Abstract
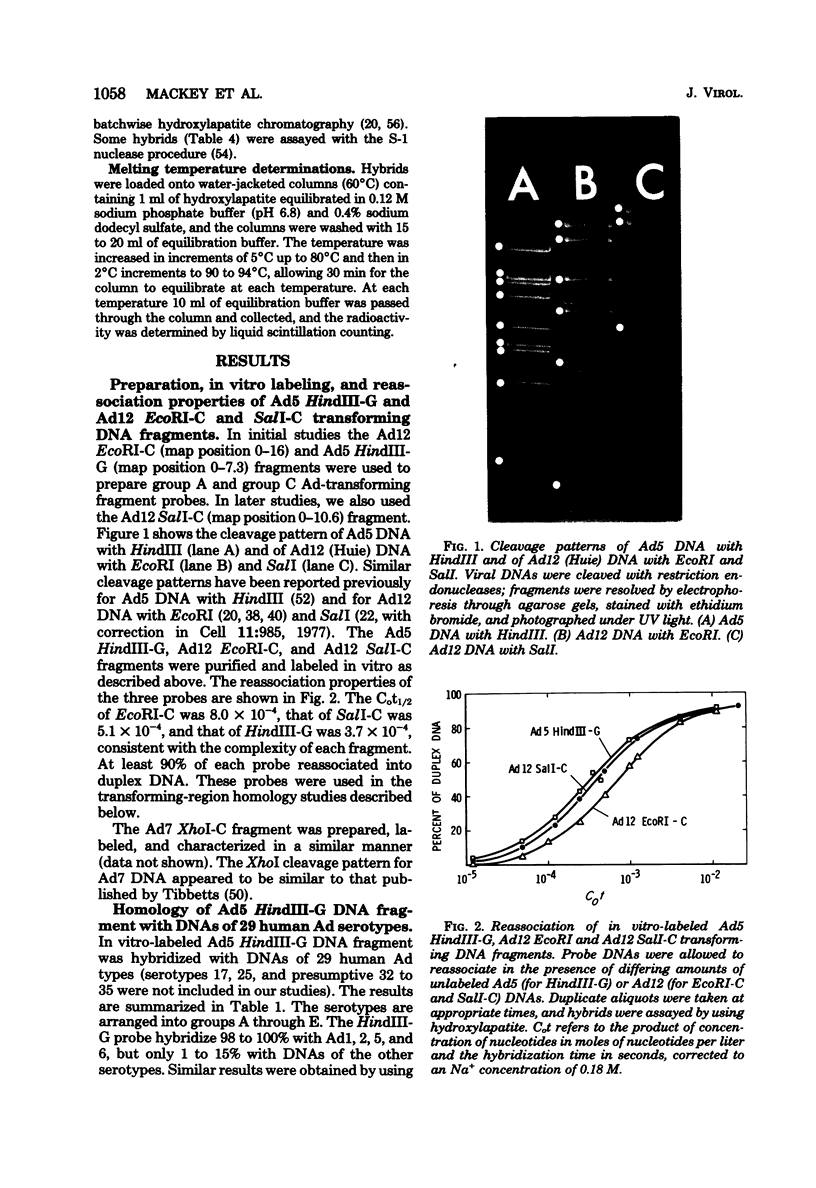
The 31 human adenovirus (Ad) serotypes form five groups based upon DNA genome homologies: group A (Ad12, 18, 31), group B (Ad3, 7, 11, 14, 16, 21), group C (Ad1, 2, 5, 6), group D (Ad8, 9, 10, 13, 15, 17, 19, 20, 22-30), and group E (Ad4) (M. Green, J. Mackey, W. Wold, and P. Rigden, Virology, in press). Group A Ads are highly oncogenic in newborn hamsters, group B Ads are weakly oncogenic, and other Ads are nononcogenic. However, most or all Ads transform cultured cells. We have studied the homology of Ad5, Ad7, and Ad12 transforming restriction endonuclease DNA fragments with DNAs of 29 Ad types. Ad5 HindIII-G (map position 0-7.3), Ad7 XhoI-C (map position 0-10.8), and Ad12 (strain Huie) EcoRI-C (map position 0-16) and SalI-C (map position 0-10.6) fragments were purified, labeled in vitro (nick translation), and annealed with DNAs of Ad1 to Ad16, Ad18 to Ad24, and Ad26 to Ad31. Hybrids were assayed by using hydroxylapatite. Ad5 HindIII-G hybridized 98 to 100% with DNAs of group C Ads, but only 1 to 15% with DNAs of other types. Ad7 XhoI-C fragment hybridized 85 to 99% with DNAs of group B Ads, but only 6 to 21% with DNAs of other types. Ad12 (Huie) EcoRI-C hybridized 53 to 68% with DNAs of five other Ad12 strains, 53% with Ad18 DNA, 56% with Ad31 DNA, but only 3 to 13% with DNAs of other types. In vitro-labeled Ad12 (Huie) SalI-C hybridized 35 to 71% with DNAs of 6 other Ad12 strains, 44% with Ad18 DNA, 52% with Ad31 DNA, but only 2 to 7% with DNAs Ad7, Ad2, Ad26, or Ad4. When assayed using S-1 nuclease, SalI-C annealed 17 to 44% with DNAs of group A Ads. The melting temperatures of the hybrids of Ad5 HindIII-G with all group C Ad DNAs were 84°C in 0.12 M sodium phosphate (pH 6.8). The melting temperature of the Ad12 (Huie) EcoRI-C hybrid with Ad12 (Huie) DNA was 83°C, but was only 71 to 77°C with DNAs of other group A Ads. Thus, group C and group B Ads both have very homologous transforming regions that are not represented in DNAs of non-group C Ads or non-group B Ads, respectively. Similarily, group A Ads have unique but less homologous transforming regions. These different transforming nucleotide sequences may be reflected in the different oncogenic properties of group A, B, and C Ads.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blacklow N. R., Hoggan M. D., Austin J. B., Rowe W. P. Observations on two new adenovirus serotypes with unusual antigenic characteristics. Am J Epidemiol. 1969 Dec;90(6):501–505. doi: 10.1093/oxfordjournals.aje.a121095. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Ludwig W., Heubner R. J., Cate T. R., Chu L. W. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA. 1966 Feb 7;195(6):445–452. [PubMed] [Google Scholar]
- Fanning E., Doerfler W. Intracellular forms of adenovirus DNA. V. Viral DNA sequences in hamster cells abortively infected and transformed with human adenovirus type 12. J Virol. 1976 Nov;20(2):373–383. doi: 10.1128/jvi.20.2.373-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sambrook J., Williams J. F., Sharp P. A. Viral nucleic acid sequences in transformed cells. IV. A study of the sequences of adenovirus 5 DNA and RNA in four lines of adenovirus 5-transformed rodent cells using specific fragments of the viral genome. Virology. 1976 Jul 15;72(2):456–470. doi: 10.1016/0042-6822(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Vanderpool E. A., Henry P. H., Austin J. B., Huebner R. J. Transformation of primary rat embryo cells by adenovirus type 2. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1205–1212. doi: 10.1073/pnas.58.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARDI A. J., HILLEMAN M. R., ZWICKEY R. E. TESTS IN HAMSTERS FOR ONCOGENIC QUALITY OF ORDINARY VIRUSES INCLUDING ADENOVIRUS TYPE 7. Proc Soc Exp Biol Med. 1964 Apr;115:1141–1150. doi: 10.3181/00379727-115-29138. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., McDougall J. K., Chen L. B. In vitro traits of adenovirus-transformed cell lines and their relevance to tumorigenicity in nude mice. Cell. 1977 Apr;10(4):669–678. doi: 10.1016/0092-8674(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Hierholzer J. C., Rose J. A. Mapping of base sequence heterologies between genomes from different adenovirus serotypes. Virology. 1973 Aug;54(2):414–426. doi: 10.1016/0042-6822(73)90153-0. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Kern J., Freeman A. E., Martin C. E., McAllister R. C., Turner H. C., Huebner R. J. T and tumour antigens of adenovirus group C-infected and transformed cells. Nature. 1968 Aug 3;219(5153):517–518. doi: 10.1038/219517a0. [DOI] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Mackey J. K., Green M. Multiple copies of human adenovirus 12 genomes are integrated in virus-induced hamster tumors. J Virol. 1977 Apr;22(1):238–242. doi: 10.1128/jvi.22.1.238-242.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Chardonnet Y., Doerfler W. Integrated viral sequences in adenovirus type 12-transformed hamster cells. Cell. 1977 Jan;10(1):101–111. doi: 10.1016/0092-8674(77)90144-1. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., LANE W. T. Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2051–2058. doi: 10.1073/pnas.48.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Atuk N. O., Gwaltney J. M., Jr New human adenovirus isolated from a renal transplant recipient: description and characterization of candiate adenovirus type 34. J Clin Microbiol. 1975 Apr;1(4):366–376. doi: 10.1128/jcm.1.4.366-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Casey M. J., Chanock R. M., Schell K. Tumors induced in hamsters by a strain of adenovirus type 3: sharing of tumor antigens and "neoantigens" with those produced by adenovirus type 7 tumors. Proc Natl Acad Sci U S A. 1965 Aug;54(2):381–388. doi: 10.1073/pnas.54.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson N., Ankerst J. Studies on adenovirus type 9-induced mammary fibroadenomas in rats and their malignant transformation. Cancer. 1977 Jun;39(6):2513–2519. doi: 10.1002/1097-0142(197706)39:6<2513::aid-cncr2820390631>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- LARSON V. M., GIRARDI A. J., HILLEMAN M. R., ZWICKEY R. E. STUDIES OF ONCOGENICITY OF ADENOVIRUS TYPE 7 VIRUS IN HAMSTERS. Proc Soc Exp Biol Med. 1965 Jan;118:15–24. doi: 10.3181/00379727-118-29744. [DOI] [PubMed] [Google Scholar]
- Lee K. C., Mak S. Adenovirus type 12 DNA sequences in primary hamster tumors. J Virol. 1977 Oct;24(1):408–411. doi: 10.1128/jvi.24.1.408-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Levine A. J. The group C adenovirus tumor antigens: identification in infected and transformed cells and a peptide map analysis. Cell. 1977 Aug;11(4):871–879. doi: 10.1016/0092-8674(77)90298-7. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rabson A. S., Levine A. S. Studies of nondefective adenovirus 2-simian virus 40 hybrid viruses. Transformation of hamster kidney cells by adenovirus 2 and the nondefective hybrid viruses. J Virol. 1974 Jun;13(6):1291–1301. doi: 10.1128/jvi.13.6.1291-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- MCBRIDE W. D., WIENER A. IN VITRO TRANSFORMATION OF HAMSTER KIDNEY CELLS BY HUMAN ADENOVIRUS TYPE 12. Proc Soc Exp Biol Med. 1964 Apr;115:870–874. doi: 10.3181/00379727-115-29060. [DOI] [PubMed] [Google Scholar]
- Mackey J. K., Brackmann K. H., Green M. R., Green M. Preparation and characterization of highly radioactive in vitro labeled adenovirus DNA and DNA restriction fragments. Biochemistry. 1977 Oct 4;16(20):4478–4483. doi: 10.1021/bi00639a023. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Lewis A. M., Jr, Macpherson I., Huebner R. J. Transformation of rat embryo cells by adenovirus type 1. J Gen Virol. 1969 Jan;4(1):29–36. doi: 10.1099/0022-1317-4-1-29. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Reed G., Kern J., Gilden R. V., Huebner R. J. Transformation of rodent cells by adenovirus 19 and other group D adenoviruses. J Natl Cancer Inst. 1969 Oct;43(4):917–923. [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA M. S., PERIERA H. G., CLARKE S. K. HUMAN ADENOVIRUS TYPE 31. A NEW SEROTYPE WITH ONCOGENIC PROPERTIES. Lancet. 1965 Jan 2;1(7375):21–23. doi: 10.1016/s0140-6736(65)90925-6. [DOI] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Pettersson U., Lindberg U. Molecular biology of adenoviruses. Virol Monogr. 1975;14:1–115. doi: 10.1007/978-3-7091-8391-5_1. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. IX. Chemical and base composition analysis of 28 human adenoviruses. Proc Natl Acad Sci U S A. 1965 Aug;54(2):547–551. doi: 10.1073/pnas.54.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN L. A hemagglutination-inhibition technique for typing adenoviruses. Am J Hyg. 1960 Jan;71:120–128. doi: 10.1093/oxfordjournals.aje.a120085. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Sekikawa K., Shiroki K., Shimojo H., Ojima S., Fujinaga K. Transformation of a rat cell line by an adenovirus 7 DNA fragment. Virology. 1978 Jul 1;88(1):1–7. doi: 10.1016/0042-6822(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Handa H., Shimojo H., Yano S., Ojima S., Fujinaga K. Establishment and characterization of rat cell lines transformed by restriction endonuclease fragments of adenovirus 12 DNA. Virology. 1977 Oct 15;82(2):462–471. doi: 10.1016/0042-6822(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Stalder H., Hierholzer J. C., Oxman M. N. New human adenovirus (candidate adenovirus type 35) causing fatal disseminated infection in a renal transplant recipient. J Clin Microbiol. 1977 Sep;6(3):257–265. doi: 10.1128/jcm.6.3.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Tibbetts C. Physical organization of subgroup B human adenovirus genomes. J Virol. 1977 Nov;24(2):564–579. doi: 10.1128/jvi.24.2.564-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Eb A. J., Mulder C., Graham F. L., Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. doi: 10.1016/0378-1119(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Williams J. F. Oncogenic transformation of hamster embryo cells in vitro by adenovirus type 5. Nature. 1973 May 18;243(5403):162–163. doi: 10.1038/243162a0. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Brackmann K. H., Cartas M. A., Devine C. Genome expression and mRNA maturation at late stages of productive adenovirus type 2 infection. J Virol. 1976 Nov;20(2):465–477. doi: 10.1128/jvi.20.2.465-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S., Ojima S., Fujinaga K., Shiroki K., Shimojo H. Transformation of a rat cell line by an adenovirus type 12 DNA fragment. Virology. 1977 Oct 1;82(1):214–220. doi: 10.1016/0042-6822(77)90044-7. [DOI] [PubMed] [Google Scholar]