Abstract
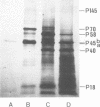
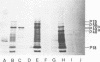
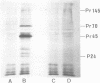
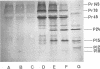
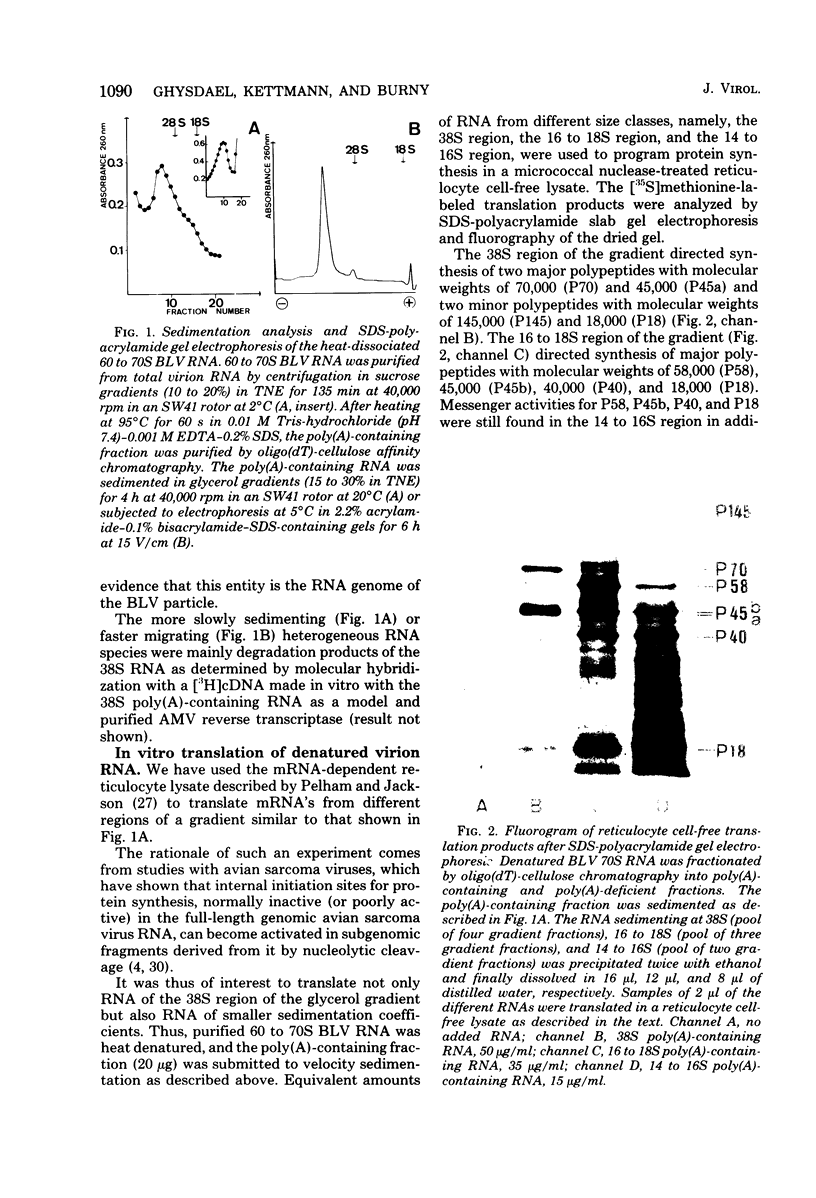
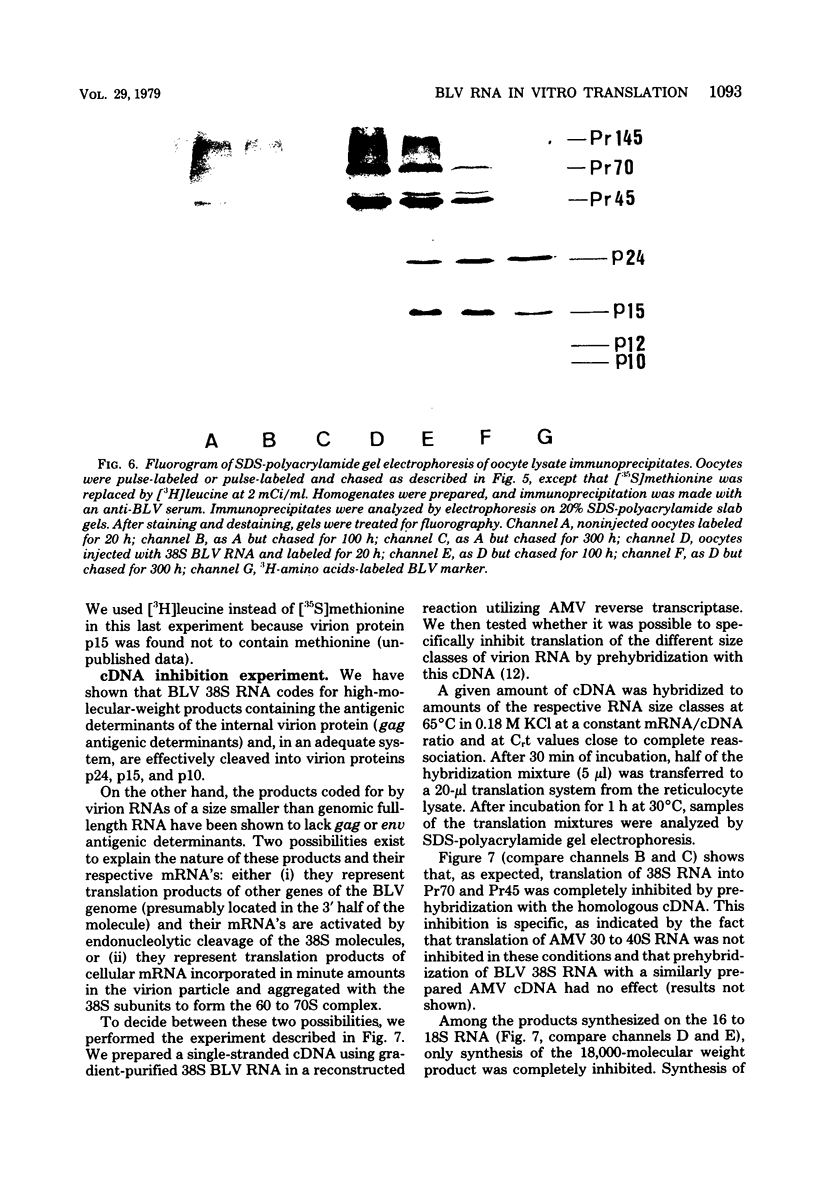
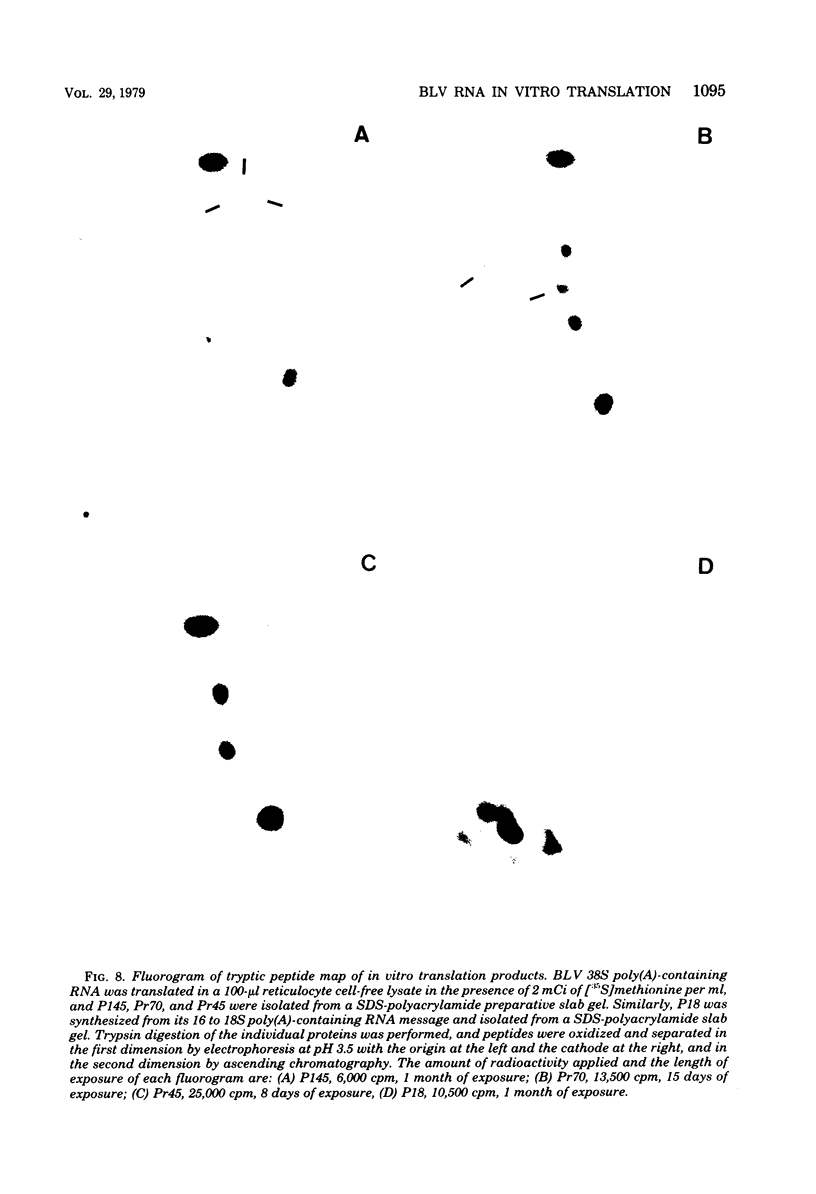
Bovine leukemia virus 60 to 70S RNA was heat denatured, the polyadenylic acid-containing species were separated by velocity sedimentation, and several size classes were translated in a micrococcal nuclease-treated cell-free system from rabbit reticulocytes. The major RNA species sedimented at 38S and migrated as a single component of molecular weight 2.95 x 10(6) when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The predominant polypeptides of the in vitro translation of bovine leukemia virus 38S RNA were products with molecular weights of 70,000 and 45,000; minor components with molecular weights of 145,000 and 18,000 were also observed. Two lines of evidence indicate that the 70,000- and 45,000-molecular weight polypeptides represent translation products of the gag gene of the bovine leukemia virus genome (Pr70gag and Pr45gag). First, they are specifically precipitated by a monospecific antiserum to the major internal protein, p24, and second, they are synthesized and correctly processed into virion proteins p24, p15, and p10 in Xenopus laevis oocytes microinjected with bovine leukemia virus 38S RNA. The 145,000-molecular weight polypeptide was immunoprecipitated by the anti-p24 serum and not by an antiserum to the major envelope glycoprotein, gp60. It contained all the tryptic peptides of Pr70gag and additional peptides unique to it, and thus represents in elongation product of Pr70gag in an adjacent gene, presumably the pol gene. The 18,000-molecular weight product was antigenically unrelated to p24 and gp60 and shared no peptides in common with Pr70gag, Pr45gag, or the 145,000-molecular weight polypeptide. It was maximally synthesized on a polyadenylic acid-containing virion 16 to 18S RNA, and we present evidence that this RNA is a 3' end-derived subgenomic fragment of the bovine leukemia virus genome rather than a contaminating cellular RNA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Robbins K. C. Rat sequences of the Kirsten and Harvey murine sarcoma virus genomes: nature, origin, and expression in rat tumor RNA. J Virol. 1976 Feb;17(2):335–351. doi: 10.1128/jvi.17.2.335-351.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burny A., Bex F., Chantrenne H., Cleuter Y., Dekegel D., Ghysdael J., Kettmann R., Leclercq M., Leunen J., Mammerickx M. Bovine leukemia virus involvement in enzootic bovine leukosis. Adv Cancer Res. 1978;28:251–311. doi: 10.1016/s0065-230x(08)60649-1. [DOI] [PubMed] [Google Scholar]
- Deshayes L., Levy D., Parodi A. L., Levy J. P. Proteins of bovine leukemia virus. I. Characterization and reactions with natural antibodies. J Virol. 1977 Mar;21(3):1056–1060. doi: 10.1128/jvi.21.3.1056-1060.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Stephenson J. R. Biochemical and immunological characterization of the major envelope glycoprotein of bovine leukemia virus. J Virol. 1977 Aug;23(2):443–447. doi: 10.1128/jvi.23.2.443-447.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Cleuter Y. Biosynthesis of bovine leukemia virus (BLV) major internal protein (p24) in infected cells and X. laevis oocytes microinjected with BLV 60-70S RNA [proceedings]. Arch Int Physiol Biochim. 1977 Dec;85(5):978–979. doi: 10.3109/13813457709053315. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Trávnícek M., Bolognesi D. P., Burny A., Cleuter Y., Huez G., Kettmann R., Marbaix G., Portetelle D. Frog oocytes synthesize and completely process the precursor polypeptide to virion structural proteins after microinjection of avian myeloblastosis virus RNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3230–3234. doi: 10.1073/pnas.74.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden R. V., Long C. W., Hanson M., Toni R., Charman H. P., Oroszlan S., Miller J. M., Van der Maaten M. J. Characteristics of the major internal protein and RNA-dependent DNA polymerase of bovine leukaemia virus. J Gen Virol. 1975 Dec;29(3):305–314. doi: 10.1099/0022-1317-29-3-305. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A. Analysis of mRNA populations by cDNA.mRNA hybrid-mediated inhibition of cell-free protein synthesis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1217–1221. doi: 10.1073/pnas.75.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kaaden O. R., Frenzel B., Dietzschold B., Weiland F., Mussgay M. Isolation of a p15 polypeptide from bovine leukemia virus and detection of specific antibodies in leukemic cattle. Virology. 1977 Apr;77(2):501–509. doi: 10.1016/0042-6822(77)90475-5. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Rohde W., Pauli G., Paulsen J., Harms E., Bauer H. Bovine and ovine leukemia viruses. I. Characterization of viral antigens. J Virol. 1978 Apr;26(1):159–164. doi: 10.1128/jvi.26.1.159-164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Maryak J., Vass W., Goldberg R. J., Parks W. P. Type C particle-positive and type C particle-negative rat cell lines: characterization of the coding capacity of endogenous sarcoma virus-specific RNA. J Virol. 1976 Dec;20(3):570–582. doi: 10.1128/jvi.20.3.570-582.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Hu S. S. The genetic structure of RNA tumor viruses. Annu Rev Genet. 1977;11:203–238. doi: 10.1146/annurev.ge.11.120177.001223. [DOI] [PubMed] [Google Scholar]