Abstract
Purpose
Loose fragments in osteochondritis dissecans (OCD) of the knee require internal fixation. On the other hand, loose fragments derived from spontaneous osteonecrosis of the knee (SONK) are usually removed. However, the difference in healing potential between OCD- and SONK-related loose fragments has not been elucidated. In this study, we investigated proliferative activity and redifferentiation potential of normal cartilage-derived and loose fragment-derived chondrocytes.
Methods
Cells were prepared from normal articular cartilages and loose fragment cartilages derived from knee OCD and SONK. Cellular proliferation was compared. Redifferentiation ability of pellet-cultured chondrocytes was assessed by real-time PCR analyses. Mesenchymal differentiation potential was investigated by histological analyses. Positive ratio of a stem cell marker CD166 was evaluated in each cartilaginous tissue.
Results
Normal and OCD chondrocytes showed a higher proliferative activity than SONK chondrocytes. Chondrogenic pellets derived from normal and OCD chondrocytes produced a larger amount of safranin O-stained proteoglycans compared with SONK-derived pellets. Expression of chondrogenic marker genes was inferior in SONK pellets. The CD166-positive ratio was higher in normal cartilages and OCD loose fragments than in SONK loose fragments.
Conclusions
The OCD chondrocytes maintained higher proliferative activity and redifferentiation potential compared with SONK chondrocytes. Our results suggest that chondrogenic properties of loose fragment-derived cells and the amount of CD166-positive cells may affect the repair process of osteochondral defects.
Introduction
Osteochondritis dissecans (OCD) is a condition affecting the subchondral bone with secondary effects on articular cartilage that results in pain, effusions, and loose fragment formation. The OCD of the knee causes knee pain and dysfunction among skeletally immature (juvenile) and young adult patients [1]. Several aetiologies of knee OCD have been proposed, including repetitive microtrauma, genetics, vascular, mechanical axis malalignment, and discoid lateral meniscus [2, 3]. Based on the histological findings, the initial change in OCD is bone necrosis or subchondral fracture [4]. Management of knee OCD includes nonoperative measures or operative procedures such as drilling, fragment fixation, and osteochondral autograft (allograft) [1]. Internal fixation of an OCD loose fragment using bioabsorbable screws, pins, and osteochondral plugs shows excellent clinical outcomes and high healing rates [1, 5]. On the other hand, osteochondral loose fragments derived from spontaneous osteonecrosis of the knee (SONK) are usually removed by surgically [6]. SONK is clinically characterised by a sudden onset of severe knee pain in elderly patients. Traumatic and vascular theories have been proposed as a causative factor of SONK involving a subchondral fracture of the femoral condyle [6]. Histological analyses have revealed that osteonecrotic lesions between the subchondral fracture line and the articular surface are observed in the advanced stage of SONK [7]. Conservative treatments that include protected weight-bearing and the use of bisphosphonate are effective in the early stage of SONK [8]. In the progressive and advanced stages of SONK, surgical treatments that include drilling, core decompression, artificial bone graft, osteochondral autograft (allograft), high tibial osteotomy, and unicompartmental or total knee arthroplasty may be required [6, 9]. We have previously demonstrated that SONK loose fragment chondrocytes have a low potential for cellular proliferation and redifferentiation [10]. However, the differences among normal cartilage-, OCD-, and SONK-derived chondrocytes remain unclear in their regeneration potentials. Human chondrocytes isolated from detached OCD fragments maintain similar cell viability to those from healthy normal cartilages [11]. On the other hand, the chondrogenic redifferentiation potential of horse OCD-derived chondrocytes seems to be inferior to that of normal chondrocytes because OCD chondrocytes show lower expression of a chondrogenic transcription factor, Sry-type high-mobility-group box (SOX) 9 [12]. In this study, we investigated proliferative activity, redifferentiation ability, and expression of chondrogenic marker genes in chondrocytes derived from normal cartilage, OCD loose fragments, and SONK loose fragments.
Methods
Tissues, cells, and cell culture
Normal articular cartilage was obtained at notchplasty in patients (13, 18, 19, and 21 years of age) undergoing anterior cruciate ligament reconstructions (n = 4). Unnecessary parts of loose fragments in knee OCD (15, 15, 15, and 21 years of age) were obtained at arthroscopy (n = 4). Osteonecrotic loose fragments were obtained at knee arthroplasty in patients (62, 64, 65, and 66 years of age) suffering from SONK (n = 4). Demographic data of the patients are shown as Table 1. This study received the approval of our Institutional Review Board and patients gave their informed consent for this research. The diagnosis was established according to clinical findings and magnetic resonance imaging. The OCD lesions were arthroscopically evaluated by the International Cartilage Repair Society (ICRS) classification (ICRS OCD II, III, IV, and IV in each patient; Table 1) [13]. Radiographic stages of SONK (stage III in all patients) were assessed by Koshino’s classification (Table 1) [9]. Articular cartilage was carefully separated from osteochondral fragments. Normal and loose fragment chondrocytes were prepared by collagenase (Sigma, St. Louis, MO) digestion [14]. Cells were maintained with Dulbecco’s modified Eagle’s medium (DMEM, Wako, Osaka, Japan) containing foetal bovine serum (HyClone, South Logan, UT) and penicillin/streptomycin (Sigma). Chondrocytes between passage one and three were used in this study. Tissue samples were fixed with 4 % paraformaldehyde-buffered solution. Decalcified paraffin-embedded tissue sections were prepared for histological analyses.
Table 1.
Demographic data
| Case | Age (years) | Sex | Location | Diagnosis | Duration until surgery (months) | Fragment size (cm2) |
|---|---|---|---|---|---|---|
| 1 | 15 | M | Lt MFC | OCD (II) | 5 | 4 |
| 2 | 15 | F | Lt MFC | OCD (III) | 4 | 3 |
| 3 | 15 | M | Lt LFC | OCD (IV) | 3 | 4 |
| 4 | 21 | M | Lt LFC | OCD (IV) | 15 | 6 |
| 5 | 62 | M | Lt MFC | SONK (3) | 12 | 6 |
| 6 | 64 | F | Lt MFC | SONK (3) | 6 | 6 |
| 7 | 65 | F | Rt MFC | SONK (3) | 10 | 3 |
| 8 | 66 | M | Lt MFC | SONK (3) | 9 | 6 |
Case 3: OCD occurred 5 years after partial excision of a discoid lateral meniscus. Case 4: OCD progressed one year after subtotal excision of discoid lateral meniscus. Knee OCD was evaluated by ICRS OCD classification (I–IV) [13]. SONK was assessed by Koshino’s radiological stage (1–4) [9]. MFC, medial femoral condyle. LFC, lateral femoral condyle
Cell proliferation assay
Cell proliferation assays were performed as described [15]. In brief, cultured cells were plated as 2.5 × 103 cells/well and incubated for 12 hours. Then, cells were harvested for 0, 24, 48, and 72 hours before addition of water soluble tetrazolium (WST)-1 reagents (Roche, Mannheim, Germany). Optical density (OD) was measured by a iMark microplate reader (Bio-Rad, Hercules, CA) after three-hour treatment of WST-1. Data were obtained by subtracting 630-nm readings from 450-nm readings for evaluation. The mean value derived from wells was evaluated.
In vitro differentiation and histological analyses
In vitro differentiation was performed as described [16]. For chondrogenic redifferentiation, pelleted micromass culture was performed. Pellet-cultured cells (5 × 105 cells/pellet) were maintained in the chondrogenic induction medium supplemented with 10 ng/ml of recombinant human bone morphogenetic protein (BMP)-2 (kindly provided from Pfizer, New York, NY) and transforming growth factor-β3 (R&D Systems, Minneapolis, MN) for three weeks [17]. Proteoglycans in pellets were observed with safranin O staining. Redifferentiation potential was assessed by the Bern pellet histology score (range, 0–9) [18, 19]. To assess the effect of tissue-derived mesenchymal stem cells (MSCs), we performed in vitro differentiation analysis for the other mesenchymal lineages. To induce adipogenic differentiation, confluent cells were cultured with adipogenesis induction and maintenance media for three weeks according to the manufacturer’s protocol (Millipore, Billerica, MA). Lipid vacuoles were stained with oil red O solution [20]. Osteogenic induction was performed using MSC osteogenesis kit (Millipore) and 1 ng/ml of BMP-2 for three weeks. Calcium deposition was visualised by von Kossa staining. Tissue specimens of osteochondral fragments were immunostained by a rabbit anti-CD166 antibody (ALCAM H-108, 1:100, Santa Cruz, Santa Cruz, CA) for detecting a cell surface marker of MSCs and progenitor cells [21]. Staining density of oil red O, von Kossa, and CD166 was quantified by Image J 1.31 [15]. Relative staining density was normalised by a mean value derived from five different images of SONK chondrocytes. The CD166-positive cell ratio was evaluated by a microscopic counting of chondrocytes in five different areas.
Quantitative real-time PCR analyses
RNAs purified from chondrogenic pellets were reverse-transcribed with ReverTra Ace (Toyobo, Osaka, Japan). Quantitative real-time PCR analyses were performed using LightCycler ST-300 and FastStart DNA Master SYBR Green I kit (Roche). Chondrogenic redifferentiation was assessed using the primers for SOX9, SOX5/6, and α1(II) collagen (COL2A1) [16, 20]. Amplification of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used for normalisation [16]. The cycle number crossing the signal threshold was selected in the linear part of the amplification curve. Relative mRNA levels were normalised with the level of SONK-derived pellets.
Statistical analysis
All experiments were repeated three times and similar results were obtained. The data presented in the Figs. 1, 2, 3, and 4 were derived from the same patients (normal, 18 years old; OCD, 15 years old; SONK, 66 years old). Data were expressed as means with standard deviations. Mean values were compared with a one-way ANOVA. Post hoc comparisons were performed using the Tukey test. Significance was set at p < 0.05.
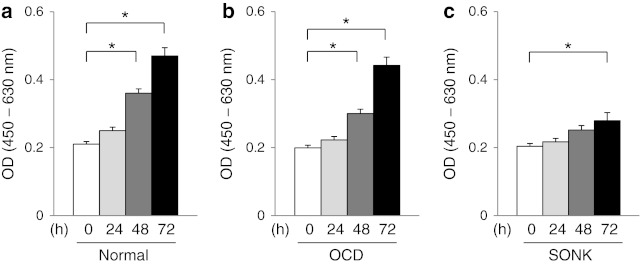
Fig. 1.
Normal chondrocytes and OCD loose fragment chondrocytes showed a higher proliferative activity than SONK loose fragment chondrocytes in cell proliferation assays. Normal chondrocytes (a). OCD-derived chondrocytes (b). SONK-derived chondrocytes (c). *p < 0.05
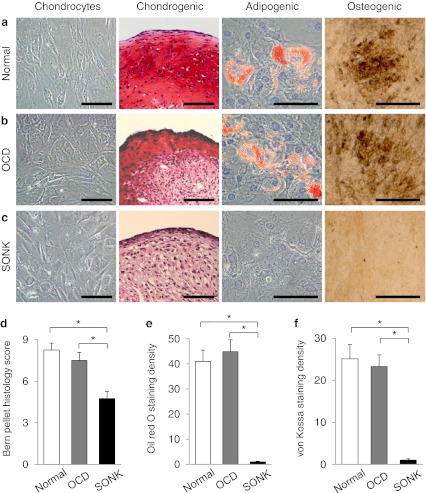
Fig. 2.
Normal and OCD chondrogenic pellets produced the larger amount of safranin O-stained proteoglycans compared with SONK pellets (a–c, red). Lipid vacuoles were observed in normal and OCD chondrocytes after adipogenic induction (a and b, red). Calcium depositions were induced by osteogenic treatments in normal cartilage- and OCD-derived cells (a and b, brown). On the other hand, neither adipogenesis nor osteogenesis was induced in SONK-derived cells (c). The Bern pellet histology score was higher in normal and OCD pellets than in SONK pellets (d). Staining density of oil red O and von Kossa was lower in SONK-derived cells than in normal and OCD chondrocytes after the treatment of adipogenic and osteogenic induction (e and f). Bars, 100 μm. *p < 0.05
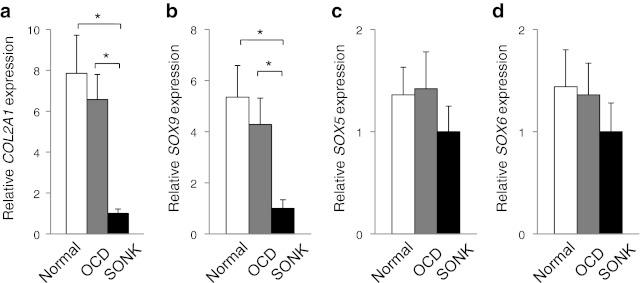
Fig. 3.
Expression pattern of chondrogenic marker genes. Expression of COL2A1 and SOX9 was more activated in normal and OCD chondrogenic pellets than in SONK pellets (a and b). However, the difference among normal, OCD, and SONK chondrogenic pellets was not observed in SOX5/6 expression (c and d). *p < 0.05
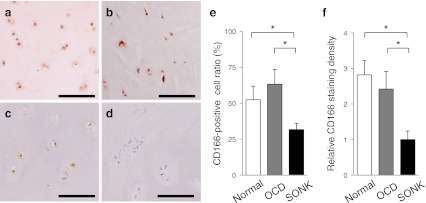
Fig. 4.
CD166, a MSC surface marker, was detected in the cells of normal and loose fragment cartilages (a–c, brown in the transitional zone of articular cartilage). Normal cartilage (a). OCD loose fragment (b). SONK loose fragment (c). Negative control in the absence of an anti-CD166 antibody (d). CD166-positive cell ratio was higher in normal cartilages and OCD fragments than in SONK fragments (e). Normal cartilages and OCD fragments showed higher staining densities of CD166 compared with SONK loose fragment cartilages (f). Bars, 100 μm. *p < 0.05
Results
Proliferative activity of chondrocytes
Chondrocytes derived from normal cartilages and OCD loose fragments showed a higher proliferative activity than SONK chondrocytes (Fig. 1a–c). OCD chondrocytes showed a similar proliferative activity to normal chondrocytes (Fig. 1a and b). On the other hand, cellular proliferation of SONK chondrocytes was inferior to that of normal chondrocytes (Fig. 1a and c).
Redifferentiation potential of chondrocytes
In vitro differentiation analyses revealed that normal and OCD chondrogenic pellets produced the larger amount of proteoglycans compared with SONK-derived pellets (Fig. 2a–c). The Bern pellet histology score was higher in normal and OCD chondrocyte-derived pellets than in SONK chondrogenic pellets (Fig. 2d). Lipid vacuoles were observed in normal and OCD chondrocytes after adipogenic induction (Fig. 2a and b). In addition, osteogenic treatments induced calcium depositions in normal cartilage-derived cells and OCD loose fragment cells (Fig. 2a and b). On the other hand, neither adipogenesis nor osteogenesis was induced in SONK loose fragment-derived cells (Fig. 2c). Staining density of oil red O and von Kossa was lower in SONK-derived cells than in normal cartilage- and OCD-derived cells after the induction for each mesenchymal lineage (Fig. 2e and f). Gene expression pattern indicated that redifferentiation potential of OCD chondrocytes was higher than that of SONK chondrocytes (Fig. 3). Expression of COL2A1 and SOX9 was more activated in normal and OCD chondrogenic pellets than SONK pellets (Fig. 3a and b). However, expression of SOX5/6 was not highly induced in normal and OCD pellets by chondrogenic treatments (Fig. 3c and d).
CD166-positive cells in loose fragment cartilages
CD166, a MSC surface marker, was detected in chondrocytes packaged in normal cartilages (Fig. 4a). In addition, CD166-positive cells were observed in loose fragment cartilages of OCD and SONK (Fig. 4b and c). However, CD166-positive cell ratio was higher in normal cartilages and OCD loose fragments than in SONK-derived fragments (Fig. 4e). Relative CD166 staining density was also higher in normal and OCD cartilage samples than in SONK loose fragments (Fig. 4f).
Discussion
Osteonecrotic loose fragment and unstable cartilage flap in SONK are usually removed at surgical treatment [6, 9]. On the other hand, fixation of OCD loose fragment shows excellent clinical outcomes and high healing rates [1, 5]. However, these reports have not mentioned the difference between SONK- and OCD-derived loose fragment chondrocytes in cellular potential. This study has demonstrated that OCD loose fragment chondrocytes maintained similar proliferative and redifferentiation potentials as normal chondrocytes and that SONK loose fragment chondrocytes showed a lower activity in cellular proliferation and redifferentiation.
Monolayer-cultured articular chondrocytes leads to a process of dedifferentiation whereby the cells acquire a fibroblastic morphology and lose their chondrocytic properties [22]. The expression of chondrocyte-specific genes, such as COL2A1, aggrecan, SOX9, and SOX5/6, is gradually down-regulated during cell multiplication in monolayer culture conditions [22]. We previously demonstrated that COL2A1 expression was not detected in monolayer-cultured (dedifferentiated) SONK chondrocytes, whereas type II collagen deposition and COL2A1 expression were observed in tissue samples of SONK loose fragment cartilages [10]. Aging also influences cellular properties. Aged human chondrocytes are inferior to juvenile chondrocytes in producing cartilage-specific extracellular matrix [23]. In cell proliferation and chondrogenic redifferentiation, aged human chondrocytes show lower cellular potentials rather than chondrocytes derived from younger donors [24]. Our study demonstrated that aged SONK chondrocytes are inferior to normal and OCD chondrocytes in cellular proliferation (Fig. 1). In addition, three-dimensional-cultured SONK chondrocytes showed lower chondrogenic phenotypes than normal cartilage- and OCD-derived chondrogenic pellets (Figs. 2 and 3). On the other hand, OCD loose fragment chondrocytes maintained similar chondrogenic phenotypes to normal articular chondrocytes (Figs. 2 and 3). These findings suggest that chondrocyte aging may be a key factor in reducing its proliferative activity and redifferentiation potential.
The MSCs are multipotent cells that differentiate into chondrogenic, adipogenic, and osteogenic lineages [25]. Several tissue-derived MSCs, such as bone marrow, synovium, adipose, ligament, and meniscus, are candidates for a cell source of regeneration for damaged tissue [16, 20, 25]. However, few reports have investigated the cellular behavior of MSCs packaged in articular cartilage [21, 26]. The literature focuses on CD166 as a biomarker to identify cartilage-derived MSCs and/or progenitor cells in both normal and osteoarthritic cartilages [21, 26]. Primary cell culture from aged osteoarthritic cartilages (ranged from 59 to 87 years old) shows a higher rate of CD105/CD166-positive progenitor cells than that from younger normal cartilages (ranged from 17 to 39 years old) [26]. On the other hand, Pretzel et al. have demonstrated that the ratio of CD105/CD166-positive progenitor cells is similar between osteoarthritic chondrocytes and normal chondrocytes using flow cytometry [21]. The percentage of CD166-positive cells is also similar between osteoarthritic and normal cartilage in immunohistological analysis [21]. In our study, CD166-positive cell ratio and relative CD166 staining density were higher in OCD fragments and normal cartilages than in SONK loose fragments (Fig. 4). These findings suggest that CD166-positive cell ratio is not determined in an age-dependent manner. The ratio of CD166-positive cells might represent the status of intrinsic repair capacity in articular cartilage. We consider that the healing potential of osteoarthritic chondrocytes might be preserved (or transiently activated) by an excessive mechanical stress. However, CD166-positive cells might not be induced in SONK detached fragment chondrocytes for lack of mechanical loading. The MSCs secrete a variety of growth factors that stimulate mitosis and differentiation of tissue-intrinsic progenitor cells [27]. The MSC-mediated trophic effect is distinct from the direct differentiation of MSCs into repairing tissue. On the other hand, cryopreserved human cartilage fragments promote chondrogenic differentiation of human bone marrow-derived MSCs in a nude mouse transplantation model [28]. These findings suggest that the interaction between CD166-positive cells and primitive MSCs may have an essential role in enhancing cartilage repair. However, further studies involved in the other MSC markers, such as CD44, 73, 90, and 105, will be required to understand the precise role of cartilage-derived progenitor cells and MSCs.
Chondrogenic differentiation and maturation are cooperatively regulated by several transcription factors and coactivators [17, 29, 30]. Chondrogenic master transcription factor SOX9 positively regulates the expression of its target genes through the association with the consensus DNA sequences (WWCAAWG) on promoters and enhancers of cartilage-specific genes, such as COL2A1, α1(IX), α2(XI) collagen, aggrecan, and cartilage link protein [30]. The SOX9-associating coactivators including p300, Smad3, and E47 have important roles in modulating SOX9-dependent transcriptional activation [30]. Our results indicated that SOX9 expression was higher in normal and OCD chondrocytes than in SONK chondrocytes during the redifferentiation procedure (Fig. 3). However, the expression of SOX9-supporting molecule SOX5/6 in normal cartilage- and OCD-derived chondrogenic pellets was similar to that in SONK-derived pellets (Fig. 3). These findings suggest that SOX9-associating molecules and other chondrogenic factors except SOX5/6 would have fundamental roles in enhancing chondrogenic redifferentiation.
In conclusion, our experimental study has demonstrated that articular chondrocytes derived from normal cartilage and detached OCD loose fragments maintained higher proliferative activity and redifferentiation potential compared with SONK loose fragment chondrocytes. Our results suggest that the proliferative and redifferentiation properties of chondrocytes and the amount of CD166-positive cells in loose fragment cartilages may affect the repair process of osteochondral defects.
Acknowledgments
We are grateful to Prof. Nobuhiro Abe, Ms. Emi Matsumoto, Ms. Aki Yoshida, and Ms. Reina Tanaka for their technical support. This work was supported by Grants from the Japan Society for the Promotion of Science (No. 24791546), the JSPS Fujita Memorial Fund for Medical Research, and the Nakatomi Foundation.
References
- 1.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J, Dandy DJ. Osteochondritis dissecans and other lesions of the femoral condyles. J Bone Joint Surg Br. 1989;71:518–522. doi: 10.1302/0301-620X.71B3.2722949. [DOI] [PubMed] [Google Scholar]
- 3.Mizuta H, Nakamura E, Otsuka Y, Kudo S, Takagi K. Osteochondritis dissecans of the lateral femoral condyle following total resection of the discoid lateral meniscus. Arthroscopy. 2001;17:608–612. doi: 10.1053/jars.2001.19979. [DOI] [PubMed] [Google Scholar]
- 4.Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E. Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med. 2009;37:2003–2008. doi: 10.1177/0363546509346542. [DOI] [PubMed] [Google Scholar]
- 5.Adachi N, Motoyama M, Deie M, Ishikawa M, Arihiro K, Ochi M. Histological evaluation of internally-fixed osteochondral lesions of the knee. J Bone Joint Surg Br. 2009;91:823–829. doi: 10.1302/0301-620X.91B6.20957. [DOI] [PubMed] [Google Scholar]
- 6.Patel DV, Breazeale NM, Behr CT, Warren RF, Wickiewicz TL, O’Brien SJ. Osteonecrosis of the knee: current clinical concepts. Knee Surg Sports Traumatol Arthrosc. 1998;6:2–11. doi: 10.1007/s001670050064. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82:858–866. doi: 10.1302/0301-620X.82B8.11194. [DOI] [PubMed] [Google Scholar]
- 8.Kraenzlin ME, Graf C, Meier C, Kraenzlin C, Friedrich NF. Possible beneficial effect of bisphosphonates in osteonecrosis of the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1638–1644. doi: 10.1007/s00167-010-1106-4. [DOI] [PubMed] [Google Scholar]
- 9.Koshino T. The treatment of spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am. 1982;64:47–58. [PubMed] [Google Scholar]
- 10.Takata N, Furumatsu T, Abe N, Naruse K, Ozaki T. Comparison between loose fragment chondrocytes and condyle fibrochondrocytes in cellular proliferation and redifferentiation. J Orthop Sci. 2011;16:589–597. doi: 10.1007/s00776-011-0128-1. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Garrido C, Tanoira I, Muscolo DL, Ayerza MA, Makino A. Viability of loose body fragments in osteochondritis dissecans of the knee. A series of cases. Int Orthop. 2010;34:827–831. doi: 10.1007/s00264-010-0951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvican ER, Vaughan-Thomas A, Redmond C, Clegg PD. Chondrocytes harvested from osteochondritis dissecans cartilage are able to undergo limited in vitro chondrogenesis despite having perturbations of cell phenotype in vivo. J Orthop Res. 2008;26:1133–1140. doi: 10.1002/jor.20602. [DOI] [PubMed] [Google Scholar]
- 13.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 14.Date H, Furumatsu T, Sakoma Y, Yoshida A, Hayashi Y, Abe N, Ozaki T. GDF-5/7 and bFGF activate integrin α2-mediated cellular migration in rabbit ligament fibroblasts. J Orthop Res. 2010;28:225–231. doi: 10.1002/jor.20981. [DOI] [PubMed] [Google Scholar]
- 15.Saiga K, Furumatsu T, Yoshida A, Masuda S, Takihira S, Abe N, Ozaki T. Combined use of bFGF and GDF-5 enhances the healing of medial collateral ligament injury. Biochem Biophys Res Commun. 2010;402:329–334. doi: 10.1016/j.bbrc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Furumatsu T, Hachioji M, Saiga K, Takata N, Yokoyama Y, Ozaki T. Anterior cruciate ligament-derived cells have high chondrogenic potential. Biochem Biophys Res Commun. 2010;391:1142–1147. doi: 10.1016/j.bbrc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. J Biol Chem. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 18.Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, Martin I, Mainil-Varlet P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto E, Furumatsu T, Kanazawa T, Tamura M, Ozaki T. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun. 2012;420:124–129. doi: 10.1016/j.bbrc.2012.02.127. [DOI] [PubMed] [Google Scholar]
- 20.Furumatsu T, Kanazawa T, Yokoyama Y, Abe N, Ozaki T. Inner meniscus cells maintain higher chondrogenic phenotype compared with outer meniscus cells. Connect Tissue Res. 2011;52:459–465. doi: 10.3109/03008207.2011.562061. [DOI] [PubMed] [Google Scholar]
- 21.Pretzel D, Linss S, Rochler S, Endres M, Kaps C, Alsalameh S, Kinne RW. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 23.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391:S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 24.Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthr Cartil. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 26.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 27.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 28.Chen CC, Liao CH, Wang YH, Hsu YM, Huang SH, Chang CH, Fang HW. Cartilage fragments from osteoarthritic knee promote chondrogenesis of mesenchymal stem cells without exogenous growth factor induction. J Orthop Res. 2012;30:393–400. doi: 10.1002/jor.21541. [DOI] [PubMed] [Google Scholar]
- 29.Furumatsu T, Shukunami C, Amemiya-Kudo M, Shimano H, Ozaki T. Scleraxis and E47 cooperatively regulate the Sox9-dependent transcription. Int J Biochem Cell Biol. 2010;42:148–156. doi: 10.1016/j.biocel.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Furumatsu T, Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama. 2010;64:351–357. doi: 10.18926/AMO/41320. [DOI] [PubMed] [Google Scholar]