Abstract
D6 is a scavenging-receptor for inflammatory CC chemokines that are essential for resolution of inflammatory responses in mice. Here, we demonstrate that D6 plays a central role in controlling cutaneous inflammation, and that D6 deficiency is associated with development of a psoriasis-like pathology in response to varied inflammatory stimuli in mice. Examination of D6 expression in human psoriatic skin revealed markedly elevated expression in both the epidermis and lymphatic endothelium in “uninvolved” psoriatic skin (ie, skin that was more than 8 cm distant from psoriatic plaques). Notably, this increased D6 expression is associated with elevated inflammatory chemokine expression, but an absence of plaque development, in uninvolved skin. Along with our previous observations of the ability of epidermally expressed transgenic D6 to impair cutaneous inflammatory responses, our data support a role for elevated D6 levels in suppressing inflammatory chemokine action and lesion development in uninvolved psoriatic skin. D6 expression consistently dropped in perilesional and lesional skin, coincident with development of psoriatic plaques. D6 expression in uninvolved skin also was reduced after trauma, indicative of a role for trauma-mediated reduction in D6 expression in triggering lesion development. Importantly, D6 is also elevated in peripheral blood leukocytes in psoriatic patients, indicating that upregulation may be a general protective response to inflammation. Together our data demonstrate a novel role for D6 as a regulator of the transition from uninvolved to lesional skin in psoriasis.
Psoriasis is a common cutaneous inflammatory disorder1 with poorly understood pathogenesis. Although there is evidence of a pre-psoriatic phenotype in “uninvolved” psoriatic skin (ie, skin that is more than 8 cm distant from psoriatic plaques),2,3 the factors regulating transition to lesion development are unknown. Chemokines4 are essential regulators of inflammatory leukocyte migration in vivo, and are important for the development of a range of inflammatory pathologies, including psoriasis.5,6 They are therefore plausible central contributors to this transition event. Accordingly, their regulation is likely to affect pathogenesis.5,6 We study the regulation of the resolution of chemokine-driven inflammatory responses, and have characterized an atypical chemokine receptor,7 D6 (a 7-transmembrane–spanning receptor) that is active both in vitro and in vivo as a “scavenging receptor” for inflammatory CC chemokines.7–11 The scavenging activity of D6 is specific for inflammatory CC chemokines, as it does not bind homeostatic CC chemokines, CXC chemokines, or XC or CX3C chemokines.7 D6 is expressed in lymphatic endothelial cells12 in the skin, gut, and lung as well as in the syncytiotrophoblast layer of the placenta.13,14 In addition, D6 is expressed by subsets of peripheral blood leukocytes.15
Consistent with its chemokine-scavenging role, D6-deficient mice are unable to efficiently resolve inflammatory responses.16–19 In the context of the skin, treatment with the phorbol ester TPA, induces an exaggerated inflammatory response in D6-deficient mice that is not seen in wild-type (WT) mice and that bears many similarities to psoriasis.16 In addition, transgenic expression of D6 in the epidermis actively suppresses cutaneous inflammatory responses.20 This suggests a role for D6 in limiting overt cutaneous chemokine action that might otherwise lead to development of inflammatory disorders such as psoriasis.
In this study, we demonstrate a key role for D6 in resolution of cutaneous inflammatory responses in mice. We also show, for the first time, that D6 is overexpressed and regulated in uninvolved psoriatic skin in a manner supportive of a role in suppressing psoriatic lesion development.
Materials and Methods
Murine Studies
WT and D6-deficient mice, TPA painting, and histological analyses have been described previously.16,20 Antibodies were administered at 200 μg i.p. per mouse in PBS every second day throughout the treatment period. In all experiments, five mice per group were used, and histology was scored by microscopically measuring 20 skin thicknesses per skin section.
Patient-Based Studies
Uninvolved biopsy samples were obtained from healthy skin at least 8 cm distant from neighboring psoriatic plaques. Lesional and peri-lesional biopsy samples were obtained from single elliptic biopsy samples collected at the margins of plaques. These elliptic biopsy samples were halved, with half designated as peri-lesional and the other half as lesional, using histological criteria. For the tape-stripping studies (using Scotch tape), patients were subjected to 10 strips of a specific area of skin, and 24 hours later a biopsy sample was taken from an uninvolved area of the patient's body, in addition to a biopsy sample at the site of tape-stripping. Biopsy samples were immediately placed on dry ice, RNA was purified, and quantitative polymerase chain reaction (qPCR) was carried out as described below.
RNA Extraction and qPCR
RNA was purified from primary human epidermal keratinocytes (HEKs) and skin biopsies and qPCR performed as previously described.15 D6 RNA levels were normalized to GAPDH, TBP, or β-actin. Apart from GAPDH,15 the qPCR primer sequences were as follows: murine, D6-5′-TTCTCCCACTGCTGCTTCAC-3′ and 5′-TTCCATCTCAACATCACAGA-3′; human, D6-5′-CTCAGCCATCAGCAGCATT-3′ and 5′-GCAAGTGAAGAAAGTGGAGGA-3′; β-actin, 5′-TAAAAACTGGAACGGTGAAGG-3′ and 5′-ATTGTGAACTTTGGGGGATG-3′; TBP, 5′-ACTGACCCCACAGCCTATTC-3′ and 5′-TGCCTTTGTTGCTCTTCCA-3′.
Immunofluorescence
Biopsy samples were embedded in paraffin and 5-μm sections cut onto superfrost slides (VWR, Leicester, UK), dewaxed, and rehydrated using xylene and decreasing ethanol concentrations. Endogenous peroxidase activity was blocked by incubating sections for 30 minutes in 1% hydrogen peroxide. Slides were then briefly washed (PBS-Tween20 (0.05%)) and antigens unmasked by boiling sections for 15 minutes in 0.01 mol/L citric acid, pH 6. Once sections cooled, they were washed (PBS-Tween20 [0.05%]) and blocked (40 minutes) using 20% equine serum in PBS-Tween20 (0.05%) containing avidin block. Slides were washed with PBS-Tween20 to remove excess avidin blocker and then incubated (4°C) with 0.5 μg/mL rabbit–anti-human D6 antibody (Sigma-Aldrich, Poole, Dorset) in Real Antibody Diluent (DAKO, Cambridgeshire, UK) containing 2.5% equine serum, 2.5% human serum, and biotin block. The next day, sections were washed with PBS-Tween20 (0.05%) and incubated for 30 minutes with biotinylated anti-rabbit secondary antibody (Vector Labs, Peterborough, UK) before further washes in PBS-Tween20 (0.05%) and incubation for 40 minutes with FITC-conjugated avidin (Vector Labs). After a final wash in PBS-Tween20 (0.05%), slides were mounted using Vectashield containing DAPI and then imaged. CCL2 staining used a monoclonal mouse anti-human CCL2 antibody (R&D Systems, Minneapolis, MN) and a biotinylated horse anti-mouse secondary antibody (Vector Labs). CCL5 staining used a polyclonal goat anti-human CCL5 antibody (R&D Systems) and a biotinylated horse anti-goat secondary antibody (Vector Labs).
Human Keratinocyte Culture and Cytokine Stimulations
HEKs were obtained from PromoCell (Heidelberg, Germany), seeded into 25-cm tissue culture flasks, and fed every second day. Before cytokine stimulation, HEKs were seeded into 12-well plates and allowed to grow to 80% confluency.
Results
D6 Is Involved in the Resolution of Cutaneous Inflammatory Responses
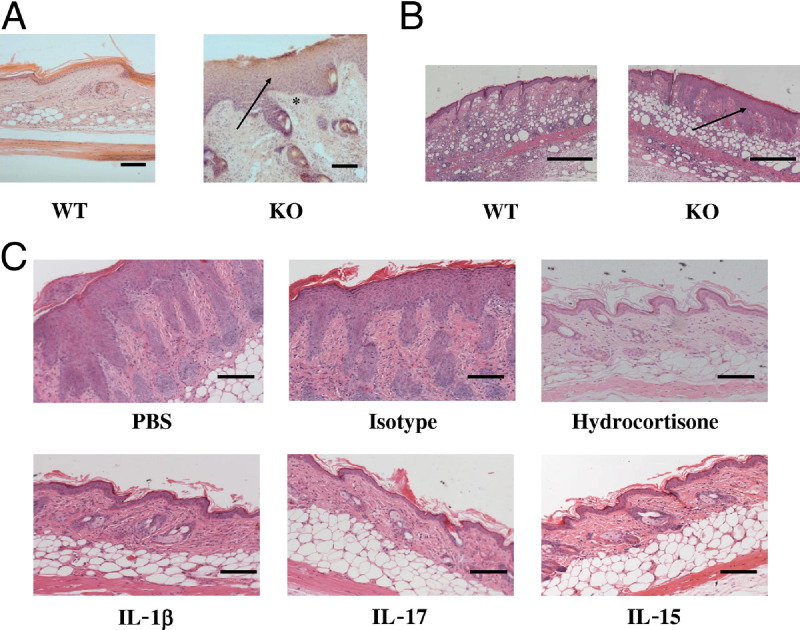
Exposure of D6-deficient mice to a variety of cutaneous insults, including dermal liposome injections (Figure 1A) and subcutaneous granuloma induction (Figure 1B), consistently led to the development of psoriasiform epidermal hyperplasia, which was not seen in similarly treated WT mice and which was identical to that seen after TPA application to D6-deficient skin. Notably, the psoriasiform pathology that developed in response to TPA application was responsive to a variety of immune-mediated interventions including glucocorticoids, TNF blockade,16 antibodies to IL-1β, and IL-17; and a receptor-based IL-15-blocker21 (Figure 1C; see also Supplemental Figure S1 at http://ajp.amjpathol.org). Together with our previous data,16 these observations support the notion that D6 is a central determining factor of inflammatory outcome on cutaneous provocation.
Figure 1.
Dynamic D6 expression is associated with psoriasis. A: D6-deficient mice displayed psoriasis-like lesions after repeated liposome injection into tail skin as compared with WT mice. The Mice were subjected to injections every second day, and tissue was collected for histology at day 21. Note the markedly increased epidermal thickening (arrow) and dermal inflammatory infiltrate (asterisk) in D6-deficient skin. Scale bar = 100 μm. B: A psoriasis-like phenotype (arrow) is seen in D6-deficient mice after subcutaneous injection of complete Freund's adjuvant (CFA). CFA was injected subcutaneously into WT and D6-deficient mice, and skin was harvested for histology 3 days later. Scale bar = 200 μm. C: The psoriasis-like pathology developing in D6-deficient mice in response to TPA application is sensitive to topical hydrocortisone treatment and systemic treatment with antibodies to IL-1β and IL-17 and a receptor-based blocker of IL-15. Scale bar = 100 μm.
D6 Is Markedly Overexpressed in Uninvolved Skin from Psoriatic Patients
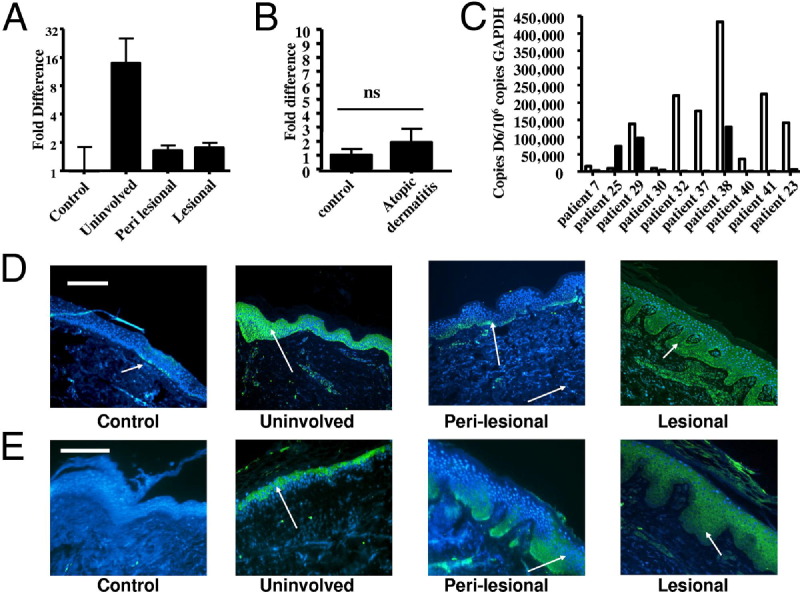
Given the association of D6 deficiency with development of psoriasis-like pathology in murine skin,16 we hypothesized a role for D6 in suppressing chemokine function, and thus lesion development, in human psoriasis. qPCR analysis of D6 expression in uninvolved, perilesional, and lesional skin in comparison to healthy control (control) skin, surprisingly revealed that uninvolved, histologically normal skin from psoriatic patients displayed markedly higher D6 expression than control skin (Figure 2A). Elevated expression also was evident in perilesional and lesional, compared to control, skin, but these levels were significantly lower than in uninvolved skin (Figure 2A). Although D6 levels were higher in skin from patients with atopic dermatitis (Figure 2B), these differences did not reach statistical significance. Examination of matched uninvolved/lesional biopsy samples from individual psoriatic patients revealed (Figure 2C) elevated expression in uninvolved, compared with lesional, skin in all but one patient, suggesting this to be a generalizable phenomenon. Thus D6 is strongly overexpressed in the apparently normal-looking uninvolved skin of psoriatic patients.
Figure 2.
D6 is highly expressed and is coincident with inflammatory CC-chemokines in the epidermis of ‘uninvolved’ psoriatic skin. A: Relative expression of D6 in uninvolved, perilesional and lesional psoriatic skin compared with healthy control (control) skin which is set as the comparator of 1. P < 0.02 between control and uninvolved, P < 0.003 between uninvolved and both perilesional and lesional, and P < 0.03 between control and both perilesional and lesional expression. B: Expression of D6 in biopsy samples from healthy control skin and lesional atopic dermatitis skin. C: Expression levels of D6 in matched uninvolved (open bars) and lesional (filled bars) biopsy samples from 10 patients. D: Immunofluorescent detection of D6 expression (arrowed) in control, uninvolved, perilesional, and lesional skin (scale bar = 100 μm). E: Immunofluorescent detection of CCL2 (arrowed) in control, uninvolved, perilesional, and lesional skin (scale bar = 100 μm).
D6 Is Prominently Expressed in Uninvolved Psoriatic Epidermis
Next we characterized cutaneous D6 protein expression to see if it mirrored the transcriptional data, and to define the cell types responsible for elevated D6 expression in uninvolved skin. We demonstrated for the first time that D6 is expressed in the epidermis in control skin (Figure 2D). This expression is sparse and is restricted to keratinocytes occupying the epidermal basal layer. Consistent with the qPCR data, D6 protein was detectable throughout the viable epidermal layers in uninvolved psoriatic skin. This expression was reduced in perilesional areas where it was again restricted to the basal layer; although, its expression in this region was more uniform than in control epidermis. Intriguingly, in lesional skin, D6 protein expression was again evident throughout all viable epidermal layers. However, this may reflect the uncoupling of differentiation seen in psoriatic epidermis, and thus represent expanded basal layer expression, rather than expression in differentiated layers as seen in uninvolved skin. Overall, these data demonstrate markedly elevated D6 expression in histologically normal, uninvolved, skin of psoriatic patients.
Elevated D6 Expression Is Coincident with Inflammatory Chemokine Expression
Elevated D6 expression in uninvolved skin suggests that it may play a protective role against lesion development by blocking local chemokine action. We therefore examined expression of the inflammatory chemokines CCL2 (Figure 2E) and CCL5 (see Supplemental Figure S2 at http://ajp.amjpathol.org), both of which are high-affinity D6 ligands,9 in patient samples. Notably, whereas CCL2 (Figure 2E) is undetectable in control skin, it is easily seen in the upper epidermal layers of uninvolved skin but is not associated with plaque development at these sites. In addition, CCL2 is seen in perilesional epidermis and at extremely high levels in lesional epidermis, where it is associated with plaque development. D6 and CCL2 show significant co-localization in lesional epidermis (see Supplemental Figure S3 at http://ajp.amjpathol.org). However it is also notable that D6 expression predominates in basal layer keratinocytes whereas CCL2 expression is maximal in upper epidermal layers. These observations support the notion that D6 presents a chemokine-scavenging barrier between the inflamed outer epidermis and the dermis. Together these data reveal a correlation between high levels of D6 expression and the presence, but apparent lack of function of, inflammation-promoting chemokine activity in uninvolved psoriatic skin. Importantly, we have previously reported that enforced epidermal D6 expression, in transgenic mice,20 suppresses cutaneous inflammatory responses. This, along with the coincidence of chemokine and D6 expression in uninvolved psoriatic skin, suggests that D6 is elevated in uninvolved skin to block initiation of chemokine-driven inflammatory responses.
D6 Is Overexpressed in Other Inflammation-Exposed Cells in Psoriatic Patients
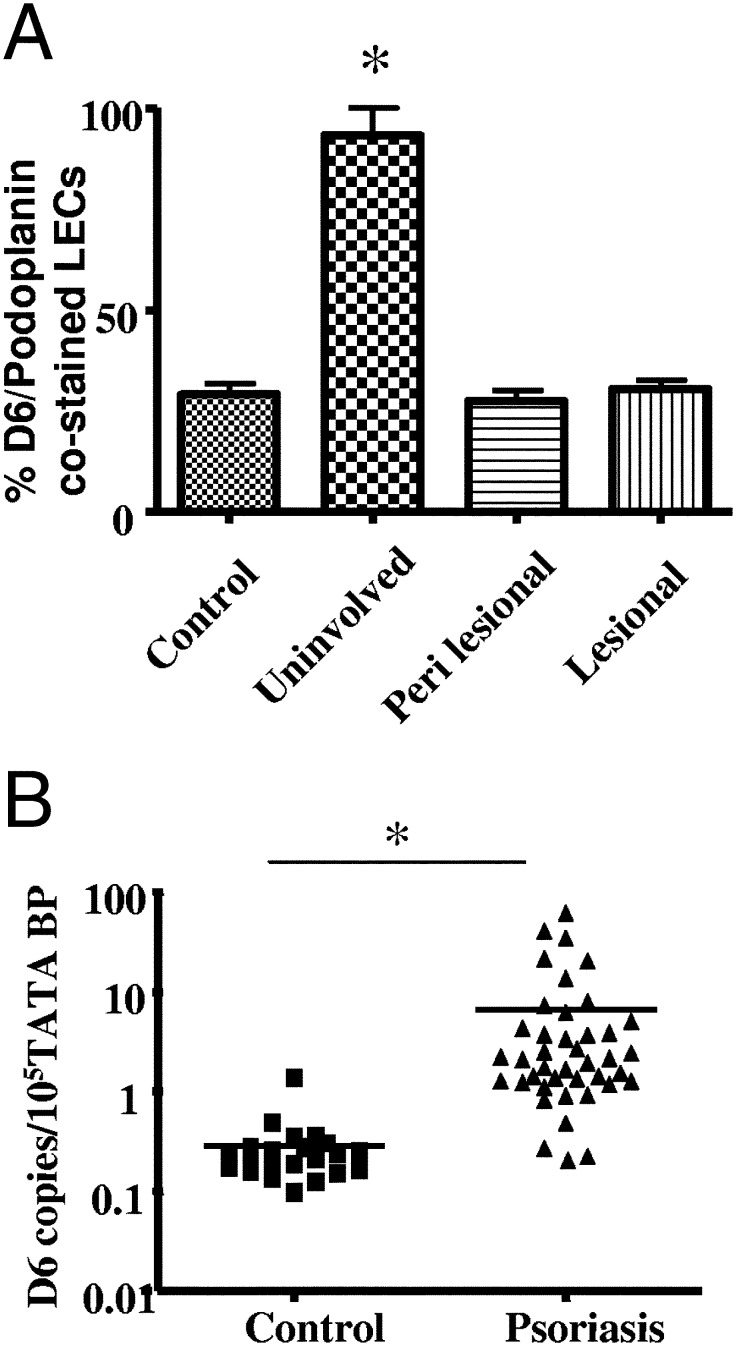
To determine whether D6 overexpression was restricted to uninvolved epidermis or whether it was also seen in other cell types, we evaluated expression in more typical D6-expressing cells ie, dermal lymphatic vessels12 and peripheral blood leukocytes (PBLs).15 D6 expression in lymphatic vessels was enumerated by counting the numbers of D6+/podoplanin+ dermal vessels, per total podoplanin+ vessels in skin sections. As with the epidermal expression, markedly upregulated D6 expression was seen in lymphatic vessels in uninvolved psoriatic skin, compared with control, or perilesional or lesional skin (Figure 3A). In addition, we found similarly upregulated D6 expression in PBLs of psoriatics compared with controls (Figure 3B). Together these observations suggest that elevated D6 expression may be a general protective cellular response to inflammation in psoriatics and that the elevated expression seen in uninvolved skin is a combination of increased epidermal and lymphatic D6 transcript numbers.
Figure 3.
D6 is expressed by a range of cell types in psoriatic patients. A: Enumeration of D6+/podoplanin+lymphatic vessels in the dermis of control and psoriatic skin biopsy samples. Levels in uninvolved skin are significantly higher (*P < 0.0001) than in control, perilesional, or lesional skin. B: qPCR analysis of D6 expression in control and psoriatic peripheral blood leukocytes. *P < 0.0001.
Cutaneous D6 Expression Is Regulated by Psoriasis-Relevant Cytokine Activity and Trauma
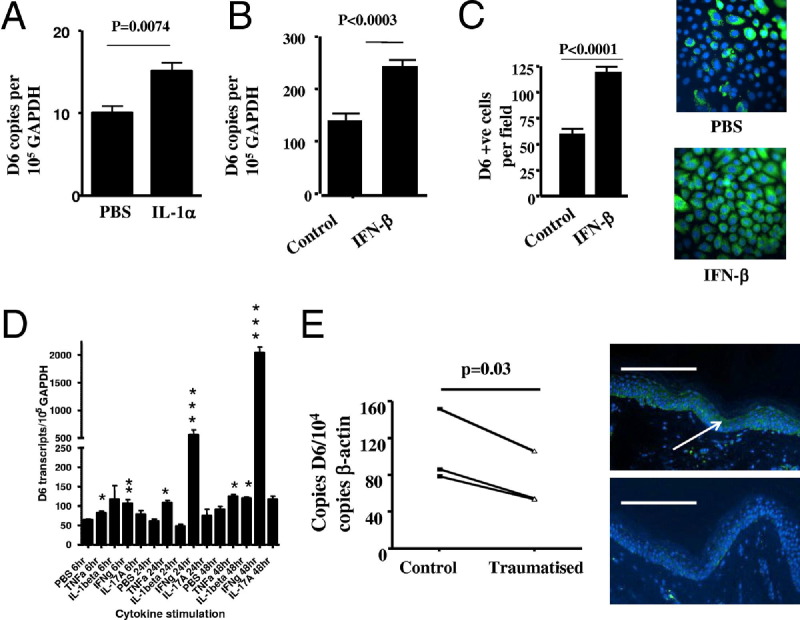
Next we examined the basis for the upregulated D6 expression in uninvolved psoriatic epidermis by measuring the effects of psoriasis-relevant cytokines on keratinocyte D6 expression. Microarray and other studies show that unaffected psoriatic skin differs from control skin in expression of pro-inflammatory cytokines22,23 including IL-1α. In addition, although plasmacytoid dendritic cells (pDCs) are absent from control skin, they are seen in both uninvolved and involved psoriatic skin.24 pDCs produce type I inteferons (IFNs), which play prominent roles in psoriasis pathogenesis. Notably, both IL-1α (Figure 4A) and IFN-β (Figure 4B) induced significant increases in D6 transcript levels in vitro (and protein levels, shown for IFN-β; Figure 4C) in cultured keratinocytes. The differences in D6 transcript levels induced by these cytokines were modest but suggested up-regulation by inflammatory cytokines. To examine this in more detail, we tested additional cytokines for their ability to affect D6 expression. As shown in Figure 4D, and similar to the data presented for IL-1α and interferon-β, TNF induced significant but modest increases in D6 expression at all time points tested and IL-1β induced a significant enhancement of D6 expression by 48 hours. Strikingly, however, IFN-γ induced significant and substantial D6 overexpression (> 20-fold by 48 hours) at all time points measured. Furthermore, treatment of keratinocytes with a mix of inflammatory cytokines (see Supplemental Figure S4 at http://ajp.amjpathol.org) induced even stronger transcriptional up-regulation (> 35-fold). Intriguingly, neither IL-17 nor IL-22 had any effect on D6 transcript levels despite their known involvement in psoriasis pathogenesis. We therefore propose that a major proportion of elevated D6 expression in uninvolved psoriatic skin results from inflammatory cytokine expression in this area.
Figure 4.
D6 expression is regulated by psoriasis-relevant cytokines and trauma. IL-1α (A) and IFN-β (B) induce up-regulation of D6 expression in cultured keratinocytes in vitro at 6 hours (IL-1α) and 48 hours (IFN-β), respectively. Cytokines were used at 100 ng/mL. C: D6 protein levels were assessed as the number of D6+ cells per ×20 microscopic field and 10 fields were counted for control and for IFN-β–treated cell monolayers (left panel). Immunofluorescent staining demonstrates elevated D6 protein expression in IFN-β–treated compared with PBS-treated keratinocytes (right panels). D: Keratinocytes were treated with each of the indicated cytokines at 100 ng/mL for 6, 24, or 48 hours, and D6 expression levels were assessed by q-PCR. *P < 0.03; **P < 0.01; ***P < 0.002. E: Trauma induced by tape stripping causes a significant reduction in D6 transcript expression in full-thickness skin biopsy samples from “uninvolved” psoriatic skin by 24 hours, compared with expression in similar biopsy samples from nontraumatized uninvolved skin from the same donors (left panel), and (right panels) intensity of D6 immunostaining in biopsy samples from traumatized compared with matched, nontraumatized uninvolved skin at 48 hours Scale bar = 100 μm.
Finally, we examined the possible basis for the transition from high to low D6 expression in uninvolved and perilesional skin, as this may prove permissive to inflammation onset. In some psoriatic patients, a phenomenon called Koebnerisation1 is observed in which trauma to uninvolved skin leads to lesion development, suggesting that uninvolved skin is primed for lesion formation, which is in keeping with the observation of expression of inflammatory chemokines in this apparently normal-looking skin. These observations raise the possibility that trauma may trigger plaque development in part by reducing D6 expression and thus unmasking chemokine activity in the skin. We therefore induced mild trauma in uninvolved psoriatic skin by tape stripping. Twenty-four hours later, we obtained a full-thickness skin biopsy sample from the traumatized area as well as a second biopsy sample from the same patient from a nontraumatized, uninvolved skin area. As can be seen in Figure 4E (left panel), trauma to uninvolved psoriatic skin significantly reduced D6 expression by 24 hours, supporting a role for mild tissue trauma in the suppression of D6 expression and the transition from uninvolved to lesional skin. This reduction in D6 transcript levels was also reflected in reduced D6 immunostaining in traumatized epidermis 48 hours after tape stripping (Figure 4E, right panels).
Thus differential D6 expression within psoriatic skin can be partly explained on the basis of psoriasis-relevant cytokine activity and trauma supportive of a role in suppressing lesion development.
Discussion
The identification and characterization of the inflammatory chemokine “scavenging” receptor D6 has shed new light on the overall regulation of the resolution phase of inflammatory responses.7 Through its ability to efficiently internalize, and degrade, inflammatory CC chemokines, D6 plays an indispensable role in the resolution of inflammatory responses at each of the body sites at which it is expressed. Our previous studies have demonstrated that D6-deficient mice respond to inflammation induced by phorbol ester by developing a psoriasiform pathology.16 Here we show that this psoriasis-like response is a common consequence of cutaneous inflammation in D6-deficient mice exposed to varied inflammatory stimuli. The remarkable similarity of the cutaneous pathology in inflamed D6-deficient skin to human psoriasis led us to examine the possible involvement of D6 in the pathogenesis of this disorder. We now report that D6 is markedly overexpressed in the epidermis and lymphatic endothelium of uninvolved compared with lesional skin of psoriatic patients as well as healthy control skin. This is coincident with elevated, but apparently nonfunctional, inflammatory CC-chemokine expression in the uninvolved areas. We have previously shown that when D6 is transgenically expressed in the murine epidermis, it can impair the development of cutaneous inflammatory responses to topically applied inflammatory stimuli.20 Therefore, the elevated D6 expression in uninvolved skin epidermis is compatible with a role in the local suppression of inflammatory chemokine action, and a block to psoriatic lesion development, at these sites.
D6 transcript levels are lower in perilesional and lesional psoriatic skin; although, as in uninvolved skin, strong D6 immunostaining is seen in lesional epidermis (presumably induced by the numerous inflammatory cytokines present at these sites), suggesting that LEC expression of D6 may significantly contribute to the relative elevation of transcript levels in uninvolved skin. Of notable interest is the observation that mild trauma, in the form of tape stripping, is able to bring about a reduction in cutaneous D6 expression. Tape stripping was selected to mimic the Koebner phenomenon,1 in which mild trauma to otherwise healthy skin of psoriatic patients can precipitate the development of lesions. Our data suggest that a trauma-induced down-regulation of D6 expression may contribute to lesion development, and explain in part the Koebner phenomenon and the transition from uninvolved to lesional skin. We propose that whereas epidermal D6 expression is again elevated at lesional sites, it is not sufficient to fully control the now-established inflammatory response.
Intriguingly, not only is D6 elevated in the epidermis and lymphatic endothelium in uninvolved skin, it is also markedly elevated in PBLs, indicating that elevated D6 expression is a generic response in patients to inflammation and one that is designed to suppress pathological inflammatory responses. Our observations of elevated D6 expression in the PBLs of systemic sclerosis patients and the demonstration of an inverse correlation between D6 expression and circulating inflammatory CC chemokine levels support this contention.25 Further analysis of PBLs in both psoriasis and systemic sclerosis indicates that D6 expression is elevated in all leukocyte subtypes, and that this elevation is not accounted for by increases in any particular leukocyte subpopulations (25) (data not shown).
To conclude, we have demonstrated for the first time that D6 is overexpressed in a range of cell types in psoriatic patients. In uninvolved psoriatic skin, expression is coincident with inflammatory CC chemokine expression and consistent with a role in suppressing chemokine-driven inflammatory responses. We suggest that where D6 expression drops in perilesional areas or after trauma, inflammatory chemokines can gain a “foot-hold” and establish inflammatory responses leading to plaque formation. We therefore propose a role for D6 as a novel suppressor of psoriasis pathogenesis, and suggest that regulation of D6 expression within psoriatic skin may be a viable therapeutic option.
Footnotes
This work was supported by grants from the Scottish Chief Scientists Office and the Medical Research Council.
Disclosures: J.M.C. is currently employed and A.R. was formerly employed by Novartis Institutes for Biomedical Research. None of the other authors report any conflicts of interest.
Supplemental material for this article can be found at http://ajp.amjapathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.06.042.
Supplementary data
Quantitation of the effects of blocking by skin thickness measurement. A: Inflammation, B: IL-1β, C: IL-17, and D: IL-15 on the development of the psoriasis-like pathology in D6-deficient mice. Control/Isotype/Mutant blocker skins are TPA treated and administered with appropriate treatment controls. The hydrocortisone, IL1β, and IL17 Ab; and IL15 blocker treated mice are also treated with TPA but received appropriate blockers as indicated. Resting skin is from mice that have not been treated with TPA. Note that all differences between the relevant controls and treated and resting skins are significant to P < 0.0001. Antibodies used (all from R&D Systems Inc., Minneapolis, MN) were as follows: anti–IL-17: rat IgG2A (clone no. 50104, MAB421); anti–IL-1β: rat IgG1 (clone no. 30311, MAB401); rat IgG2A isotype control (clone no. 54447, MAB006); rat IgG1 isotype control (clone no. 43414, MAB002).
Immunofluorescence analysis of CCL5 staining (green) in healthy control (A), uninvolved (B), perilesional (C), and lesional (D) psoriatic skin. Cell nuclei are stained with DAPI. Scale bar = 100 μm.
A: co-staining for D6 (green) and CCL2 (red) reveal co-localization in the epidermis. Scale bar = 100 μm. B: Scatter-plot analysis of co-localization of D6 and CCL2 (left panel) and background fluorescence (right panel). Note that co-localization, as shown in B, is significant with a correlation coefficient (Pearson's) of 0.612 and an overlap coefficient (Mander's) of 0.978.
Keratinocytes were treated with (A) IL-20 or IL-22 for 24 and 48 hours, respectively, or (B) a mixture of cytokines including IL-1β, IL-17, IFNγ, and TNF, all at 100 ng/mL. D6 transcript levels were measured by quantitative PCR. All differences between PBS control and Cytomix D6 expression levels are significant at P = 0.0001.
References
- 1.Nickoloff B.J., Qin J.Z., Nestle F.O. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- 2.van de Kerkhof P.C. The evolution of the psoriatic lesion. Br J Dermatol. 2007;157:4–15. doi: 10.1111/j.1365-2133.2007.07907.x. [DOI] [PubMed] [Google Scholar]
- 3.Gudjonsson J.E., Ding J., Li X., Nair R.P., Tejasvi T., Qin Z.S., Ghosh D., Aphale A., Gumucio D.L., Voorhees J.J., Abecasis G.R., Elder J.T. Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol. 2009;129:2795–2804. doi: 10.1038/jid.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rot A., von Andrian U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 5.Homey B., Meller S. Chemokines and other mediators as therapeutic targets in psoriasis vulgaris. Clin Dermatol. 2008;26:539–545. doi: 10.1016/j.clindermatol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Nickoloff B.J., Xin H., Nestle F.O., Qin J.Z. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568–573. doi: 10.1016/j.clindermatol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Graham G.J. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol. 2009;39:342–351. doi: 10.1002/eji.200838858. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A., Bonecchi R., Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 9.Nibbs R.J., Wylie S.M., Yang J., Landau N.R., Graham G.J. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J Biol Chem. 1997;272:32078–32083. doi: 10.1074/jbc.272.51.32078. [DOI] [PubMed] [Google Scholar]
- 10.Fra A.M., Locati M., Otero K., Sironi M., Signorelli P., Massardi M.L., Gobbi M., Vecchi A., Sozzani S., Mantovani A. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J Immunol. 2003;170:2279–2282. doi: 10.4049/jimmunol.170.5.2279. [DOI] [PubMed] [Google Scholar]
- 11.Weber M., Blair E., Simpson C.V., O'Hara M., Blackburn P.E., Rot A., Graham G.J., Nibbs R.J. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol Biol Cell. 2004;15:2492–2508. doi: 10.1091/mbc.E03-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nibbs R.J., Kriehuber E., Ponath P.D., Parent D., Qin S., Campbell J.D., Henderson A., Kerjaschki D., Maurer D., Graham G.J., Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madigan J., Freeman D.J., Menzies F., Forrow S., Nelson S.M., Young A., Sharkey A., Moffett A., Graham G.J., Greer I.A., Rot A., Nibbs R.J. Chemokine scavenger d6 is expressed by trophoblasts and AIDS the survival of mouse embryos transferred into allogeneic recipients. J Immunol. 2010;184:3202–3212. doi: 10.4049/jimmunol.0902118. [DOI] [PubMed] [Google Scholar]
- 14.Martinez de la Torre Y., Buracchi C., Borroni E.M., Dupor J., Bonecchi R., Nebuloni M., Pasqualini F., Doni A., Lauri E., Agostinis C., Bulla R., Cook D.N., Haribabu B., Meroni P., Rukavina D., Vago L., Tedesco F., Vecchi A., Lira S.A., Locati M., Mantovani A. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci USA. 2007;104:2319–2324. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKimmie C.S., Fraser A.R., Hansell C., Gutierrez L., Philipsen S., Connell L., Rot A., Kurowska-Stolarska M., Carreno P., Pruenster M., Chu C.C., Lombardi G., Halsey C., McInnes I.B., Liew F.Y., Nibbs R.J., Graham G.J. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J Immunol. 2008;181:3353–3363. doi: 10.4049/jimmunol.181.5.3353. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson T., Cook D.N., Nibbs R.J., Rot A., Nixon C., McLean P., Alcami A., Lira S.A., Wiekowski M., Graham G.J. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 17.Martinez de la Torre Y., Locati M., Buracchi C., Dupor J., Cook D.N., Bonecchi R., Nebuloni M., Rukavina D., Vago L., Vecchi A., Lira S.A., Mantovani A. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 18.Vetrano S., Borroni E.M., Sarukhan A., Savino B., Bonecchi R., Correale C., Arena V., Fantini M., Roncalli M., Malesci A., Mantovani A., Locati M., Danese S. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead G.S., Wang T., DeGraff L.M., Card J.W., Lira S.A., Graham G.J., Cook D.N. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med. 2007;175:243–249. doi: 10.1164/rccm.200606-839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nibbs R.J., Gilchrist D.S., King V., Ferra A., Forrow S., Hunter K.D., Graham G.J. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruchatz H., Leung B.P., Wei X.Q., McInnes I.B., Liew F.Y. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 22.Johnston A., Xing X., Guzman A.M., Riblett M., Loyd C.M., Ward N.L., Wohn C., Prens E.P., Wang F., Maier L.E., Kang S., Voorhees J.J., Elder J.T., Gudjonsson J.E. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J Immunol. 2011;186:2613–2622. doi: 10.4049/jimmunol.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshinaga Y., Higaki M., Terajima S., Ohkubo E., Nogita T., Miyasaka N., Kawashima M. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158–164. doi: 10.1007/BF01262325. [DOI] [PubMed] [Google Scholar]
- 24.Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y.J., Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codullo V., Baldwin H.M., Singh M.D., Fraser A.R., Wilson C., Gilmour A., Hueber A.J., Bonino C., McInnes I.B., Montecucco C., Graham G.J. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis. 2011;70:1115–1121. doi: 10.1136/ard.2010.137349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitation of the effects of blocking by skin thickness measurement. A: Inflammation, B: IL-1β, C: IL-17, and D: IL-15 on the development of the psoriasis-like pathology in D6-deficient mice. Control/Isotype/Mutant blocker skins are TPA treated and administered with appropriate treatment controls. The hydrocortisone, IL1β, and IL17 Ab; and IL15 blocker treated mice are also treated with TPA but received appropriate blockers as indicated. Resting skin is from mice that have not been treated with TPA. Note that all differences between the relevant controls and treated and resting skins are significant to P < 0.0001. Antibodies used (all from R&D Systems Inc., Minneapolis, MN) were as follows: anti–IL-17: rat IgG2A (clone no. 50104, MAB421); anti–IL-1β: rat IgG1 (clone no. 30311, MAB401); rat IgG2A isotype control (clone no. 54447, MAB006); rat IgG1 isotype control (clone no. 43414, MAB002).
Immunofluorescence analysis of CCL5 staining (green) in healthy control (A), uninvolved (B), perilesional (C), and lesional (D) psoriatic skin. Cell nuclei are stained with DAPI. Scale bar = 100 μm.
A: co-staining for D6 (green) and CCL2 (red) reveal co-localization in the epidermis. Scale bar = 100 μm. B: Scatter-plot analysis of co-localization of D6 and CCL2 (left panel) and background fluorescence (right panel). Note that co-localization, as shown in B, is significant with a correlation coefficient (Pearson's) of 0.612 and an overlap coefficient (Mander's) of 0.978.
Keratinocytes were treated with (A) IL-20 or IL-22 for 24 and 48 hours, respectively, or (B) a mixture of cytokines including IL-1β, IL-17, IFNγ, and TNF, all at 100 ng/mL. D6 transcript levels were measured by quantitative PCR. All differences between PBS control and Cytomix D6 expression levels are significant at P = 0.0001.