Abstract
Cranial primary afferent sensory neurons figure importantly in homeostatic control of visceral organ systems. Of the two broad classes of visceral afferents, the role of unmyelinated or C-type class remains poorly understood. This review contrasts key aspects of peripheral discharge properties of C-fiber afferents and their glutamate transmission mechanisms within the solitary tract nucleus (NTS). During normal prevailing conditions, most information arrives at the NTS through myelinated A-type nerves. However, most of visceral afferent axons (75–90%) in NTS are unmyelinated, C-type axons. Centrally, C-type solitary tract (ST) afferent terminals have presynaptic transient receptor potential vanilloid type 1 (TRPV1) receptors. Capsaicin activation of TRPV1 blocks phasic or synchronous release of glutamate but facilitates release of glutamate from a separate pool of vesicles. This TRPV1-operated pool of vesicles is active at normal temperatures and is responsible for actively driving a 10-fold higher release of glutamate at TRPV1 compared with TRPV1− terminals even in the absence of afferent action potentials. This novel TRPV1 mechanism is responsible for an additional asynchronous release of glutamate that is not present in myelinated terminals. The NTS is rich with presynaptic G protein-coupled receptors, and the implications of TRPV1-operated glutamate offer unique targets for signaling in C-type sensory afferent terminals from neuropeptides, inflammatory mediators, lipid metabolites, cytokines, and cannabinoids. From a homeostatic view, this combination could have broad implications for integration in chronic pathological disturbances in which the numeric dominance of C-type endings and TRPV1 would broadly disturb multisystem control mechanisms.
Keywords: autonomic, brain stem, solitary tract nucleus, synaptic, transmitter
Two Classes of Cranial Visceral Afferents: Conduction and Discharge
at the dawn of modern electrophysiology, Lord Adrian recorded impulses traveling along peripheral nerve trunks that included the vagus and aortic depressor nerve (ADN) (2). The flow of afferent impulses is a fundamental building block of organism-wide homeostatic regulation. The central nervous system (CNS) collects moment-to-moment status reports of the ongoing conditions within visceral organ sites and integrates them for homeostatic control of the milieu intérieur. Electrophysiologists discovered early on that nerve fibers were not homogeneous. Recordings showed fast and slowly conducted volleys of action potentials (51, 53, 56). The two major divisions differentiated by conduction velocity are rapidly conducting, myelinated, A-fibers and slowly conducting, unmyelinated, C-fibers (49). Recognition of these two broad classes presaged the discovery of the rich spectrum of phenotypic heterogeneity of primary afferent neurons, but the consequences of this dichotomy for cranial visceral afferent pathways to a large extent remain poorly understood. Goals of this review include highlighting important aspects of how the characteristics of C-fiber afferents intersect with our understanding of the neural mechanisms of reflex performance and new findings about central afferent synaptic transmission within the solitary tract nucleus (NTS) and the contributions of the TRPV1 receptor in presynaptic control of neurotransmitter release. Many of the specific examples in the review will rely on cardiorespiratory neurons, but the principal conclusions likely apply much more broadly.
Nearly a century ago, Adrian recorded bursts of action potentials with each pulse of arterial pressure from the ADN (1). The ADN contains thousands of aortic baroreceptor axons and generates a blur of action potentials (Fig. 1) with each systolic pressure pulse. Individual signals from active A- and C-fiber baroreceptors are impossible to discern in whole nerve recordings (14). Physically “splitting” the nerve trunk reveals the discharge from single baroreceptor afferents (Fig. 1). Such unit recordings indicated that some afferents discharged at high, regular frequencies (A-fiber), which faithfully encoded arterial pressure pulses (Fig. 2), whereas C-fiber baroreceptors generated sparse, irregular patterns of activity with limited fidelity of pressure encoding (107–109, 114). The intuitive appeal of the high fidelity signaling from A-fibers has consequently shaped an overwhelmingly A-fiber centric point of view. Accordingly, this has led to the anticipation that cardiovascular-related central neurons should show a cardiac-related rhythm (3, 35, 55, 83). However, this expectation overlooks the highly variable and often low fidelity encoding of most cardiovascular afferents (i.e., C-fibers, Fig. 2). As a whole, the variation in discharge characteristics across individual primary afferents provides a diverse stream of information flowing into the CNS, but the impact of this C-fiber primary afferent information and its utility to reflex control are poorly understood within the context of an integrated system.
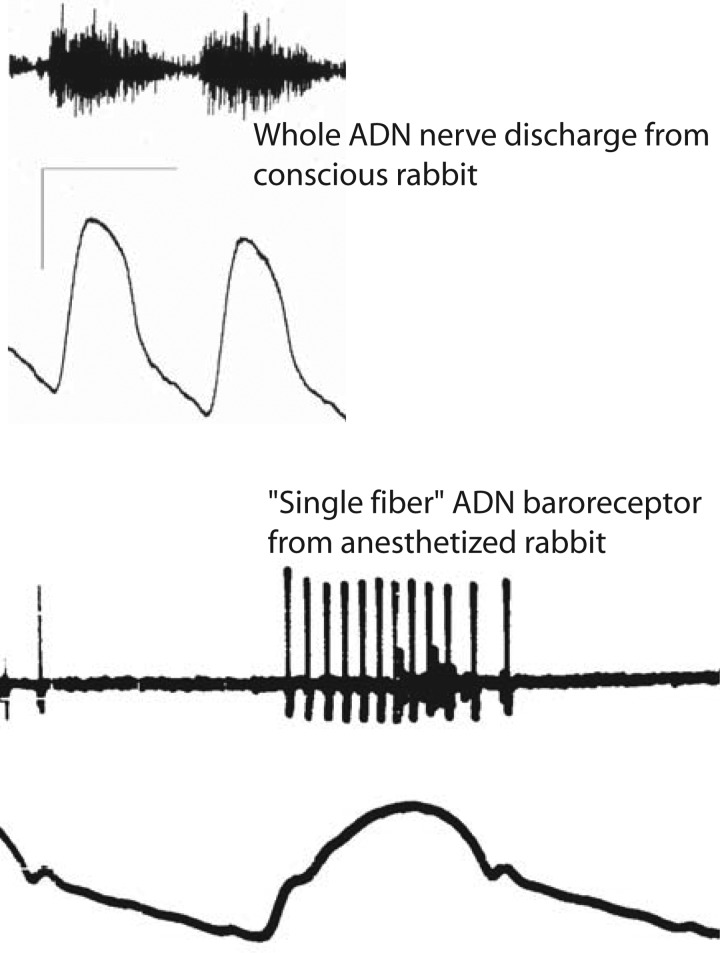
Fig. 1.
Whole nerve activity and single fiber nerve activity from the aortic depressor nerve (ADN). Top: a whole ADN activity recorded for two cardiac cycles from a conscious rabbit while occlusion of the descending aorta forced arterial pressure higher. Bottom: recorded activity from a thin, split fiber divided from the ADN of an anesthetized rabbit. Whole nerve recording registers activity from a nerve trunk containing thousands of axons but reflects electrical signal interactions from an unknown number of these axons (14). The single fiber recording shows the phasic activation of a regularly discharging aortic baroreceptor whose instantaneous frequency encodes and reports details of the arterial pressure typical of myelinated, A-type baroreceptors. A cluster of small amplitude spikes likely from C-type baroreceptors fires sparsely only at the peak of systole.
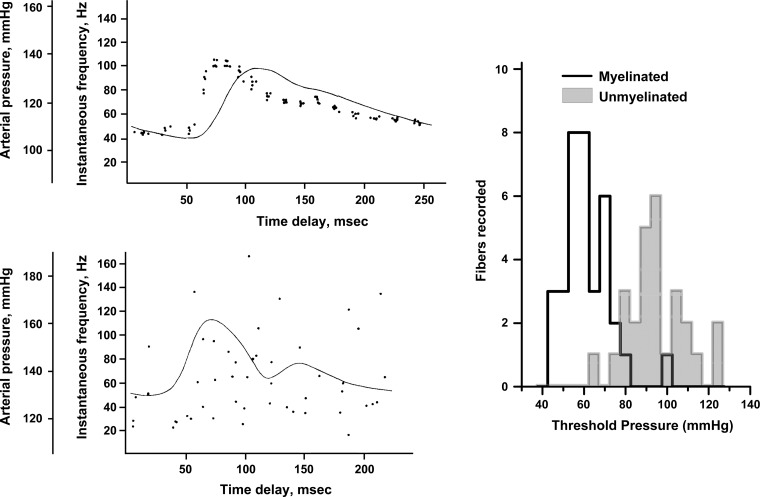
Fig. 2.
Myelinated aortic baroreceptors have highly reproducible and detailed pressure responses, but C-fiber aortic baroreceptors have quite sparse firing patterns with variable discharge. Recordings and data modified with permission from Yao and Thoren (114). Left: Five consecutive responses from a representative A-fiber baroreceptor (top) and C-fiber baroreceptor (bottom) were R-wave averaged overlays of action potential time of occurrence. The solid line is arterial pressure in each case. Right: averaged data from this study was replotted to display the distribution of pressure thresholds for 35 myelinated and 28 unmyelinated fibers. Note that nearly all unmyelinated fibers required greater pressures than the highest myelinated baroreceptor threshold.
Aortic baroreceptors are fairly representative of differences in discharge (high fidelity vs. sparse) and physiological thresholds (low vs. high) across A- and C-fiber axons, respectively, regardless of organ origin. Thus cranial visceral afferents repeat similar patterns whether from the heart, blood vessels, airways, lungs, or gastrointestinal visceral regions, and all send their information to the caudal NTS (17, 29, 37, 106). Conduction velocity and differences in discharge characteristics are two functional aspects that subdivide primary afferent neurons, but phenotypic cellular and molecular differences also segregate across these two classes. Such divisions in cranial visceral afferent neurons resemble the broad phenotypic separation of somatosensory primary afferents located in the spinal dorsal root ganglia that includes the expression of key ion channels and receptors (73, 96, 97). C-type neurons often express a different mixture of particular ion channels than myelinated afferents [e.g., tetradotoxin (TTX)-resistant sodium channels (5)], and these differences extend to ligand gated channels (e.g., TRPV1).
Cranial Visceral Afferents: Mostly Physiologically Quiet and C-Fibers
The C-fiber class is overwhelmingly the dominant phenotype of primary afferents from any given organ, a fact that is perhaps under appreciated, despite the starkly contrasting numbers. The relationship or ratios of the numbers of myelinated and unmyelinated afferents varies with species and organ. For example, aortic baroreceptors (10) and lung afferents (70, 94) are ∼90% unmyelinated. Physiologically, however, if one considers normal prevailing conditions that might activate afferents, then the functional activity of these groups flips this relationship to A-fibers dominating transduction of ongoing local conditions. In somatosensory neurons (8, 11, 12, 71), A-fibers are associated with fine discriminating sensation (e.g., knee flexion), whereas somatic C-fibers tend to be associated with nociception and near damaging stimuli (e.g., noxious heat). In cranial visceral afferents, C-fibers often have supraphysiological thresholds and sparse, irregular discharge. A-fiber afferents in contrast regularly discharge during basal physiological circumstances (e.g., normal ventilation or ambient resting blood pressure). Since visceral thresholds are so high, the stimuli required to activate C-fiber afferents correspond to harsh organ-level conditions (e.g., high distending pressures) and may thus correspond to potentially damaging stimuli, paralleling spinal cord afferents in the dorsal root ganglia (4, 17, 29, 30, 73). Visceral C-fibers are also, on average, less sensitive to mechanical, chemical, or thermal stimuli, and their discharge remains sporadic even with intense stimuli. The relatively mild stimuli that activate myelinated cranial visceral afferents has resulted in a greater knowledge of A-fibers and their pathways, again contributing to an A-fiber centric view (69). One ramification is that C-type afferents and their role in homeostatic regulation may be under recognized.
An illustrative example of how a common set of prevailing conditions differently activates A-/C-fiber afferent subtypes is aortic baroreceptors. Nearly all myelinated baroreceptors (90% of the A-type baroreceptor population) actively discharge at resting blood pressures (i.e., suprathreshold), and this information is conducted into the CNS (Fig. 2). At the same time, C-fiber baroreceptors are physiologically silent at these same prevailing blood pressures (114). Since most cranial afferents are C-type and they are likely to send little activity to the CNS, this prompts us to reconsider our understanding of their function in homeostatic control under normal conditions. In the arterial baroreflex for example, experimental lesions using the ultra-potent TRPV1 agonist resiniferatoxin (RTX) resulted in loss of neural structures: TRPV1 labeling in the aortic arch, nodose ganglion, and solitary tract (ST). RTX had no effect on blood pressure challenges below 125 mmHg, but RTX reduced the baroreflex responses above 125 mmHg, a deficit corresponding to the minimal pressure range for C-type aortic baroreceptors (102). Thus, TRPV1 and C-fibers had no measurable contribution unless pressure was forced well above normal prevailing pressures, effectively a silent deficit that required a specific stimulus to reveal. Afferent cellular phenotype strongly impacts the physiological activation thresholds and sensitivity above threshold, and these in turn determine the number of afferent action potentials sent to the CNS for reflex action. This example represents an interesting corollary that points out that very different adequate stimuli are required to assess A- and C-type functional contributions. Such phenotypic distinctions are an integral aspect of assessing the fate of their excitatory signals within the central “wiring,” and these pathway distinctions may well strongly influence their functional contributions to CNS integration.
Silent Majority and NTS Neurons
Afferent discharge activates central terminals which begins the translation and integration of that information. Cranial visceral primary afferents send their axons via the IXth and Xth cranial nerves directly to the brain stem and synapse on second-order sensory neurons within the caudal portions of the NTS (11, 12). These transmission lines have an interesting substructure: the peripheral nerve trunks are composed of smaller bundles of primary visceral afferents that contain a mix of 1–3 myelinated axons with 5 or more C-fiber axons. The peripheral and central nerve endings share the pattern that as the nerve fascicle approaches the target, e.g., the visceral organ, the A-fiber primary afferent axon loses its myelin and branches into final sensory arbors. The accompanying C-fiber cohort spreads across the same general region as the A-fibers [e.g., aortic baroreceptors (67, 105, 111) or pulmonary afferents (68)]. At the central ends within the NTS, A- and C-fiber synaptic terminal distributions share similar subregions (6, 59–61) and A-type axons become “postmyelinated” (60). Central mapping efforts detailed single afferent branching and terminal fields and yielded remarkably similar maps: whether for A- or C-type, or for baroreceptor or chemoreceptor, or for pulmonary or carotid sinus afferents (42, 46–48). Since single cranial visceral afferent axons commonly branch to cover similar NTS topologies, the proximity creates the potential for overlap and convergence (42, 47, 48, 69). This concept of convergence is an important one, but precisely where and how afferent information is combined was less clear. Tests on the same or different nerves suggest that convergence at NTS neurons was remarkably low (25, 45, 82, 84). In vivo data demonstrated that even maximal stimulation (i.e., activating perhaps >50,000 axons) failed to activate more than one input in ∼85% of single NTS neurons. Thus, contrary to anatomical impressions, each second-order neuron directly received limited afferent input. Even among seemingly similar modalities (e.g., cardiovascular), arterial baroreceptors rarely (<13%) activated single NTS neurons that were also activated by cardiac mechanoreceptors (85, 98). The centralis region and its gastrointestinal inputs, however, may present a more mixed profile with respect to TRPV1 and other presynaptic markers (27). Most single second-order NTS neurons receive a stream of primary afferent excitation that is limited and focused and thus they resemble “labeled lines” dedicated to parsing select information.
Synaptic Transmission From Cranial Visceral Afferents in Brain Stem Slices
Some of the highest experimental resolution is afforded by in vitro approaches. Slices that are cut along the alignment of the visceral afferent inflow tract, the ST, produce horizontal brain stem slices for study. This preserves contiguous segments of cranial visceral afferents (i.e., the ST) that course caudally and medially from cranial nerve rootlets to second-order neurons in the caudal one-third of the NTS (11, 12). In these slices, minimal intensity shocks to ST axons reliably activate excitatory postsynaptic currents (EPSCs) through non-N-methyl-d-aspartate (NMDA) glutamate receptors (15, 50) (Fig. 3). The EPSCs activate with a nearly invariant latency from the ST shock and the standard deviation of the latency (jitter <200 μs) serves as a metric to discriminate monosynaptic pathways to the neuron from those activated by an indirect, polysynaptic route. Anatomical tracers applied to peripheral afferent nerves are transported centrally to fluorescently mark ST terminals located on the soma and proximal dendrites of NTS neurons and anatomically confirm that direct contacts correlate with low jitter ST-EPSCs (13, 50, 66, 99). Small increments in intensity outline a recruitment profile with a sharp, minimum threshold and constant suprathreshold EPSC amplitude that indicates that a single ST axon is responsible for these monosynaptic ST-EPSCs. The high amplitude of ST-EPSCs means that single afferent axons generate highly reliable, excitatory inputs that almost always excite an action potential in the postsynaptic neuron with a high safety factor for transmission of afferent activity (16, 21).
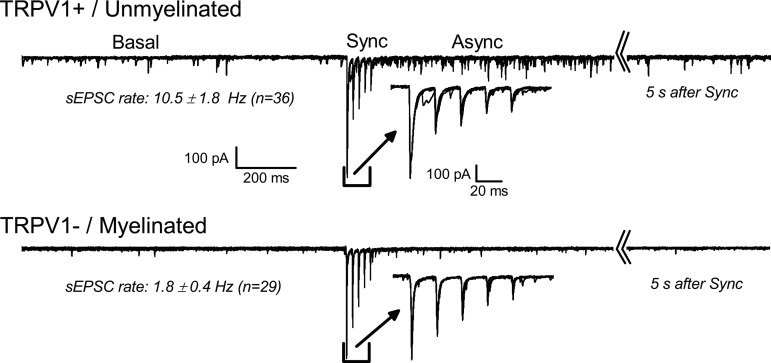
Fig. 3.
Central transmission for transient receptor potential vanilloid 1 (TRPV1)+ and TRPV1− solitary tract (ST) afferents. Recordings are from a rat horizontal solitary tract nucleus (NTS) slice. Panels show responses to ST activation in two different representative second-order NTS neurons with TRPV1+ ST input (top) and TRPV1− ST input (bottom). Labels mark the separate regions of the record analyzed to measure the specified features. Traces for five stimulation trials are overlaid in each case. Basal activity is measured for 1 s before ST activation (Sync, expanded in inset). ST activation delivered five 100 ms shocks at 50 Hz. Sampling continued for 6 s before repeating (note the broken axis). The 1 s following the ST synced responses is the asynchronous period (Async). The typical TRPV1+ neuron has high Basal spontaneous excitatory postsynaptic current (sEPSC) rate before ST activation and the Async period has elevated EPSC activity that decays in frequency back to the Basal rate by the end of 6 s. ST-EPSC activity was blocked from the TRPV1+ afferent during capsaicin exposure (not shown). TRPV1− afferents do not have additional EPSC activity following identical ST shocks and capsaicin does not block the ST-EPSC activity.
In most neurons, only a single monosynaptic ST afferent connection can be detected (13, 80), echoing in vivo findings. Our ability to discriminate single axons allowed detailed investigations of the mechanisms responsible for ST synaptic transmission. The variations in EPSC amplitudes across many trials provide an index of the probability of vesicle release. In this variance-mean analysis (V-M), the probability of vesicle release changed with external Ca2+ and V-M estimated that ST-evoked transmission averaged ∼90% in 2 mM Ca2+ with remarkably low failure rates to ST shocks (i.e., <1%) (13, 22, 88). Overall, afferents evoked remarkably similar release rates from a readily releasable pool (RRP) of docked vesicles, and we calculated that this arose from an average of about 20 release sites. These synaptic characteristics were similar for A- and C-type afferents and together suggest a uniformity of basic excitatory transmission via synchronous release of glutamate. This is a surprising finding given the substantial differences in ion channels and action potential characteristics between A- and C-type primary visceral afferent neurons (74, 77, 96, 97).
Active TRPV1 on Primary Afferents in NTS
Capsaicin has long been associated with activation of unmyelinated afferents including cranial sensory neurons (38, 39). The responsiveness to capsaicin viewed from current knowledge implies the expression of TRPV1 and is correlated with afferent activation under extraordinary conditions such as over-stretch, anoxic conditions or irritating chemicals (38, 39). TRPV1 cloning (26, 31, 79) advanced the close association between TRPV1 and nociceptive primary afferents. TRPV1 receptors are cation-selective ion channels that are activated by a canonical trio of stimuli including thermal (>43°C), H+ (pH <∼5.0), or phosholipid (vanilloids). Thus a single receptor complex underwrites multivalent integration (58). TRPV1's location, selectivity for calcium (∼10 × Ca2+/Na+), and depolarizing action (31) had potential ramifications when extended to afferent synaptic terminals within the NTS.
Shortly before the cloning of TRPV1, we began to study whether capsaicin might alter ST synaptic transmission in slices of NTS (9). Early surveys demonstrated TRPV1 at the central terminals of vagal afferents within the NTS (110), and recent genetic approaches confirmed that they are presynaptic (32). Superfusion of brain stem slices with nanomolar concentrations of capsaicin robustly increased “spontaneous” glutamate vesicle release (sEPSCs). Within 1–5 min exposure to capsaicin, ST shocks failed to activate synchronized EPSCs despite continued high frequencies of random sEPSC events. Not all afferents were capsaicin sensitive (TRPV1+) indicating that transmission from single ST axons was either TRPV1+ or completely capsaicin insensitive (TRPV1−). This all-or-nothing finding suggested that ST afferents segregate and single NTS neurons received either only TRPV1+ or only TRPV1− inputs (86). All capsaicin actions were consistent with presynaptic mechanisms controlling glutamate release, and there was no evidence of postsynaptic TRPV1-mediated currents in NTS neurons. Parallel tests in nodose neurons indicated that capsaicin activated inward currents only in cells with C-type conduction velocities and capsaicin-resistant nodose neurons had A-type conduction velocities (57). Thus capsaicin responses in ST-EPSC transmission to NTS neurons indicated that TRPV1+ responses were from C-fibers and TRPV1− from A-fibers. Using this method of separating TRPV1+ from TRPV1− ST inputs, no differences in the detailed characteristics of evoked synchronous glutamate release (ST-EPSCs) could be identified, a finding that suggests that TRPV1 does not participate in synchronous release (13). Likewise, dye-labeled baroreceptive NTS neurons had synaptic machinery equivalent to that of adjacent unlabeled and presumably nonbaroreceptor neurons whether A- or C-type ST afferents (13). Thus synchronous glutamate transmission by all cranial visceral afferents within the medial NTS is remarkably uniform despite different afferent phenotypes and different visceral organ sources.
TRPV1 Activity Generates Spontaneous EPSCs
One key synaptic difference has emerged from the analyses of A- and C-type cranial afferents. The “spontaneous” EPSC rate without any afferent stimulation averaged nearly 10-fold higher at neurons with TRPV1+ afferents compared with those receiving TRPV1− afferents under similar conditions (87). This basal EPSC activity difference persisted when action potentials were blocked by TTX. Together, the pairing of higher spontaneous transmission in TRPV1+ neurons, despite synchronous transmission resembling TRPV1− neurons, was quite surprising. In landmark work, Katz (62) originally discovered that the neurotransmitter released following an action potential (synchronous release) was composed of many small vesicles or quanta and that these same quantal vesicles were also released spontaneously, albeit at a very low rate. These quantal vesicles were contained in the synaptic terminals and were released rapidly when calcium entered the terminals during action potentials. The pool of quanta available for synchronized release, the readily releasable pool (RRP), was thought to contain the same quanta as those spontaneously released in the presence of TTX. The synaptic events in TTX lacked the coordinating influence of action potentials and were termed quantal or miniature EPSCs (mEPSCs). This idea of a common source for both synchronous and the spontaneous releases has recently been challenged by results from several brain areas together with noncanonical soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins for differential control of release (20, 64, 91, 92). In the NTS, evoked ST-EPSCs from TRPV1+ and TRPV1− sources are quite similar (Fig. 3). The only sign thus far that may reflect the characteristically different excitability, action potentials, and ion channels arising from TRPV1+ and TRPV1− nodose neurons (43, 44, 74–76, 96) may be the increased transmission failures in C-type ST inputs with high frequencies (13). Since evoked EPSCs were indistinguishable and yet the same neurons expressed substantially different basal spontaneous EPSC rates, then glutamate release from ST afferents differs from the conventional view. Bursts of ST shocks depressed synchronous EPSC amplitudes from all afferents in a manner that is consistent primarily with vesicle depletion (87). However, in TRPV1+ afferent neurons, bursts of synchronous EPSCs and depletion of the RRP are followed by an elevated rate of spontaneous EPSCs trailing immediately after the evoked responses, a phenomenon termed asynchronous release (Fig. 3). Thus high basal release and asynchronous release are synaptically diagnostic of TRPV1+ ST afferent transmission and suggested that TRPV1+ ST afferent terminals utilized two distinct pools of glutamate vesicles (54).
Unconventional Modes of Glutamate Release
Our studies suggested that myelinated cranial afferent terminals release glutamate synchronously and at a very low spontaneous rate. In contrast, TRPV1+ terminals possess an additional form of release from a separate, TRPV1-operated pool that is responsible for most of spontaneous release in basal conditions. Since vesicular release is promoted by rises in intracellular calcium, changes in external calcium, addition of specific calcium channel blockers or exposure to membrane permeant calcium buffers depressed evoked and asynchronous release similarly (87). However, TRPV1 antagonists decreased both the basal and asynchronous release EPSC rates without affecting the amplitude of the ST-evoked EPSCs (87). In TTX, we found that cadmium, a broad-spectrum blocker of voltage-activated calcium channels, did not affect the rate of mEPSCs in TRPV1+ neurons. This suggests that the calcium influx responsible for basal and asynchronous release enters through TRPV1. Interestingly, this also indicated that TRPV1-related calcium influx did not contribute to synchronous release from TRPV1+ ST terminals. Together, the evidence suggested that TRPV1 controlled or “operated” a distinct pool of glutamate vesicles that were not released by action potentials but rather their release depended on the activity of TRPV1. The independence of ST-EPSCs from TRPV1 activity may mean that TRPV1 and the TRPV1-operated pool of vesicles are localized close together, potentially within a presynaptic-restricted nanodomain such as a lipid raft (28, 95). The high rate of spontaneous EPSCs may thus be due to calcium entry through TRPV1 to release glutamate vesicles not released by action potentials.
Heat can also activate TRPV1+. The canonical thresholds for gating TRPV1 suggested that conditions were inappropriate in our slices for activating TRPV1. Nonetheless, to test whether temperature could be influencing TRPV1 in ST afferent terminals in our slices, we varied bath temperature while monitoring sEPSC rate. Temperatures well below the conventional threshold for TRPV1 activation (Fig. 4) continuously modulated sEPSC rates in TRPV1+ but in not TRPV1− NTS neurons. Blockade of voltage-dependent sodium and calcium channels did not alter this TRPV1-driven release (87, 101). Therefore, at normal temperatures, calcium enters synaptic ST terminals through TRPV1 to activate release of vesicles from the TRPV1-operated pool without affecting conventional synchronous transmission from the same afferents. TRPV1 is absent in myelinated ST afferents and neurons receiving those afferents are therefore remarkably quiet at rest, i.e., their synchronous pool appears to avidly hold on to its synaptic vesicles and have little temperature sensitivity (101). These experiments suggest that at physiological temperatures, C-type afferents have a unique mode of releasing glutamate that actively drives basal release of vesicles and thus is autonomous of afferent action potential activity. This TRPV1-operated mode of release may offer a novel tonic signaling mechanism within those afferent pathways.
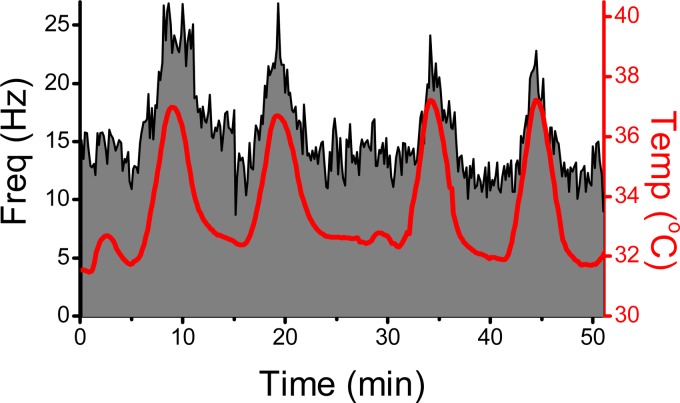
Fig. 4.
For neurons with TRPV1+ solitary tract afferents, the rate of sEPSCs increases when bath temperature is raised. The histogram of sEPSC rate over time is depicted in grey and black outline. Recording from a second-order TRPV1+ neuron in a horizontal slice of rat NTS identified as outlined in Fig. 3. Red line and axis indicates bath temperature controlled by an in-line heater. Without electrical stimulation, the spontaneous EPSC rate was high and reproducibly tracked increases in bath temperature (red) between 31°C and 37°C. Such temperature responses persist in tetrodotoxin (TTX) or calcium channel blockers such as cadmium but are attenuated by TRPV1 antagonists such as SB-366791. Similar recordings in TRPV1− second-order neurons showed low sEPSC rates and little change with similar temperature shifts (not shown).
TRPV1 in the CNS
TRPV1 function within the CNS has long been suggested but is controversial. Certainly, TRPV1 is expressed at primary sensory afferent neurons, and this extends to their central terminal portions, but this presence of TRPV1 within the CNS arises overwhelmingly from peripheral sensory neurons. A number of studies using immunolabeling and autoradiography have suggested that CNS neurons intrinsically express low levels of TRPV1 in cortex, amygdala, thalamus, hypothalamus, and other areas (104). TRPV1 agonists and antagonists acting on hippocampal neurons suggested that TRPV1 was required for long-term synaptic depression (34, 63). Such actions in cortical areas might be related to alterations in cognitive and emotional responses in TRPV1 knockout mice (78). In a different vein, TRPV1 in vasopressin-releasing hypothalamic neurons is linked to central thermosensory transduction (100). The issue of localization of central TRPV1 has recently been revisited, however, using molecular genetics methods incorporating a highly sensitive, TRPV1 reporter mouse, and these results counter the view of widespread TRPV1 expression and instead detected no central TRPV1 expressing cell bodies of neurons except for minimal TRPV1 expression in quite discrete brain regions including the caudal hypothalamus (32, 33). The high TRPV1 detection sensitivity of this approach did reveal a wider distribution of TRPV1+ primary afferents than expected (32, 33). At this point, TRPV1 of CNS origin and a broad participation in plasticity remain to be reconciled.
How Could TRPV1 Shape NTS Performance and Impact Reflex Control?
Several surprising aspects have emerged from our work linking TRPV1 activity and ST synaptic transmission. First, TRPV1 is vigorously active at normal brain temperatures. Second, calcium entry through TRPV1 is coupled to glutamate vesicles that are distinct from those mediating action potential-synchronized EPSCs. Third, terminal depolarization facilitates release from the TRPV1-operated pool of glutamate vesicles but not vice versa. Thus TRPV1 generates a stochastic signal related to afferent endings in the CNS that does not require peripheral sensory activation. At 37°C, TRPV1 channel activity stochastically generates EPSCs from TRPV1+ afferents. This TRPV1-derived, synaptic activity triggered a tonic level of action potential activity in the postsynaptic NTS neurons that was rapidly removed by cooling (87, 101). Recall that ∼80–90% of ST terminals are C-type cranial afferents and TRPV1+. Accordingly, most NTS neurons have a tonic basal drive that represents TRPV1 actively driving central activity. Many of these autonomous events trigger action potentials that may contribute a random drive of C-type afferent reflex pathways. This TRPV1 signal may have broad implications but, at this time, it remains largely untested.
Is There a Physiological Role for Central TRPV1 Signaling in the NTS?
Physiological activation of cranial visceral afferents trigger synchronized EPSCs that excite NTS. The function of TRPV1-operated glutamate is uncertain. A recent respiratory reflex example may indicate a physiological impact of TRPV1 actions within the NTS. Laryngeal afferents activated by fluid infused into the larynx of neonatal animals trigger a pronounced airway-protective reflex, the laryngeal chemoreflex (LCR). This reflex is characterized by disrupted respiration, prolonged apnea, coughing, and swallowing (41). Relatively modest elevations in body temperature (2°C) enhanced the LCR in neonatal pigs triggering an abnormally prolonged reflex apnea (41). The LCR may be important in sudden infant death syndrome and hyperthermia may increase this risk (65). Experimentally, the influence of temperature on the LCR is unlikely to reside at the peripheral sensory endings since selectively changing the temperature of the distilled water infused into the larynx failed to alter the LCR (41). In contrast, focal warming of the NTS altered the LCR despite holding body temperature constant and suggested that the NTS regional temperature is critical in the response (113). Furthermore, bilateral injection of the TRPV1 blocker 5′-iodoresiniferatoxin blocked the enhancement of the LCR during body temperature elevation and this result suggested that TRPV1 receptors were essential for the reflex in neonatal pigs (112). Interestingly, respiratory frequency rose following introduction of the TRPV1 blocker regardless of NTS temperature levels (38.6°C or 40.7°C). Such temperatures are below the canonical TRPV1 threshold (>43°C) but well within the range of temperatures at which TRPV1 strongly promotes glutamate release from C-type afferent endings in rat NTS (87, 101). The evidence suggests that the TRPV1-operated vesicles in C-type primary afferent endings within NTS may be responsible for an important aspect of both basal activity and evoked responses in this respiratory circuit. While this example concerns respiratory afferents, our work in slices suggests that the influence of TRPV1 mechanisms in NTS is unlikely to be limited to the LCR.
Signal Targets of Multimodal Glutamate Release
In the broad view of excitatory signaling from cranial afferents, glutamate in TRPV1+ terminals has at least three modes of vesicular release depending on calcium source (Fig. 3): 1) synchronous in response to action potentials and voltage dependent calcium channel activation; 2) autonomous in response to calcium entering through TRPV1; and 3) interactive, which results from an unknown mechanism linking action potential-gating of calcium entry to temporary facilitation of the TRPV1-operated release. TRPV1-negative terminals, in contrast, have very low spontaneous release rates (e.g., “reluctant” vesicles) despite comparable numbers of active zones and similar synchronous release. One additional potential role of TRPV1-operated tonic glutamate release might be in maintaining synaptic connections in otherwise generally silent C-type pathways (81, 89, 103).
The NTS is rich with GPCRs that have signal transduction cascades that modify conventional voltage-dependent ion channels. The presence of TRPV1-operated glutamate adds an additional effector for GPCRs in C-type sensory afferent terminals. C-fiber neurons are strongly associated with neuropeptides and GPCRs (7, 11). GABAB is a widespread GPCR that inhibits presynaptic glutamate release, and we recently tested whether its actions extended to the TRPV1-operated pool (52, 87, 101). Baclofen, a GABAB receptor agonist, inhibited synchronous EPSCs in both TRPV1+ and TRPV1− ST afferents and reduced the rates of basal and asynchronous EPSCs associated with TRPV1. In isolation of action potential-mediated release, baclofen strongly suppressed temperature-gated mEPSCs in TRPV1+ neurons. Thus presynaptic GPCRs can act on both the synchronous release mechanism, likely at calcium and/or potassium channels, as well as the TRPV1-operated mechanism. Alternative players in this realm expand beyond amino acid and peptide transmitters to include inflammatory mediators, lipid metabolites, cytokines, and cannabinoids. This raises the question of whether other GPCRs might differentially affect fast synchronous transmission or the TRPV1 mechanism separately. For example, CB1 and TRPV1 are colocalized and have structurally similar endogenous ligands (endocannabinoids and endovanilloids), suggesting that some lipid mediators may affect both receptors (90). A wide variety of mediators modify synchronous EPSCs (18, 19, 22–24, 40, 72, 88, 93), but the modulation of TRPV1-operated release remains largely untested. As in many targeting stratagems, it is also possible that particular GPCRs might act to selectively modulate TRPV1-operated release and tonic activation of C-type pathways without affecting phasic transmission. From a homeostatic view, this latter possibility could have broad implications in chronic pathological disturbances in which the numeric dominance of C-type endings and TRPV1 would broadly disturb multisystem control mechanisms.
Perspectives and Significance
Two old facts offer a provocative context for a new and intriguing view of afferent transmission in NTS. First, an overwhelming majority of primary visceral afferents in cranial nerves reaching NTS are unmyelinated. This suggests that perhaps most of the glutamate available for excitation in caudal NTS is locked within the terminals of unmyelinated afferents, an idea little appreciated but potentially profound in its implications. Second, the rather harsh physiological stimuli that are “adequate” to activate most C-fiber afferents have invited a conclusion that C-type glutamate terminals may be of less consequence under normal physiological circumstances. Our recent work may help to disconnect these two ideas. We found that TRPV1 on C-type glutamate terminals actively triggers the release of glutamate at a tonic rate corresponding to the local temperature. Thus normal temperature is sufficient to gate calcium entry through TRPV1, and this calcium triggers release from a unique pool of synaptic vesicles, a process that does not require action potentials and is thus intrinsically autonomous. The ramifications of the high rates of spontaneous glutamate release via this TRPV1 mechanism are not well understood, but TRPV1 may be critical in some reflex pathways such as the laryngeal chemoreflex. Since this ongoing TRPV1-mediated release of glutamate can be modulated by GPCRs, the regulation of vesicle release by TRPV1 represents a potential new source of pathway plasticity and complex behaviors (36).
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant HL-105703 (to M. C. Andresen). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C.A., M.E.H., and J.A.F. conception and design of research; M.C.A., M.E.H., and J.A.F. interpreted results of experiments; M.C.A. and J.A.F. prepared figures; M.C.A., M.E.H., and J.A.F. drafted manuscript; M.C.A., M.E.H., and J.A.F. edited and revised manuscript; M.C.A., M.E.H., and J.A.F. approved final version of manuscript; M.E.H. and J.A.F. performed experiments; M.E.H. and J.A.F. analyzed data.
REFERENCES
- 1. Adrian ED. The impulses produced by sensory nerve endings. Part I. J Physiol 61: 49–72, 1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adrian ED. Nobel Lecture: The activity of the nerve fibres (1932). In: Nobel Lectures: Physiology or Medicine 1922–1941 Amsterdam: Elsevier, 1965 [Google Scholar]
- 3. Agarwal SK, Calaresu FR. Reciprocal connections between nucleus tractus solitarii and rostral ventrolateral medulla. Brain Res 523: 305–308, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Akopian AN, Abson NC, Wood JN. Molecular genetic approaches to nociceptor development and function. Trends Neurosci 19: 240–246, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379: 257–262, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Anders K, Ohndorf W, Dermietzel R, Richter DW. Synapses between slowly adapting lung stretch receptor afferents and inspiratory beta-neurons in the nucleus of the solitary tract of cats: a light and electron microscopic analysis. J Comp Neurol 335: 163–172, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Andresen MC. Cardiovascular integration in the nucleus of the solitary tract. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston, MA: Kluwer Academic, 2004, p. 59–80 [Google Scholar]
- 8. Andresen MC, Doyle MW, Bailey TW, Jin YH. Differentiation of autonomic reflex control begins with cellular mechanisms at the first synapse within the nucleus tractus solitarius. Braz J Med Biol Res 37: 549–558, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Andresen MC, Fan W, Yang M. Capsaicin depresses rat baroreceptor afferents peripherally as well as synaptic responses to tract activation in medial nucleus tractus solitarius (mNTS) (Abstract). 25th Annual Meeting of Society for Neuroscience 21: 888, 1995 [Google Scholar]
- 10. Andresen MC, Krauhs JM, Brown AM. Relationship of aortic wall baroreceptor properties during development in normotensive and spontaneously hypertensive rats. Circ Res 43: 728–738, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Andresen MC, Paton JF. The nucleus of the solitary tract: Processing information from viscerosensory afferents. In: Central Regulation of Autonomic Functions, edited by Llewellyn-Smith IJ, Verberne AJ. London: Oxford, 2011, p. 23–46 [Google Scholar]
- 13. Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the solitary tract nucleus. Am J Physiol Heart Circ Physiol 295: H2032–H2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andresen MC, Yang M. Interaction among unitary spike trains: implications for whole nerve measurements. Am J Physiol Regul Integr Comp Physiol 256: R997–R1004, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Andresen MC, Yang M. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 259: H1307–H1311, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Andresen MC, Yang M. Dynamics of sensory afferent synaptic transmission in aortic baroreceptor regions of nucleus tractus solitarius. J Neurophysiol 74: 1518–1528, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol 2: 650–656, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 25: 3578–3585, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. J Neurosci 27: 13292–13302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci 28: 10151–10166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey TW, Hermes SM, Aicher SA, Andresen MC. Target-specific, dynamic pathway tuning by A-type potassium channels in solitary tract nucleus: cranial visceral afferent pathways to caudal ventrolateral medulla or paraventricular hypothalamus. J Physiol 582: 613–628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailey TW, Jin YH, Doyle MW, Smith SM, Andresen MC. Vasopressin inhibits glutamate release via two distinct modes in the brainstem. J Neurosci 26: 6131–6142, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol 284: R1340–R1353, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Bloom FE, Koda LY. Chemical circuitry and central cardiovascular regulation. Clin Exp Hypertens A 4: 529–541, 1982 [DOI] [PubMed] [Google Scholar]
- 25. Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus tractus solitarius. Am J Physiol Heart Circ Physiol 264: H1674–H1685, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Bráz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience 163: 1220–1232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Browning KN, Wan S, Baptista V, Travagli RA. Vanilloid, purinergic and CCK receptors activate glutamate release on single neurons of the nucleus tractus solitarius centralis. Am J Physiol Regul Integr Comp Physiol 301: R394–R401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca(2+) channels and Ca(2+) sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron 57: 536–545, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152: 223–242, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Cavanaugh DJ, Chesler AT, Bráz JM, Shah NM, Julius D, Basbaum AI. Restriction of Transient Receptor Potential Vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci 31: 10119–10127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci 13: 1511–1518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen CY, Bonham AC. Non-NMDA and NMDA receptors transmit area postrema input to aortic baroreceptor neurons in NTS. Am J Physiol Heart Circ Physiol 275: H1695–H1706, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Civelli O. Orphan GPCRs and neuromodulation. Neuron 76: 12–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coleridge HM, Coleridge JCG. Cardiovascular afferents involved in regulation of peripheral vessels. Annu Rev Physiol 42: 413–427, 1980 [DOI] [PubMed] [Google Scholar]
- 38. Coleridge HM, Coleridge JCG, Dangel A, Kidd C, Luck J, Sleight P. Impulses in slowly conducting vagal fibers from afferent endings in the veins, atria, and arteries of dogs and cats. Circ Res 33: 87–97, 1973 [DOI] [PubMed] [Google Scholar]
- 39. Coleridge HM, Coleridge JCG, Luck JC. Pulmonary afferent fibres of small diameter stimulated by capsaicin and by hyperinflation of the lungs. J Physiol 179: 248–262, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cui RJ, Li X, Appleyard SM. Ghrelin inhibits visceral afferent activation of catecholamine neurons in the solitary tract nucleus. J Neurosci 31: 3484–3492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol 98: 780–786, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol 373: 63–86, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol 514: 125–138, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci 24: 3335–3343, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol 360: 261–273, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Donoghue S, Garcia M, Jordan D, Spyer KM. Identification and brain-stem projections of aortic baroreceptor afferent neurones in nodose ganglia of cats and rabbits. J Physiol 322: 337–353, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donoghue S, Garcia M, Jordan D, Spyer KM. The brain-stem projections of pulmonary stretch afferent neurones in cats and rabbits. J Physiol 322: 353–363, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Douglas WW, Ritchie JM. Mammalian nonmyelinated nerve fibers. Physiol Rev 42: 297–334, 1962 [DOI] [PubMed] [Google Scholar]
- 50. Doyle MW, Andresen MC. Reliability of monosynaptic transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Erlanger J, Gasser HS. The action potential in fibers of slow conduction in spinal roots and somatic nerves. Am J Physiol 90: 338–339, 1929 [Google Scholar]
- 52. Fawley JA, Peters JH, Andresen MC. GABAB-mediated inhibition of multiple modes of glutamate release in the nucleus of the solitary tract. J Neurophysiol 106: 1833–1840, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gasser HS. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol 33: 651–690, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glitsch MD. Spontaneous neurotransmitter release and Ca2+–how spontaneous is spontaneous neurotransmitter release? Cell Calcium 43: 9–15, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Hayward LF, Felder RB. Cardiac rhythmicity among NTS neurons and its relationship to sympathetic outflow in rabbits. Am J Physiol Heart Circ Physiol 269: H923–H933, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Hinsey JC, Gasser HS. The component of the dorsal root mediating vasodilatation and the Sherrington contracture. Am J Physiol 92: 679–689, 1930 [Google Scholar]
- 57. Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals. J Neurosci 24: 4709–4717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Kalia M, Richter DW. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. I. A light microscopic analysis. J Comp Neurol 241: 503–520, 1985 [DOI] [PubMed] [Google Scholar]
- 60. Kalia M, Richter DW. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. II. An ultrastructural analysis. J Comp Neurol 241: 521–535, 1985 [DOI] [PubMed] [Google Scholar]
- 61. Kalia M, Richter DW. Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius. J Comp Neurol 274: 560–573, 1988 [DOI] [PubMed] [Google Scholar]
- 62. Katz B. Quantal mechanism of neural transmitter release. Science 173: 123–126, 1971 [DOI] [PubMed] [Google Scholar]
- 63. Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 32: 215–224, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DM. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology (Bethesda) 26: 45–53, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med 361: 795–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 88: 2736–2744, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Krauhs JM. Structure of rat aortic baroreceptors and their relationship to connective tissue. J Neurocytol 8: 401–414, 1979 [DOI] [PubMed] [Google Scholar]
- 68. Krauhs JM. Morphology of presumptive slowly adapting receptors in dog trachea. Anat Rec 210: 73–85, 1984 [DOI] [PubMed] [Google Scholar]
- 69. Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kubin L, Davies RO. Central pathways of pulmonary and airway vagal afferents. In: Regulation of Breathing, edited by Dempsey JA, Pack AI. New York: Marcel Dekker, 1995, p. 219–284 [Google Scholar]
- 71. Kunze DL, Andresen MC. Arterial baroreceptors: excitation and modulation. In: Reflex Control of the Circulation, edited by Zucker IH, Gilmore JP. Boca Raton, FL: CRC, 1991, p. 141–166 [Google Scholar]
- 72. Laaris N, Weinreich D. Prostaglandin E2 depresses solitary tract-mediated synaptic transmission in the nucleus tractus solitarius. Neuroscience 146: 792–801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol 87: 239–244, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Li BY, Feng B, Tsu HY, Schild JH. Unmyelinated visceral afferents exhibit frequency dependent action potential broadening while myelinated visceral afferents do not. Neurosci Lett 421: 62–66, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Li BY, Glazebrook P, Kunze DL, Schild JH. KCa1.1 channel contributes to cell excitability in unmyelinated but not myelinated rat vagal afferents. Am J Physiol Cell Physiol 300: C1393–C1403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li BY, Qiao GF, Feng B, Zhao RB, Lu YJ, Schild JH. Electrophysiological and neuroanatomical evidence of sexual dimorphism in aortic baroreceptor and vagal afferents in rat. Am J Physiol Regul Integr Comp Physiol 295: R1301–R1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods 164: 75–85, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in Transient Receptor Potential Vanilloid Type 1 Receptor-deficient mice. J Neurosci 27: 832–839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matta JA, Ahern GP. TRPV1 and synaptic transmission. Curr Pharm Biotechnol 12: 95–101, 2011 [DOI] [PubMed] [Google Scholar]
- 80. McDougall SJ, Peters JH, Andresen MC. Convergence of cranial visceral afferents within the solitary tract nucleus. J Neurosci 29: 12886–12895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci 2: 44–49, 1999 [DOI] [PubMed] [Google Scholar]
- 82. Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol 271: R870–R880, 1996 [DOI] [PubMed] [Google Scholar]
- 83. Nosaka S, Murase S, Murata K, Inui K. ‘Aortic baroreceptor’ neurons in the nucleus tractus solitarius in rats: Convergence of cardiovascular inputs as revealed by heartbeat-locked activity. J Auton Nerv Syst 55: 69–80, 1995 [DOI] [PubMed] [Google Scholar]
- 84. Ootani S, Umezaki T, Shin T, Murata Y. Convergence of afferents from the SLN and GPN in cat medullary swallowing neurons. Brain Res Bull 37: 397–404, 1995 [DOI] [PubMed] [Google Scholar]
- 85. Paton JFR. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998 [DOI] [PubMed] [Google Scholar]
- 86. Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLos One 6: e25015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32, 2001 [DOI] [PubMed] [Google Scholar]
- 90. Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol 141: 1118–1130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol 21: 275–282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ramirez DM, Kavalali ET. The role of non-canonical SNAREs in synaptic vesicle recycling. Cell Logist 2: 20–27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci 26: 12642–12646, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sant'Ambrogio G. Nervous receptors of the tracheobronchial tree. Annu Rev Physiol 49: 611–627, 1987 [DOI] [PubMed] [Google Scholar]
- 95. Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci 31: 5378–5382, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schild JH, Clark JW, Hay M, Mendelowitz D, Andresen MC, Kunze DL. A- and C-type nodose sensory neurons: Model interpretations of dynamic discharge characteristics. J Neurophysiol 71: 2338–2358, 1994 [DOI] [PubMed] [Google Scholar]
- 97. Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol 78: 3198–3209, 1997 [DOI] [PubMed] [Google Scholar]
- 98. Seagard JL, Dean C, Hopp FA. Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol 520: 243–253, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol 552: 547–559, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharif-Naeini R, Ciura S, Bourque CW. TRPV1 gene required for thermosensory transduction and anticipatory secretion from vasopressin neurons during hyperthermia. Neuron 58: 179–185, 2008 [DOI] [PubMed] [Google Scholar]
- 101. Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 30: 14470–14475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sun H, Li DP, Chen SR, Hittelman W, Pan HL. Sensing of blood pressure increase by Transient Receptor Potential Vanilloid 1 Receptors on baroreceptors. J Pharmacol Exp Ther 331: 851–859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science 304: 1979–1983, 2004 [DOI] [PubMed] [Google Scholar]
- 104. Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51: 159–212, 1999 [PubMed] [Google Scholar]
- 105. Tatalovic M, Glazebrook PA, Kunze DL. Expression of the P/Q (Cav2.1) calcium channel in nodose sensory neurons and arterial baroreceptors. Neurosci Lett 520: 38–42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thoren PN. Role of cardiac vagal c-fibers in cardiovascular control. Rev Physiol Biochem Pharmacol 86: 1–94, 1979 [DOI] [PubMed] [Google Scholar]
- 107. Thoren PN, Andresen MC, Brown AM. Resetting of aortic baroreceptors with non-myelinated afferent fibers in spontaneously hypertensive rats. Acta Physiol Scand 117: 91–97, 1983 [DOI] [PubMed] [Google Scholar]
- 108. Thoren PN, Munch PA, Brown AM. Mechanisms for activation of aortic baroreceptor C-fibres in rabbits and rats. Acta Physiol Scand 166: 167–174, 1999 [DOI] [PubMed] [Google Scholar]
- 109. Thoren PN, Saum WR, Brown AM. Characteristics of rat aortic baroreceptors with nonmedullated afferent nerve fibers. Circ Res 40: 231–237, 1977 [DOI] [PubMed] [Google Scholar]
- 110. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998 [DOI] [PubMed] [Google Scholar]
- 111. Wladyka CL, Feng B, Glazebrook PA, Schild JH, Kunze DL. The KCNQ/M-current modulates arterial baroreceptor function at the sensory terminal in rats. J Physiol 586: 795–802, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xia L, Bartlett D, Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol 176: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming in the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol 102: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- 114. Yao T, Thoren PN. Characteristics of brachiocephalic and carotid sinus baroreceptors with non-medullated afferents in rabbit. Acta Physiol Scand 117: 1–8, 1983 [DOI] [PubMed] [Google Scholar]