Summary
CBA mouse macrophages effectively control Leishmania major infection, yet are permissive to Leishmania amazonensis. It has been established that some Leishmania species are destroyed by reactive oxygen species (ROS). However, other species of Leishmania exhibit resistance to ROS or even down-modulate ROS production. We hypothesized that L. amazonensis–infected macrophages reduce ROS production soon after parasite–cell interaction. Employing a highly sensitive analysis technique based on chemiluminescence, the production of superoxide (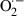 ) and hydrogen peroxide (H2O2) by L. major- or L. amazonensis-infected CBA macrophages were measured. L. major induces macrophages to release levels of
) and hydrogen peroxide (H2O2) by L. major- or L. amazonensis-infected CBA macrophages were measured. L. major induces macrophages to release levels of 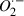 3·5 times higher than in uninfected cells. This
3·5 times higher than in uninfected cells. This 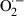 production is partially dependent on NADPH oxidase (NOX) type 2. The level of accumulated H2O2 is 20 times higher in L. major-than in L. amazonensis-infected cells. Furthermore, macrophages stimulated with L. amazonensis release amounts of ROS similar to uninfected cells. These findings support previous studies showing that CBA macrophages are effective in controlling L. major infection by a mechanism dependent on both
production is partially dependent on NADPH oxidase (NOX) type 2. The level of accumulated H2O2 is 20 times higher in L. major-than in L. amazonensis-infected cells. Furthermore, macrophages stimulated with L. amazonensis release amounts of ROS similar to uninfected cells. These findings support previous studies showing that CBA macrophages are effective in controlling L. major infection by a mechanism dependent on both 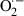 production and H2O2 generation. Furthermore, these data reinforce the notion that L. amazonensis survive inside CBA macrophages by reducing ROS production during the phagocytic process.
production and H2O2 generation. Furthermore, these data reinforce the notion that L. amazonensis survive inside CBA macrophages by reducing ROS production during the phagocytic process.
Keywords: Leishmania, macrophage, reactive oxygen intermediates
Introduction
Leishmania are obligate intracellular parasites that cause either visceral or cutaneous leishmaniases. To understand the mechanisms involved in host response to Leishmania, studies using several mouse strains have been carried out (1). The outcome of Leishmania infection is determined by the early events occurring during innate immune response. The main initial events in Leishmania–macrophage interaction are recognition, followed by parasite internalization (2–4). Parasite recognition may induce macrophages to release reactive oxygen species (ROS), such as superoxide (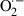 ).
). 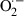 production is dependent on the recruitment of NADPH oxidase (NOX) subunits to the membrane of nascent phagosome, resulting in NOX assembly.
production is dependent on the recruitment of NADPH oxidase (NOX) subunits to the membrane of nascent phagosome, resulting in NOX assembly.
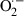 and nitric oxide (NO) are key molecules known to be involved in the macrophage-mediated innate host defence against protozoan parasites (5–7).
and nitric oxide (NO) are key molecules known to be involved in the macrophage-mediated innate host defence against protozoan parasites (5–7). 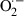 can be produced by macrophages even without any previous activation during the early contact of parasites with the host cell (5). On the other hand, NO is a molecule produced only by activated macrophages. Depending on Leishmania species both
can be produced by macrophages even without any previous activation during the early contact of parasites with the host cell (5). On the other hand, NO is a molecule produced only by activated macrophages. Depending on Leishmania species both 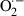 and NO play a crucial role in controlling infections (8–10). In addition to its own toxicity,
and NO play a crucial role in controlling infections (8–10). In addition to its own toxicity, 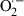 is precursor of other ROS, such as hydrogen peroxide (H2O2), hydroxyl radical (HO˙), hypochlorite (HOCl−) (5,6,11). These molecules can combine with NO to produce peroxynitrite (ONOO−) that exhibited a high toxic effect against Leishmania parasites (11). A recent in vitro study has demonstrated that there is an association between high levels of
is precursor of other ROS, such as hydrogen peroxide (H2O2), hydroxyl radical (HO˙), hypochlorite (HOCl−) (5,6,11). These molecules can combine with NO to produce peroxynitrite (ONOO−) that exhibited a high toxic effect against Leishmania parasites (11). A recent in vitro study has demonstrated that there is an association between high levels of 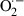 production and the significant leishmanicidal capacity of host cells (12). Nonetheless, some Leishmania species adopt various defence mechanisms to cope with oxidative stress, such as decrease in
production and the significant leishmanicidal capacity of host cells (12). Nonetheless, some Leishmania species adopt various defence mechanisms to cope with oxidative stress, such as decrease in 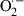 production, inhibition of NOX assembly, as well as by expression of antioxidant molecules (11,13–15).
production, inhibition of NOX assembly, as well as by expression of antioxidant molecules (11,13–15).
CBA mice, while known to be resistant to Leishmania major, are susceptible to Leishmania amazonensis. This model allows the trigger mechanisms involved in Leishmania infection to be identified because of the static genetic background of the host (16). Additionally, CBA macrophages control L. major infection, while they are permissive to L. amazonensis infection (17). We have previously shown that interferon-gamma (IFN-γ)-stimulated CBA macrophages produce similar amounts of NO in response to L. major or L. amazonensis infection (17). However, using this model, NO produced in response to IFN-γ only played a role in controlling L. major infection, which suggests that L. amazonensis modulates or is resistant to factors that control L. major infection. We hypothesized that L. amazonensis modulates the production of microbicidal molecules other than NO, such as ROS, soon after infection, allowing parasites to survive inside CBA macrophages.
A comparative study endeavouring to evaluate the ability of macrophages to release distinct levels of ROS in response to two distinct Leishmania species has not been previously performed. As the 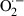 production at early stages of infection can be crucial to efficient intracellular parasite killing (12), we aimed to characterize ROS production by measuring the levels of
production at early stages of infection can be crucial to efficient intracellular parasite killing (12), we aimed to characterize ROS production by measuring the levels of 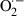 released and H2O2 generated by CBA mouse peritoneal, thioglycolate-elicited macrophages in response to L. major or L. amazonensis stimulation. The data herein show that CBA macrophages exposed to L. major produced high levels of ROS, yet in response to L. amazonensis very low levels of ROS were generated during the phagocytic process.
released and H2O2 generated by CBA mouse peritoneal, thioglycolate-elicited macrophages in response to L. major or L. amazonensis stimulation. The data herein show that CBA macrophages exposed to L. major produced high levels of ROS, yet in response to L. amazonensis very low levels of ROS were generated during the phagocytic process.
Materials and Methods
Reagents
Lucigenin (bis-N-methylacridinium nitrate), luminol (5-amino-2,3 dihydro-1,4-phthalazinedione sodium salt), microperoxidase, apocynin (4-hydroxy-methoxyacetophenone), Schneider’s medium, superoxide dismutase (SOD), thioglycolate and latex beads were obtained from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), foetal bovine serum (FBS), L-glutamine and HEPES were purchased from Invitrogen (Carlsbad, NM, USA), and ciprofloxacin was from HalexIstar (Goiânia, Brazil).
Parasites
L. amazonensis (MHOM/Br88/Ba-125) and L. major (MHOM/RI/−/WR-173) parasites were provided by Dr. Aldina Barral (CPqGM/FIOCRUZ). L. major and L. amazonensis promastigotes were maintained in Schneider’s medium plus 10% FBS for up to six passages and were expanded for 3–5 days in Schneider’s medium plus 10% FBS to reach the stationary phase, then washed with a saline solution as previously described (16) and finally adjusted to a ratio of ten parasites per macrophage (10:1 ratio).
Thioglycolate-elicited peritoneal macrophages
All experiments were performed accordingly to the standards of the Ethics Committee on Animal Experimentation at the Oswaldo Cruz Foundation (CPqGM/FIOCRUZ). Macrophages were harvested from the 4-day thioglycolate-elicited peritoneal cavity of CBA mice as previously described (17). Briefly, macrophages were cultivated in DMEM medium at a concentration of 5 × 105 cells/mL and then plated in 35-mm Petri dishes at 37°C in 5% CO2/95% humidified air. After 4 h, the nonadherent cells were removed and the cell cultures were incubated overnight.
ROS production by Leishmania-stimulated macrophages
The ROS production by peritoneal inflammatory macrophages response to Leishmania stimulation was estimated using a photon-counting device monitoring chemiluminescence (CL) incorporating a gallium arsenide photomultiplier tube (Hamamatsu R943, Hamamatsu Photonics K.K., Hamamatsu City, Japan). CL emissions from sample dishes, incubated at 37°C in a sealed chamber, were reflected and focused onto the photomultiplier tube. The emitted signal was fed directly to a frequency counter unit, and data were collected in units of photon counts per second (8).
Macrophage cultures were set aside for 3 min to allow for temperature stabilization before sampling. The 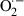 production and H2O2 formation were measured using CL. To quantify
production and H2O2 formation were measured using CL. To quantify 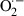 production, thioglycolate-elicited peritoneal CBA macrophages (5 × 105 cells/mL) were stimulated with L. major or L. amazonensis promastigotes (10:1 ratio) during the first 30 min of parasite–host cell interaction at 37°C in the presence of lucigenin (25 μm). Macrophage cultures were maintained for 30 min at 37°C in the presence of lucigenin (25 μm) to evaluate basal
production, thioglycolate-elicited peritoneal CBA macrophages (5 × 105 cells/mL) were stimulated with L. major or L. amazonensis promastigotes (10:1 ratio) during the first 30 min of parasite–host cell interaction at 37°C in the presence of lucigenin (25 μm). Macrophage cultures were maintained for 30 min at 37°C in the presence of lucigenin (25 μm) to evaluate basal 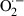 production (negative control). Opsonized zymosan particles (10:1 ratio) were used as positive (18), and latex beads (0·9 μm; 10:1 ratio) as negative controls. The rapid decay values of photon emission in response to the addition of SOD (2·5 UI/mL) were verified at the end of each assay, confirming that photon released was as a result of
production (negative control). Opsonized zymosan particles (10:1 ratio) were used as positive (18), and latex beads (0·9 μm; 10:1 ratio) as negative controls. The rapid decay values of photon emission in response to the addition of SOD (2·5 UI/mL) were verified at the end of each assay, confirming that photon released was as a result of 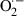 production. For H2O2 measurement, CBA macrophages were incubated with luminol (25 μm) and immediately exposed to L. major or L. amazonensis (10:1 ratio) at 37°C. After 30 min, cell supernatants were collected, and the supernatants were stored at -20°C, centrifuged at 200×g for 3 min prior to peroxide determination using a luminol-dependent CL assay (19). Briefly, luminol (25 μm) was added to cell supernatants, followed by microperoxidase (80 nm). The microperoxidase-dependent H2O2 decay was determined for the next 2 min.
production. For H2O2 measurement, CBA macrophages were incubated with luminol (25 μm) and immediately exposed to L. major or L. amazonensis (10:1 ratio) at 37°C. After 30 min, cell supernatants were collected, and the supernatants were stored at -20°C, centrifuged at 200×g for 3 min prior to peroxide determination using a luminol-dependent CL assay (19). Briefly, luminol (25 μm) was added to cell supernatants, followed by microperoxidase (80 nm). The microperoxidase-dependent H2O2 decay was determined for the next 2 min.
NOX inhibition using apocynin
Apocynin acts as an inhibitor of 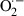 production by blocking the phosphorylation and translocation of the p47phox and p67phox subunits of NOX to phagosome membrane, resulting in inhibition of NOX assembly (20). To evaluate the role NOX plays in
production by blocking the phosphorylation and translocation of the p47phox and p67phox subunits of NOX to phagosome membrane, resulting in inhibition of NOX assembly (20). To evaluate the role NOX plays in 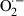 production induced by Leishmania spp. promastigotes, macrophage cultures (5 × 105 cells/mL) were treated with apocynin (500 μm) for 18 h at 37°C and then infected with L. major or L. amazonensis promastigotes at a 10:1 ratio.
production induced by Leishmania spp. promastigotes, macrophage cultures (5 × 105 cells/mL) were treated with apocynin (500 μm) for 18 h at 37°C and then infected with L. major or L. amazonensis promastigotes at a 10:1 ratio. 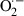 production by L. major- and L. amazonensis-infected cells treated with apocynin was measured for 10 min at 37°C in the presence of lucigenin (25 μm). Apocynin treatment (250–1000 μm) did not alter macrophage viability for 48-h culture (data not shown).
production by L. major- and L. amazonensis-infected cells treated with apocynin was measured for 10 min at 37°C in the presence of lucigenin (25 μm). Apocynin treatment (250–1000 μm) did not alter macrophage viability for 48-h culture (data not shown).
Sequential phagocytosis assays
To test whether the parasite-induced effect on 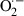 production is an active and specific L. amazonensis-induced mechanism, a sequential stimulation assay was used and Leishmania-infected macrophages were incubated with a second stimulus. Macrophages were initially incubated with L. major or L. amazonensis promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L.
amazonensis or L. major promastigotes (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μm) at 37°C and
production is an active and specific L. amazonensis-induced mechanism, a sequential stimulation assay was used and Leishmania-infected macrophages were incubated with a second stimulus. Macrophages were initially incubated with L. major or L. amazonensis promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L.
amazonensis or L. major promastigotes (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μm) at 37°C and 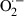 production was measured by determining photon counts emitted by stimulated cells.
production was measured by determining photon counts emitted by stimulated cells.
Data presentation and statistical analyses
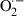 release is represented as the average level of ROS production (n = 11 experiments) by inflammatory macrophages following the addition of L.
major or L. amazonensis promastigotes.
release is represented as the average level of ROS production (n = 11 experiments) by inflammatory macrophages following the addition of L.
major or L. amazonensis promastigotes. 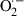 production by infected and control cells were also expressed as Rmax, which represents average of the highest CL response from stimulated cells. H2O2 accumulation is illustrated by a representative experiment (one of five identical experiments). The equation R= Rmax/(Tmax − Ti) was used to estimate the amount of H2O2 detected in culture supernatants of Leishmania-infected cells (21). Rmax= average of the highest CL response from stimulated cells, Tmax = the point in time (seconds) at which the maximum number of photons is emitted by cells and Ti = the time point (seconds) at which cells begin to emit photons. All statistical tests were performed using graphpad prism 4·00 (San Diego, CA, USA), and analyses used were Student’s t-test with Welch’s correction, Mann–Whitney U-test or one-way anova with Newman–Keuls post-test. Differences with P<0·05 were considered statistically significant.
production by infected and control cells were also expressed as Rmax, which represents average of the highest CL response from stimulated cells. H2O2 accumulation is illustrated by a representative experiment (one of five identical experiments). The equation R= Rmax/(Tmax − Ti) was used to estimate the amount of H2O2 detected in culture supernatants of Leishmania-infected cells (21). Rmax= average of the highest CL response from stimulated cells, Tmax = the point in time (seconds) at which the maximum number of photons is emitted by cells and Ti = the time point (seconds) at which cells begin to emit photons. All statistical tests were performed using graphpad prism 4·00 (San Diego, CA, USA), and analyses used were Student’s t-test with Welch’s correction, Mann–Whitney U-test or one-way anova with Newman–Keuls post-test. Differences with P<0·05 were considered statistically significant.
Results and Discussion
L. major but not L. amazonensis induces 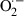 production in CBA macrophage cultures.
production in CBA macrophage cultures.
The present study aimed to evaluate ROS production by macrophages in response to different stimuli. Uninfected macrophages released very low levels of 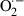 that ranged from 10 to 122 photon counts (n = 10) and were similar to those detected in macrophage cultures stimulated with latex beads (38·4–99·10 photon counts) (n = 1). By contrast, the positive control cultures stimulated with zymosan particles released high levels of
that ranged from 10 to 122 photon counts (n = 10) and were similar to those detected in macrophage cultures stimulated with latex beads (38·4–99·10 photon counts) (n = 1). By contrast, the positive control cultures stimulated with zymosan particles released high levels of 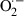 (581·2–7072·2, n = 11).
(581·2–7072·2, n = 11).
Kinetics analysis of 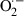 production shows an increase in the
production shows an increase in the 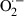 amount when L. major promastigotes were added to cells (Figure 1a). By contrast, L. amazonensis promastigotes fail to induce the release of significant amounts of
amount when L. major promastigotes were added to cells (Figure 1a). By contrast, L. amazonensis promastigotes fail to induce the release of significant amounts of 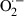 (Figure 1a) which was similar to levels in control nonstimulated macrophages or stimulated with latex beads (data not shown). When dead L. major promastigotes were added to macrophage cultures, no increase in photon counts was observed (Figure 1a), which supports the notion that
(Figure 1a) which was similar to levels in control nonstimulated macrophages or stimulated with latex beads (data not shown). When dead L. major promastigotes were added to macrophage cultures, no increase in photon counts was observed (Figure 1a), which supports the notion that 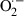 production is dependent on L. major viability. The addition of SOD (2·5 UI/mL) at the end of each assay confirms that photon released is as a result of
production is dependent on L. major viability. The addition of SOD (2·5 UI/mL) at the end of each assay confirms that photon released is as a result of 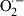 production (Figure 1b).
production (Figure 1b).
Figure 1.
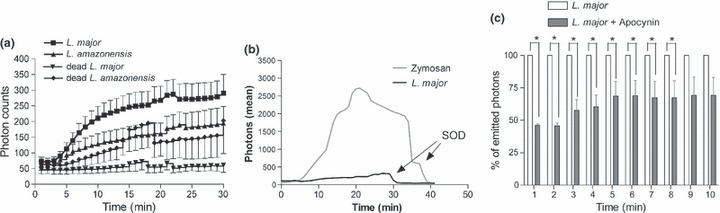
Leishmania major promastigotes induce NOX-dependent 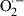 production. Thioglycolate-elicited peritoneal macrophages were incubated with L.
major or L. amazonensis promastigotes at a 10:1 ratio at 37°C for 30 min in the presence of lucigenin (25 μm). Control cells were incubated with dead L.
major or dead L. amazonensis promastigotes, as well as zymosan, under the same conditions.
production. Thioglycolate-elicited peritoneal macrophages were incubated with L.
major or L. amazonensis promastigotes at a 10:1 ratio at 37°C for 30 min in the presence of lucigenin (25 μm). Control cells were incubated with dead L.
major or dead L. amazonensis promastigotes, as well as zymosan, under the same conditions. 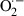 production was measured using lucigenin-based chemiluminescence (CL), expressed in photon counts. L. major promastigotes induce the release of significantly higher amounts of
production was measured using lucigenin-based chemiluminescence (CL), expressed in photon counts. L. major promastigotes induce the release of significantly higher amounts of 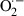 in comparison with L. amazonensis (n = 11, P<0·001, One-way anova and Newman–Keuls), but these levels did not differ significantly from those produced by control macrophage cultures stimulated with dead parasites (a). Lucigenin-based CL decreased in stimulated cell cultures in response to superoxide dismutase (SOD). SOD (2·5 U/mL) was added at the end of each assay, which confirms that photon released in response to L. major or zymosan is dependent on
in comparison with L. amazonensis (n = 11, P<0·001, One-way anova and Newman–Keuls), but these levels did not differ significantly from those produced by control macrophage cultures stimulated with dead parasites (a). Lucigenin-based CL decreased in stimulated cell cultures in response to superoxide dismutase (SOD). SOD (2·5 U/mL) was added at the end of each assay, which confirms that photon released in response to L. major or zymosan is dependent on 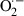 production (one representative experiment out of eight similar experiments) (b). NOX inhibition by apocynin cause partial reduction in lucigenin-based CL. L. major-infected cells were pretreated for 18 h with apocynin (500 μm) prior to the addition of parasites.
production (one representative experiment out of eight similar experiments) (b). NOX inhibition by apocynin cause partial reduction in lucigenin-based CL. L. major-infected cells were pretreated for 18 h with apocynin (500 μm) prior to the addition of parasites. 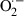 production was detected at 37°C for 10 min and was partially reduced by apocynin. Results are expressed as the percentage of the number of photons emitted by apocynin-treated cells (ranging from 57·6 to 193·9 photons) in relation to untreated macrophages considered as 100% (ranging from 126·2 to 318·7 photons) (n = 4, P=0·02, Mann–Whitney U-test) (c).
production was detected at 37°C for 10 min and was partially reduced by apocynin. Results are expressed as the percentage of the number of photons emitted by apocynin-treated cells (ranging from 57·6 to 193·9 photons) in relation to untreated macrophages considered as 100% (ranging from 126·2 to 318·7 photons) (n = 4, P=0·02, Mann–Whitney U-test) (c).
Next, the participation of NOX assembly in 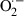 production was evaluated in cells pretreated with apocynin (500 μm). First, pretreatment of L. major-infected cells with apocynin was performed and induced a partial reduction on
production was evaluated in cells pretreated with apocynin (500 μm). First, pretreatment of L. major-infected cells with apocynin was performed and induced a partial reduction on 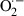 production (n = 4, P = 0·02, Mann–Whitney; Figure 1c). This partial inhibition of
production (n = 4, P = 0·02, Mann–Whitney; Figure 1c). This partial inhibition of 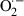 production by apocynin indicates that L. major-induced release of
production by apocynin indicates that L. major-induced release of 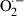 production is dependent on NOX2 and also on a different NOX, such as NOX4. NOX4 is an NADPH-dependent oxidase that is not inhibited by apocynin (20). It is highly expressed in numerous cell types including endothelial cells (22) and embryonic stem cells (23). Although it has been described that NOX4 is involved in other cell functions (24,25), its role in innate immunity has been suggested (26), so it is possible that this oxidase also participates in the
production is dependent on NOX2 and also on a different NOX, such as NOX4. NOX4 is an NADPH-dependent oxidase that is not inhibited by apocynin (20). It is highly expressed in numerous cell types including endothelial cells (22) and embryonic stem cells (23). Although it has been described that NOX4 is involved in other cell functions (24,25), its role in innate immunity has been suggested (26), so it is possible that this oxidase also participates in the 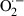 production involved in the control of Leishmania infection. Then, L. amazonensis-infected macrophages were pretreated with apocynin that did not modify
production involved in the control of Leishmania infection. Then, L. amazonensis-infected macrophages were pretreated with apocynin that did not modify 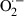 production by these cells (data not shown). This finding suggests that the
production by these cells (data not shown). This finding suggests that the 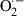 production by CBA macrophages detected during the assay was not dependent upon NOX.
production by CBA macrophages detected during the assay was not dependent upon NOX.
The average value of the maximum number of lucigenin-derived photons released (Rmax) by macrophages in response to L. major was then calculated and shown to be 276·10 ± 98·08 photon counts, a value 3·5 times higher (P<0·05; n = 6; Kruskal–Wallis) than the Rmax detected in uninfected macrophage cultures (40·70 ± 10·39 photon counts; P>0·05; n = 6, Kruskal–Wallis). In addition, the Rmax values of lucigenin-derived photons in macrophage cultures stimulated with L. amazonensis (177·30 ± 73·54 photon counts) was not statistically different (P>0·05; n = 6, Kruskal–Wallis) from those in control macrophages. These findings show that, different from L. major, L. amazonensis did not trigger 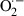 production during phagocytosis.
production during phagocytosis.
Next, we hypothesized that L. amazonensis inhibits 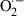 production in response to L. major infection. To test this hypothesis, sequential phagocytic assays were then performed by incubating cells with L. amazonensis promastigotes for 30 min, followed by a 30-min period of incubation with L. major. As expected, macrophages uniquely infected with L. amazonensis produced very low levels of
production in response to L. major infection. To test this hypothesis, sequential phagocytic assays were then performed by incubating cells with L. amazonensis promastigotes for 30 min, followed by a 30-min period of incubation with L. major. As expected, macrophages uniquely infected with L. amazonensis produced very low levels of 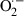 (Figure 2). The addition of L. major promastigotes to L. amazonensis-stimulated cells reverted the relatively low levels of
(Figure 2). The addition of L. major promastigotes to L. amazonensis-stimulated cells reverted the relatively low levels of 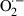 production, which were increased to levels similar to those produced by cells uniquely stimulated with L. major (Figure 2). Thereafter, cells were primarily stimulated with L. major promastigotes for 30 min, followed by a 30-min period of incubation with L. amazonensis. Interestingly, the addition of L. amazonensis promastigotes to macrophages previously stimulated with L. major did not reverse the L. major-induced enhancement of
production, which were increased to levels similar to those produced by cells uniquely stimulated with L. major (Figure 2). Thereafter, cells were primarily stimulated with L. major promastigotes for 30 min, followed by a 30-min period of incubation with L. amazonensis. Interestingly, the addition of L. amazonensis promastigotes to macrophages previously stimulated with L. major did not reverse the L. major-induced enhancement of 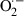 production.
production. 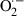 levels remained similar to those produced by macrophages which were exclusively stimulated with L. major (Figure 2), showing that L. amazonensis promastigotes did not additionally stimulate
levels remained similar to those produced by macrophages which were exclusively stimulated with L. major (Figure 2), showing that L. amazonensis promastigotes did not additionally stimulate 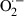 production by macrophages, even when NOX complex was already assembled in response to L. major stimulation. In sum, these findings suggest that the events, regarding
production by macrophages, even when NOX complex was already assembled in response to L. major stimulation. In sum, these findings suggest that the events, regarding 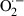 production in response to L. major and lack of production in response to L. amazonensis, are independent of each other.
production in response to L. major and lack of production in response to L. amazonensis, are independent of each other.
Figure 2.
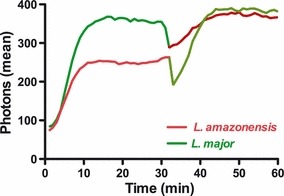
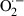 production by macrophages sequentially stimulated with L. major or L. amazonensis promastigotes. Phagocytic assays were performed by incubating cells with L. major (green) or L. amazonensis (red) promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L. amazonensis or L. major promastigote (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μm) at 37°C and
production by macrophages sequentially stimulated with L. major or L. amazonensis promastigotes. Phagocytic assays were performed by incubating cells with L. major (green) or L. amazonensis (red) promastigotes for 30 min. Next, the parasite stimuli were switched, and the cells were incubated for a second 30-min period, with either L. amazonensis or L. major promastigote (10:1), respectively. These sequential stimulations were performed in the presence of lucigenin (25 μm) at 37°C and 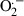 production was measured via lucigenin-based chemiluminescence emitted by cells. L. amazonensis did not revert the
production was measured via lucigenin-based chemiluminescence emitted by cells. L. amazonensis did not revert the 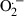 production induced by L. major in macrophage cultures, yet incubation with L.
major in cultures previously stimulated with L. amazonensis did revert relative low levels of
production induced by L. major in macrophage cultures, yet incubation with L.
major in cultures previously stimulated with L. amazonensis did revert relative low levels of 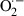 production (one representative experiment out of three similar experiments).
production (one representative experiment out of three similar experiments).
The mechanism involved in the failure of 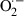 production in L. amazonensis-infected cells remained to be elucidated. It is possible that L. amazonensis alters ROS production by host cells, using one of the mechanisms that have been previously described for several microbes: (i) Leishmania donovani promastigotes delay
production in L. amazonensis-infected cells remained to be elucidated. It is possible that L. amazonensis alters ROS production by host cells, using one of the mechanisms that have been previously described for several microbes: (i) Leishmania donovani promastigotes delay 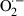 production by preventing NOX assembly and phagosome maturation (5,13,14), subsequent to maintenance of a periphagosomal coat of F-actin (5,13,14,27); (ii) Leishmania pifanoi amastigotes avoid
production by preventing NOX assembly and phagosome maturation (5,13,14), subsequent to maintenance of a periphagosomal coat of F-actin (5,13,14,27); (ii) Leishmania pifanoi amastigotes avoid 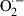 production by inducing an increase in haeme degradation. This results in blockage of the maturation of gp91phox subunit of NOX, and, subsequently, prevents assembly of the NOX complex (15); (iii) Salmonella typhimurium reduces
production by inducing an increase in haeme degradation. This results in blockage of the maturation of gp91phox subunit of NOX, and, subsequently, prevents assembly of the NOX complex (15); (iii) Salmonella typhimurium reduces 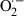 production by removing cytochrome b558 subunit from the phagosomal membrane of infected macrophages (16,28); and (iv) Helicobacter pylori recruits to nascent phagosomes cytochrome b558, yet does not efficiently acquire or retain p47phox or p67phox components of NOX. This results in disruption of NOX, lack of ROS accumulation inside phagosomes and
production by removing cytochrome b558 subunit from the phagosomal membrane of infected macrophages (16,28); and (iv) Helicobacter pylori recruits to nascent phagosomes cytochrome b558, yet does not efficiently acquire or retain p47phox or p67phox components of NOX. This results in disruption of NOX, lack of ROS accumulation inside phagosomes and 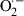 release into the cytoplasm (29).
release into the cytoplasm (29).
L. major induces H2O2 accumulation in macrophage cultures
ROS generation is a process involving a cascade of events that begins with 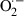 production, which dismutates into H2O2 either spontaneously, especially at low pH levels, or via a mechanism dependent on SOD (30,31). In vitro experiments demonstrated a dose-dependent leishmanicidal effect of H2O2 against L. donovani, Leishmania tropica and Leishmania chagasi promastigotes (11). Using the phenol red method, we described previously that L. amazonensis induced the accumulation of half as much H2O2 as was accumulated in L. major-infected macrophages (17). To confirm this, we measured peroxide levels using the more sensitive luminol-based CL method to determine microperoxidase-induced decay of H2O2 (19). The highest amount of H2O2 was detected in supernatants from live L. major-infected macrophages (Figure 3). To illustrate the differences in H2O2 accumulation between L. major- and L. amazonensis-infected cells, the maximal oxidative responses for a specific time interval were calculated using the equation R= Rmax/(Tmax − Ti) (21). Figure 3(b) illustrates the R values corresponding to H2O2 accumulation in supernatants of L. major- or L. amazonensis-stimulated macrophages. These findings reveal that H2O2 accumulation in supernatants of L. major-stimulated macrophages was 20 times greater than in L. amazonensis-stimulated cells (P=0·04, Student’s t-test with Welch’s correction; Figure 3b).
production, which dismutates into H2O2 either spontaneously, especially at low pH levels, or via a mechanism dependent on SOD (30,31). In vitro experiments demonstrated a dose-dependent leishmanicidal effect of H2O2 against L. donovani, Leishmania tropica and Leishmania chagasi promastigotes (11). Using the phenol red method, we described previously that L. amazonensis induced the accumulation of half as much H2O2 as was accumulated in L. major-infected macrophages (17). To confirm this, we measured peroxide levels using the more sensitive luminol-based CL method to determine microperoxidase-induced decay of H2O2 (19). The highest amount of H2O2 was detected in supernatants from live L. major-infected macrophages (Figure 3). To illustrate the differences in H2O2 accumulation between L. major- and L. amazonensis-infected cells, the maximal oxidative responses for a specific time interval were calculated using the equation R= Rmax/(Tmax − Ti) (21). Figure 3(b) illustrates the R values corresponding to H2O2 accumulation in supernatants of L. major- or L. amazonensis-stimulated macrophages. These findings reveal that H2O2 accumulation in supernatants of L. major-stimulated macrophages was 20 times greater than in L. amazonensis-stimulated cells (P=0·04, Student’s t-test with Welch’s correction; Figure 3b).
Figure 3.
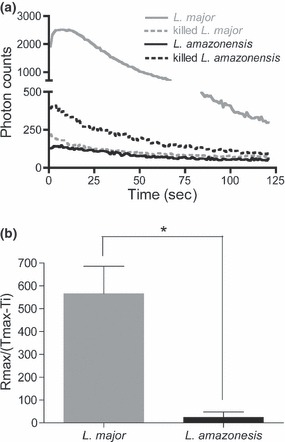
L. major promastigotes induce higher levels of H2O2 accumulation. Thioglycolate-elicited peritoneal macrophages were incubated with L. amazonensis or L. major promastigotes (10:1) for 30 min at 37°C. H2O2 accumulation was measured in cell supernatants by chemiluminescence decay in the presence of luminol (25 μm) and microperoxidase (80 nm) for an additional two min. The highest amount of H2O2 was detected in supernatants collected from live L. major-stimulated macrophages (one representative experiment out of five similar experiments) (a). Differences in H2O2 accumulation between L. major- and L. amazonensis-infected cells are expressed as the maximal oxidative responses for a given time interval [R] (as described in Materials and Methods). L. major-infected macrophages accumulated twenty times more H2O2 than L. amazonensis-infected cells (n = 3, P=0·04, Student’s t-test with Welch’s correction) (b).
The luminol-microperoxidase method was used to distinguish H2O2 accumulation from the production of others ROS, which are also detected by luminol-based CL, such as 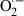 (32). In addition to the highest levels of
(32). In addition to the highest levels of 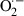 produced by L. major-infected macrophages, it is also conceivable that the initial cellular production of
produced by L. major-infected macrophages, it is also conceivable that the initial cellular production of 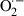 , followed by subsequent dismutation into H2O2, was likely responsible for the elevated levels of ROS detected in supernatants (Figure 3). The data presented herein do not rule out the possibility that other ROS besides H2O2 are released in L. major-infected cultures. In fact, there is evidence that inside macrophages, H2O2 can be converted in a variety of other ROS, such as •OH, HOCl− (33) and ONOO−. As ONOO− exhibited a great toxic effect against Leishmania, it is possible that this compound plays a crucial role in L. major killing inside macrophages from CBA mice (6). Furthermore, the cellular and molecular mechanisms whereby ROS exert their cytotoxic activities are not yet fully described for Leishmania (11). Regarding
, followed by subsequent dismutation into H2O2, was likely responsible for the elevated levels of ROS detected in supernatants (Figure 3). The data presented herein do not rule out the possibility that other ROS besides H2O2 are released in L. major-infected cultures. In fact, there is evidence that inside macrophages, H2O2 can be converted in a variety of other ROS, such as •OH, HOCl− (33) and ONOO−. As ONOO− exhibited a great toxic effect against Leishmania, it is possible that this compound plays a crucial role in L. major killing inside macrophages from CBA mice (6). Furthermore, the cellular and molecular mechanisms whereby ROS exert their cytotoxic activities are not yet fully described for Leishmania (11). Regarding 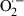 and H2O2 microbicidal activity (30,34), we suggest that these molecules may contribute to intracellular events resulting in L. major killing inside CBA macrophages.
and H2O2 microbicidal activity (30,34), we suggest that these molecules may contribute to intracellular events resulting in L. major killing inside CBA macrophages.
Concluding Remarks
Previous study using the phenol red method showed that L. amazonensis induced the accumulation of half as much H2O2 as was accumulated in L. major-infected inflammatory macrophages (17). These data are in accordance with this study which employed comparative and real-time CL assay, a high-sensitive approach that evaluates ROS production in cell cultures (35,36). A recent study has demonstrated that Leishmania mexicana, a parasite species closely related to L. amazonensis, diminished ROS production in PMA-stimulated macrophages from both BALB/c and C57BL/6 mice (37). Nonetheless, this study is the first report, which demonstrates that two distinct species of Leishmania markedly triggered the production of different levels of ROS in macrophages from a unique mouse strain. The fact that L. amazonensis-infected cells release lower amounts of ROS, in comparison with either uninfected or L. major-infected macrophages, suggests that the inability of CBA macrophages to destroy L amazonensis parasites (7) depend, at least partially, on inefficient ROS production. It has been recently demonstrated by Khouri et al. (12) that exposition of Leishmania braziliensis- or L. amazonensis-infected cells to increasing levels of 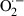 induced a severe reduction in the number of intracellular parasites, demonstrating an effective role for
induced a severe reduction in the number of intracellular parasites, demonstrating an effective role for 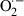 in intracellular parasite killing. Other authors have shown that a low ROS production by Leishmania-infected macrophages is a result of the parasite antioxidative response for ROS production (38,39). However, we present evidence against this idea, because L. major and L. amazonensis parasites did not exhibit any
in intracellular parasite killing. Other authors have shown that a low ROS production by Leishmania-infected macrophages is a result of the parasite antioxidative response for ROS production (38,39). However, we present evidence against this idea, because L. major and L. amazonensis parasites did not exhibit any 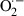 production and H2O2 formation when incubated alone with lucigenin or luminol, respectively (data not shown) and also did not exhibit any anti-oxidative responses when incubated with
production and H2O2 formation when incubated alone with lucigenin or luminol, respectively (data not shown) and also did not exhibit any anti-oxidative responses when incubated with 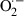 and H2O2 donors (data not shown). Alternatively, the inability of CBA macrophages to kill L. amazonensis may depend on interactions between parasite surface molecules and macrophage receptors (38–40), which may lead to the modulation of host-cell signalling pathways (41) and a macrophage deficiency in the activation of parasite innate killing mechanisms (42). Also viable parasites can express different surface molecules able to interact with macrophage’s surface receptors necessary to induce ROS production. We showed that the genetic background of the host determines the relative degree in which the parasite could be modulating the oxidative response, but further experiments need to be performed to determine the exact mechanism involved in the impairment of ROS production in L. amazonensis-infected CBA macrophages.
and H2O2 donors (data not shown). Alternatively, the inability of CBA macrophages to kill L. amazonensis may depend on interactions between parasite surface molecules and macrophage receptors (38–40), which may lead to the modulation of host-cell signalling pathways (41) and a macrophage deficiency in the activation of parasite innate killing mechanisms (42). Also viable parasites can express different surface molecules able to interact with macrophage’s surface receptors necessary to induce ROS production. We showed that the genetic background of the host determines the relative degree in which the parasite could be modulating the oxidative response, but further experiments need to be performed to determine the exact mechanism involved in the impairment of ROS production in L. amazonensis-infected CBA macrophages.
Acknowledgments
We would like to thank Dr. Washington Luis Conrado dos Santos who provided assistance with statistical analysis and Andris K. Walter for providing English revision and consulting services.
References
- 1.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Russell DG, Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989;10:328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967;46:19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Channon JY, Roberts MB, Blackwell JM. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunol. 1984;53:345–355. [PMC free article] [PubMed] [Google Scholar]
- 6.Murray HW, Cartelli DM. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983;72:32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray HW, Nathan CF. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assreuy J, Cunha FQ, Epperlein M, et al. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol. 1994;24:672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- 9.Giudice A, Camada I, Leopoldo PT, et al. Resistance of Leishmania Leishmania amazonensis and Leishmania Viannia braziliensis to nitric oxide correlates with disease severity in Tegumentary Leishmaniasis. BMC Infect Dis. 2007;7:7. doi: 10.1186/1471-2334-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukbel RM, Patten C, Jr, et al. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Amer J Trop Med Hyg. 2007;76:669–675. [PubMed] [Google Scholar]
- 11.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Rad Biol Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Khouri R, Bafica A, Silva Mda P, et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 13.Lodge R, Descoteaux A. Phagocytosis of Leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of NADPH oxidase activation. Eur J Immunol. 2006;36:2735–2744. doi: 10.1002/eji.200636089. [DOI] [PubMed] [Google Scholar]
- 14.Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 15.Pham NK, Mouriz J, Kima PE. Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing haeme degradation. Inf immun. 2005;73:8322–8333. doi: 10.1128/IAI.73.12.8322-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemos de Souza V, Ascencao Souza J, Correia Silva TM, Sampaio Tavares Veras P, Rodrigues de-Freitas LA. Different Leishmania species determine distinct profiles of immune and histopathological responses in CBA mice. Microbes Infect. 2000;2:1807–1815. doi: 10.1016/s1286-4579(00)01340-x. [DOI] [PubMed] [Google Scholar]
- 17.Gomes IN, Calabrich AF, Tavares Rda S, Wietzerbin J, de Freitas LA, Veras PS. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes Infect. 2003;5:251–260. doi: 10.1016/s1286-4579(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 18.Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- 19.Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS. Mitochondrial superoxide production during oxalate-mediated oxidative stress in renal epithelial cells. Free Rad Biol Med. 2002;32:1339–1350. doi: 10.1016/s0891-5849(02)00846-8. [DOI] [PubMed] [Google Scholar]
- 20.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldefie-Chezet F, Walrand S, Moinard C, Tridon A, Chassagne J, Vasson MP. Is the neutrophil reactive oxygen species production measured by luminol and lucigenin chemiluminescence intra or extracellular? Comparison with DCFH-DA flow cytometry and cytochrome c reduction. Clin Chim Acta. 2002;319:9–17. doi: 10.1016/s0009-8981(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 22.Ago T, Kitazono T, Ooboshi H, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Stansbury KH, Zhu H, Trush MA. Biochemical characterization of lucigenin (Bis-N-methylacridinium) as a chemiluminescent probe for detecting intramitochondrial superoxide anion radical production. Biochem Biophys Res Commun. 1999;262:80–87. doi: 10.1006/bbrc.1999.1174. [DOI] [PubMed] [Google Scholar]
- 24.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 25.Mahadev K, Motoshima H, Wu X, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm A, Tejle K, Magnusson KE, Descoteaux A, Rasmusson B. Leishmania donovani lipophosphoglycan causes periphagosomal actin accumulation: correlation with impaired translocation of PKCalpha and defective phagosome maturation. Cell Microbiol. 2001;3:439–447. doi: 10.1046/j.1462-5822.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 28.Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 29.Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 30.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 31.Scandalios JG. The rise of ROS. Trends Biochem Sci. 2002;27:483–486. doi: 10.1016/s0968-0004(02)02170-9. [DOI] [PubMed] [Google Scholar]
- 32.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 33.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. Faseb J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 34.Linares E, Giorgio S, Mortara RA, Santos CX, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Rad Biol Med. 2001;30:1234–1242. doi: 10.1016/s0891-5849(01)00516-0. [DOI] [PubMed] [Google Scholar]
- 35.Hothersall JS, Gordge M, Noronha-Dutra AA. Inhibition of NADPH supply by 6-aminonicotinamide: effect on glutathione, nitric oxide and superoxide in J774 cells. FEBS Lett. 1998;434:97–100. doi: 10.1016/s0014-5793(98)00959-4. [DOI] [PubMed] [Google Scholar]
- 36.Yamashoji S. Determination of viable mammalian cells by luminol chemiluminescence using microperoxidase. Anal Biochem. 2009;386:119–120. doi: 10.1016/j.ab.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Delgado-Dominguez J, Gonzalez-Aguilar H, Aguirre-Garcia M, et al. Leishmania mexicana lipophosphoglycan differentially regulates PKCalpha-induced oxidative burst in macrophages of BALB/c and C57BL/6 mice. Parasite Immunol. 2010;32:440–449. doi: 10.1111/j.1365-3024.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 38.Descoteaux A, Matlashewski G, Turco SJ. Inhibition of macrophage protein kinase C-mediated protein phosphorylation by Leishmania donovani lipophosphoglycan. J Immunol. 1992;149:3008–3015. [PubMed] [Google Scholar]
- 39.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes IN, Palma LC, Campos GO, et al. The scavenger receptor MARCO is involved in Leishmania major infection by CBA/J macrophages. Parasite Immunol. 2009;31:188–198. doi: 10.1111/j.1365-3024.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- 41.Martiny A, Meyer-Fernandes JR, de Souza W, Vannier-Santos MA. Altered tyrosine phosphorylation of ERK1 MAP kinase and other macrophage molecules caused by Leishmania amastigotes. Mol Biochem Parasitol. 1999;102:1–12. doi: 10.1016/s0166-6851(99)00067-5. [DOI] [PubMed] [Google Scholar]
- 42.Bogdan C. Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol. 2008;10:1221–1234. doi: 10.1111/j.1462-5822.2008.01146.x. [DOI] [PubMed] [Google Scholar]


