Abstract
Purpose
The aim of this study was to assess the stability of the glycosaminoglycan (GAG) content in the long term after matrix-associated autologous chondrocyte transplantation (MACT) with Hyalograft C in the knee over a follow-up period of one year.
Methods
In this cross-sectional evaluation, 11 patients after MACT of the knee consented to delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) measurements. The mean post-operative interval before the first MR examination was 40.6 ± 22.0 months, and the second MR examination was carried out after another 12 months. The Lysholm score was assessed for clinical evaluation. Quantitative T1 measurements after intravenous negatively charged MR contrast agent administration were performed. Global post-contrast T1 of the reference cartilage and the repair tissue and a relative post-contrast T1 value were calculated.
Results
The Lysholm score improved significantly from 59.8 ± 12.9 at baseline to 86.1 ± 15.7 at the second visit (p < 0.01). The mean global T1 of the repair tissue (1st visit 581.3 ± 126.4 ms; 2nd visit 684.1 ± 169.9 ms; p = 0.104) and the mean relative T1 value showed stable results over one year (1st visit 0.81 ± 0.28; 2nd visit 0.76 ± 0.32; p = 0.4).
Conclusions
The study demonstrated stable glycosaminoglycan content of the repair tissue after MACT at midterm.
Introduction
The treatment of deep cartilage defects remains a complex and demanding field in orthopaedic surgery. There is a wide variety of different surgical techniques for the treatment of singular defects available; however, despite partly encouraging results, we still lack a strategy to restore full joint function in the long term. Cell-based techniques originating from autologous chondrocyte implantation (ACI) technique introduced by Brittberg et al. [1] still have the best potential to introduce repair tissue (RT) into the defect in sufficient quality to withstand joint loading in the long term in order to relieve symptoms and, above all, to significantly delay the onset of osteoarthritis (OA) [2].
Among others, matrix-associated ACT (MACT) with a hyaluronan matrix (Hyalograft C) has been widely applied and the technique shows good midterm results [3, 4]. In a study by Marcacci et al., 71.4 % of patients reported that they had unlimited joint function in everyday activities three years after surgery and 76 % stated that they had no pain [5]. Several studies demonstrated that the major adverse events associated with the periosteal flap in the original ACI technique such as hypertrophy (2.4–20 %) and adhesions (4.8–19.6 %) have been significantly reduced in MACT [5, 6].
In addition to ligament stability and orthograde joint axis, the long-term clinical outcome will depend on RT quality. Glycosaminoglycans (GAG) are the main source of fixed charge density (FCD) in articular cartilage, which are thought to be the first component of the extracellular matrix to be lost in early cartilage degeneration. Several studies have demonstrated that cases with hyaline cartilage, regardless of the respective technique that is used to introduce the RT, will have a lower probability of failure than cases with fibrous RT [7–10]. Knutsen et al. suggested that good two-year results can predict good outcome at the five-year follow-up [8, 11]. Nehrer et al. reported a more fibrous composition in failed cartilage repair before 18 months [10]. Therefore, it is of interest to assess the GAG content, since it seems that a stable GAG content has a positive predictive value for the future clinical outcome.
Histological evaluation after cartilage repair remains the gold standard to evaluate cartilage repair techniques; however, it is restricted by its invasiveness. Quantitative magnetic resonance (MR) mapping techniques have become a valid alternative to histology for cartilage repair monitoring [12, 13]. In particular, delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) allows one to assess the GAG content both of cartilage and RT. Briefly, the technique is based on the principle that cartilage GAG confers a negative FCD to the cartilage matrix; negatively charged contrast agent (gadolinium diethylenetriamine pentaacetate anion, Gd-DTPA2−) will therefore accumulate in areas with lower GAG content after administration. This specific mechanism can be quantified by MR T1 mapping and has been shown to be feasible for the assessment of cartilage RT GAG content [14–17]. The major advantage of quantitative MR mapping is that it allows one to directly compare different case series in terms of RT quality, provided that the MR protocols are adequate. dGEMRIC is still the most commonly used technique to evaluate cartilage RT GAG content. In a study by Trattnig et al., the authors demonstrated a maturation of the graft over time in 15 patients after MACT in the knee [16].
With the availability of dGEMRIC in clinics, we aimed to obtain additional data on the RT quality after Hyalograft C. The specific aim of this study was to determine if the GAG content was constant over a follow-up period of one year in patients two to four years after surgery.
Methods
Patient population
Out of a case series of 53 patients treated with Hyalograft C between 2001 and 2006 [4], 11 cases consented to undergo repeated dGEMRIC measurements for this cross-sectional evaluation. The surgical technique and post-operative rehabilitation protocol have been reported on in detail [4, 18].
Briefly, surgery took place in two steps. First, arthroscopy was performed to harvest chondrocytes from a non-weight-bearing area and to measure the exact defect size. After this procedure, the harvested material was stored in a sterile transport medium and shipped to the laboratory, where the chondrocytes were expanded in culture and the MACT implant was prepared. In the second operation the autograft was implanted through an arthrotomy.
After surgery, all patients underwent rehabilitation to restore function while protecting the RT. Immediately after the surgery, all patients wore a brace. On the next day, continuous passive motion from 0 to 30° of flexion was started and continued until full flexion was possible after four weeks. Crutch-assisted non-weight-bearing ambulation was ordered for six weeks. During weeks seven to 12 patients walked with touch-down weight-bearing until full weight-bearing was achieved. Afterwards, rehabilitation was not monitored. Moderate exercise such as cycling and swimming was started three to six months after surgery, but sports with stop-and-go activity and jumping were prohibited for the first year.
Regarding the specific study group, four patients were female (36.4 %) and seven male (63.6 %); the average age (± standard deviation) was 31.8 ± 10.3 years, the body mass index (BMI) was 24.5 ± 3.7 kg/m2 and the average lesion size was 4.0 ± 1.9 cm2. The defect was located on the medial femoral condyle (MFC) in ten and on the lateral femoral condyle (LFC) in one case. All defects were classified as Outerbridge grade III or IV [19]. The modality of the onset of symptoms was gradual (n = 5), osteochondritis dissecans (n = 4), trauma (n = 1) and not otherwise specified trauma (n = 1). There were eight patients (72.7 %) who had surgical procedures before MACT surgery: anterior cruciate ligament reconstruction (n = 3), partial meniscectomy (n = 2), subchondral drilling (n = 2), microfracturing (n = 2), spongiosaplasty (n = 1) and corrective osteotomy (n = 1).
The minimum post-operative interval before the first MR examination was 12 months (mean 40.6 ± 22.0 months). The second MR examination was carried out after a further 12 months.
Clinical examination
For clinical examination the Lysholm score was evaluated [20]. The Lysholm score was assessed before surgery and at both MR visits. The main focus of this score is on instability, pain, locking, swelling, possibility of stair climbing, limping, need for a walker and if a crouch is possible.
MR technique
The recommendations given by Burstein et al. [14] were followed: after an intravenous bolus injection of 0.2 mmol gadolinium per kilogram body weight, 20 minutes of exercise was carried out and the interval until the MR measurement was 90 minutes. The images were obtained in the sagittal plane with a 3 T MR unit (Magnetom TIM Trio, Siemens, Erlangen, Germany) using an eight-channel (phased array) flexible multipurpose coil (Noras, Würzburg, Germany). For quantitative T1 mapping a dual flip angle (24 and 4°) 3D gradient echo (GRE) sequence, volumetric interpolated breath-hold examination (VIBE) with a repetition time (TR) of 50 ms, an echo time (TE) of 3.6 ms, a field of view (FOV) of 183 × 200 and a matrix size of 317 × 384 mm was used, resulting in a resolution in plane of 0.6 × 0.5 mm with a slice thickness of 1 mm. The slab had 36 slices with 20 % distance factor. The bandwidth was 130 Hz/pixel, and the scan time was six minutes 53 seconds [16, 21].
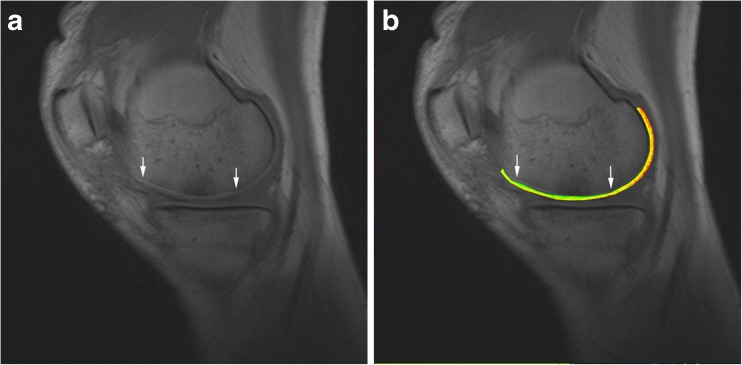
In all patients, three contiguous slices were assessed to cover the majority of the graft (two contiguous slices at small grafts) and were taken for further analyses. The slab was positioned in a way that the graft was in the middle of the slab [17]. The regions of interest (ROI) for assessment of T1 relaxation time values were manually drawn to cover the region of cartilage RT and in an area of morphologically intact reference cartilage (RC) of the femoral condyle. At the repair site the whole cartilage layer was covered; however, care was taken not to include subchondral bone or joint fluid (Fig. 1). Quantitative T1 measurements were performed for all examinations.
Fig. 1.
a Sagittal T1-weighted image of a 25-year-old male patient. The borders of the implant are marked with the arrows. b Corresponding colour-coded contrast-enhanced T1 map
According to the report of Trattnig et al., only T1 after contrast administration was considered for the evaluation of the RT [16]. The global T1 both of the reference (T1 RC) and of the repair site (T1 RT) was calculated under consideration of all ROIs. A relative T1 value (rT1) was then calculated to account for individual differences in cartilage GAG and for differences in contrast agent (CA) dynamics: rT1 = T1 RT/T1 RC. In contrast to the relative ΔR1 [22], rT1 needs no pre-contrast T1.
Statistical analyses
The statistical analyses were carried out with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA). Paired Student’s t tests were used to test for the differences between RC and RT and between rT1 at the different MR examinations. The level of significance was p < 0.05.
Results
The mean baseline preoperative Lysholm score was 59.8 ± 12.9. It improved at the first visit to 83.3 ± 13.03 points and was 86.1 ± 15.7 points at the second visit. The differences were statistically significant (visit 1 p < 0.01; visit 2 p < 0.01) in comparison to the preoperative Lysholm score. The mean values and the results for every patient are shown in Table 1.
Table 1.
Single case data for the Lysholm score and rT1 values
| Patient no. | Lysholm | Lysholm | Lysholm | rT1 | rT1 |
|---|---|---|---|---|---|
| Pre-op | Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| 1 | 80 | 80 | 90 | 1.28 | 1.51 |
| 2 | 45 | 100 | 100 | 0.36 | 0.59 |
| 3 | 50 | 81 | 76 | 0.93 | 0.85 |
| 4 | 61 | 87 | 94 | 0.52 | 0.45 |
| 5 | 66 | 74 | 95 | 0.97 | 0.70 |
| 6 | 69 | 90 | 92 | 0.97 | 0.55 |
| 7 | 56 | 62 | 43 | 0.85 | 0.53 |
| 8 | 37 | 71 | 82 | 1.03 | 1.12 |
| 9 | 69 | 100 | 91 | 0.72 | 0.78 |
| 10 | 53 | 72 | 90 | 0.88 | 0.76 |
| 11 | 72 | 99 | 94 | 0.44 | 0.50 |
| Mean | 59.8 | 83.3 | 86.1 | 0.81 | 0.76 |
| ± SD | 12.9 | 13.03 | 15.7 | 0.28 | 0.32 |
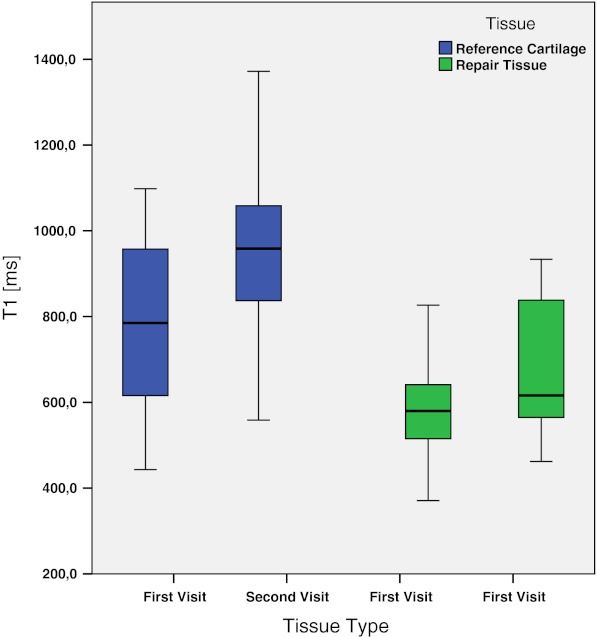
The global post-contrast T1 of the RC was significantly higher than the global post-contrast T1 of the RT at both time points. As seen in Table 2, the mean T1 of the RC at the first visit was 778.2 ± 231.3 ms and the T1 of the RT at the first visit was 581.3 ± 126.4 ms. The difference was statistically significant (p < 0.05). When compared at the second visit, the mean T1 of the RC was 959.8 ± 216.4 ms and the T1 of the RT was 684.1 ± 169.9 ms (Fig. 2). The difference was again statistically significant (p = 0.01). The mean values of the RT showed no statistically significant difference between the first and the second visit when tested with the paired t test (p = 0.104).
Table 2.
Mean global T1 of the repair tissue and the reference cartilage
| T1 RC | T1 RT | T1 RC | T1 RT | |
|---|---|---|---|---|
| Visit 1 | Visit 1 | Visit 2 | Visit2 | |
| Mean | 778.2 | 581.3 | 959.8 | 684.1 |
| ± SD | 231.3 | 126.4 | 216.4 | 169.9 |
Fig. 2.
Box plot showing T1 values for RC and RT at the first and second visits
The mean relative T1 value at the first visit was 0.81 ± 0.28, whereas on the second visit it was 0.76 ± 0.32. The results were stable over one year; the difference was not statistically significant (p = 0.4).
Discussion
The aim of this study was to further evaluate the GAG content of the RT after Hyalograft C, which has been shown in several studies to yield good midterm results: In the study described by Nehrer et al. [4] with a follow-up period up to seven years, all clinical scores improved significantly [Lysholm score, objective International Knee Documentation Committee (IKDC) knee form, subjective IKDC knee form, modified Cincinnati score]. In another study with a seven year follow-up period by Filardo et al. [3], all clinical scores [subjective and objective IKDC knee form, Tegner score, EuroQol visual analogue scale (EQ VAS)] improved significantly again. Marcacci et al. [5] demonstrated in a study on 192 patients treated with Hyalograft C that 71.4 % of the patients reported they could do everything or nearly everything with their joint (at a mean follow-up of 38 months) compared to 4.3 % prior to surgery. In a study by Gobbi et al. [6], where 32 patients with patellofemoral full-thickness chondral defects were treated with Hyalograft C, a clinical improvement was found in 90.7 % of the patients after a follow-up of 24 months. All authors concluded that Hyalograft C provides good clinical improvement in healthy young patients with singular defects. The cases included in this study were comparable to the other Hyalograft C case series in terms of the clinical outcome and the clinical baseline data: Lysholm score improved significantly from a preoperative score of 59.8 ± 12.9 up to 86.1 ± 15.7 at the second assessment.
dGEMRIC has proven its feasibility to assess the cartilage GAG content in several studies [14, 15, 17]. The dGEMRIC technique used in this study had already been applied in previous studies [16, 21]. In a study on 30 patients after MACT, with a mean follow-up of 19.61 ± 8.81 months, Trattnig et al. [16] found a mean T1 RT of 427 ± 159 ms and a mean T1 RC of 636 ± 189 ms. Multanen et al. depicted that dGEMRIC imaging is a reliable instrument for cartilage assessment with a day-to-day reproducibility of 7 % for full-thickness ROIs [23]. Our study demonstrated comparable RT with relatively high GAG contents (T1 RT 684.1 ± 169.9 ms, T1 RC 959.8 ± 216.4 ms, mean follow-up 52.8 ± 21.7 months). Furthermore, rT1 showed stable GAG content in the RT over a follow-up period of one year. The study demonstrated a wide range of rT1 values from 0.36 to 1.51 resulting from a wide range of T1 values in both RC and transplanted cartilage, coinciding with former dGEMRIC analyses of Hyalograft C transplants [13, 17]. Further long-term studies have to show whether rT1 values will remain stable.
The results substantiate the notion that the technique produces RT with relatively high GAG content, but also that there is a wide range of tissue quality, as seen in other cartilage repair techniques [11]. Knutsen et al. [8] reported that ACI samples tended to have more hyaline repair cartilage then microfracture (MFX) samples; however, this was not statistically significant. In a later study with the same study population, Knutsen et al. [11] demonstrated that after a follow-up period of five years none of the patients with the best-quality cartilage (predominantly hyaline-like) had a treatment failure. Therefore, the histological results after two years [8] demonstrated a positive predictive value for the results after five years [11]. Also Saris et al. [9, 24] stated that ACI results in superior structural repair compared with MFX, which was statistically significant. It is further worth noting, regardless of the respective technique that is used to introduce the RT, that cases with hyaline-like RT will have a better outcome than cases with fibrous RT [7–9].
In light of these findings we believe it is of substantial interest to evaluate if the RT after a particular cartilage repair technique has a high GAG content, and if the quality of the RT is stable over time. The outcome after MFX has been reported to deteriorate after 18 months, and it seems that the period between 18 and 36 months is decisive for the mid- and long-term outcome [8, 11, 25]. Therefore, it was our aim to demonstrate constant GAG values after MACT over this period of time.
We are aware of the limitations of this study regarding the small number of cases, the lack of a histological control and possible selection bias; however, we think it is worthwhile to add these data to the existing knowledge on Hyalograft C.
In accordance with the clinical outcome, Hyalograft C yielded RT with a relatively high GAG content that was stable over time at midterm. Further follow-up will be required to determine if there is a predictive value of dGEMRIC for the long-term outcome.
References
- 1.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 3.Filardo G, Kon E, Di Martino A, Iacono F, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am J Sports Med. 2011;39:2153–2160. doi: 10.1177/0363546511415658. [DOI] [PubMed] [Google Scholar]
- 4.Nehrer S, Dorotka R, Domayer S, Stelzeneder D, Kotz R. Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years’ follow-up. Am J Sports Med. 2009;37(Suppl 1):81S–87S. doi: 10.1177/0363546509350704. [DOI] [PubMed] [Google Scholar]
- 5.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Stefani G, Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 6.Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763–1773. doi: 10.1177/0363546506288853. [DOI] [PubMed] [Google Scholar]
- 7.Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253–261. doi: 10.1097/01.blo.0000238829.42563.56. [DOI] [PubMed] [Google Scholar]
- 8.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S–19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 10.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–162. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 12.Trattnig S, Domayer S, Welsch GW, Mosher T, Eckstein F. MR imaging of cartilage and its repair in the knee–a review. Eur Radiol. 2009;19:1582–1594. doi: 10.1007/s00330-009-1352-3. [DOI] [PubMed] [Google Scholar]
- 13.Trattnig S, Mamisch TC, Pinker K, Domayer S, Szomolanyi P, Marlovits S, Kutscha-Lissberg F, Welsch GH. Differentiating normal hyaline cartilage from post-surgical repair tissue using fast gradient echo imaging in delayed gadolinium-enhanced MRI (dGEMRIC) at 3 Tesla. Eur Radiol. 2008;18:1251–1259. doi: 10.1007/s00330-008-0859-3. [DOI] [PubMed] [Google Scholar]
- 14.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::AID-MRM1006>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Gillis A, Bashir A, McKeon B, Scheller A, Gray ML, Burstein D. Magnetic resonance imaging of relative glycosaminoglycan distribution in patients with autologous chondrocyte transplants. Invest Radiol. 2001;36:743–748. doi: 10.1097/00004424-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Trattnig S, Burstein D, Szomolanyi P, Pinker K, Welsch GH, Mamisch TC. T1(Gd) gives comparable information as Delta T1 relaxation rate in dGEMRIC evaluation of cartilage repair tissue. Invest Radiol. 2009;44:598–602. doi: 10.1097/RLI.0b013e3181b4c236. [DOI] [PubMed] [Google Scholar]
- 17.Trattnig S, Marlovits S, Gebetsroither S, Szomolanyi P, Welsch GH, Salomonowitz E, Watanabe A, Deimling M, Mamisch TC. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) for in vivo evaluation of reparative cartilage after matrix-associated autologous chondrocyte transplantation at 3.0 T: preliminary results. J Magn Reson Imaging. 2007;26:974–982. doi: 10.1002/jmri.21091. [DOI] [PubMed] [Google Scholar]
- 18.Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3–8. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 20.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 21.Domayer SE, Welsch GH, Nehrer S, Chiari C, Dorotka R, Szomolanyi P, Mamisch TC, Yayon A, Trattnig S. T2 mapping and dGEMRIC after autologous chondrocyte implantation with a fibrin-based scaffold in the knee: preliminary results. Eur J Radiol. 2010;73:636–642. doi: 10.1016/j.ejrad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, Moriya H. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239:201–208. doi: 10.1148/radiol.2383050173. [DOI] [PubMed] [Google Scholar]
- 23.Multanen J, Rauvala E, Lammentausta E, Ojala R, Kiviranta I, Häkkinen A, Nieminen MT, Heinonen A. Reproducibility of imaging human knee cartilage by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5 Tesla. Osteoarthritis Cartilage. 2009;17:559–564. doi: 10.1016/j.joca.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 25.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]