Abstract
Purpose
The number of shoulder arthroplasties has increased over the last decade, which can partly be explained by the increasing use of the reverse total shoulder arthroplasty technique. However, the options for revision surgery after primary arthroplasty are limited in cases of irreparable rotator cuff deficiency, and tuberosity malunion, nonunion, or resorption. Often, conversion to a reverse design is the only suitable solution. We analysed the functional outcome, complication rate and patient satisfaction after the revision of primary shoulder arthroplasty using an inverse design.
Methods
Over a ten-year period 57 patients underwent revision surgery for failed primary shoulder arthroplasty using a reverse design. Of the 57 patients, 50 (mean age, 64.2 years) were available after an average follow-up of 51 months. Clinical evaluation included the Constant Murley Score, the UCLA score, and the Simple Shoulder Test, whereas radiological evaluation included plain radiographs in standard projections. Patients were also requested to rate their subjective satisfaction of the final outcome as excellent, good, satisfied or dissatisfied.
Results
Compared to the preoperative status, the overall functional outcome measurements based on standardised outcome shoulder scores improved significantly at follow-up. The overall mean Constant Murley score improved from 18.5 to 49.3 points, the mean UCLA score improved from 7.1 to 21.6 points, and the mean simple shoulder test improved from 1.2 to 5.6 points. The average degree of abduction improved from 40 to 93° (p < 0.0001), and the average degree of anterior flexion improved from 47 to 98° (p < 0.0001). The median VAS pain score decreased from 7 to 1. Complications occurred in 12 cases (24 %).A total of 32 (64 %) patients rated their result as good or excellent, six (12 %) as satisfactory and 12 (24 %) as dissatisfied.
Conclusion
In revision shoulder arthroplasty after failed primary shoulder arthroplasty an inverse design can improve the functional outcome, and patient satisfaction is usually high. However, the complication rate of this procedure is also high, and patient selection and other treatment options should be carefully considered.
Introduction
The number of shoulder arthroplasties has increased over the last decade, which can partly be explained by the increasing use of the reverse total shoulder arthroplasty. With this rapidly increasing rate of shoulder arthroplasties, the number of revision arthroplasties will also increase. [1].
In 1985, Paul Grammont [2] introduced the reverse prosthetic design. With its large hemisphere on the glenoid and its small polyethylene cup on the humeral metaphysis in a non-anatomical inclination, the Grammont design provides a fixed medialised centre of rotation and causes a caudalisation of the humeral component. This orientation increases the lever arm of the deltoid muscle and recruits more deltoid fibres for abduction, resulting in proper tensioning of the deltoid fibres to achieve restoration of stability and mobility in patients with advanced, irreparable rotator cuff pathologies combined with glenohumeral osteoarthritis.
Anatomical total shoulder arthroplasties, in the presence of severe, irreparable rotator cuff pathologies, are associated with high failure rates due to eccentric loading and loosening of the glenoid component [3, 4]. Outcome data for hemiarthroplasties in combination with severe rotator cuff deficiencies are variable, with instability and glenoid bone loss occurring in some instances [5–9]. Tuberosity malunion, nonunion or resorption after hemiarthroplasty for proximal humeral fractures can cause severe pain and loss of function and often requires revision surgery [10–15].
For those conditions, reverse shoulder arthroplasty (RSA) is described as a salvage procedure.
Despite the great popularity of the RSA, a high complication rate, ranging from 19 to 68 %, has been reported [16, 17]. For revision RSA, a complication rate as high as 50 % has been reported [18].
However, the options for revision surgery after primary arthroplasty are limited in cases of irreparable rotator cuff deficiency, and tuberosity malunion, nonunion, or resorption. Often, conversion to a reverse design is the only suitable solution.
Limited information is available regarding the outcomes of revision shoulder arthroplasty, and most studies report inferior results of revision shoulder arthroplasty compared with primary arthroplasty [19, 20]. However, revision RSA is reported as an effective treatment for failed primary shoulder arthroplasty [21].
The goal of our study was to analyse the functional outcome, complication rate and patient satisfaction after the revision of primary shoulder arthroplasty using an inverse design. The patient groups who receive the most benefit from this procedure are highlighted to improve future patient selection.
Materials and methods
Patient population
In this retrospective outcome study, 57 patients (57 consecutive shoulders) who underwent revision surgery for failed primary shoulder arthroplasty using a reverse design between 2000 and 2010 were included, with a minimum follow-up time of 24 months. All of the surgical procedures were performed by three experienced shoulder surgeons.
Three patients were lost to follow-up, and four patients died due to causes unrelated to the shoulder, resulting in the inclusion of 50 shoulders in 50 patients (follow-up rate of 88 %).
There were 34 females and 16 males, and the mean age at revision surgery was 64.2 years (range, 44–84 years). The dominant side was affected in 47 patients (94 %).
The indication for revision surgery was rotator cuff insufficiency and pseudoparalysis in 23 patients; instability due to severe rotator cuff deficiency or global decoaptation in ten patients; infection in combination with or without severe, irreparable rotator cuff deficiency in nine patients; component loosening in four patients; and tuberosity resorption in four patients.
The types of primary arthroplasties were hemiarthroplasty (HA) in 23 (11 for fracture sequelae and 12 for primary osteoarthritis), total shoulder arthroplasty (TSA) in 13 (for primary osteoarthritis), and reverse shoulder arthroplasty (RSA) in 14 patients (11 for cuff tear arthropathy and three for primary fracture treatment).
Data collection
Preoperative data were available from the institution’s shoulder database. The mean follow-up was 51 months (range, 24–101 months) and consisted of a clinical and radiological evaluation.
For the clinical evaluation, the Constant Murley score [22], the UCLA score [23], and the simple shoulder test [24] were used, and the visual analog pain scale [25] was used to rate the patients’ subjective pain. A goniometer was used to determine the range of motion. Patients were also requested to rate their subjective satisfaction of the final outcome as excellent, good, satisfied or dissatisfied.
The clinical evaluation was performed by an independent examiner.
Operative technique
All procedures were performed in the beach chair position through a deltopectoral approach. General anaesthesia in combination with an interscalene block was used, and all patients received perioperative intravenous antibiotic prophylaxis. Intraoperative cultures were taken and incubated for at least 14 days to exclude low-grade bacteriological infection.
Using small chisels and osteotomes, efforts were made to remove the stem without splitting the humeral shaft by dismantling the humeral component from the proximal aspects of the shaft, which was successful in 30 cases. In the remaining 20 shoulders, removal of the humeral component was performed by splitting the humeral shaft anteriorly. Osteosynthesis of the shaft was performed using metallic cerclages (Figs. 1 and 2).
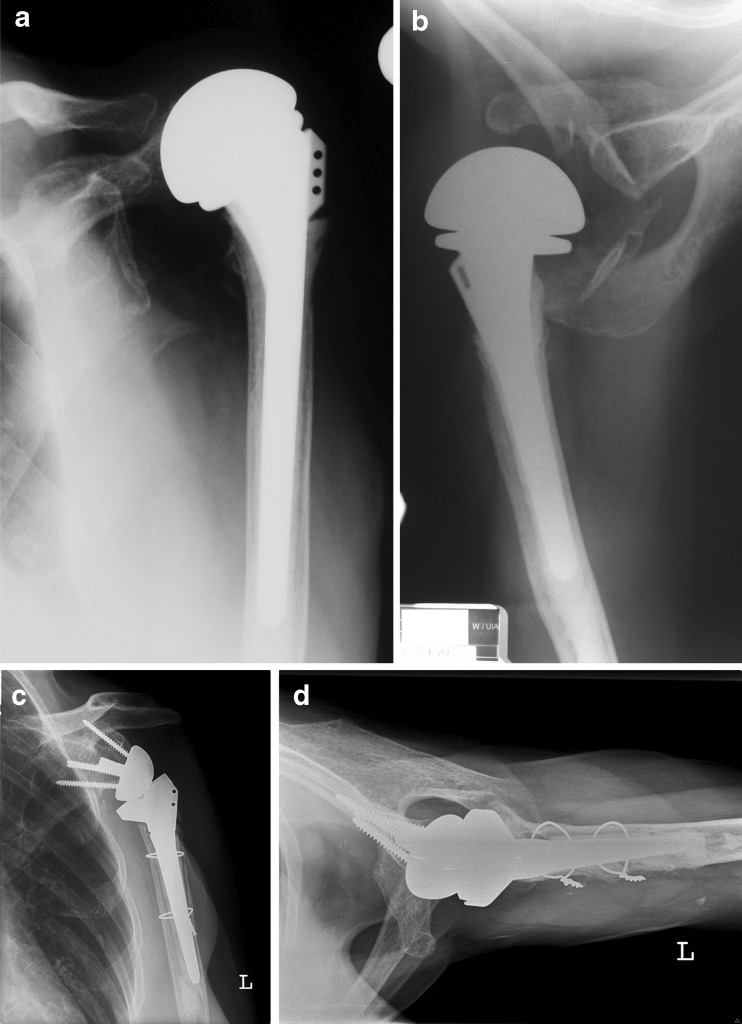
Fig. 1.
A 74-year-old woman presented with severe restriction of motion and pain of the shoulder after hemiarthroplasty (HA) for fracture treatment. (a) Anteroposterior and axillary (b) radiographs show anterosuperior migration of the prosthesis. c Anteroposterior and axillary (d) radiographs after revision reverse shoulder arthroplasty (rRSA) at four-year follow up
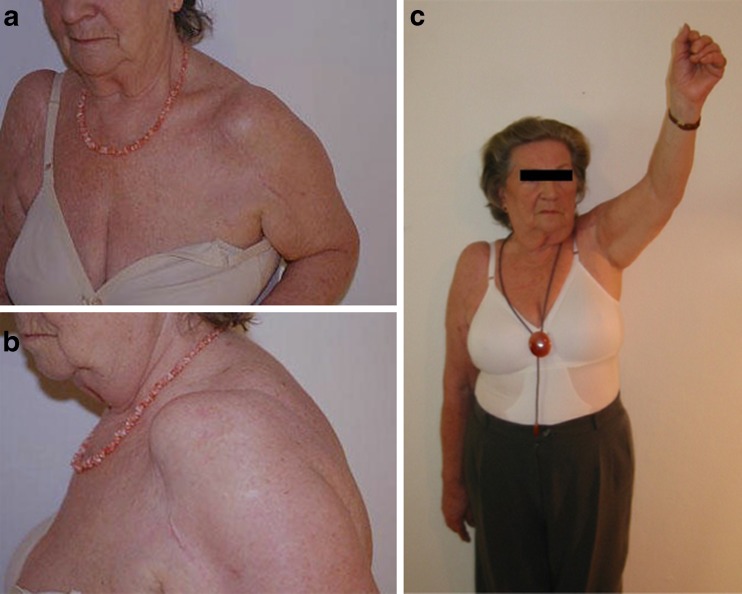
Fig. 2.
Preoperative and postoperative range of motion in the 74-year-old woman presented in Fig. 1. a Pseudoparlaysis of the shoulder with no abduction, anterior flexion and outer rotation. b Anterosuperior migration of the prosthesis. c Range of motion after revision reverse shoulder arthroplasty at four-year follow up
Due to poor bone quality, 43 (86 %) of the revision prostheses were cemented. Bone grafting for severe glenoid bone loss was performed in five (10 %) patients. In all cases, autologous iliac crest bone grafts were used. A single-stage procedure was performed in four cases, and a two-stage procedure was performed in one case.
In all cases, a Delta III or Delta Xtend reverse shoulder prosthesis (De Puy-Johnson & Johnson, Warsaw, IN) was implanted.
Postoperative protocol
The postoperative protocol included sling immobilisation for three to six weeks. In patients in whom no SSC repair was possible, during the first three postoperative weeks, continuous sling immobilisation was necessary. Patients were treated with finger, elbow, and pendulum exercises, as well as isometric exercises. After the third week, the sling was removed and an increased active range of motion was allowed. In patients who underwent additional SSC repair, sling immobilisation and abstention from abduction and external rotation for six weeks were necessary.
Statistical analysis
Repeated measures ANOVA with one fixed factor (HA, TEP and Inverse) was used to compare means among the three groups pre- and postoperatively. The 95 % confidence intervals for means, as well as the differences between means, were computed. Tukey HSD tests were used as post-hoc tests. VAS scores and differences in VAS scores over time were compared using a Kruskal-Wallis ANOVA. Whisker plots were created to illustrate results. A p-value less than 5 % indicated a statistically significant result. All computations were performed using STATISTICA 10.0 (StatSoft, Tulsa, OK).
Results
Compared with the preoperative status, the overall functional outcome measurements based on standardised outcome shoulder scores improved significantly at follow-up (Table 1). The overall mean Constant Murley score improved from 18.5 to 49.3 points (p < 0.0001), the mean UCLA score improved from 7.1 to 21.6 points (p < 0.0001), and the mean SST score improved from 1.2 to 5.6 points (p < 0.0001). The average degree of abduction improved from 40 to 93° (p < 0.0001), and the average degree of anterior flexion improved from 47 to 98° (p < 0.0001). The median VAS pain score decreased from 7 to 1 (p < 0.0001).
Table 1.
Overall functional outcome measurements
| Overall outcome (n = 50) | Pre-op | Post-op | Improvement | P value |
|---|---|---|---|---|
| CMS | 18.5 ± 9.3 | 49.3 ± 13.9 | 30.8 | <0.0001 |
| UCLA | 7.1 ± 3.0 | 21.6 ± 6.6 | 14.5 | <0.0001 |
| SST | 1.2 ± 0.8 | 5.6 ± 2.6 | 4.4 | <0.0001 |
| VAS | 7.0 | 1.0 | 6.0 | <0.0001 |
| Abduction | 39.7 ± 26.1 | 92.5 ± 32.4 | 52.8 | <0.0001 |
| Anterior flexion | 47.1 ± 26.4 | 98.4 ± 28.7 | 51.3 | <0.0001 |
CMS Constant Murley score, SST simple shoulder test, VAS visual analogue pain scale, UCLA score University of California, Los Angeles shoulder rating scale
Data given as value ± standard deviation
According to the UCLA score, 42 (84 %) and 8 (16 %) results were graded as poor and good, respectively. A total of 32 (64 %) patients rated their results as good or excellent, six (12 %) rated them as satisfactory and 12 (24 %) rated them as dissatisfied.
Among the 12 (24 %) dissatisfied patients, the average Constant Murley score was 34 points. The average degrees of abduction and anterior flexion were 27° and 63°, respectively. Four of the dissatisfied patients suffered from postoperative complications (two infections, one instability, and one aseptic glenoid loosening) after revision RSA and had to undergo further revision surgery. The patients named revision surgery and poor function as their reasons for dissatisfaction. Six of the unsatisfied patients received revision RSA in a two-stage procedure to combat deep prosthetic infection. These patients quoted poor functional outcome, persistent pain and extended suffering as the causes of their dissatisfaction.
A subgroup outcome analysis of primary arthroplasty types (HA, TEP, and RSA) was performed. In all groups, improvement in functional outcome and a decrease in pain level were noted between the pre- and postoperative data.
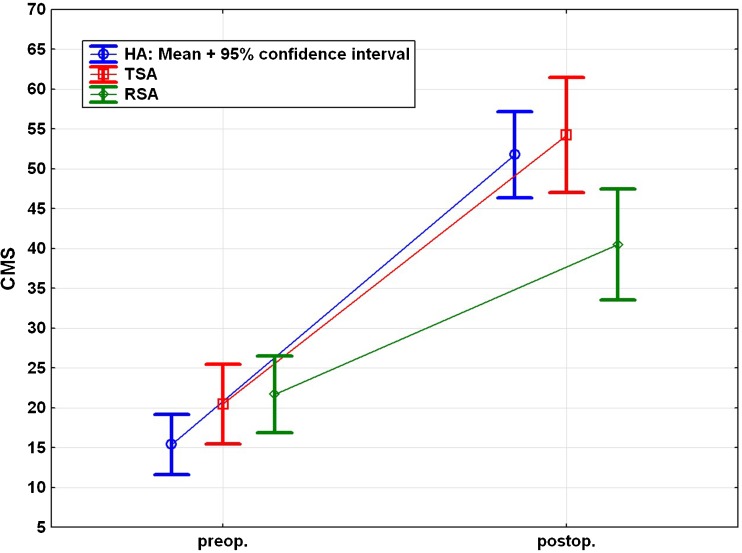
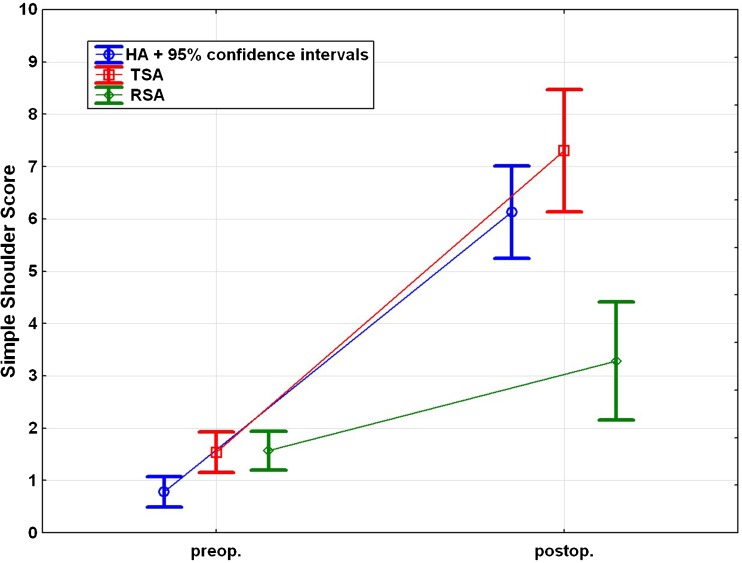
Comparison of the pre- and postoperative Constant Murley scores revealed a significant improvement (p < 0.001) in all groups after surgery. However, the revision RSA group exhibited statistically significantly lower CMS scores than the HA and TEP groups (p < 0.05) (Fig. 3).
Fig. 3.
Pre- and postoperative Constant Murley score for each subgroup
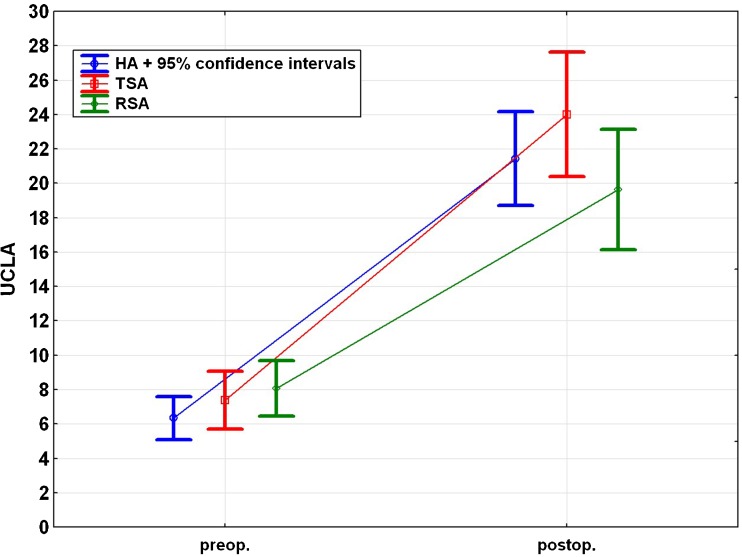
The UCLA score demonstrated significant improvement from pre- to postsurgery in every group. However, no significant difference was found among the groups (Fig. 4).
Fig. 4.
Pre- and postoperative University of California, Los Angeles shoulder rating scale (UCLA score) for each subgroup
The SST improved significantly in all groups between the pre- and postoperative data (p < 0.05). The postoperative SST was significantly worse in the revision RSA group compared with the HA and TSA groups (p < 0.001) (Fig. 5).
Fig. 5.
Pre- and postoperative simple shoulder test for each subgroup
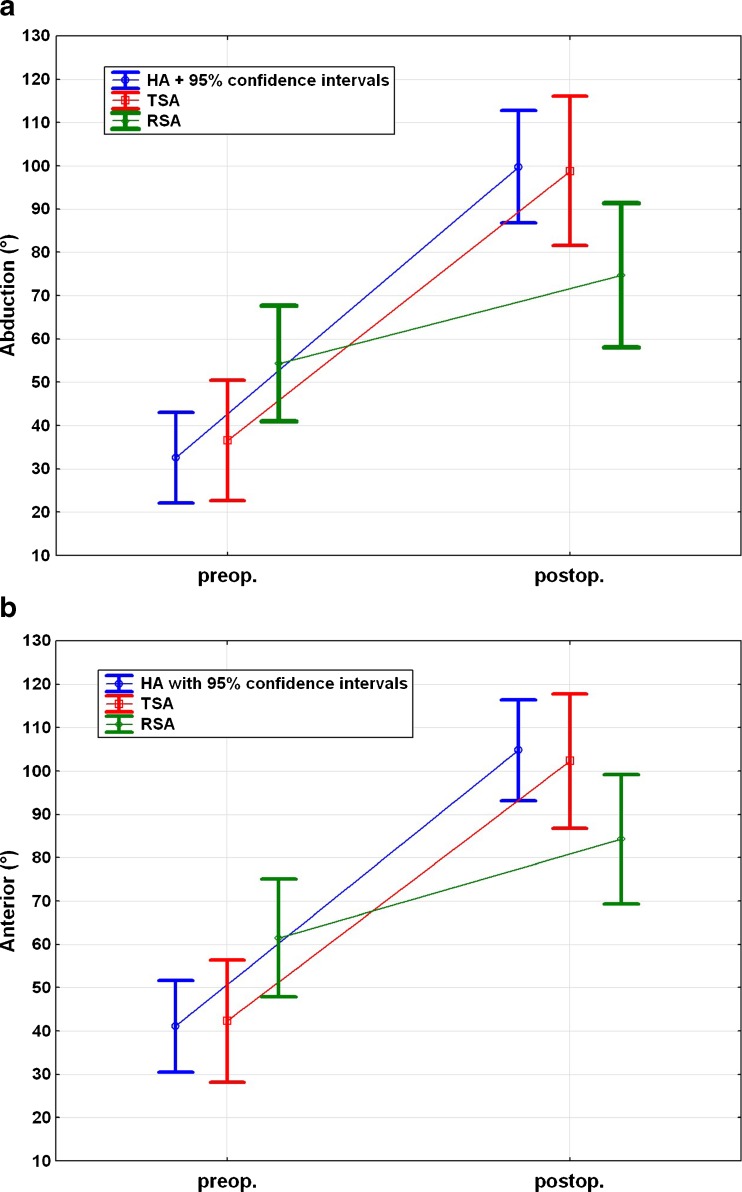
Abduction and flexion improved significantly in the HA and TSA groups (p < 0.001), but no statistically significant improvement was observed in the reverse RSA group (p = 0.069 and p = 0.090, respectively). No significant difference could be observed between the groups both pre- and postoperatively (Fig. 6).
Fig. 6.
a Pre- and postoperative abduction for each subgroup. b Pre- and postoperative anterior flexion for each subgroup
The VAS pain score decreased significantly in all groups (p < 0.001), but no significant difference among the groups was observed (p = 0.53).
Complications
Complications occurred in 12 cases (24 %). In one patient who underwent revision RSA after a two-stage revision for deep infection, postoperative haematoma occurred, requiring revision surgery on the fifth postoperative day. Nevertheless, at the final follow-up, this patient had a relatively good outcome (Constant Murley score 67, UCLA 27, SST 6, VAS 3, abduction 80°, anterior flexion 140°). In five patients, postoperative instability occurred, and a change of the polyethylene inlay was performed. Three of those patients rated their outcome as good, one as satisfied and one as dissatisfied.
One patient sustained postoperative anterolateral deltoid insufficiency due to partial axillary nerve injury during the revision operation. The patient was treated with an inverse pectoralis major transfer [26] and six months after revision RSA, at the final follow-up, the patient reported the result as good. The examination revealed abduction of 50° and anterior flexion of 100° (CMS 51, UCLA 23, SST 5 and VAS 2). Two patients sustained a Vancouver B2 periprosthetic fracture one and four years after revision RSA, and both were treated operatively to revise the long cemented humeral stem and cable cerclages. Both patients rated their final outcome as satisfactory. In one patient, aseptic glenoid loosening was observed. In two patients, late prosthetic infections occurred, one 13 months and the other 16 months after revision. Propionibacterium acnes was the causative bacteria, and both patients were treated by two-stage revision and rated their results as poor at the final follow-up. Clinical investigation revealed 40° and 60° of abduction and 60° of anterior flexion for both patients. The CMS scores were low at 26 and 32 points.
Discussion
Revision arthroplasty represents a challenge for orthopaedic surgeons in all fields, including shoulder surgery. Previously published studies indicate that the outcomes after revision shoulder arthroplasty are inferior to those of primary shoulder arthroplasty [19, 20]. However, the overall clinical and functional outcomes of this study are in accordance with results published in the literature [21, 27].
Levy et al. [28] reported the outcomes of reverse shoulder arthroplasty for the treatment of failed hemiarthroplasty performed after proximal humeral fracture. In 29 patients, they retrospectively found significant improvements in the simple shoulder test from 0.9 to 2.6 (p < 0.004), forward flexion from 38.1 to 72.7 (p < 0.001), and abduction from 34.1 to 70.4 (p < 0.001). The complication rate was 28 %.
Treatment of failed hemiarthroplasty after fracture of the proximal humerus is challenging and requires a very clear indication and proper patient selection. In cases of isolated complications, such as glenoid erosion in combination with an intact rotator cuff, treatment with secondary glenoid implantation should be performed. In cases of malunion or nonunion of the tuberosities and rotator cuff deficiency, conversion to a reverse shoulder prosthesis is often the only suitable option. The indication for revision reverse shoulder arthroplasty should be based on multifactorial complications, as described by Levy et al. [28]: “Only patients with combined rotator cuff deficiency and glenoid arthritis accompanied with severe pain and loss of function are candidates.” The treatment of substantial humeral bone loss is often very difficult. Therefore, the authors [28] used a humeral allograft to reconstruct the proximal portion of the humerus. In our cohort, the use of such allografts was not necessary.
In another study by Levy et al. [29], the authors retrospectively investigated the outcome of reverse shoulder arthroplasty after failed hemiarthroplasty in patients with glenohumeral arthritis and cuff deficiency. The flexion and abduction improved from 49.7 to 76.1° (p = 0.0062) and 42.2 to 77.2° (p = 0.0005), respectively. Prosthesis-related complications led to re-operation in 32 % of patients.
In another study, Walker et al. [30] evaluated 24 patients who underwent RSA after failure of TSA. The simple shoulder test score improved from 1 to 5 (p < 0.006), and the forward flexion and abduction improved from 50 to 130° and 45 to 100° (p < 0.001), respectively. The overall complication rate was 22.7 %.
In a retrospective, multicentre cohort study of 34 patients who underwent revision RSA for failed TSA, Melis et al. [31] found that the Constant Murley score improved from 24 to 55 (p < 0.0001) and that the active anterior flexion improved from 68 to 121° (p < 0.0001). The postoperative complication rate was 30 %, and 22 % of these patients needed re-revision surgery.
The results, including the complication rate, from our study are similar to those reported in other studies in the literature [21, 27–31]. The best results were achieved in the TSA group, a trend which is also suggested in the study by Walker et al. [30] and Melis et al. [31], who obtained better pre- and postoperative functional outcome data compared with the studies of Levy et al. [28, 29].
Significantly worse CMS and SST scores were observed in revision RSA compared with HA and TSA revisions. In the revision RSA group, no significant improvements in abduction or anterior flexion were achieved, and the outcome in this group was worse than those in the revision HA and TSA groups. These data illustrate the main causes of low functional score results.
In this study, the reason for the inferior results of revision RSA cannot be directly related to the implant design. Instead, the poor outcomes and patient dissatisfaction are probably due to the fact that in eight out of nine revision RSAs, two-stage revision surgery was required due to deep infection. Infection in reverse shoulder arthroplasty is a disastrous condition, leading to severe bone loss, which causes difficulties in proper fixation and positioning of the glenoid component. In some cases, glenoid bone grafting may become necessary. In this cohort, bone grafting for deep infection was performed in five patients, using a two-stage procedure in four patients and a one-stage procedure in one patient. Chronic infection often harms the soft tissue around the joint and causes scar tissue formation. We also observed that only one-third of those patients who required two-stage revision for deep infection were pain-free and that patients in the revision RSA group were older than those in the HA and TSA groups (average ages: RSA 78.6 years; TSA 69.9 years; and HA 52.2 years).
Several studies, however, have shown that the use of reverse shoulder arthroplasty (RSA) is a suitable option associated with moderate complication rates in the treatment of primary rotator cuff arthropathy [32, 33]. The complication rates described in the revision setting are high [27–31], again often due to severe glenoid or humeral bone loss [29].
In addition to infection, instability is a complication following shoulder arthroplasty. In our study, five shoulders had to be revised because of instability due to inadequate deltoid tensioning. In all cases, changing of the polyethylene inlay was successful and led to stable joint. Generally, the cause of instability is insufficient deltoid tensioning, glenoid notching or infection. On the other hand, overly high tension can lead to nerve injuries or acromial fractures. For the surgeon, generating the proper tension of the deltoid muscle is challenging, which emphasises the importance of the surgeon’s experience in revision arthroplasty. Inadequate tensioning can be treated by using a thicker inlay or a lateraliser, as well as a glenosphere with a greater diameter [19, 34].
Aseptic glenoid loosening occurred in one patient in our cohort. To avoid glenoid loosening, it is mandatory to place the metaglene onto a sufficient bone stock and in an inferior position [35]. In the revision setting, insufficient removal of cement on the glenoid side could prevent bleeding and ingrowth of the glenoid component [30].
Postoperative infections occurred in two patients (over 12 months postoperatively), and both were treated by two-stage revision. Management strategies for this condition range from debridement and intravenous antibiotic therapy to single or two-stage reimplantation to partial component removal and resection arthroplasty. In revision arthroplasty, attention should be paid to low-virulence organisms, such as Propionibacterium acnes and Staphylococcus epidermidis. Intraoperative samples should be taken and incubated for at least 14 days to exclude infection [36–38].
One patient suffered from postoperative anterolateral deltoid insufficiency and was treated with pectoralis major inverse plasty [26] six months postoperatively. Muscle insufficiency was verified with electromyography. Nerve injury appeared to be caused by the lengthening of the arm during reverse shoulder arthroplasty. Nerve injuries seem to occur more often in reverse shoulder arthroplasty than in anatomical shoulder arthroplasties [39]. In the revision setting, the incidence of nerve injuries seems to be even higher because of difficult preparation due to scar tissue formation and difficulties during prosthesis removal.
There were some limitations of this study, including its retrospective design. Due to a complete and accurate shoulder database, however, preoperative data are available and reliable. Another limitation is the small patient sample size in each subgroup, which limits the statistical analysis of indications and risk factors for revision surgery.
Conclusion
The functional outcomes improved in each subgroup, and high patient satisfaction was seen, demonstrating that reverse shoulder arthroplasty is a suitable treatment option for failed primary shoulder arthroplasty. However, the complication rate of this procedure is relatively high, and patient selection and other treatment options should be carefully considered. Revision arthroplasty for deep infection is associated with poor outcomes, which must be discussed with the patient.
References
- 1.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 2.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 3.Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA., 3rd Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69:865–872. [PubMed] [Google Scholar]
- 4.Franklin JL, Barrett WP, Jackins SE, Matsen FA., 3rd Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3:39–46. doi: 10.1016/S0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 5.Arntz CT, Jackins S, Matsen FA., 3rd Prosthetic replacement of the shoulder for the treatment of defects in the rotator cuff and the surface of the glenohumeral joint. J Bone Joint Surg Am. 1993;75:485–491. doi: 10.2106/00004623-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Field LD, Dines DM, Zabinski SJ, Warren RF. Hemiarthroplasty of the shoulder for rotator cuff arthropathy. J Shoulder Elbow Surg. 1997;6:18–23. doi: 10.1016/S1058-2746(97)90066-5. [DOI] [PubMed] [Google Scholar]
- 7.Berth A, Pap G. Hemi- versus bipolar shoulder arthroplasty for chronic rotator cuff arthropathy. Int Orthop. 2008;32:735–740. doi: 10.1007/s00264-007-0394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman JD, Scott AJ, Gallagher MA. Hemiarthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2000;9:169–172. doi: 10.1016/S1058-2746(00)90050-8. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001;83-A:1814–1822. doi: 10.2106/00004623-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Compito CA, Self EB, Bigliani LU (1994) Arthroplasty and acute shoulder trauma. Reasons for success and failure. Clin Orthop Relat Res 307:27–36 [PubMed]
- 11.Robinson CM, Page RS, Hill RMF, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A:1215–1223. doi: 10.2106/00004623-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Plausinis D, Kwon YW, Zuckerman JD. Complications of humeral head replacement for proximal humeral fractures. Instr Course Lect. 2005;54:371–380. [PubMed] [Google Scholar]
- 13.Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA., 3rd Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11:431–441. doi: 10.1067/mse.2002.125806. [DOI] [PubMed] [Google Scholar]
- 14.Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12:569–577. doi: 10.1016/S1058-2746(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 15.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11:401–412. doi: 10.1067/mse.2002.124527. [DOI] [PubMed] [Google Scholar]
- 16.Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19:439–449. [PubMed] [Google Scholar]
- 17.Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34:1075–1082. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly JD, 2nd, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21:1516–1525. doi: 10.1016/j.jse.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Dines JS, Fealy S, Strauss EJ, Allen A, Craig EV, Warren RF, Dines DM. Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg Am. 2006;88:1494–1500. doi: 10.2106/JBJS.D.02946. [DOI] [PubMed] [Google Scholar]
- 21.Flury MP, Frey P, Goldhahn J, Schwyzer H-K, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure—midterm results. Int Orthop. 2011;35:53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Constant CR, Murley AH (1987) A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 214:160–164 [PubMed]
- 23.Amstutz HC, Sew Hoy AL, Clarke IC (1981) UCLA anatomic total shoulder arthroplasty. Clin Orthop Relat Res 7–20 [PubMed]
- 24.Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 25.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/S0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 26.Resch H, Povacz P, Maurer H, Koller H, Tauber M. Pectoralis major inverse plasty for functional reconstruction in patients with anterolateral deltoid deficiency. J Bone Joint Surg Br. 2008;90:757–763. doi: 10.1302/0301-620X.90B6.19804. [DOI] [PubMed] [Google Scholar]
- 27.Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 28.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89:292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 29.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89:189–195. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 30.Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:514–522. doi: 10.1016/j.jse.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Melis B, Bonnevialle N, Neyton L, Lévigne C, Favard L, Walch G, Boileau P. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21:342–349. doi: 10.1016/j.jse.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14:147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 34.Gallo RA, Gamradt SC, Mattern CJ, Cordasco FA, Craig EV, Dines DM, Warren RF. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20:584–590. doi: 10.1016/j.jse.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Nyffeler RW, Werner CML, Simmen BR, Gerber C. Analysis of a retrieved delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86:1187–1191. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 36.Zavala JA, Clark JC, Kissenberth MJ, Tolan SJ, Hawkins RJ (2012) Management of deep infection after reverse total shoulder arthroplasty: a case series. J Shoulder Elbow Surg 21(10):1310–1315 [DOI] [PubMed]
- 37.Beekman PDA, Katusic D, Berghs BM, Karelse A, De Wilde L. One-stage revision for patients with a chronically infected reverse total shoulder replacement. J Bone Joint Surg Br. 2010;92:817–822. doi: 10.1302/0301-620X.92B6.23045. [DOI] [PubMed] [Google Scholar]
- 38.Dodson CC, Craig EV, Cordasco FA, Dines DM, Dines JS, Dicarlo E, Brause BD, Warren RF. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19:303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 39.Lädermann A, Lübbeke A, Mélis B, Stern R, Christofilopoulos P, Bacle G, Walch G. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93:1288–1293. doi: 10.2106/JBJS.J.00369. [DOI] [PubMed] [Google Scholar]