti-sheep IgG horseradish peroxidase conjugate (no. 12-342) were all purchased from EMD Millipore (Billerica, MA). Anti-phospho AS160 Thr642 (pAS160Thr642; no. 3028-P1) was from B-Bridge International (Cupertino, CA). Anti-phospho Akt Thr308 (pAktThr308; no. 9275), anti-phospho Akt Ser473 (pAktSer473; no. 9272), and anti-rabbit IgG horseradish peroxidase conjugate (no. 7074) were from Cell Signaling Technology (Danvers, MA). Anti-Akt2 (no. AF23151) was from R&D Biosystems (Minneapolis, MN). Anti-phospho Filamin CSer2213 (pFLNcSer2213; PB-131) was from Kinasource (Dundee, Scotland, UK). 2-Deoxy-d-[3H]glucose (2-[3H]DG), [14C]mannitol, and γ-[32P]ATP were from Perkin Elmer (Boston, MA). Sepharose-A beads (no. 17-0469-01) were from GE Healthcare (Piscataway, NJ). Phosphatidylinositol was from Avanti Polar Lipids (Alabaster, AL). TLC plates (no. 4865-821) were from Whatman (Piscataway, NJ).
Animal treatment.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer 344 × Brown Norway rats, both CR rats and their AL controls were obtained at 8 mo of age from National Institute of Aging (NIA) Calorie Restricted Rodent Colony. The CR protocol was initiated at 14 wk of age in the CR group by the NIA as previously described (34). Upon arrival in Ann Arbor, rats were housed at the University of Michigan for ∼1 mo before experimentation. During this time the rats were housed individually in shoebox cages and maintained on a 12–12 h light-dark cycle (lights out at 17:00 h) in specific pathogen-free conditions and had free access to water. Rats were provided chow (AL: NIH31 chow; CR: NIH31/NIA Fortified chow) and maintained on their respective feeding protocol (AL: free access to chow; CR: ∼60–65% of AL consumption).
Muscle dissection and incubation.
Rats were euthanized when they were ∼9 mo of age. Food was removed from the cages of all rats on the morning of the experimental day between 07:00 and 08:00 h. Soleus muscle dissection was performed as previously described (34), and the resultant soleus strips were placed in vials containing the appropriate media as described below, shaken at 45 rpm, continuously gassed (95% O2-5% CO2), and heated (35°C) in a water bath. In the first incubation step, all muscles were incubated in vials containing 2 ml Krebs-Henseleit buffer (KHB) supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, 6 mM mannitol as a rinse step for 30 min. In the second incubation step, muscles were incubated in vials containing 2 ml KHB supplemented with 0.1% BSA, 2 mM sodium pyruvate, 6 mM mannitol, and either 0 nM (basal) or 1.2 nM insulin for 30 min. Muscles were either transferred to a third vial containing 2 ml of KHB-BSA solution, the same insulin concentration as the previous step with 0.1% BSA, 1 mM 2-DG, and 9 mM mannitol, or to assay for 2-DG uptake, transferred to a third vial containing 2 ml of KHB-BSA solution, the same insulin concentration as the previous step, 1 mM 2-DG (including a final specific activity of 2.25 mCi/mmol 2-[3H]DG), and 9 mM mannitol (including a final specific activity of 0.022 mCi/mmol [14C]mannitol) for 20 min. After the third incubation step, the muscles were rapidly blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80°C for later processing and analysis.
Muscle lysate preparation.
Frozen muscles were weighed, transferred to microfuge tubes, and homogenized in ice-cold lysis buffer (1 ml/muscle) using a TissueLyser II (Qiagen, Valencia, CA). The lysis buffer contained tissue protein extraction reagent (TPER) supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Homogenates were transferred to microfuge tubes, rotated for 1 h at 4°C, and then centrifuged (15,000 g) for 15 min (4°C) to remove insoluble material. Protein concentration was measured using the BCA method.
Immunoprecipitation.
Evaluation of Akt2 phosphorylation at either the Thr308or Ser473 residue was performed as has been previously described (34).
Immunoblotting.
Western blotting procedures were performed as have been previously described (34). Immunoreactive proteins were quantified by densitometry (AlphaEase FC; Alpha Innotech, San Leandro, CA). Values are expressed relative to the normalized average of the basal samples on each blot.
Multiplex analysis.
Multiplex analysis was performed by the Luminex L200 instrument (Luminex, Austin, TX), as described previously (36).
IRS-1-associated PI3K activity.
IRS1-phosphatidylinositol 3-kinase (PI3K) activity in the soleus muscle was determined as previously described (10, 34).
2-DG uptake.
The calculation of 2-[3H]DG uptake from skeletal muscle lysates was performed as has been previously described (2, 19).
Statistical analysis.
Two-way ANOVA was used to determine the main effects of insulin (0 or 1.2 nM) and diet (AL or CR) and interactions, and a Student's t-test was used to compare body masses between AL and CR groups (SigmaPlot version 11.0; Systat Software, San Jose, CA). Data are presented as means ± SE. A P value ≤0.05 for was considered statistically significant.
RESULTS
Proximal insulin signaling.
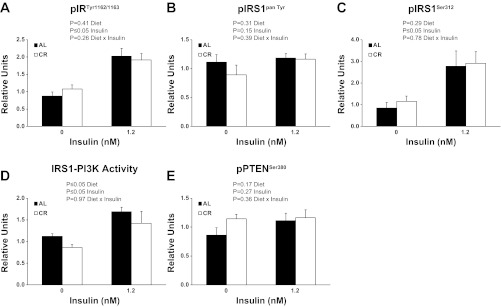
There was a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) for pIRTyr1162/1163 (Fig. 1A). There was no significant main effect of diet or insulin for pIRS1pan Tyr (Fig. 1B). There was a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) for pIRS1Ser312 (Fig. 1C). There were significant (P ≤ 0.05) main effects of diet (CR < AL) and insulin (1.2 nM > 0 nM) for IRS-1 associated PI3K activity (Fig. 1D). There was no significant main effect of diet or insulin for pPTENSer380(Fig. 1E).
Fig. 1.
Phosphorylation of insulin receptor substrate (IR)Tyr1162/1163 (A), IRS1pan Tyr (B), IRSSer312 (C), IRS1-associated phosphatidylinositol 3-kinase (PI3K) activity (D), and phosphorylation phosphatase and tension homologue (PTEN)Ser380 (E). CR, calorie-restricted group; AL, ad libitum food consumption group. Main effects of diet, insulin, and diet × insulin interactions are reported from two-way ANOVA analysis. P ≤ 0.05 is considered statistically significant. Values are means ± SE; n = 7–18 per treatment group.
Akt phosphorylation.
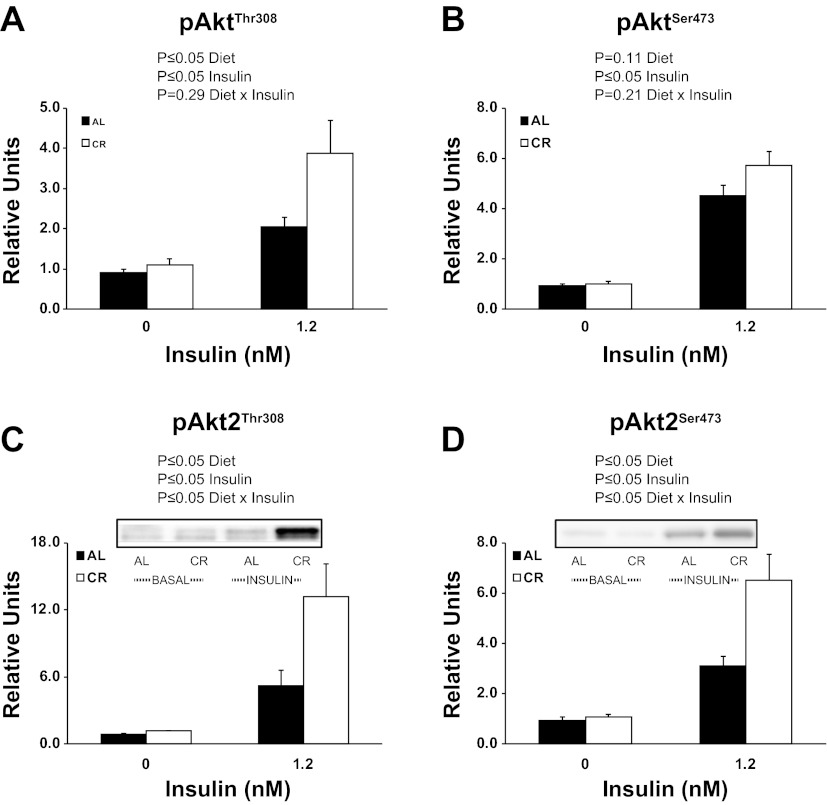
There were significant (P ≤ 0.05) main effects of diet (CR > AL) and insulin (1.2 nM > 0 nM) for pAktThr308 (Fig. 2A). There was a significant (P ≤ 0.05) main effect of insulin for pAktSer473 (Fig. 2B). There were significant (P ≤ 0.05) main effects of diet (CR > AL), insulin (1.2 nM > 0 nM), and an interaction (AL and CR groups are similar with 0 nM insulin, however, CR is much greater than AL with 1.2 mM insulin) for pAkt2Thr308 (Fig. 2C). There were significant (P ≤ 0.05) main effects of diet (CR > AL), insulin (1.2 nM > 0 nM), and an interaction (AL and CR groups are similar with 0 nM insulin, however, CR is much greater than AL with 1.2 mM insulin) for pAkt2Ser473(Fig. 2D).
Fig. 2.
Phosphorylation of AktThr308 (A), AktSer473 (B), Akt2Thr308 (C), and Akt2Ser473 (D). Main effects of diet, insulin, and diet × insulin interactions are reported from two-way ANOVA analysis. P ≤ 0.05 is considered statistically significant. Values are means ± SE; n = 7–18 per treatment group.
Akt substrate phosphorylation.
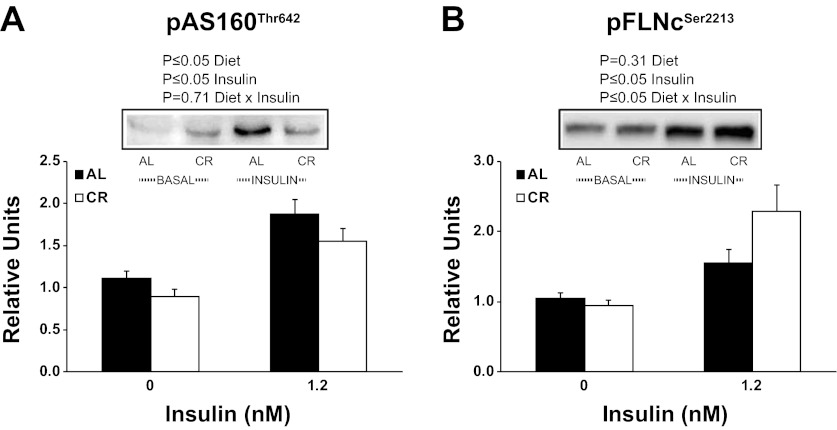
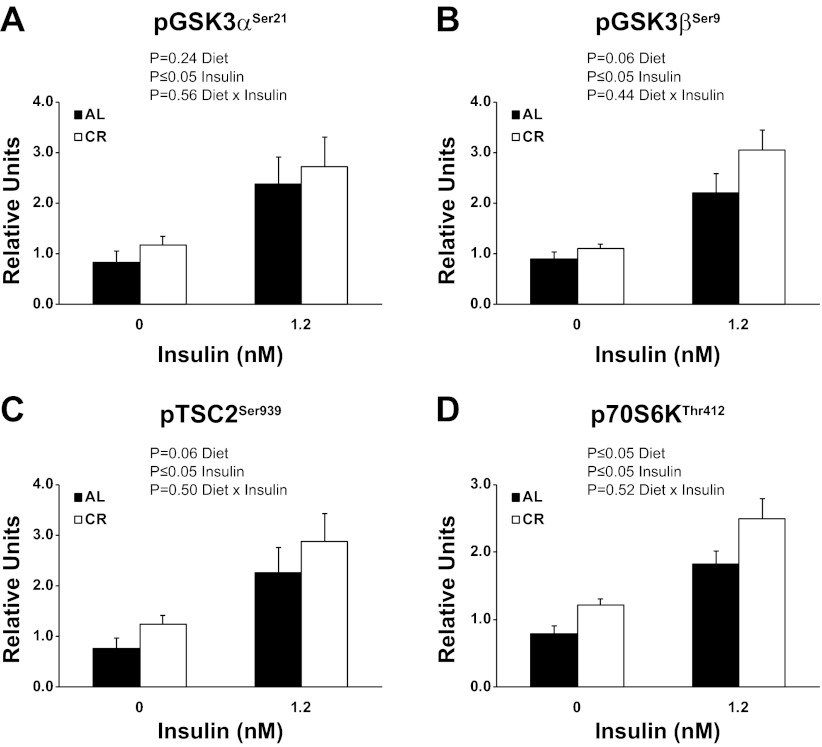
There were significant (P ≤ 0.05) main effects of diet (CR < AL) and insulin (1.2 nM > 0 nM) for pAS160Thr642 (Fig. 3A). There was a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) and an interaction (AL and CR groups are similar with 0 nM insulin, however, CR is much greater than AL with 1.2 mM insulin) for pFLNcSer2213 (Fig. 3B). When Δinsulin values were calculated (insulin stimulated − basal values), there was a significant (P ≤ 0.05) effect of CR on pFLNcSer2213. There was a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) for pGSK3αSer21 (Fig. 4A). There was a statistically nonsignificant trend for a main effect of diet (P = 0.06) and a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) for pGSK3βSer9 (Fig. 4B). There was a statistically nonsignificant trend for a main effect of diet (P = 0.06) and a significant (P ≤ 0.05) main effect of insulin (1.2 nM > 0 nM) for pTSC2Ser939 (Fig. 4C). There were significant (P ≤ 0.05) main effects of diet (CR > AL) and insulin (1.2 nM > 0 nM) for pP70S6KThr412 (Fig. 4D).
Fig. 3.
Phosphorylation of AS160Thr642 (A) and Filamin CSer2213 (B). Main effects of diet, insulin, and diet × insulin interactions are reported from two-way ANOVA analysis. P ≤ 0.05 is considered statistically significant. Values are means ± SE; n = 8–14 per treatment group.
Fig. 4.
Phosphorylation of GSK3αSer21 (A), GSK3βSer9 (B), TSC2Ser939 (C), P70S6KThr412 (D). Main effects of diet, insulin, and diet × insulin interactions are reported from two-way ANOVA analysis. P ≤ 0.05 is considered statistically significant. Values are means ± SE; n = 8 per treatment group.
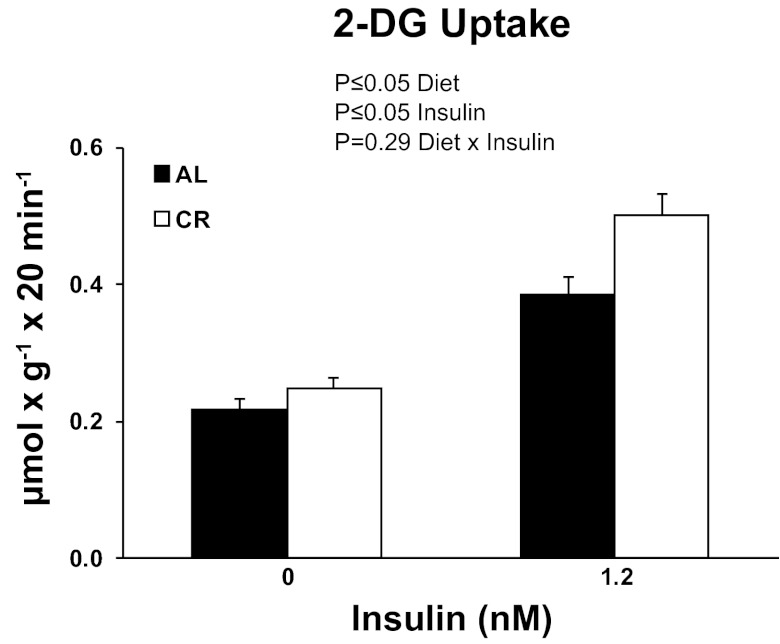
2-DG uptake.
There were significant (P ≤ 0.05) main effects of diet (CR > AL) and insulin (1.2 nM > 0 nM) for 2-DG uptake (Fig. 5).
Fig. 5.
Rates of 2-deoxy-d-glucose (2-DG) uptake in soleus muscle strips incubated without (0 nM) or with (1.2 nM) insulin. Main effects of diet, insulin, and diet × insulin interactions are reported from two-way ANOVA analysis. P ≤ 0.05 is considered statistically significant. Values are means ± SE; n = 23–24 per treatment group.
DISCUSSION
The current study reinforced and extended the results of prior research that has consistently reported that CR enhances insulin-stimulated Akt phosphorylation in isolated rat skeletal muscle (25, 26, 34, 35). Earlier studies indicated that the effects of CR on Akt in isolated rat skeletal muscle were not accompanied by significantly greater activation of key upstream insulin signaling steps (tyrosine phosphorylation of IR or IRS1, and IRS1-associated PI3K activity) that regulate Akt phosphorylation (7, 8, 34). In the current study we further advanced previous knowledge by also reporting for the first time in isolated rat soleus muscles that CR did not induce a greater activation of IRS1Ser312 phosphorylation or PTENSer380 phosphorylation. These novel results suggest that the mechanism for the CR-induced increase in Akt activation is not dependent on greater activation of these important proximal insulin signaling steps that have been demonstrated to regulate Akt.
The lack of a diet effect on insulin-stimulated IRS1-associated PI3K activity of the soleus is similar to our previous results in a different cohort of rats undergoing identical dietary treatment (34). Other work from our group using predominantly fast-twitch rat muscles has also shown no CR effect on insulin-stimulated PI3K activity (7, 9, 10). However, it has been reported that CR enhanced insulin-stimulated pY-associated PI3K activity in isolated soleus muscles from mice (32), as well as IRS1-associated PI3K activity in vastus lateralis muscles from monkeys under hyperinsulinemic-euglycemic clamp conditions (40). It is unclear if the differing results for CR effects on PI3K are related to differences in species, insulin concentration, or other experimental differences. Nonetheless, our group previously reported that the activity of atypical PKC (which, along with Akt, is a downstream target of PI3K) was not affected by CR, consistent with the idea that CR does not appear to enhance IRS1-PI3K in isolated rat muscle (34).
PTEN is another protein that could potentially influence Akt phosphorylation. Phosphorylation of PTEN on Ser380 reduces PTEN-mediated dephosphorylation of (3,4,5)-trisphosphate (20, 24, 42), thus favoring greater activation of Akt. PTEN mutations in humans have been demonstrated to enhance insulin sensitivity in skeletal muscle (30). However, in the current study, CR did not alter pPTENSer380 indicating that its activity is unlikely to account for the CR effects on Akt phosphorylation in the soleus.
The lack of evidence for significant CR effects on proximal insulin signaling steps in isolated rat skeletal muscle begs the question, what might account for the greater Akt that is consistently found in rat skeletal muscle? One possibility is that CR may influence the subcellular localization of Akt. Full activation requires Akt insertion into the plasma membrane (1). CR may increase the amount of Akt at the plasma membrane leading to greater Akt activation. However, it is unclear what might account for such a change in Akt localization with CR. There is evidence that CR may alter the binding of Akt to proteins that lead to greater phosphorylation thus enhancing Akt signaling. In epitrochlearis muscles from 9-mo-old rats, CR versus AL animals had greater heat shock protein (HSP)90-bound to Akt (based on coimmunoprecipitation) concomitant with increased Akt phosphorylation (34). However, there was no evidence of increased HSP90-Akt association in the soleus of CR rats in this earlier study. It remains possible that CR may be improving Akt signaling in the soleus through alterations in the binding to other protein partners. Because phosphorylation of proteins depends on the balance between the actions of kinases and phosphatases, another possibility is that CR reduces the rate of Akt dephosphorylation. However, Sharma et al. (34) found in soleus and epitrochlearis muscles from 9-mo-old rats that there was no CR-related difference in Akt association with protein phosphatase 2A, a key Ser/Thr protein phosphatase that dephosphorylates Akt (28). Identification of CR-mediated factors that associate with Akt and enhance its actions should be a target of future study.
We measured the phosphorylation status of several insulin-responsive Akt substrates. Phosphorylation of AS160 on its Thr642 site is important for insulin-stimulated GLUT4 translocation and glucose uptake (31). CR did not further enhance insulin-stimulated AS160Thr642 phosphorylation, which is consistent with a prior publication in isolated soleus muscles (34). We have previously reported that CR enhanced insulin-stimulated AS160Thr642 phosphorylation in isolated epitrochlearis (predominantly fast-twitch) muscles (34, 35). The current findings support the idea that there may be a muscle-specific, and possibly a fiber-type specific, response to the effect of CR on AS160Thr642 phosphorylation. The Thr642 phosphosite along with the Ser588 phosphosite on AS160 is crucial for insulin-stimulated glucose uptake (31). We also previously reported that CR does not enhance insulin-stimulated AS160Ser588 phosphorylation in isolated rat soleus muscle (34). The increased insulin-stimulated glucose uptake and Akt phosphorylation for the soleus of CR compared with AL rats was also consistent with earlier results for the isolated rat soleus muscle (34). Taken together, it seems likely that CR may be influencing glucose uptake in the soleus as the result actions on phosphorylation of Akt substrates other than AS160.
FLNc is an actin-binding protein that is believed to stabilize and anchor three-dimensional actin filament networks with cell membranes or act as a scaffolding protein (29). FLNc is a muscle-specific isoform that has been identified as a substrate of Akt (29) that can be phosphorylated on Ser2213 in response to insulin (11). In the current study, insulin-stimulated pFLNcSer2213 of isolated soleus muscles was enhanced with CR. This result is similar to our previous reports in epitrochlearis muscles from 9-mo-old rats (35). The functional role of insulin-mediated FLNc phosphorylation remains to be elucidated, but our data identify FLNc as an Akt substrate that is responsive to both CR and insulin in the predominantly slow-twitch soleus muscle.
We also measured phosphorylation of other important insulin-responsive Akt substrates. As expected, we found that a physiological insulin dose was able to induce site-specific phosphorylation of GSK3αSer21 and GSK3βSer9, serine/threonine kinases that have diverse cellular functions (6, 41); TSC2Ser939, a protein with an important role in the control of cell size (23); and pP70S6KThr412, a serine/threonine kinase that is key regulator of protein synthesis (44). Our novel observations included a significant CR effect on pP70S6KThr412 and strong trends (P = 0.06) for CR effects on pGSK3βSer9 and pTSC2Ser939, along with no evidence for a CR effect on pGSK3αSer21. Together with the results for pAS160Thr642 and pFLNcSer2213, these data clearly demonstrate that CR has a nonuniform influence on several key Akt substrates in rat soleus muscles.
Our group has previously observed that CR effects on insulin signaling are not identical in all skeletal muscles. McCurdy et al. (25) found that CR led to increased insulin-stimulated glucose uptake in both the predominantly fast-twitch extensor digitorum longus (EDL) and the soleus muscles of wild-type mice. In Akt2-null mice, the CR effect was eliminated completely in the EDL, but only partly reduced in the soleus of male mice, suggesting a possible role for an Akt2-independent mechanism only in the soleus. We have also studied 9-mo-old rats and found that despite no diet effects on either pIR or IRS1-associated PI3K activity with a submaximally effective insulin dose, Akt phosphorylation was increased for CR versus AL groups in both epitrochlearis and soleus, concomitant to an increase in glucose uptake. However, a CR-induced increase in pAS160 was observed in the epitrochlearis but not in the soleus (34). The results of the current study are consistent with our earlier study of 9-mo-old rats (34), which also found a significant diet effect of pAkt (CR > AL) on insulin-stimulated Akt phosphorylation concomitant with an increase in glucose uptake, despite no increases pIRTyr1162/1163 or pAS160Thr642.
In summary, the current study extends earlier research by demonstrating that the CR-induced increase in pAkt in isolated soleus muscles from 9-mo-old rats can occur without concomitant enhancement of several important insulin signaling events that are proximal to Akt activation, including pIRTyr1162/1163, pIRS1Tyr, pIRS1Ser312, IRS1-associated PI3K activity, or pPTENSer380. These results suggest that the greater pAkt in the soleus muscles from CR rats were attributable to an alternative mechanism. Our working hypothesis is that the improved Akt activation with CR is related to altered Akt binding to protein partners that regulate Akt phosphorylation. It will be important for future research to test this idea by evaluating Akt binding to proteins that have been reported to favor greater Akt phosphorylation [e.g., PHLDB1 (45) and ClipR-59 (12)] as well Akt's binding to carboxy-terminal modulator protein (CTMP) (14) and Tribble 3 (TRB3) (13) that have been reported to favor less pAkt. The signaling link between Akt and glucose uptake accounting for CR's ability to elevate insulin-stimulated glucose uptake remains to be determined, as CR did not enhance insulin-stimulated pAS160Thr642 but did lead to greater insulin-stimulated pFLNcSer2213 in the soleus. Notably, CR effects were not uniform for phosphorylation of six important insulin-regulated Akt substrates in the soleus. This result, using a physiologically relevant dietary model, is consistent with other studies that have used other experimental approaches (e.g., Akt inhibitors or gene knockouts) in cultured cells to probe the degree to which modulating Akt activation results in changes in phosphorylation of various Akt substrates (39). The differential response that we observed for CR effects on phosphorylation of Akt substrates likely has important implications for explaining complex effects of CR on diverse cellular functions.
GRANTS
This research was supported by National Institute on Aging Grants AG-010026, AG-013283, and T32-AG000114.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.S., D.A.S., and G.D.C. conception and design of research; N.S., D.A.S., and E.B.A. performed experiments; N.S., D.A.S., E.B.A., and G.D.C. analyzed data; N.S., D.A.S., and G.D.C. interpreted results of experiments; N.S. and D.A.S. prepared figures; N.S., D.A.S., and G.D.C. drafted manuscript; N.S., D.A.S., E.B.A., and G.D.C. edited and revised manuscript; N.S., D.A.S., E.B.A., and G.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dane Schils for technical assistance in animal care.
REFERENCES
- 1. Alessi DR, Downes CP. The role of PI 3-kinase in insulin action. Biochim Biophys Acta 1436: 151–164, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Cartee GD, Dean DJ. Glucose transport with brief dietary restriction: heterogenous responses in muscles. Am J Physiol Endocrinol Metab 266: E946–E952, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37: 188–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cohen P, Alessi DR, Cross DA. PDK1, one of the missing links in insulin signal transduction? FEBS Lett 410: 3–10, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Davidson RT, Arias EB, Cartee GD. Calorie restriction increases muscle insulin action but not IRS-1-, IRS-2-, or phosphotyrosine-PI 3-kinase. Am J Physiol Endocrinol Metab 282: E270–E276, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Dean DJ, Brozinick JT, Jr, Cushman SW, Cartee GD. Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol Endocrinol Metab 275: E957–E964, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Dean DJ, Cartee GD. Brief dietary restriction increases skeletal muscle glucose transport in old Fischer 344 rats. J Gerontol A Biol Sci Med Sci 51: B208–B213, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Dean DJ, Cartee GD. Calorie restriction increases insulin-stimulated tyrosine phosphorylation of insulin receptor and insulin receptor substrate-1 in rat skeletal muscle. Acta Physiol Scand 169: 133–139, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and Filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Ding J, Du K. ClipR-59 interacts with Akt and regulates Akt cellular compartmentalization. Mol Cell Biol 29: 1459–1471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300: 1574–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Franke TF. Intracellular signaling by Akt: bound to be specific. Sci Signal 1: pe29, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Fujita M, Mitsuhashi H, Isogai S, Nakata T, Kawakami A, Nonaka I, Noguchi S, Hayashi YK, Nishino I, Kudo A. Filamin C plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev Biol 361: 79–89, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Gazdag AC, Tucker MZ, Turcotte LP, Dean DJ, Cartee GD. Effect of extracellular palmitate on 2-deoxy-d-glucose uptake in muscle from Ad libitum fed and calorie restricted rats. Biochem Biophys Res Commun 252: 733–737, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Gazdag AC, Wetter TJ, Davidson RT, Robinson KA, Buse MG, Yee AJ, Turcotte LP, Cartee GD. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol Regul Integr Comp Physiol 278: R504–R512, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5: 87–90, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76: 979–985, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Hu Z, Wang H, Lee IH, Modi S, Wang X, Du J, Mitch WE. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 59: 1312–1320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 136: 1552–1560, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47: 443–467, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378, 1998 [DOI] [PubMed] [Google Scholar]
- 25. McCurdy CE, Cartee GD. Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54: 1349–1356, 2005 [DOI] [PubMed] [Google Scholar]
- 26. McCurdy CE, Davidson RT, Cartee GD. Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle. Am J Physiol Endocrinol Metab 285: E693–E700, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCurdy CE, Davidson RT, Cartee GD. Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle. Am J Physiol Endocrinol Metab 288: E996–E1001, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci 24: 186–191, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Murray JT, Campbell DG, Peggie M, Mora A, Cohen P. Identification of filamin C as a new physiological substrate of PKBalpha using KESTREL. Biochem J 384: 489–494, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, Cooper NS, Linden H, Levy JC, Wakelam MJ, Walker L, Karpe F, Gloyn AL. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med 367: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Schenk S, McCurdy CE, Philp A, Chen MZ, Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K, Olefsky JM. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J Clin Invest 121: 4281–4288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sequea DA, Sharma N, Arias EB, Cartee GD. Calorie restriction enhances insulin-stimulated glucose uptake and Akt phosphorylation in both fast-twitch and slow-twitch skeletal muscle of 24-month-old rats. J Gerontol A Biol Sci Med Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma N, Arias EB, Bhat AD, Sequea DA, Ho S, Croff KK, Sajan MP, Farese RV, Cartee GD. Mechanisms for increased insulin-stimulated Akt phosphorylation and glucose uptake in fast- and slow-twitch skeletal muscles of calorie-restricted rats. Am J Physiol Endocrinol Metab 300: E966–E978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma N, Arias EB, Sequea DA, Cartee GD. Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction's effect on glucose uptake in skeletal muscle. Biochim Biophys Acta 1822: 1735–1740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sharma N, Castorena CM, Cartee GD. Tissue-specific responses of IGF-1/insulin and mTOR signaling in calorie restricted rats. PLos One 7: e38835, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Standaert ML, Ortmeyer HK, Sajan MP, Kanoh Y, Bandyopadhyay G, Hansen BC, Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-zeta/lambda/iota. Diabetes 51: 2936–2943, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ. Signaling pathways mediating insulin-stimulated glucose transport. Ann NY Acad Sci 892: 169–186, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Tan SX, Ng Y, Meoli CC, Kumar A, Khoo PS, Fazakerley DJ, Junutula JR, Vali S, James DE, Stockli J. Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J Biol Chem 287: 6128–6138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang ZQ, Floyd ZE, Qin J, Liu X, Yu Y, Zhang XH, Wagner JD, Cefalu WT. Modulation of skeletal muscle insulin signaling with chronic caloric restriction in cynomolgus monkeys. Diabetes 58: 1488–1498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol 93: 384–392, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene 22: 3113–3122, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 413: 201–215, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Zanchi NE, Lancha AH., Jr Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur J Appl Physiol 102: 253–263, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Zhou QL, Jiang ZY, Mabardy AS, Del Campo CM, Lambright DG, Holik J, Fogarty KE, Straubhaar J, Nicoloro S, Chawla A, Czech MP. A novel pleckstrin homology domain-containing protein enhances insulin-stimulated Akt phosphorylation and GLUT4 translocation in adipocytes. J Biol Chem 285: 27581–27589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]