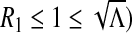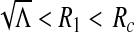Abstract
The aim of this paper is to analyze the recruitment effects of susceptible and infected individuals in order to assess the productivity of an organizational labor force in the presence of HIV/AIDS with preventive and HAART treatment measures in enhancing the workforce output. We consider constant controls as well as time-dependent controls. In the constant control case, we calculate the basic reproduction number and investigate the existence and stability of equilibria. The model is found to exhibit backward and Hopf bifurcations, implying that for the disease to be eradicated, the basic reproductive number must be below a critical value of less than one. We also investigate, by calculating sensitivity indices, the sensitivity of the basic reproductive number to the model’s parameters. In the time-dependent control case, we use Pontryagin’s maximum principle to derive necessary conditions for the optimal control of the disease. Finally, numerical simulations are performed to illustrate the analytical results. The cost-effectiveness analysis results show that optimal efforts on recruitment (HIV screening of applicants, etc.) is not the most cost-effective strategy to enhance productivity in the organizational labor force. Hence, to enhance employees’ productivity, effective education programs and strict adherence to preventive measures should be promoted.
Keywords: Human immunodeficiency virus (HIV), Productivity, Optimal control
Introduction
The profitability of any organizational venture depends largely on the productivity of its workforce. The main determining factors include skill level, motivation, satisfaction, and schedule pressure. The question now is how would an ineffective employee recruitment policy affect the organization in the face of HIV/AIDS infection?
HIV/AIDS is not only a health issue but a substantial threat to socio-economic development, imposing a heavy burden on families, communities, and economies. The pandemic has affected most countries of the world. In 2003, UNAIDS estimated that 38 million people were HIV-positive worldwide of which about 26 million were workers between the ages of 15 and 49 (the most productive age group). This has great implications for families and economies in terms of employment, productivity, and labor-market changes [1].
In 2005, about 3.1 million people died from AIDS (acquired immunodeficiency syndrome) and 4.9 million people became infected with HIV, bringing the number to 40.3 million of people living with the virus across the world (UNAIDS 2005). HIV infection, which can cause AIDS, is a global problem and has shown a high degree of prevalence in populations all over Sub-Saharan Africa. The most susceptible individuals at risk of acquiring infection include people having sexual contacts with the HIV infected, homosexual and bisexual men, intravenous drug abusers, and persons transfused with contaminated blood.
HIV is preventable but not curable. Highly active antiretroviral therapy (HAART) is not a cure but the use of drugs to halt the decline in immune deficiency and prevent disease progression and death. ART suppresses viral replication and successful treatment helps in slowing or halting the disease progression, prevention of drug resistance, and improvement in the quality of life. Although the number of infected people receiving ART in low- and middle-income countries has increased dramatically, optimal disease management is not well defined. Despite substantial progress in the access to treatment, only 20% of adults who needed ART were receiving it [2] as of 2012.
There is still an intensive search going on for an anti-HIV vaccine. The use of chemotherapy is aimed at killing or halting the pathogen, but treatment that can boost the immune system can serve to help the body fight infection on its own [3]. The new treatments are aimed at reducing the viral population and improving the immune response. This brings new hope to the treatment of HIV infection and we are exploring strategies for such treatments using optimal control techniques.
A host of social, economic, cultural, and political factors facilitate the spread of HIV through populations. HIV spreads more widely where a wrong perspective or attitudes about HIV are common, for example, extensive organizational (office) sexual networks, where a person is having sex with more than one partner in an office location. Having multiple partners concurrently creates a node of transfer from one sexual network to another. Where sexual networks are smaller and more circumscribed, HIV can spread but less widely. The challenges posed by peoples’ attitude and ignorance to the disease dynamics call for a better understanding of its transmission and the development of effective and optimal strategies for the prevention and control of the spread of HIV/AIDS. Research has also shown that a behavioral or attitudinal change has a great influence on disease spread [4].
Most organizational workplaces recruit individuals without knowing their HIV/AIDS status or whether the individual would become an asset (productive) to the company or a liability (non-productive). The non-productive employees can be associated with their lifestyle (absenteeism, passive/aggressive behavior, not meeting deadlines, carefree attitude, and negative effect of multiple sexual partners) [5]. It is clear from various studies that interventions designed to support employment, or economic policy, or a national HIV/AIDS strategy cannot be implemented without considering the particular impact HIV and AIDS are having on the economically active and productive population [1]. As very little is known about the impact of HIV/AIDS on the labor force structure and productivity, this study then seeks to fill some of the gaps in this knowledge.
The impact of AIDS is significant on most economic sectors. For instance, agriculture, being the largest sector in most African economies, which accounts for a large portion of production and large employment opportunities, bears the brunt of HIV/AIDS. Studies performed in some countries have shown that AIDS will continue to have adverse effects on agriculture, including loss of labor supply and remittance income. The loss of a few workers at crucial periods of planting and harvesting can significantly reduce the size of the harvest [6]. AIDS-related illnesses and deaths to employees affect a firm by both increasing expenditures and reducing revenues. Expenditures are increased for health care costs, burial fees and training and recruitment of replacement employees. Revenues may be decreased because of absenteeism due to illness or attendance at funerals and time spent on training. Labor turnover can lead to a less experienced labor force that is less productive [7].
Mathematical models have been very useful tools in understanding the transmission dynamics of infectious diseases including HIV and in designing control measures to contain the diseases. Furthermore, they offer a better understanding of the epidemiological patterns for disease control as they provide short- and long-term predictions of HIV and AIDS incidence. Mathematical models have been used extensively in research into the epidemiology of HIV/AIDS to help improve our understanding of the major contributing factors.
Some of the notable studies include Anderson et al. [8], who presented a simple mathematical HIV transmission model to investigate the effects of various factors on the overall pattern of the AIDS epidemic. While Nikolaos et al. [9] proposed a detailed analysis of a dynamical model to describe the pathogenesis of HIV infection, and Christopher and Jorge [10] derived a simple two-dimensional SIS (susceptible-infected-susceptible) model with vaccination and multiple endemic states. Guihua and Zhen [11] studied the global dynamics of an SEIR (susceptible-exposed-infected-recovered) epidemic model in which latent and immune states were infective. Agraj et al. [12] proposed a mathematical model to study the effect of screening of unaware infectives on the spread of HIV infection. Mukandavire et al. [13] proposed and examined a deterministic model for the co-infection of HIV and malaria in a community.
Karrakchou et al. [14] investigated the fundamental role of chemotherapy treatment in controlling the virus reproduction in an HIV patient, while Adams et al. [15] derived HIV therapeutic strategies by formulating and analyzing an optimal control problem using two types of dynamic treatments. Gul et al. [16] studied the stability and optimal vaccination of an SIR epidemic model. Okosun et al. [4] investigated the effectiveness of HIV/AIDS preventive and treatment measures (treatment of HIV individuals, treatment of AIDS individuals, education campaign in the presence of carefree susceptibles) in reducing the spread of the disease. For optimal controls applied in other epidemic models, we refer the reader to [3, 17–19].
Very little or no attention has been paid to study models that incorporate classes of productive and non-productive individuals (susceptibles and infected) and that may be helpful in determining the productivity level of the labor force in organizations in the presence of HIV/AIDS. The model we consider in this paper is an improved dynamical system model but with the inclusion of a productive susceptible class, productive HIV-infected class, and time-dependent control parameters. We study and determine the possible impact of optimal education and HAART treatment for enhancing productivity on the spread of HIV. We carried out a detailed qualitative optimal control analysis of the resulting model and we determined the necessary conditions for optimal control of the disease using Pontryagin’s maximum principle in order to identify optimal strategies for controlling the spread of the disease.
Our main goal is to set up an optimal control problem related to the model. In order to do this, we use the following recruitment strategy: (effective screening of applicants) u1, preventive measures (abstinence, being faithful, condom use) parameters on susceptibles u3, education or educational parameter u2 and HAART treatment parameter for individuals with HIV u4 as time-dependent controls in the model. Hence, we investigate the role of productive susceptibles, productive infected, optimal education through counseling, mentoring, training, screening, educational campaigns, and HAART treatment of HIV/AIDS on the spread of HIV/AIDS.
The paper is organized as follows. In Section 2, we present a model consisting in ordinary differential equations (ODEs) that describes the dynamics of the disease and the underlying assumptions. The analysis of an endemic equilibrium is presented in Section 3. In Section 4 we use Pontryagin’s maximum principle to investigate the analysis of control strategies and to find the necessary conditions for the optimal control of the disease. In Section 5, we show the simulation results and then present the cost-effectiveness analysis in Section 6. Our conclusions are discussed in Section 7.
Model formulation
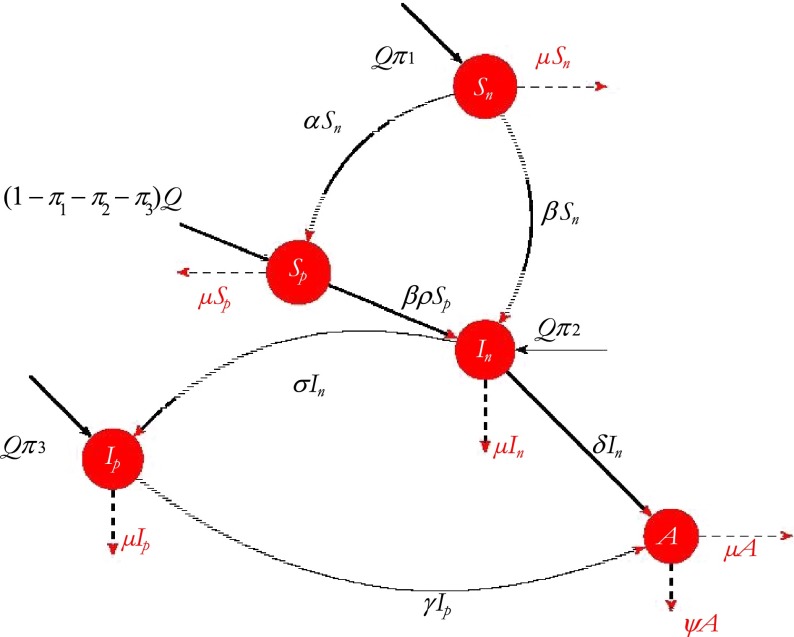
The model that we propose here is a standard compartmental model of HIV/AIDS in which we incorporated three time-dependent control measures simultaneously: (i) preventive measures (abstinence, being faithful, condom use), (ii) an education campaign, (iii) HAART treatment of HIV individuals for enhanced productivity. The model sub-divides the total human population at time t, denoted by N(t), into the following sub-populations of susceptible productive workers (Sp(t)), susceptible non-productive workers (Sn(t)), infected non-productive workers (In(t)), infected productive individuals on HAART treatment (Ip(t)), and that of full-blown AIDS individuals A(t), so that
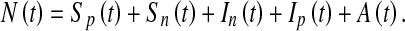 |
The susceptibles are individuals who have not contracted the infection but may be infected through sexual contacts. The susceptible non-productive are individuals whose lifestyle places them at high risk of contracting the disease.
The organization recruits workers at the rate of Q, where the proportion of susceptible non-productive, infected non-productive and infected productive workers are recruited at rates π1, π2 and π3 respectively. The parameter β is the per-capita contact rate for susceptible non-productive individuals. The susceptible productive workers have a per- capita contact rate of β1 = ρβ < β, where ρ is the modification parameter due to the right value perception of the susceptible productive workers. When susceptible non-productive individuals are educated (through counseling, mentoring, or monitoring) and their attitude changes, they progress to the susceptible productive class at a rate u2α, where u2 is the education control effort. The infected non-productive individuals on the HAART treatment progress at a rate u3σ to the infected productive individuals class, where σ is the proportion of the infected non-productive individuals on the HAART treatment for enhanced productivity and u3 is the control effort on the HAART treatment to enhance productivity.
Infected non-productive workers not on the HAART treatment progress into the full-blown AIDS class at a rate δ, while γ is the rate of progression of infected productive individuals into full-blown AIDS, here γ < δ. The term η is the modification parameter due to the HAART treatment and right value perceptions of the infected productive individuals. The disease-induced death rate of AIDS individuals is denoted by ψ and μ is the natural mortality rate unrelated to HIV/AIDS. We also assume the AIDS class to be weakly sexually active and non-productive. We denote by τ the transmission probability of an AIDS individual infecting susceptible humans (Fig. 1).
Fig. 1.
Flow diagram for HIV/AIDS disease transmission
The resulting system of the equation for the constant control case is as follows:
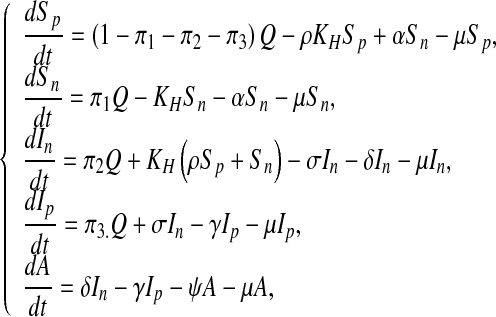 |
1 |
where
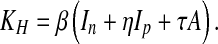 |
Analysis of the endemic equilibrium
Calculating the endemic equilibrium point, we obtain
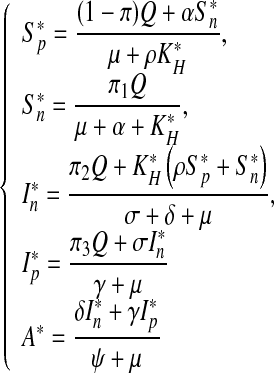 |
2 |
where
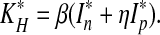 |
The endemic equilibrium satisfies the following polynomial,
 |
3 |
where
 |
4 |
We shall examine the endemic equilibrium polynomial (3) under three scenarios; (i) when there is a recruitment of productive susceptibles only (i.e., π1 = π2 = π3 = 0); (ii) when there is a recruitment of susceptibles only (i.e., 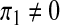 , π2 = π3 = 0) and (iii) when there is a recruitment of productive individuals only (π1 = π2 = 0,
, π2 = π3 = 0) and (iii) when there is a recruitment of productive individuals only (π1 = π2 = 0, 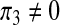 ).
).
The case of recruitment of productive susceptibles only (i.e., π1 = π2 = π3 = 0)
Setting π1 = π2 = π3 = 0, the DFE of the HIV/AIDS model (1) exists in the absence of the recruitment of infected individuals and is given by
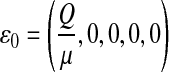 |
and the endemic equilibrium coefficients then become
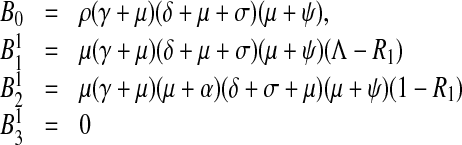 |
5 |
where
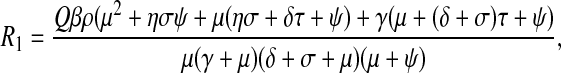 |
6 |
is the basic reproductive number under this scenario and
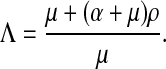 |
7 |
Λ > 1 if and only if
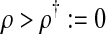 |
We obtain the following result:
Proposition 1
Proof
- If ρ < ρ
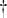 , then Λ < 1. In this case, we have
, then Λ < 1. In this case, we have
-
i.If R1 ≥ 1, then B2 ≤ 0, which implies that (3) has a unique positive solution.
-
ii.If
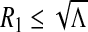 , then B1 ≥ 0 and B2 > 0. This implies that (3) has no positive solution.
, then B1 ≥ 0 and B2 > 0. This implies that (3) has no positive solution. -
iii.If
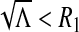 , we consider the discriminant of
, we consider the discriminant of 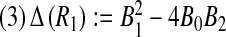 . One can see that
. One can see that 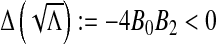 and
and 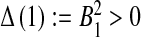 . Therefore, there exists
. Therefore, there exists 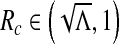 such that Δ (Rc) = 0 and Δ < 0 for
such that Δ (Rc) = 0 and Δ < 0 for 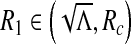 and Δ > 0 for R1 ∈ (Rc, 1). In this case, we have
and Δ > 0 for R1 ∈ (Rc, 1). In this case, we have
-
i.
Proposition 1 establishes the existence of two endemic equilibria for Λ in (Rc, 1).
The case of the recruitment of susceptibles only (i.e., 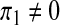 , π2 = π3 = 0)
, π2 = π3 = 0)
Setting 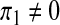 , π2 = π3 = 0, the DFE of the HIV/AIDS model (1) exists in the absence of the recruitment of infected individuals and is given by
, π2 = π3 = 0, the DFE of the HIV/AIDS model (1) exists in the absence of the recruitment of infected individuals and is given by
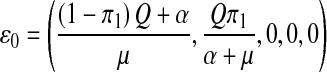 |
The endemic equilibrium coefficients become
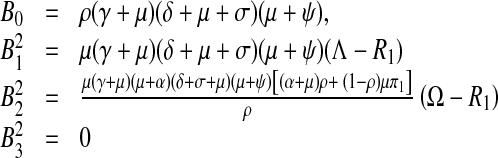 |
8 |
where
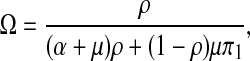 |
9 |
The proof is similar to (5) above if Ω = 1. But Ω = 1 if and only if
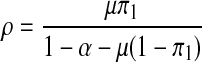 |
Hence, the model exhibits a backward bifurcation in the presence of the recruitment of non-productive susceptibles.
The case of the recruitment of productive individuals only (π1 = π2 = 0, 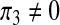 )
)
Setting π1 = π2 = 0, 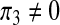 the coefficients of the endemic equilibrium (3) become,
the coefficients of the endemic equilibrium (3) become,
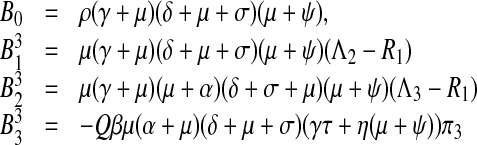 |
10 |
here
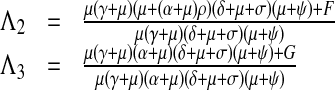 |
11 |
F = Qβρ(γ(μ(1 − τ) + ψ) + (1 − η)μ(μ + ψ) + δ(μτ − η(μ + ψ))) π3, G = Qβ((α + μ)ρ(μ(1 − τ) + ψ + (1 − η)μ(μ + ψ) + δ(μτ − η(μ + ψ))) − μ(δ + μ + σ)(γτ + η(μ + ψ))) π3.
By Routh–Hurwith criteria, the roots of (10) have negative real parts if and only if B0 > 0, 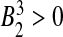 and
and 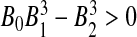 . It is clear from (10) that
. It is clear from (10) that 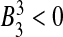 , so the disease free equilibrium is unstable.
, so the disease free equilibrium is unstable.
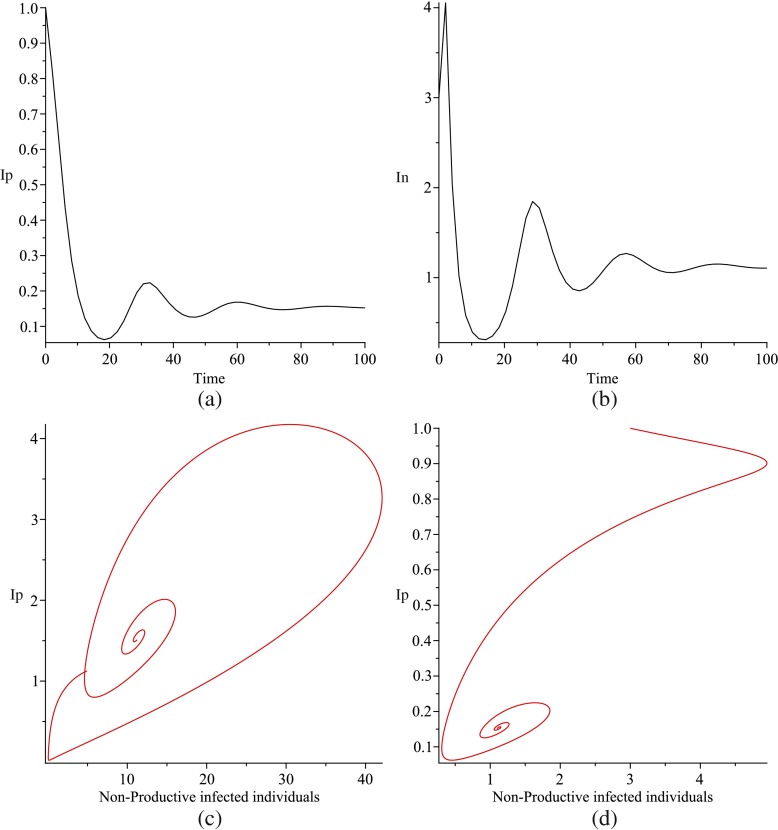
Numerically, we shall show that periodic solutions bifurcate from the endemic equilibrium, implying that the endemic equilibrium is unstable (Fig. 2).
Fig. 2.
Simulations of system (1) for Q = 100, μ = 0.04, π3 = 0.03, γ = 0.288, ρ = 0.2, σ = 0.04, δ = 0.8, α = 0.81. (a) is the solution of Ip versus time (days) and (b) is In versus time t, (c) is the projected In − Ip phase plane of the phase space and in (d) we use Q = 10 while all other parameters remain the same
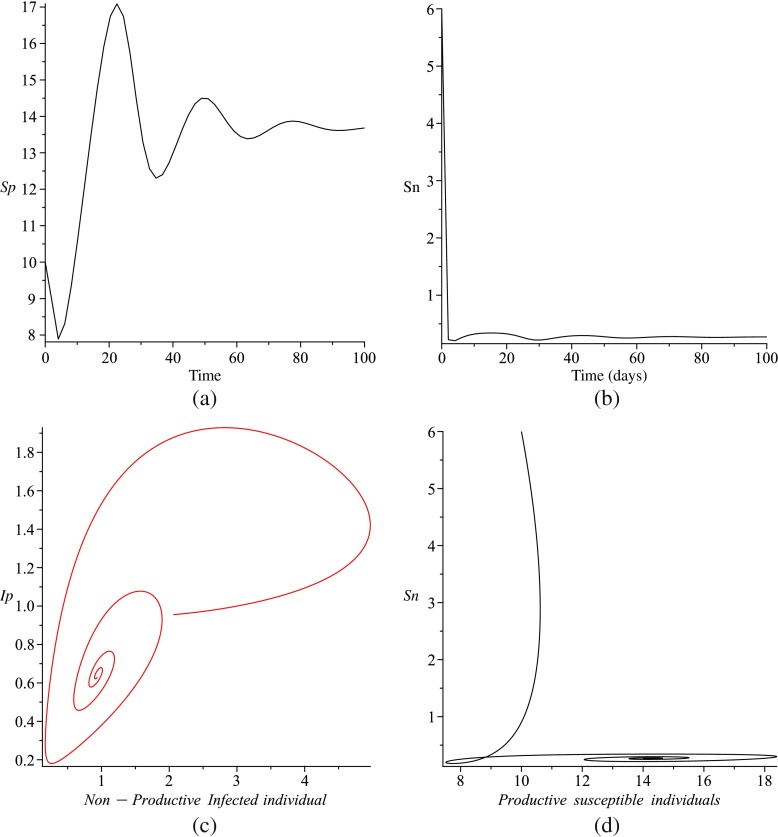
In Fig. 3c, the endemic equilibrium loses its stability and the periodic solutions bifurcate from the endemic equilibrium. This is an explicit example of the existence of a Hopf bifurcation using ρ as a bifurcation parameter and variation of initial values of state variables.
Fig. 3.
Simulations of system (1) for Q = 100, μ = 0.04, π3 = 0.03, γ = 0.288, ρ = 0.02, σ = 0.04, δ = 0.8, α = 0.81. (a) is the solution of Sp versus time (days) and (b) is Sn versus time t. (c) is the projected Ip − In phase plane space and (d) is the projected Sn − Sp phase plane of the phase space
Sensitivity analysis
Due to uncertainties associated with the estimation of certain parameter values, it is useful to investigate how sensitive the basic reproductive number is with respect to its parameters. This will also allow us to identify the parameters that cause the most reduction in R1 and therefore determine the control measure that is the most effective in controlling the disease. For this, we compute the normalized forward sensitivity index of the reproduction number with respect to these parameters. This index measures the relative change in a variable with respect to relative changes in its parameters (see [20] for the application of this index to a malaria transmission model).
Definition
The normalized forward sensitivity index of a variable, h, that depends differentiably on a parameter, l, is defined as:
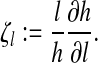 |
The sensitivity index of R1 with respect to β and Q is equal to 1. For the other parameters, we obtain
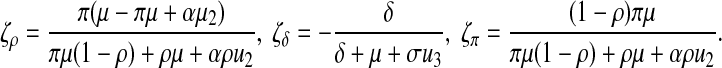 |
As μ, α, γ and σ have complex expressions, we find the sensitivity indices using the parameter values in Table 2 that are always constant (even though their expressions depend on u2, u3). Therefore, we calculate the sensitivity indices of π, μ, ρ, α, γ, η, σ, δ. Their values are given in Table 1.
Table 2.
Description of variables and parameters of the HIV/AIDS model (1)
| Parameter | Description | Value | Ref |
|---|---|---|---|
| β | Probability of susceptible non-productive contact with infectives | 0.344 | [12] |
| μ | Natural mortality unrelated to HIV/AIDS | 0.02 | [12] |
| ψ | AIDS-related death | 1 | [12] |
| ρ | Modification parameter due to productivity mindset | 0.02 | [12] |
| δ | Rate of progression into AIDS | 0.1 | [12] |
| σ | Proportion of infected non-productive workers on the HAART treatment | 0.002 | Assumed |
| π | Proportion of susceptible non-productive workers recruited | 0.002 | Assumed |
| α | Proportion of educated susceptible non-productive workers | 0.034 | Assumed |
| η | Modification parameter on infected productive class contact rate | 0.4 | Assumed |
| b1 | Cost per blood unit screened | $15.30 | [27] |
| b2 | Cost per person educated | $12.01, 47.13 | [26, 27] |
| b3 | Cost per unit condom | $1.09 | [27] |
| b4 | Cost of drug per person reached | $964.83 | [27] |
Table 1.
Sensitivity indices of R1
| Parameter | Parameter description | Sensitivity index |
|---|---|---|
| ρ | Modification parameter | +0.96 |
| δ | Progression to AIDS | −0.82 |
| μ | Mortality rate | −0.38 |
| π | Proportion of non-productive susceptibles recruited | +0.035 |
| u2 | Control effort through education | −0.0221 |
| α | Education rate | −0.02215 |
| u3 | Control effort on susceptibles | −0.0128 |
| σ | HAART treatment rate | −0.0128 |
| η | Modification parameter on the infected productive | +0.00362 |
| γ | Progression to full-blown AIDS from the infected productive | −0.00329 |
The interpretation of the sensitivity index values of R1 given in Table 1 is that an increase/decrease of 1% in any of the parameter values in the first column results in a percentage increase/decrease given by the corresponding value in the third column.
The table above implies that a decrease in the modification parameter due to productivity mindset ρ or an increase in the progression rate to AIDS δ has a positive impact in controlling HIV/AIDS in the community. The parameters are arranged from the most sensitive to the least sensitive, the most sensitive parameters are the transmission and recruitment rates β and Q. Since 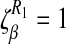 , increasing (or decreasing) the transmission probability rate β by 10% increases (or decreases) R1 by 10%. Similarly, increasing (or decreasing) the recruitment rate, Q, by 10% increases (or decreases) R1 by 10%. Increasing (or decreasing) the modification parameter rate ρ by 10% increases (or decreases) R1 by 9.6%. In the same way, increasing (or decreasing) the progression to AIDS rate δ by 10% decreases (or increases) R1 by 8.1%.
, increasing (or decreasing) the transmission probability rate β by 10% increases (or decreases) R1 by 10%. Similarly, increasing (or decreasing) the recruitment rate, Q, by 10% increases (or decreases) R1 by 10%. Increasing (or decreasing) the modification parameter rate ρ by 10% increases (or decreases) R1 by 9.6%. In the same way, increasing (or decreasing) the progression to AIDS rate δ by 10% decreases (or increases) R1 by 8.1%.
In the next section, we determine the necessary conditions for optimal control of the recruitment regime for effective productivity within the organization by using Pontryagin’s maximum principle.
Analysis of optimal control
We now incorporate time-dependent controls into the model (1) to obtain the following,
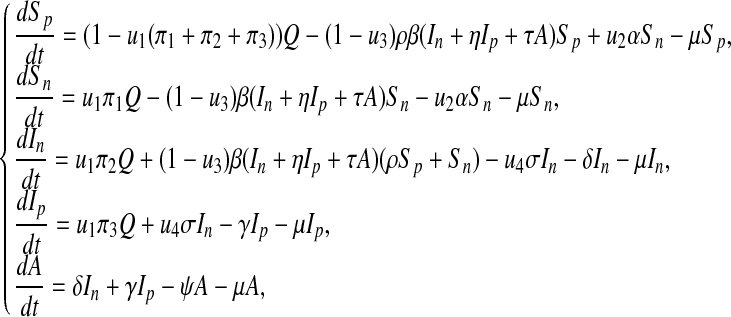 |
12 |
where u1, u2, u3, u4 are time-dependent controls. u1 is the control effort to minimize the recruitment of non-productive and infected individuals (e.g., screening, etc), u2 is the education/monitoring control on the non-productive susceptible in order to enhance its productivity, u3 is a preventative control measure on the susceptible from becoming infected with HIV/AIDS and u4 is the treatment strategy on the non-productive infected individuals.
We investigate the optimal level of effort that would be needed to control the disease and ensure productivity, which is to minimize the number of non-productive susceptibles and infectious individuals and the cost of applying the controls u1, u2, u3 and u4 over a finite time interval. We achieve this by defining the objective functional J by choosing a quadratic cost on the controls, which is similar to other literature on epidemic controls [3, 15, 17, 18, 21].
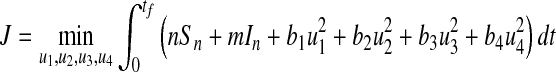 |
13 |
where n, m, b1, b2, b3, b4 are positive weights. The term 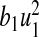 is the cost of control efforts on minimizing the recruitment of non-productive and infected individuals,
is the cost of control efforts on minimizing the recruitment of non-productive and infected individuals, 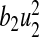 is the cost of control efforts on education/monitoring of non-productive susceptibles,
is the cost of control efforts on education/monitoring of non-productive susceptibles, 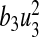 is the cost of control efforts on the preventive control on susceptibles and
is the cost of control efforts on the preventive control on susceptibles and 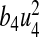 is the cost of control efforts on the treatment of non-productive infected individuals. With the given objective function J(u1, u2, u3, u4), our goal is to minimize the number of susceptible non-productive individuals Sn, while minimizing the cost of controls u1(t), u2(t), u3(t) and u4(t). We seek an optimal control quadruple
is the cost of control efforts on the treatment of non-productive infected individuals. With the given objective function J(u1, u2, u3, u4), our goal is to minimize the number of susceptible non-productive individuals Sn, while minimizing the cost of controls u1(t), u2(t), u3(t) and u4(t). We seek an optimal control quadruple  and
and 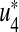 such that
such that
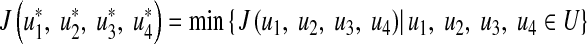 |
14 |
where U = {(u1, u2, u3, u4) such that u1, u2, u3, u4 is measurable with 0 ≤ ui ≤ 1 and i = 1, 2, 3, 4 for t ∈ [0, tf ]} is the control set. The necessary conditions that an optimal pair (u ∗ , x ∗ ), where 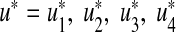 and
and 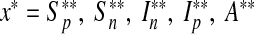 , must satisfy come from Pontryagin’s maximum principle [22]. This principle converts (12) and (13) into a problem of minimizing pointwise Hamiltonian H, with respect to u1, u2, u3 and u4
, must satisfy come from Pontryagin’s maximum principle [22]. This principle converts (12) and (13) into a problem of minimizing pointwise Hamiltonian H, with respect to u1, u2, u3 and u4
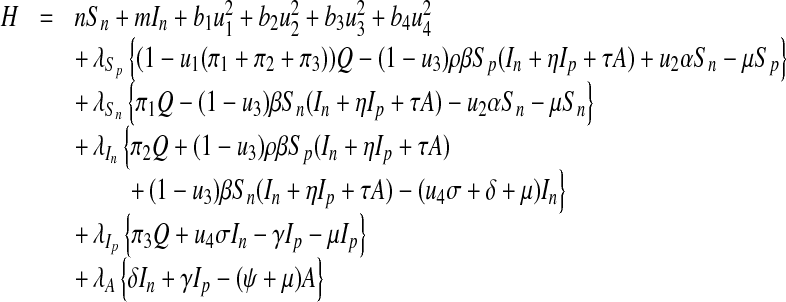 |
15 |
where 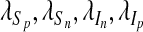 and λA are the adjoint variables or co-state variables. By applying Pontryagin’s maximum principle [22] and the existence result for the optimal control from [23], we obtain
and λA are the adjoint variables or co-state variables. By applying Pontryagin’s maximum principle [22] and the existence result for the optimal control from [23], we obtain
Proposition 2
For the optimal control quadruples 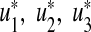 and
and 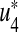 that minimize J(u1, u2, u3, u4) over U, then there exist adjoint variables
that minimize J(u1, u2, u3, u4) over U, then there exist adjoint variables 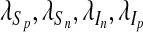 , λA satisfying
, λA satisfying
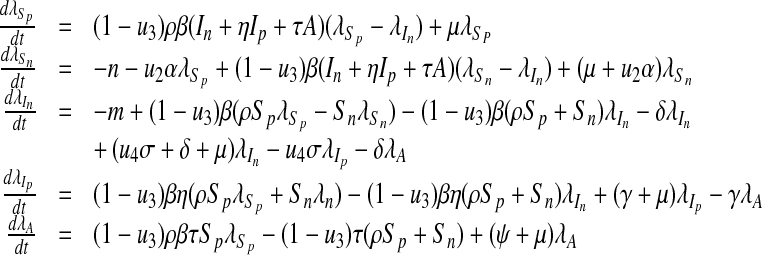 |
16 |
and with transversality conditions
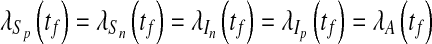 |
17 |
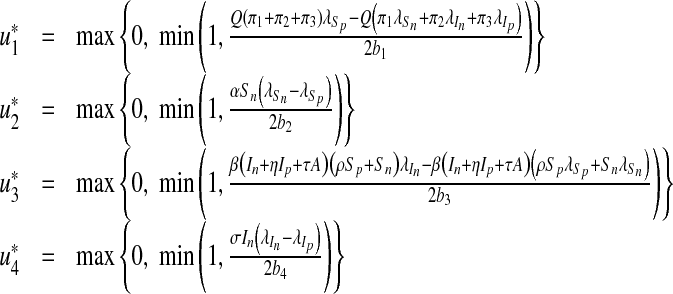 |
18 |
Proof
From Corollary 4.1, Fleming and Rishel [23] gave the existence of an optimal control due to the convexity of the integrand of J with respect to u1, u2, u3 and u4, a priori boundedness of the state solutions and the Lipschitz property of the state system with respect to the state variables [23]. The differential equations governing the adjoint variables are obtained by differentiation of the Hamiltonian function evaluated at the optimal control.
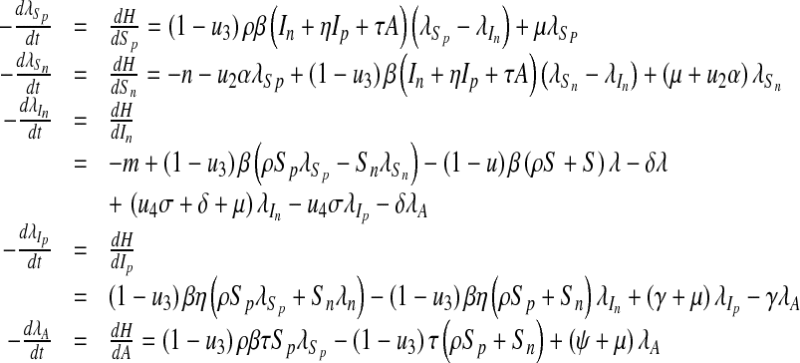 |
19 |
Furthermore, by equating to zero the derivatives of the Hamiltonian with respect to the controls we obtain (see Lenhart and Workman [24])
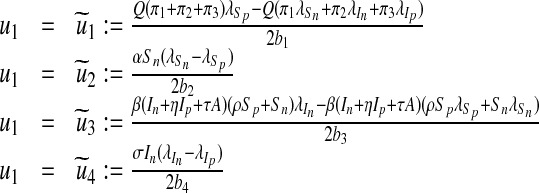 |
20 |
By standard control arguments involving the bounds on the controls, we conclude
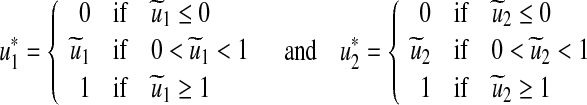 |
21 |
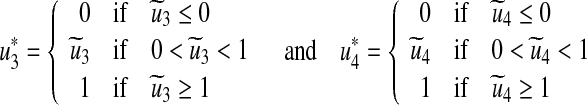 |
22 |
Due to the a priori boundedness of the state system, the adjoint system and the resulting Lipschitz structure of the ODEs, we obtain the uniqueness of the optimal control for small tf. The uniqueness of the optimal control follows from the uniqueness of the optimality system that consists in (16) and (17) with characterization (18). There is a restriction on the length of the time interval in order to guarantee the uniqueness of the optimality system.
This smallness restriction on the length of time is due to the opposite time orientations of (16) and (17); the state problem has initial values and the adjoint problem has final values. This restriction is very common in control problems [3, 17, 18, 21].□
Numerical results and discussion
In this section, we examine a deterministic model with non-productive susceptibles and we study numerically the effects of prevention, education, and the HAART treatment on the spread of HIV/AIDS. The optimal control is obtained by solving the optimality system, consisting in the state and adjoint systems. An iterative scheme is used for solving the optimality system. We start to solve the state equations with a guess for the controls over the simulated time using the fourth-order Runge–Kutta scheme. Because of transversality conditions (17), the adjoint equations are solved by a backward fourth-order Runge–Kutta scheme using the current iteration’s solutions of the state equations. Then the controls are updated by using a convex combination of the previous controls and the values from the characterizations (18). This process is repeated and iterations stopped if the values of the unknowns at the previous iterations are very close to the ones at the present iterations [24].
We explore a simple model with screening, prevention, education, and treatment as control measures to study the effects of control practices and transmission of HIV. We use various combinations of the four controls, three controls and two controls at a time. This is done under the following scenarios to compare numerical results.
Strategy A: Using screening (u1) and education (u2) without prevention (u3 = 0) and no treatment of infectives (u4 = 0)
Strategy B: Using screening (u1) and treatment of infectives (u4) without education (u2 = 0) and no prevention (u3 = 0)
Strategy C: Using screening (u1) and prevention (u3) without education (u2 = 0) and no treatment of infectives (u4 = 0)
Strategy D: Using education (u2) and prevention (u3) without screening (u1 = 0) and no treatment of infectives (u4 = 0)
Strategy E: Using education (u2) and treatment of infectives (u4) without screening (u1 = 0) and no prevention (u3 = 0)
Strategy F: Using prevention (u3) and treatment of infectives (u4) without screening (u1 = 0) and no education (u2 = 0)
Strategy G: Using screening (u1), education (u2) and prevention (u3) with no treatment of infectives (u4 = 0)
Strategy H: Using screening (u1), education (u2) and treatment of infectives (u4) with no prevention (u3 = 0)
Strategy I: Using screening (u1), prevention (u3) and treatment of infectives (u4) with no education (u2 = 0)
Strategy J: Using education (u2), prevention (u3) and treatment of infectives (u4) with no screening (u1 = 0)
Strategy K: Using all four control measures (u1, u2, u3 and u4)
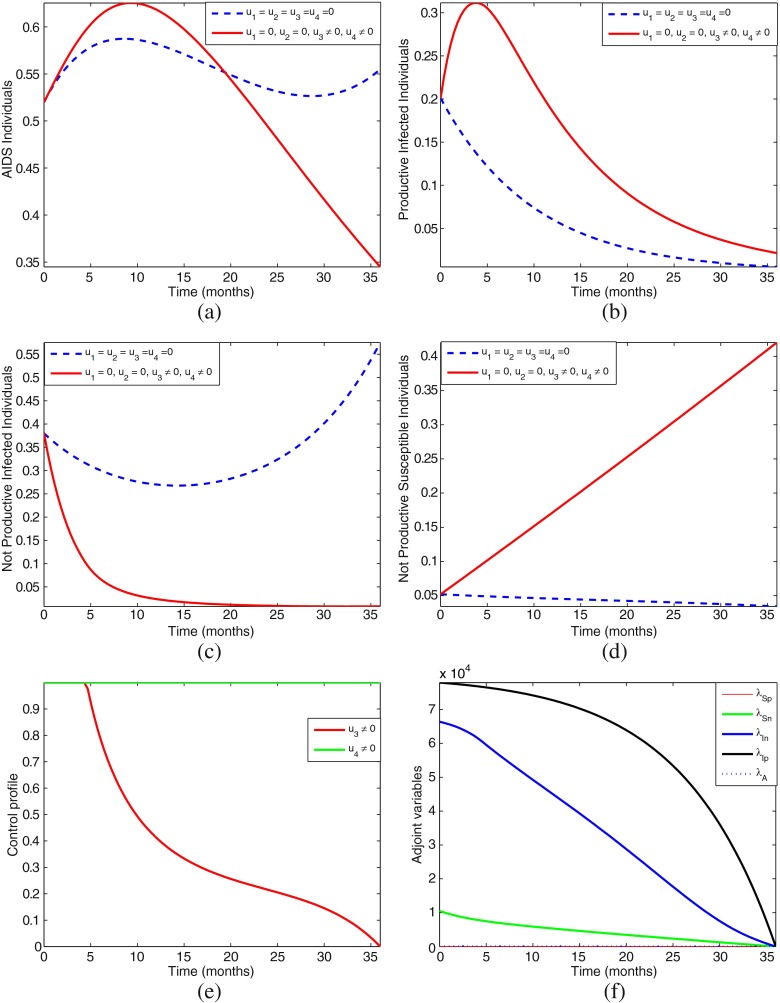
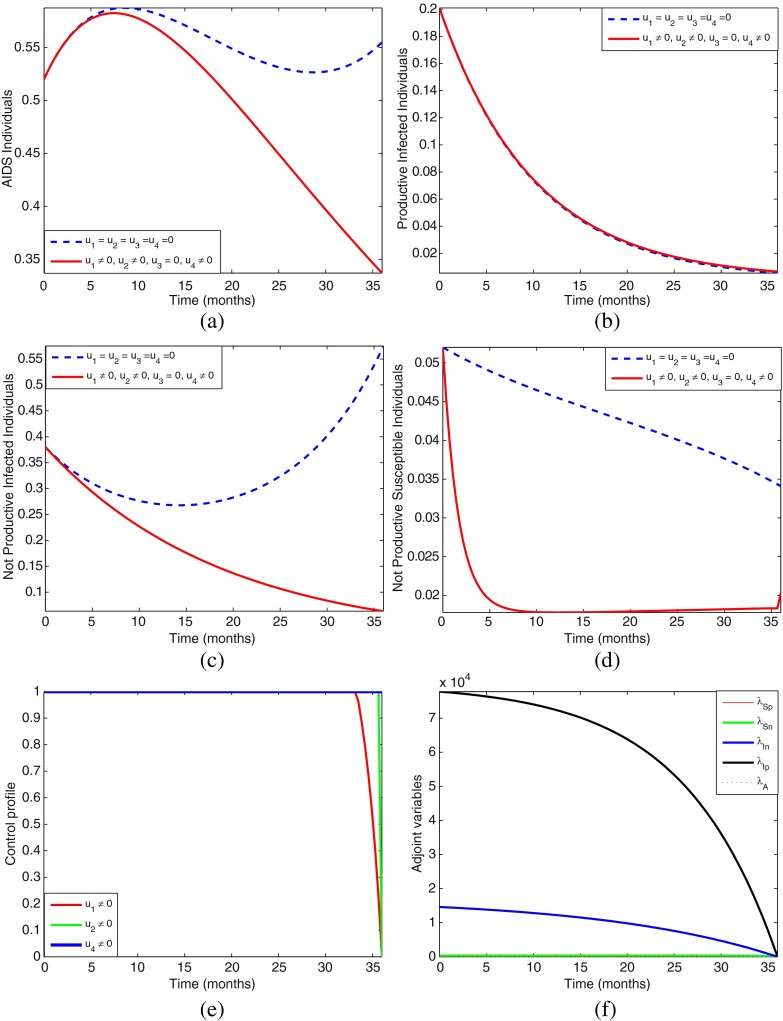
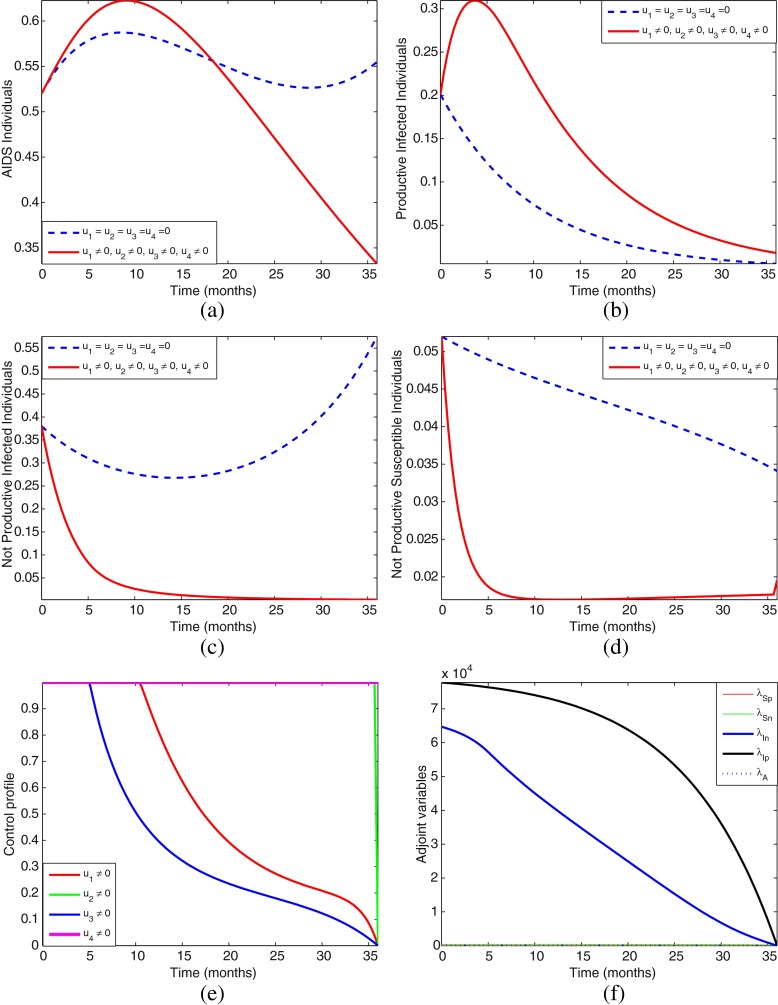
However, because of space, we shall only present numerical simulations for the four most effective strategies, that is, strategies D, F, H, and K.
Strategy D: Using education (u2) and prevention (u3) without screening (u1 = 0) and no treatment of infectives (u4 = 0)
Strategy F: Using prevention (u3) and treatment of infectives (u4) without screening (u1 = 0) and no education (u2 = 0)
Strategy H: Using screening (u1), education (u2) and treatment of infectives (u4) with no prevention (u3 = 0)
Strategy K: Using all four control measures (u1, u2, u3 and u4)
From the numerical simulations, Figs. 4, 5, 6, and 7, we can see that one cannot easily conclude which control strategy gives optimal results. Most of the strategies produce almost a similar pattern and effects, therefore we need to further ascertain which of these four most effective strategies is the most cost-effective and efficient. In the next section, we carry out the cost-effectiveness analysis.
Fig. 4.
Simulations of the model showing the effect of control strategy D on HIV/AIDS transmission. The control profile suggests control u2 to be at the upper bound for 36 months before dropping gradually to the lower bound, while control u3 to be at the upper bound for just 5 months before dropping gradually to the lower bound
Fig. 5.
Simulations of the model showing the effect of control strategy F on HIV/AIDS transmission. The control profile suggests control u3 be at the upper bound for just 4 months before dropping gradually to the lower bound and control u4 to be maintained at the upper bound throughout the intervention period
Fig. 6.
Simulations of the model showing the effect of control strategy H on HIV/AIDS transmission. The control profile suggests control u1 and u2 to be at the upper bound for 33 months and 36 months before dropping gradually to the lower bound, respectively, while control u4 to be maintained at the upper bound throughout the intervention period
Fig. 7.
Simulations of the model showing the effect of control strategy K on HIV/AIDS transmission. The control profile suggests control u1 and u2 to be at the upper bound for 11 months and 36 months before dropping gradually to the lower bound, respectively, while control u3 to be at the upper bound for just 5 months before dropping gradually to the lower bound and control u4 to be maintained at the upper bound throughout the intervention period
Cost-effectiveness analysis
To quantify the cost-effectiveness of the control measures, we examine the cost-effectiveness ratio of the strategies so that we can draw our conclusions. There are three types of cost-effectiveness ratios: (1) Average Cost-Effectiveness Ratio (ACER), which deals with a single intervention and evaluates that intervention against its baseline option (e.g., no intervention or current practice). It is calculated by dividing the net cost of the intervention by the total number of health outcomes prevented by the intervention. (2) Marginal Cost-Effectiveness Ratio (MCER) for the assessment of the specific changes in cost and effect when a program is expanded or contracted. (3) Incremental Cost-Effectiveness Ratio (ICER) used to compare the differences between the costs and health outcomes of two alternative intervention strategies that compete for the same resources and is generally described as the additional cost per additional health outcome (see [25]).
We assume that the costs of the controls are directly proportional to the number of controls deployed. This assumption is based on the understanding that the primary goal of providing preventive measures, education programs, and treatment of infective individuals is to reduce infection.
We calculated the total number of infections averted by estimating the difference between the total infectious individuals without control and the total infectious individuals with control. We used the parameter values in Table 2. The total cost in ($) USD is calculated by estimating the cost of controls 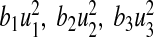 and
and 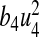 over time, respectively.
over time, respectively.
For the purpose of our study, we consider the incremental cost-effectiveness ratio (ICER). It allows us to compare the cost-effectiveness of the combination of at least two of the control strategies, screening, prevention, education, and treatment of infective individuals. In ICER, when comparing two competing intervention strategies incrementally, one intervention should be compared with the next-less-effective alternative. The ICER numerator includes the differences in intervention costs, averted disease costs, costs of prevented cases, and averted productivity losses if applicable. The ICER’s denominator is the difference in health outcomes (e.g., total number of infection averted, number of susceptibility cases prevented).
Based on the model simulation results, we rank the strategies in order of increasing effectiveness
| Strategies | Total infections averted | Total costs ($) | ICER |
|---|---|---|---|
| No strategy | 0 | 0 | – |
| Strategy H | 22.328 | $242070 | 10841.544 |
| Strategy F | 23.856 | $57468 | −120812.83 |
The ICER is calculated as follows:
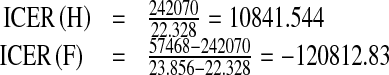 |
The comparison between strategies H and F shows a cost savings of $120812.83 for strategy F over strategy H. The negative ICER for strategy F indicates that strategy H is more costly and less effective than strategy F. Therefore, strategy H is excluded from the set of alternatives so it does not consume limited resources.
We recalculate the ICER
| Strategies | Total infections averted | Total costs ($) | ICER |
|---|---|---|---|
| Strategy F | 23.856 | $57468 | 2408.95 |
| Strategy K | 24.522 | $17339 | − 59804.77 |
The comparison between strategies F and K shows a cost savings of $59804.77 for strategy K over strategy F. Similarly, the high ICER for strategy F indicates that strategy F is more costly and less effective than strategy K. Therefore, strategy F is excluded from the set of alternatives so it does not consume limited resources.
We recalculate the ICER
| Strategies | Total infections averted | Total costs ($) | ICER |
|---|---|---|---|
| Strategy K | 24.527 | $17339 | 706.935 |
| Strategy D | 24.972 | $16266 | − 2411.236 |
The comparison between strategies K and D shows a cost savings of $2411.236 for strategy D over strategy K. Similarly, the high ICER for strategy K indicates that it is more costly and less effective than strategy D. Therefore, strategy K is excluded from the set of alternatives so it does not consume limited resources.
With this result, we conclude that strategy D (combination of education and preventive measures) has the least ICER and therefore is the more cost-effective strategy.
Conclusions
In this paper, we derived and analyzed a deterministic model for the transmission of the HIV/AIDS disease to examine the recruitment effect of the following, non-productive susceptible individuals, non-productive infected individuals, productive susceptible individuals, and productive infected individuals into an organizational workforce. We carried out a stability analysis of the equilibrium and the model was found to exhibit backward and Hopf bifurcations under different scenarios. Furthermore, we also performed an optimal control analysis of the model. Using Pontryagin’s maximum principle we derived and analyzed the conditions for optimal control of the disease with an effective HAART and ABC treatment regime and education of non-productive susceptible and infectious individuals. The results also suggest that the effective recruitment strategy, education/monitoring of employees to ensure productivity, has a significant impact in reducing the non-productivity of an infected employee (labor force) in the presence of HIV/AIDS. From the cost-effectiveness analysis, the results suggest that it is not cost-effective to screen applicants for HIV infection during recruitment and that the effective education/monitoring of employees and adherence to preventive measures against infection is the most cost-effective strategy to combat the spread of HIV/AIDS.
Contributor Information
Kazeem O. Okosun, Email: kazeemoare@gmail.com
Oluwole D. Makinde, Email: makinded@cput.ac.za
References
- 1.Coulibaly, I.: The Impact of the HIV/AIDS on the Labour Force in Sub-Saharan Africa: A Preliminary Assessment. International Labour Organisation. Website: www.ilo.org/aids (2003)
- 2.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-effectiveness of HIV monitoring strategies in resource-limited settings: a southern African analysis. Arch. Intern. Med. 2008;168(16):1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi HR. Optimal control of an HIV immunology model. Optim. Control Appl. Math. 2002;23:199–213. doi: 10.1002/oca.710. [DOI] [Google Scholar]
- 4.Okosun, K.O., Makinde, O.D., Abiodun, G.J.: Transmission dynamics of HIV/AIDS with optimal control in the presence of carefree susceptibles and treatment. BIOMAT: International Symposium on Mathematical and Computational Biology: 2011. A Chapter in BIOMAT book series, pp. 131–152. World Scientific Publishing Co. Pte. Ltd. (2012)
- 5.Bullert, B.: Effectively Managing Team Conflict. Global Knowledge Training LLC (www.globalknowledge.com) (2006)
- 6.Lori, B., John, S., Eleni, S.: The Economic Impact of AIDS in Ethiopia. The Future Group International, with collaboration with Research triangle Institute and The Centre for Development and Population Activities (1999)
- 7.Vass, J.: A Review of Labour Markets in South Africa: The Impact of HIV/AIDS on the Labour Market. Employment and Economis Policy Research Programme, Human Sciences Research Council (HSRC) (2005)
- 8.Anderson RM, Medly GF, May RM, Johnson AM. A preliminary study of the transmission dynamics of the human immunodeficiency virus (HIV), the causative agent of AIDS. IMA J. Math. Appl. Med. Biol. 1986;3:229–263. doi: 10.1093/imammb/3.4.229. [DOI] [PubMed] [Google Scholar]
- 9.Nikolaos IS, Dietz K, Schenzle D. Analysis of a model for the pathogenesis of AIDS. Math. Biosci. 1997;145:27–46. doi: 10.1016/S0025-5564(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 10.Christopher MK, Jorge XV. A simple vaccination model with multiple endemic states. Math. Biosci. 2000;164:183–201. doi: 10.1016/S0025-5564(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 11.Guihua L, Zhen J. Global stability of a SEIR epidemic model with infectious force in latent, infected and immune period. Chaos, Solutions Fractals. 2005;25:1177–1184. doi: 10.1016/j.chaos.2004.11.062. [DOI] [Google Scholar]
- 12.Agraj T, Ram N, Dileep S. Modelling the effect of screening of unaware infectives on the spread of HIV infection. Appl. Math. Comput. 2007;184:1053–1068. doi: 10.1016/j.amc.2006.07.007. [DOI] [Google Scholar]
- 13.Mukandavire Z, Gumel AB, Garira W, Tchuenche JM. Mathematical analysis of a model for HIV-malaria co-infection. Math. Biosci. Eng. 2010;6:333–362. doi: 10.3934/mbe.2009.6.333. [DOI] [PubMed] [Google Scholar]
- 14.Karrakchou, Rachik M, Gourari S. Optimal control and infectiology: application to an HIV/AIDS model. Appl. Math. Comput. 2006;177:807–818. doi: 10.1016/j.amc.2005.11.092. [DOI] [Google Scholar]
- 15.Adams BM, Banks HT, Kwon H, Tran HT. Dynamic multidrug therapies for HIV: optimal and STI control approaches. Math. Biosci. Eng. 2004;1(2):223–241. doi: 10.3934/mbe.2004.1.223. [DOI] [PubMed] [Google Scholar]
- 16.Gul Z, Yong HK, Il HJ. Stability analysis and optimal vaccination of an SIR epidemic model. BioSystems. 2008;93:240–249. doi: 10.1016/j.biosystems.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Felippe de Souza, J.A.M., Marco, A.L.C., Yoneyama, T.: Optimal control theory applied to the anti viral treatment of AIDS. In: Proceedings of the 39th Conference on Decision and Control (CDC’2000). Sydney, Australia (2000)
- 18.Lenhart SM, Yong J. Optimal control for degenerate parabolic equations with logistic growth. Nonlinear Anal. 1995;25:681–698. doi: 10.1016/0362-546X(94)00179-L. [DOI] [Google Scholar]
- 19.Magombedze G, Chiyaka C, Mukandavire Z. Optimal control of malaria chemotherapy. Nonlinear Anal. Modell. Contr. 2011;16(4):415–434. [Google Scholar]
- 20.Makinde OD, Okosun KO. Impact of chemotherapy on optimal control of malaria disease with infected immigrants. BioSystems. 2011;104(1):32–41. doi: 10.1016/j.biosystems.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Kirschner D, Lenhart S, Serbin S. Optimal control of the chemotherapy of HIV. J. Math. Biol. 1997;35:775–792. doi: 10.1007/s002850050076. [DOI] [PubMed] [Google Scholar]
- 22.Pontryagin LS, Boltyanskii VG, Gamkrelidze RV, Mishchenko EF. The Mathematical Theory of Optimal Processes. New York: Wiley; 1962. [Google Scholar]
- 23.Fleming WH, Rishel RW. Deterministic and Stochastic Optimal Control. New York: Springer; 1975. [Google Scholar]
- 24.Lenhart, S., Workman, J.T.: Optimal Control Applied to Biological Models. Chapman and Hall (2007)
- 25.Okosun KO, Ouifki R, Marcus N. Optimal control analysis of a malaria disease transmission model that includes treatment and vaccination with waning immunity. BioSystems. 2011;106:136–145. doi: 10.1016/j.biosystems.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Costing guidelines for HIV prevention strategies, UNAIDS (2000)
- 27.HIV/AIDS unit cost database, Future Institute, http://futuresinstitute.org/ (2010)